Introduction
Numerous nuclear and cytoplasmic proteins have been
found modified with O-β-N-acetylglucosamine (O-GlcNAc) at the
hydroxyl moiety of serine or threonine residues. This moiety is
dynamically added and removed by the O-GlcNAc transferase (OGT) and
the O-GlcNAcase (OGA) enzymes, respectively (1). UDP-GlcNAc is the donor substrate of
OGT, and is biosynthesized through the hexosamine pathway (HBP).
The HBP flux is highly dependent on glucose and glutamine, with
~3–5% of total glucose entering this pathway (2). O-GlcNAc is a sensor of intracellular
glucose metabolism, since the intracellular level of UDP-GlcNAc is
the main regulatory factor for the activity of OGT (3). O-GlcNAcylation is altered in
metabolism-associated diseases, such as type II diabetes (4–6),
Alzheimer’s disease (7) and cancer
(8). Therefore, abnormal
O-GlcNAcylation may play critical roles in these pathological
processes.
Aberrant metabolism is a hallmark of cancer. Most
cancer cells show increased rates of glucose and glutamine
utilization, up to 200-fold higher than those observed in the
healthy cells they originate from, and predominantly produce energy
through glycolysis followed by lactic acid fermentation (9,10).
As a glycolytic pathway, HBP can enhance the level of UDP-GlcNAc,
and thus, the activity of OGT, which may lead to an increase in the
global O-GlcNAc level (summed over all proteins) in cancer cells.
We have previously demonstrated that the global O-GlcNAc level is
increased in breast, lung and colon cancer tissues as compared to
respective adjacent tissues (11,12).
In addition, other research groups have shown that O-GlcNAcylation
is increased in breast, prostate and pancreatic cancer cell lines
(13–15). However, whether increased
O-GlcNAcylation is universal in cancer is unknown.
In this study, we examined the O-GlcNAc level in
prostate, pancreatic and liver cancer tissues, and the results
indicated that global O-GlcNAcylation is specifically increased in
prostate cancer tissues compared to corresponding adjacent tissues.
Furthermore, we investigated the roles of O-GlcNAc in prostate
cancer cell proliferation and invasion, along with the underlying
mechanism.
Materials and methods
Specimens and ethics
Liver, pancreatic and prostate cancer tissue
microarrays (TMAs; OD-CT-DgLiv03-002, OD-CT-DgPan03-002, and
OD-CT-UrPrt03-001 respectively) were purchased from Shanghai Outdo
Biotech Co. (Shanghai, China). The liver cancer tissue TMA was
constructed with 31 formalin-fixed, paraffin-embedded
hepatocellular carcinoma tissues and their corresponding adjacent
liver tissues, the pancreatic cancer tissue TMA was constructed
with 31 formalin-fixed, paraffin-embedded pancreatic ductal
adenocarcinoma tissues and their corresponding adjacent pancreatic
tissues, and the prostate cancer tissue TMA was constructed with 29
formalin-fixed, paraffin-embedded prostate cancer tissues and their
corresponding adjacent benign prostatic hyperplasia (BPH) tissues.
In addition, 26 prostate cancer tissues and 19 BPH tissues, which
were fixed with formalin and embedded with paraffin, were obtained
from the Department of Urology Surgery, Qingdao Municipal Hospital
(QMH). The tissues were cut into 6-μm-thick sections and mounted on
glass slides. Written informed consent was acquired from all
patients and/or guardians for the use of their tissue samples. The
present study was reviewed and approved by the Research Ethics
Committee of Qingdao Municipal Hospital (Qingdao, China).
Immunohistochemistry
Immunohistochemistry was performed on the specimens
using the DAKO Liquid DAB Substrate Chromogen system (Dako North
America, Inc., Carpinteria, CA, USA) and a monoclonal mouse
antibody targeting O-GlcNAc (RL2; Affinity BioReagents, Inc.,
Golden, CO, USA), used at a 1:200 dilution. The Fromowitz standard
was used to semi-quantitatively assess the staining intensity of
O-GlcNAc (16,17).
Cell cultures
The human BPH-1 line is representative of benign
prostatic hyperplasia, while the PC3 and DU145 prostate cancer cell
lines are representative of the earlier type-I ADI prostate
cancers. All cell lines were maintained in RPMI-1640 medium (Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; Corning Cellgro, Manassas, VA, USA). To increase the O-GlcNAc
level, cells were treated for 24 h or the indicated time period
with 10 μM thiamet-G, a selective OGA inhibitor, synthesized as
previously described (18).
Immunoblotting (IB)
Cells were lysed in lysis buffer [50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% NP40, 1 mM EDTA, 1 mM
Na3VO4, 10 mM NaF] containing a protease
inhibitor cocktail (Roche Applied Science, Mannheim, Germany), 50
mM GlcNAc and 5 μM PUGNAc (both from Toronto Research Chemicals
Inc., North York, ON, Canada). Protein samples (30 μg) were
separated by 7.5% dodecyl sulfate (SDS)-polyacrylamide gel
electrophoresis (PAGE) and transferred to Immobilon-P membranes
(Millipore, Billerica, MA, USA). Antibodies targeting O-GlcNAc
(CTD110.6; Abcam, Cambridge, UK), glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), OGT (F-12) and OGA (all from Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), were used along with the
Amersham ECL Prime (GE Healthcare, Buckinghamshire, UK).
Soft agar assay
The soft agar assay was performed as described in
(19). Briefly, cells
(1×104) were suspended in 1 ml top agar medium
(RPMI-1640 medium supplemented with 10% FBS in 0.4% agar). The cell
suspension was then overlaid onto 1.5 ml bottom agar medium (the
corresponding medium in 0.8% agar) in 6-well tissue culture plates
in triplicate. Fresh medium was added to the plates every 3 days as
a feeder layer. On the 5th day, images were acquired from 6 random
fields of the colonies at ×40 magnification.
Cell invasion and migration assay
The cell invasion and migration assays were
performed as described in (19).
Transwell chambers (6.5 mm; Corning, New York, NY, USA) with 8
μm-pore membranes were used for the cell migration assay. The lower
chamber was filled with RPMI-1640 medium (supplemented with 20%
FBS) and thiamet-G, except for control samples. Cells
(5×104) were suspended in 100 μl upper medium (RPMI-1640
medium with 1% FBS) and placed into the upper chamber with or
without thiamet-G. After 16 h, the number of cells detected by
crystal violet staining on the undersurface of the polycarbonate
membranes was visually scored in five random fields at ×100
magnification using a light microscope.
The invasion assay was performed using the same
procedure as the migration assay, except that the upper surface of
the membrane was covered with 70 μl of 1 mg/ml Matrigel (BD
Biosciences, San José, CA, USA) and the incubation time was
prolonged to 24 h.
RNA isolation and quantitative reverse
transcription (RT)-PCR
Total RNA was isolated from the cells using an RNA
isolation kit (BioTeke Corporation, Beijing, China), and reverse
transcription was carried out using the M-MLV reverse transcriptase
(Takara Biotechnology Co., Ltd., Dalian, China). Quantitative PCR
assays on the resulting cDNA products were carried out in
triplicate using the SYBR® Green PCR Master mix (Applied
Biosystems/Life Technologies, Carlsbad, CA, USA) on a 7500
Real-time PCR system (Applied Biosystems/Life Technologies). The
levels of the tested genes were normalized to that of the
endogenous control gene GAPDH. The primers for amplification
used were the following: human E-cadherin forward, 5′-GCC ACC CTG
GCT TTG ACG-3′, and reverse, 5′-CCA TCT GTG CCC ACT TTG AAT C-3′;
human GAPDH forward, 5′-CAG GGC TGC TTT TAA CTC TGG T-3′, and
reverse 5′-CCT GGA AGA TGG TGA TGG GAT-3′.
Extraction of cytoskeleton-binding
proteins
To separate cytoskeleton-binding proteins from other
cell constituents, BPH-1 and DU145 cells were washed twice with
phosphate-buffered saline, lysed on ice for 15 min in Triton X-100
lysis buffer [300 mM sucrose, 10 mM PIPES (pH 6.8), 50 mM NaCl, 3
mM MgCl2, 0.5% Triton X-100, 0.1 mg/ml DNase, 0.1 mg/ml
RNase, 1.2 mM phenylmethanesulfonyl fluoride and protease inhibitor
mix cocktail], passed through a 26 gauge needle four times, then
subjected to centrifugation at 48,000 × g for 10 min at 4°C. The
pellet was solubilized in loading buffer containing 2% SDS, and the
supernatant and pellet fractions were loaded onto separate gels and
processed as previously described (20).
Statistical analysis
Data were analyzed by Student’s t-tests using the
SPSS 11.0 software (IBM, Armonk, NY, USA). P<0.05 was considered
to indicate statistically significant differences. Data were
expressed as the mean ± SD.
Results
The O-GlcNAc is increased in prostate
cancer tissues
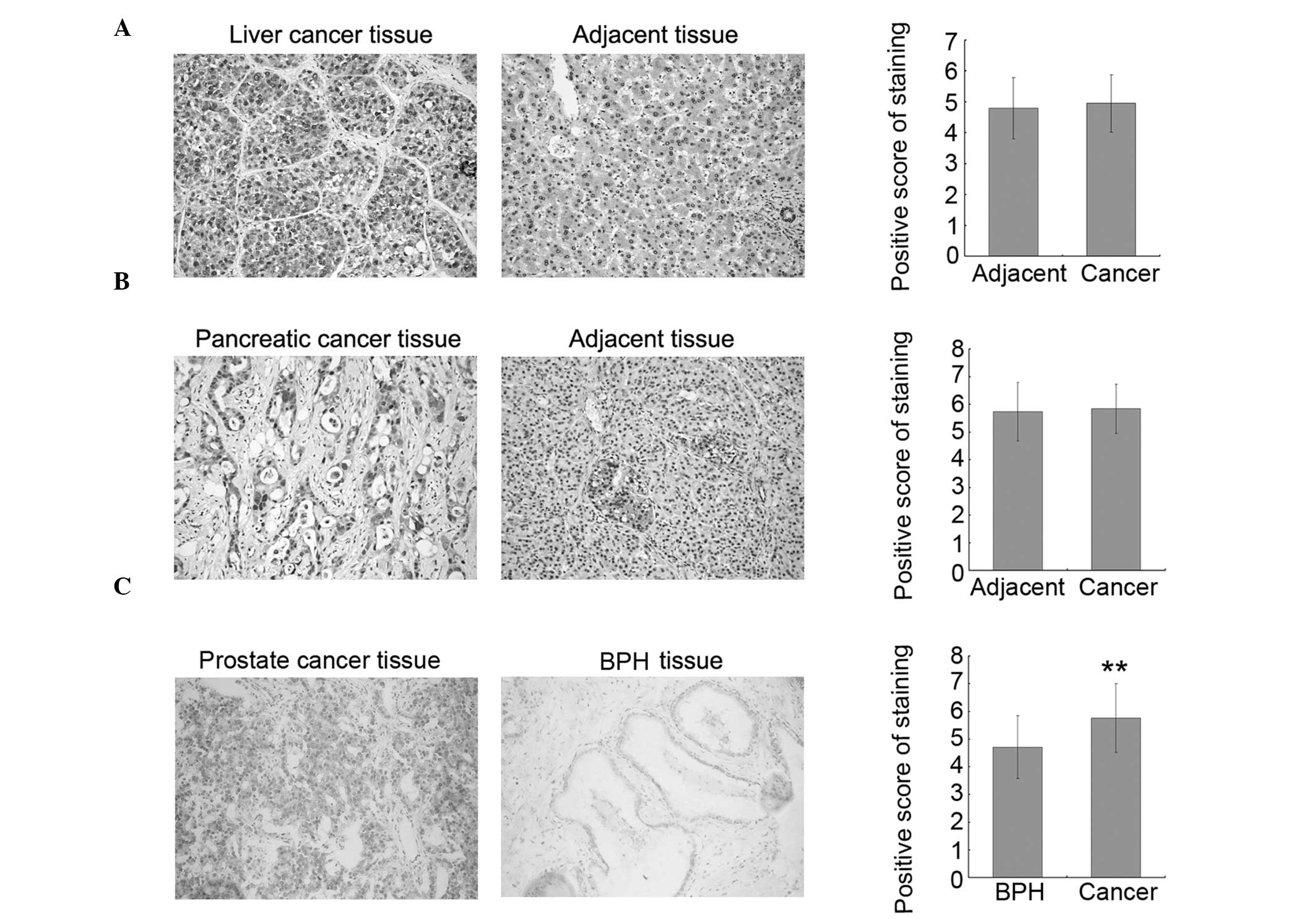
To investigate whether increased O-GlcNAcylation is
universal in cancer, TMAs were used to detect the O-GlcNAc level in
31 hepatocellular carcinoma tissues (liver cancer), 31 pancreatic
ductal adenocarcinoma tissues (pancreatic cancer), 29 prostate
cancer tissues and their corresponding adjacent BPH tissues by
immunohistochemistry, using an antibody targeting O-GlcNAc (RL2).
Representative stained tissue sections are shown in the left and
middle panels of Fig. 1. The
intensity of O-GlcNAc immunostaining was analyzed by the Fromowitz
standard. The results indicated that O-GlcNAcylation is increased
in prostate cancer tissues as compared to the adjacent tissues;
however, there was no significant difference between the
liver/pancreatic cancer tissues and their corresponding adjacent
tissues. To confirm the results observed in the prostate cancer
tissues, 26 additional prostate cancer tissues and 19 BPH tissues
were collected and assayed for O-GlcNAc by immunohistochemistry. In
accordance with the results from the TMA samples, the level of
O-GlcNAc was increased in prostate cancer tissues as compared to
BPH tissues, and statistical analysis on the combined dataset from
TMAs and collected tissues showed that this increase is significant
(Fig. 1C, right panel). These
results suggested that O-GlcNAc may be a good molecular marker of
prostate cancer and may play important roles in prostate cancer
progression.
O-GlcNAc and OGT levels are increased in
prostate cancer cells
To investigate whether O-GlcNAcylation is increased
in all types of prostate cancer compared to BPH cells, we detected
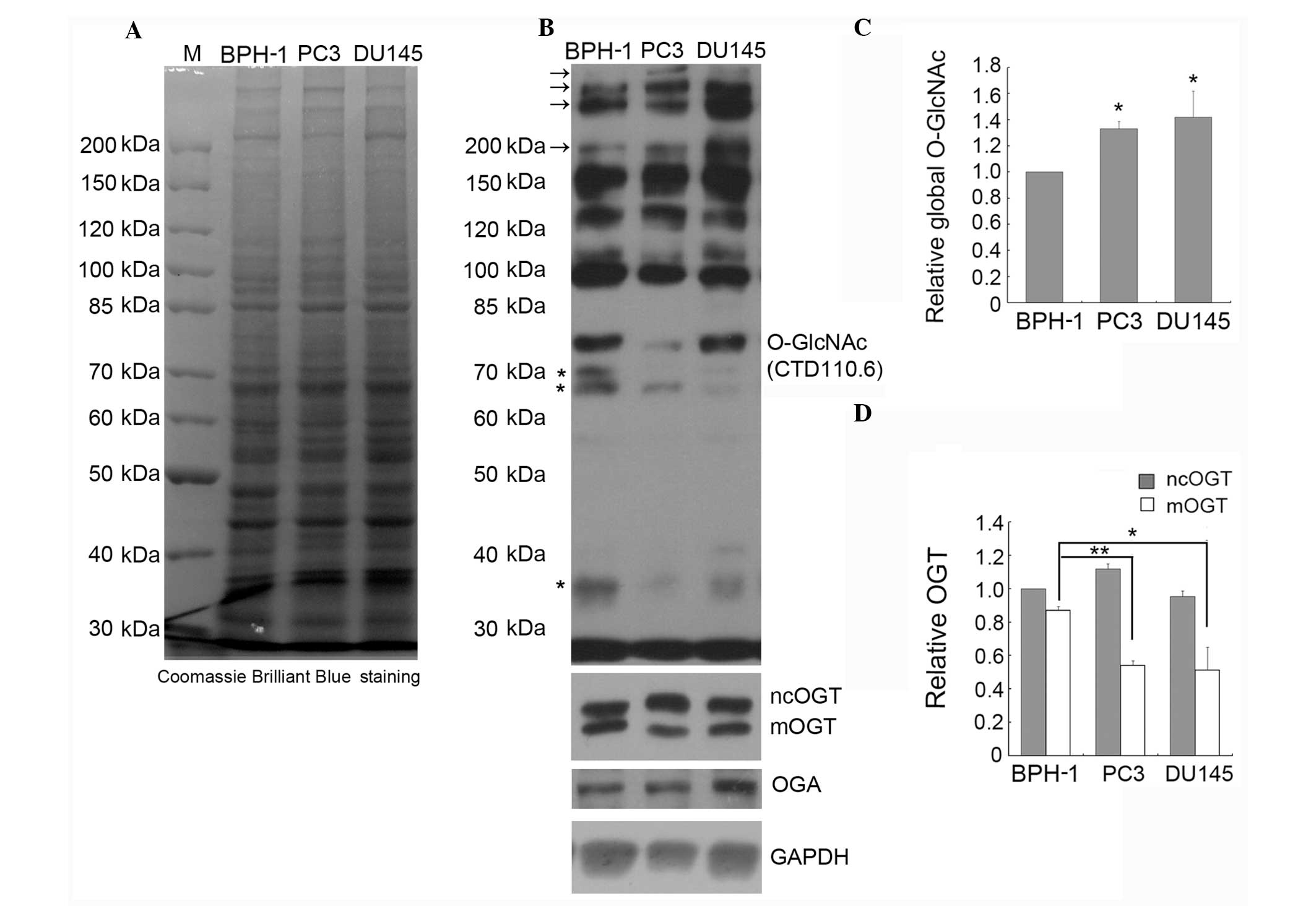
the global O-GlcNAc level in PC3, DU145 and BPH-1 cells by IB using
the CTD110.6 antibody. Comparison of the global protein expression
patterns in BPH-1, PC3 and DU145 cells by SDS-PAGE indicated that
there is no obvious difference between these three cell lines
(Fig. 2A). However, as shown in
Fig. 2B and C, where immunoblots
were treated and analyzed by ECL, the global O-GlcNAc level was
significantly increased in the PC3 and DU145 compared to the BPH-1
cells, especially the staining intensity of the high molecular
weight bands (marked with arrows, Fig.
2B); however, the intensity of the lower molecular weight bands
(marked with stars, Fig. 2B) was
weaker in the prostate cancer cell lines compared to the BPH-1
line.
Although there is only one OGT-encoding gene in
mammalian genomes, alternative promoter usage and/or alternative
splicing produce three OGT isoforms, which differ in the length of
their amino-terminal tetratricopeptide repeats (TPRs) (21). The nucleocytoplasmic OGT (ncOGT)
contains 13 TPRs, the mitochondrial OGT (mOGT) contains a
mitochondrial-targeting sequence at its N-terminus and nine TPRs,
and the shortest OGT isoform (sOGT) contains only two TPRs.
Previous studies have indicated that the expression of OGT is
increased in certain cancer types, but the OGT isoforms were not
discriminated in these reports (11,12,14,22).
Here, we detected by IB the expression of OGT using specific
antibodies that recognize all three OGT isoforms, and the results
showed that ncOGT and mOGT, but not sOGT, are detectable in all
tested cell lines (Fig. 2B). The
expression of ncOGT was not significantly different between the
prostate cancer and BPH cell lines, but the expression of mOGT was
markedly decreased in prostate cancer cells (Fig. 2B and D). To our knowledge, this is
the first report on decreased expression of the pro-apoptotic
protein mOGT (21,23) in cancer cells.
We also examined the expression of OGA in PC3, DU145
and BPH-1 cells, and the results indicated that the expression of
OGA is increased in DU145 cells (Fig.
2B). Considering that the level of O-GlcNAc in DU145 cells was
found increased, the increased expression of OGA may be due to
feedback regulation.
O-GlcNAc enhances the
anchorage-independent growth of BPH-1 and prostate cancer
cells
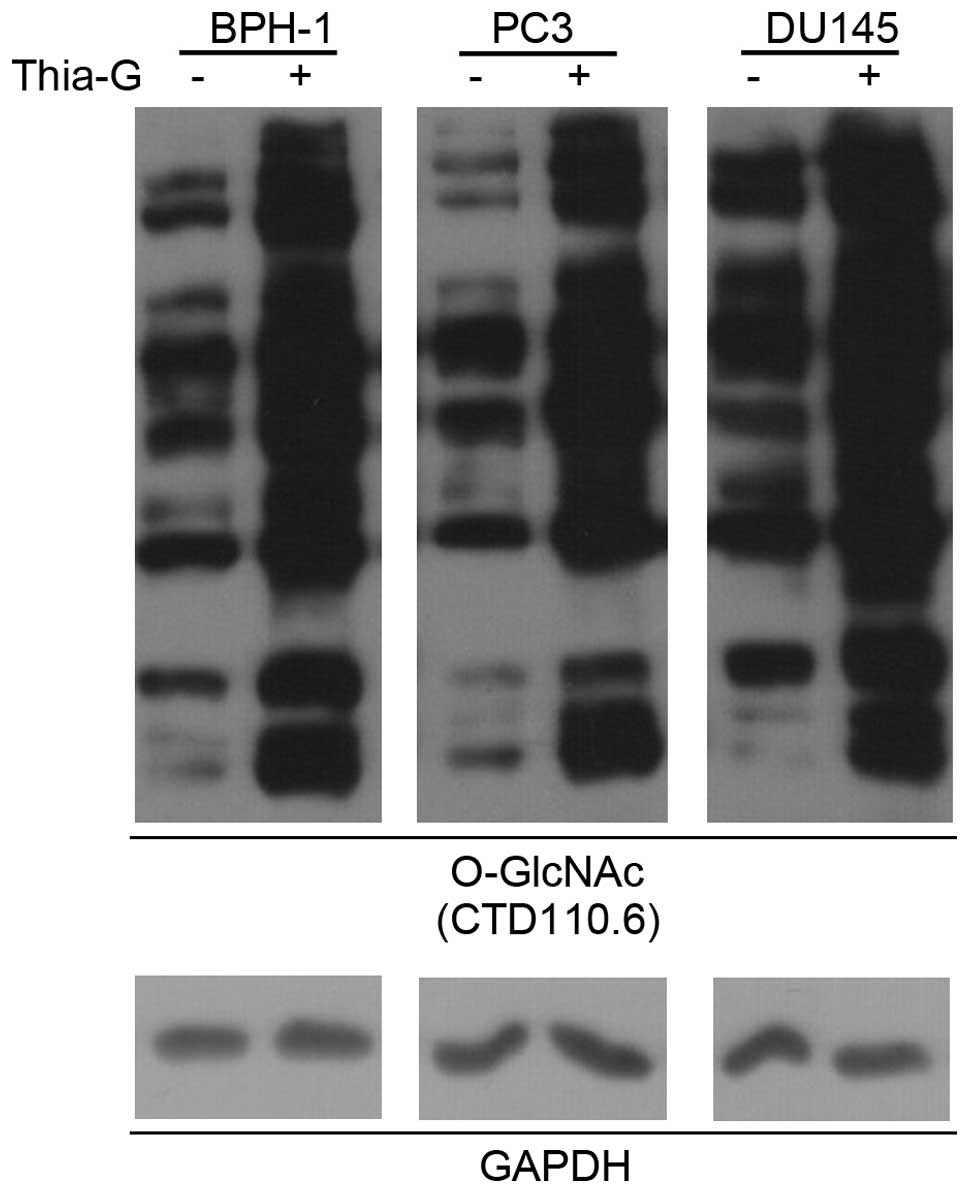
To determine whether O-GlcNAc is involved in
prostate cancer malignancy, a highly potent and selective
O-GlcNAcase inhibitor (thiamet-G) was used to increase the O-GlcNAc
level in BPH-1, PC3 and DU145 cells (Fig. 3).
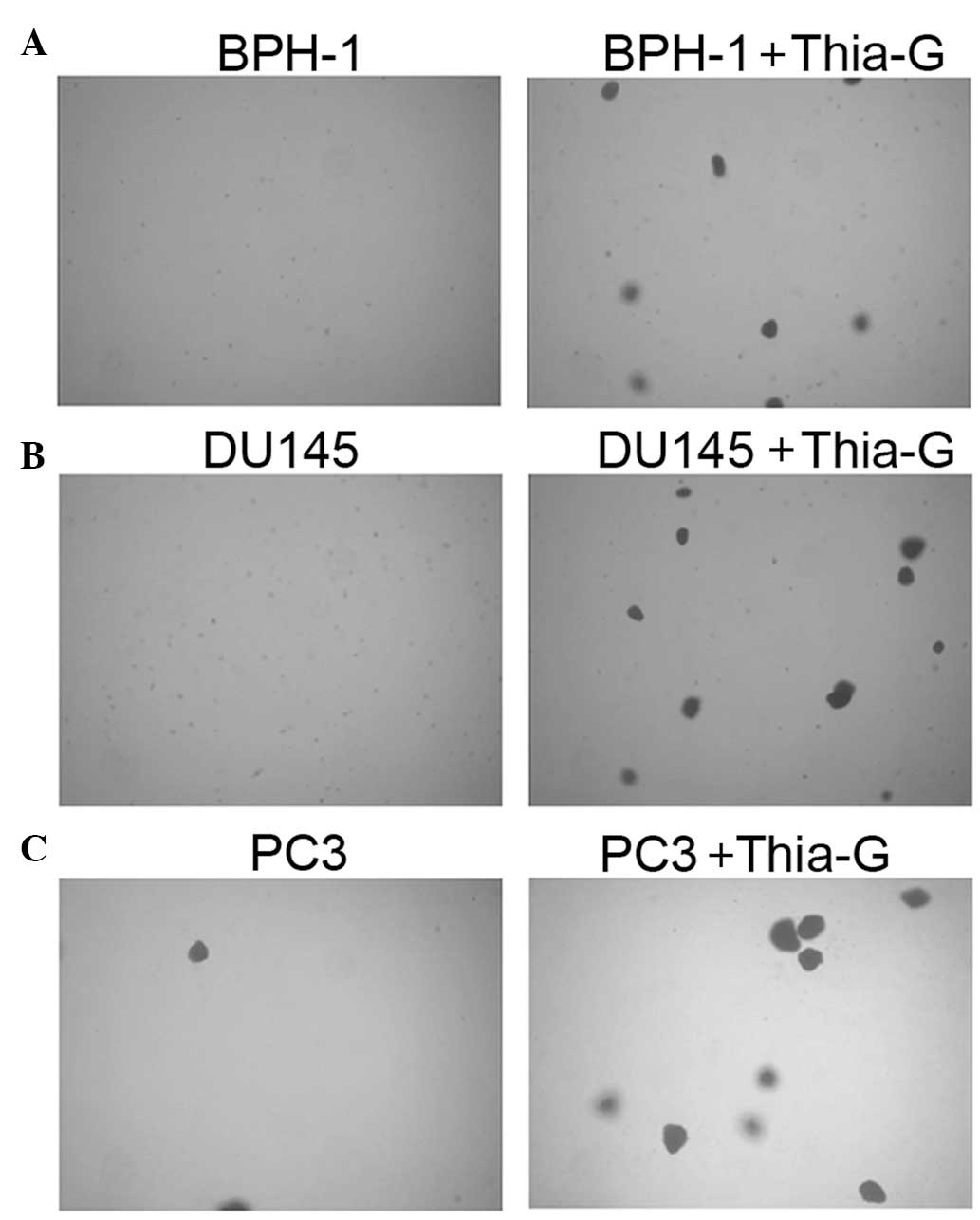
To investigate whether O-GlcNAc affects the
proliferation and anchorage-independent growth of prostate cancer
and BPH-1 cells, soft agar colony assays were performed. Thiamet-G
treatment did not significantly affect the proliferation of the
cells (MTT assay, data not shown). Soft agar colony assays showed
that in the presence of thiamet-G, BPH-1, DU145 and PC3 cells form
colonies at days 5–7 (Fig. 4);
however, without thiamet-G, DU145 and PC3 cells were able to form
colonies only two weeks later, while BPH-1 cells were not able to
form colonies (data not shown).
Thiamet-G treatment not only enhanced colony
formation in the prostate cancer cell lines DU145 and PC3, but
further endowed BPH-1 cells with the ability to form colonies.
These results indicated that O-GlcNAc is important for the
anchorage-independent growth of prostate cancer cells, suggesting
that increased O-GlcNAcylation may be sufficient for the malignant
transformation of BPH cells.
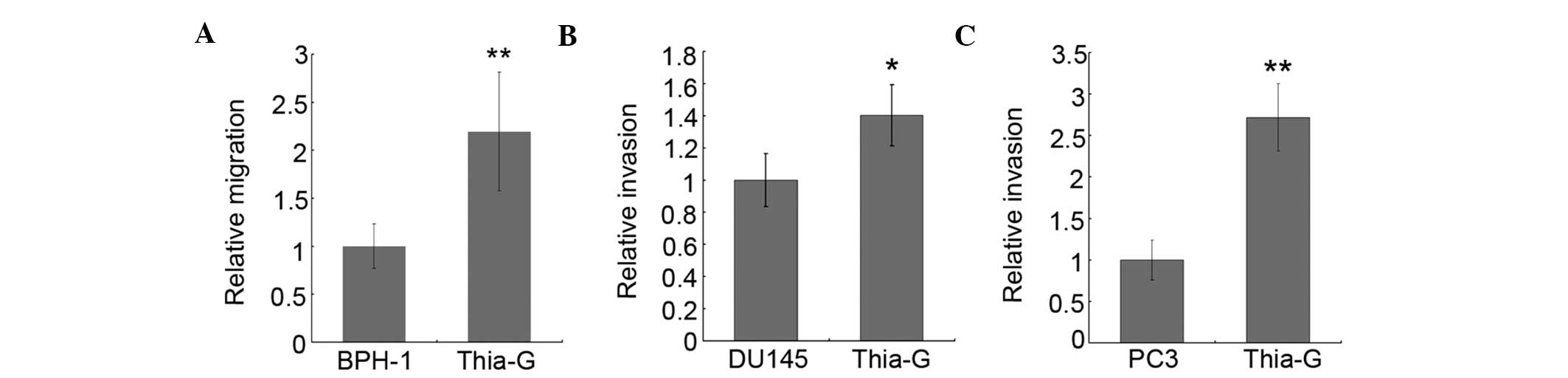
O-GlcNAc enhances the invasion of
prostate cancer cells
To investigate whether O-GlcNAc affects the invasive
ability of prostate cancer cells, invasion assays were performed.
As shown in Fig. 5, the invasive
ability of DU145 (Fig. 5B) and PC3
cells (Fig. 5C) was significantly
enhanced by thiamet-G treatment. However, neither BPH-1 nor the
thiamet-G-treated BPH-1 cells showed invasive ability in our assays
(data not shown); we thus next investigated the role of O-GlcNAc in
BPH-1 migration. The results showed that O-GlcNAc markedly enhanced
migration in BPH-1 cells (Fig.
5A). Overall, these results suggested that O-GlcNAcylation may
play important roles in prostate cancer metastasis.
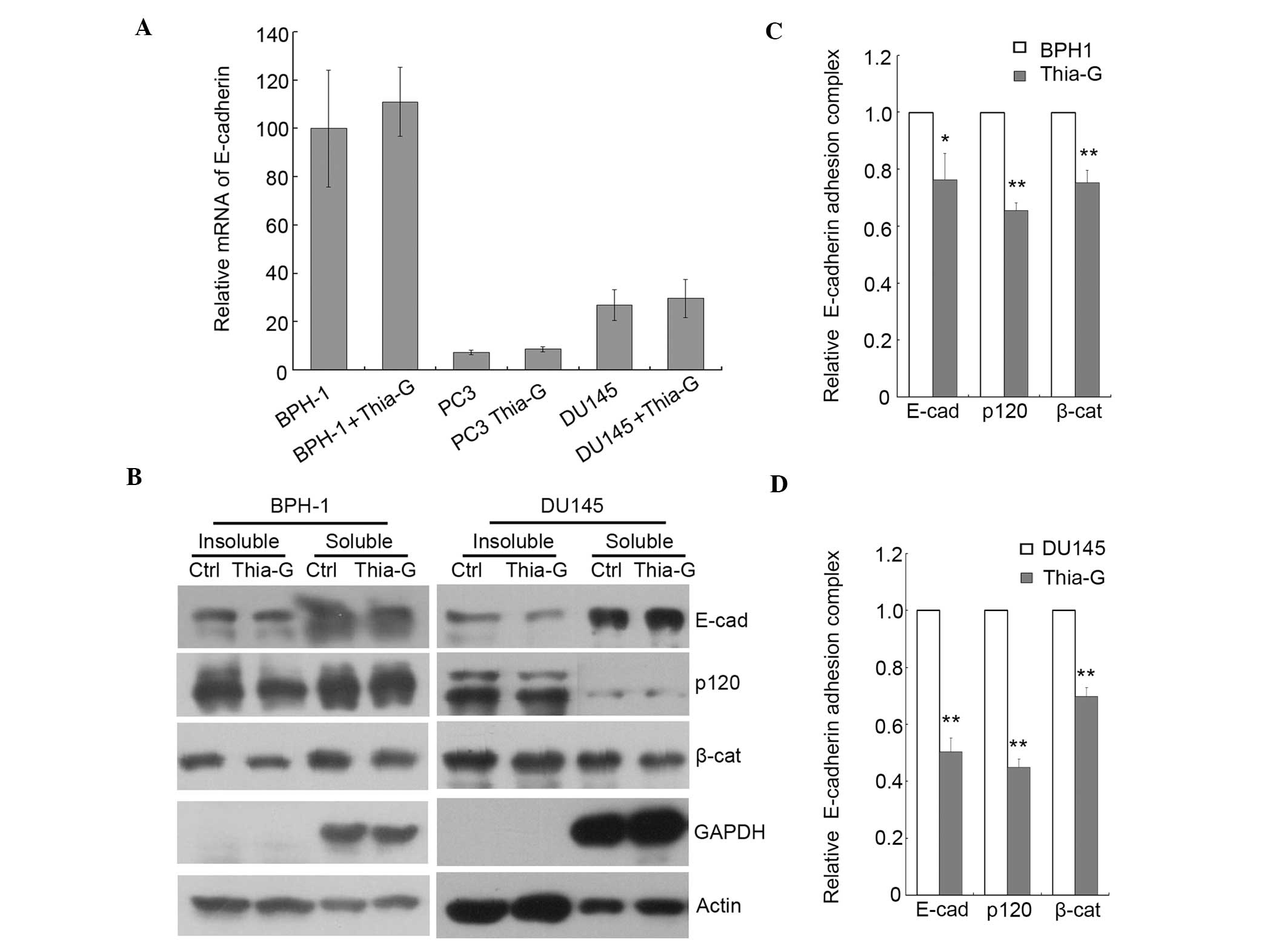
O-GlcNAc inhibits the formation of the
E-cadherin/catenin complex
E-cadherin-mediated cel-cell adhesion is generally
reduced in human cancers, and loss of E-cadherin is associated with
tumor invasiveness, metastatic dissemination, and poor patient
prognosis (24). In addition,
Snail1 was reported to be stabilized by O-GlcNAcylation and thus,
inhibit the transcription of E-cadherin (25). We therefore investigated whether
O-GlcNAc can affect the expression of E-cadherin in prostate cancer
cells. BPH-1, PC3 and DU145 cells were treated with thiamet-G for
24 h and the mRNA level of E-cadherin was detected by
quantitative RT-PCR. The results indicated that the elevation of
O-GlcNAc does not affect the transcription of E-cadherin in
any of the three cell lines (Fig.
6A). The mRNA level of E-cadherin was lower in DU145 and
PC3 compared to BPH-1 cells.
We previously reported that increased
O-GlcNAcylation reduces the level of the cell surface protein
E-cadherin, which has the potential to enhance cell invasion and
metastasis in breast cancer cells (12). The binding of the
E-cadherin/catenin complex to the cytoskeleton is essential for
strong cell-cell adhesion (26),
and we demonstrated that an increase in the O-GlcNAc level inhibits
the formation of the E-cadherin/catenin/cytoskeleton complex
(12). Here, the
E-cadherin/catenin/cytoskeleton complex was isolated from BPH-1 and
DU145 cell extracts in the Triton X-100-insoluble fraction. The
levels of the cytoskeleton-associated proteins E-cadherin and p120
were reduced in the thiamet-G-treated BPH-1 (Fig. 6B and C) and DU145 (Fig. 6B and D) cells, suggesting that the
reduction in the level of the cell surface protein E-cadherin may
cause the enhanced migratory and invasive ability of prostate
cancer cells.
Discussion
In previous studies, we analyzed histological
sections from breast, lung and colon tumors and demonstrated that
the O-GlcNAc level is increased in these tissues compared to
matched adjacent tissues (11,12).
Similarly, in patients with chronic lymphocytic leukaemia, the
O-GlcNAc level was increased when compared to healthy lymphocytes
(27). Similar results were also
obtained in breast (14), prostate
(13) and pancreatic cancer cell
lines (15) by IB analysis. A most
likely reason for the elevation of O-GlcNAcylation in cancer
tissues/cells is the increased rate of glucose utilization, which
may enhance the HBP pathway, and thus increase the concentration of
UDP-GlcNAc in cancer cells. In line with this hypothesis, it has
been reported that the concentration of UDP-GlcNAc is increased in
chronic lymphocytic leukemia cells (27). Based on all the above, we argue
that the increase in O-GlcNAc may be a universal event in different
types of cancer that show alterations in glucose metabolic pathways
(Warburg effect). However, we found that the levels of O-GlcNAc are
not increased in liver and pancreatic cancer tissues. Two
explanations for this contradictory result may be postulated:
first, there is no report on the systematic comparison of the
concentration of UDP-GlcNAc in solid cancer tissues or cells and
their healthy counterparts. Therefore, we can not confirm whether
UDP-GlcNAc is increased in different types of cancer. Second, a
previous study reported that the apparent Km (Kmapp) values of OGT
for UDP-GlcNAc vary depending on the protein substrate, suggesting
that certain OGT substrates are nutrient-responsive, whereas others
are constitutively modified (28).
The constitutively modified proteins are more easily modified by
O-GlcNAc, and thus have a higher O-GlcNAc stoichiometry; these
proteins may be the main contributors of the increased global
O-GlcNAc level observed by immunohistochemistry in cancer
tissues/cells. In conclusion, the increase in global
O-GlcNAcylation may not be a suitable marker for cancer; it may be
more relevant to consider O-GlcNAcylation in specific proteins.
In contrast to our results, a recent report
indicated that O-GlcNAcylation is globally increased in pancreatic
ductal adenocarcinoma cell lines compared to non-transformed human
pancreatic duct epithelial cells. In addition, the study also
reported that pancreatic cancer tissue exhibits increased O-GlcNAc
staining compared to healthy pancreatic ducts (15). However, this study was restricted
to a limited number and type of cells and only used one tissue
specimen. A potential explanation for the different results with
our study may be the screening of different tissues.
Zhu et al compared the O-GlcNAc levels of
forty hepatocellular carcinoma (HCC) tissues to ten healthy liver
tissues by immunohistochemistry analysis. The authors found that
global O-GlcNAcylation is significantly increased in HCC tissues
compared to healthy liver tissues (29). By contrast, our study did not find
a significant difference in the global O-GlcNAc level between
hepatocellular carcinoma tissues and their matched adjacent
tissues. One reason for the contradiction may be the fact that
liver cancer adjacent tissues used in our study were chronic
hepatitis, but not healthy liver tissues. Liver tissue inflammation
may cause the deregulation in O-GlcNAcylation.
In this study, we found that global O-GlcNAc levels
are increased in prostate cancer tissues compared to the BPH
tissues, which was consistent with our previous finding that
O-GlcNAc levels are increased in prostate cancer cell lines
compared to the healthy prostate epithelium. As discussed above,
the increase in the UDP-GlcNAc level may be the main cause for the
upregulation of global O-GlcNAcylation in prostate cancer. However,
another study showed that the increased expression of OGT may be an
additionally important positive regulator of O-GlcNAcylation in
prostate cancer (22). In this
study, we detected the global O-GlcNAc and OGT levels in BPH-1, PC3
and DU145 cells. Although the global O-GlcNAc level was slightly
increased in prostate cancer cells, the O-GlcNAc staining
intensities were not homogeneously increased in all bands: the
intensities of some O-GlcNAc-stained bands from prostate cancer
cells were increased, while the intensities of other bands were
decreased compared to BPH-1 cells. This might be explained by the
different target protein preference of OGT in prostate cancer and
BPH-1 cells.
Our study also demonstrated that O-GlcNAc can
enhance the malignancy of several cancer types, and that the OGT
level is increased in certain cancer cell lines. However, we found
that the expression of mOGT is decreased in prostate cancer cells
compared to BPH-1 cells. Considering that mOGT has pro-apoptotic
roles, the reduced expression of mOGT may enhance the survival and
anti-apoptotic abilities of prostate cancer cells. The roles of
mOGT in cancer formation and progression need to be further
investigated.
The human prostatic epithelial cell line BPH-1,
derived from BPH tissue and immortalized with the SV40 large T
antigen, is normally nontumorigenic in nude mice (30) and can not form colonies in the soft
agar transformation assay. BPH-1 can be induced to form tumors by
various factors, such as association with a tumorigenic stromal
microenvironment, treatment with hormonal carcinogens or by
oncogene transformation (31,32).
In this study, we found that the increase in O-GlcNAcylation by
thiamet-G induced anchorage-independent growth and migration in
BPH-1 cells, which is the first direct evidence that O-GlcNAc can
induce malignant transformation in bening cells. It is notable that
thiamet-G treatment induced colony formation in BPH-1, PC3 and
DU145 cells on the 4th-7th day during the soft agar assay, while
two weeks were required for colony formation in the non-treated PC3
and DU145 cells (data not shown). Moreover, we found that thiamet-G
treatment enhances invasion in PC3 and DU145 cells. Consistent with
our results, Lynch et al reported that the OGT
knock-down in prostate cancer cells inhibits anchorage-independent
cell growth, cell invasion and angiogenesis (13). Based on these results, we propose
that O-GlcNAc is involved in all steps of prostate cancer formation
and progression.
E-cadherin is a well-known cell-cell adhesion
molecule, which acts as a suppressor of cancer cell invasion; its
expression is downregulated in numerous types of advanced human
cancer. There have been some reports that provided evidence on the
regulation of E-cadherin by O-GlcNAc. For example, O-GlcNAc
inhibited the transcription of E-cadherin by modifying and
stabilizing Snail1 (25); our
group also reported that the increase in the O-GlcNAc level can
inhibit the formation of the E-cadherin/catenin/cytoskeleton
complex, and thus induce invasion and metastasis in breast cancer
cells (12). In the present study,
we found that the increase in the O-GlcNAc level does not affect
the mRNA level of E-cadherin in BPH-1, PC3 and DU145 cells, but
that O-GlcNAc inhibited the formation of the
E-cadherin/catenin/cytoskeleton complex. Therefore, regulation of
E-cadherin might constitute the mechanism underlying the induction
of prostate cancer invasion. However, the invasive ability of PC3
cells, in which E-cadherin is expressed at low levels, was also
significantly enhanced in our study. Furthermore, it was reported
that reducing the expression of OGT leads to increased degradation
of the FoxM1 protein, and thus regulates the progression of breast
and prostate cancer cells (13).
Moreover, there are numerous proteins related to tumor progression
that are modified and regulated by O-GlcNAc (8). Overall, previous reports and present
findings suggest that the mechanisms involved in the roles of
O-GlcNAc in cancer progression may be multidimensional and/or
highly context-dependent.
In summary, we showed that global O-GlcNAcylation is
increased in prostate, but not liver and pancreatic cancer tissues
compared to their corresponding adjacent tissues, and that the
expression of mOGT, but not ncOGT, is decreased in prostate cancer
cell lines; moreover, we found that O-GlcNAc can enhance the
malignancy of prostate cancer cells, at least partially through
inhibition of the formation of the E-cadherin/catenin/cytoskeleton
complex.
Acknowledgements
We would like to thank Dr Xinzhi Lu, Feng Han and
Qianhong Gong for numerous discussions. This work was supported by
the National Natural Science Foundation of China (grant nos.
81101505, 81172013 and 81272264) and the Open Research Fund Program
of the Key Laboratory of Marine Drugs [grant no.
KLMD(OUC)201308].
References
|
1
|
Banerjee PS, Hart GW and Cho JW: Chemical
approaches to study O-GlcNAcylation. Chem Soc Rev. 42:4345–4357.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshall S, Bacote V and Traxinger RR:
Discovery of a metabolic pathway mediating glucose-induced
desensitization of the glucose transport system. Role of hexosamine
biosynthesis in the induction of insulin resistance. J Biol Chem.
266:4706–4712. 1991.
|
|
3
|
Kreppel LK and Hart GW: Regulation of a
cytosolic and nuclear O-GlcNAc transferase. Role of the
tetratricopeptide repeats. J Biol Chem. 274:32015–32022. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SY, Ryu J and Lee W: O-GlcNAc
modification on IRS-1 and Akt2 by PUGNAc inhibits their
phosphorylation and induces insulin resistance in rat primary
adipocytes. Exp Mol Med. 37:220–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang X, Ongusaha PP, Miles PD, et al:
Phosphoinositide signalling links O-GlcNAc transferase to insulin
resistance. Nature. 451:964–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buse MG: Hexosamines, insulin resistance,
and the complications of diabetes: current status. Am J Physiol
Endocrinol Metab. 290:E1–E8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu F, Iqbal K, Grundke-Iqbal I, Hart GW
and Gong CX: O-GlcNAcylation regulates phosphorylation of tau: a
mechanism involved in Alzheimer’s disease. Proc Natl Acad Sci USA.
101:10804–10809. 2004.PubMed/NCBI
|
|
8
|
Slawson C and Hart GW: O-GlcNAc
signalling: implications for cancer cell biology. Nat Rev Cancer.
11:678–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pecqueur C, Oliver L, Oizel K, Lalier L
and Vallette FM: Targeting metabolism to induce cell death in
cancer cells and cancer stem cells. Int J Cell Biol.
2013:8059752013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi W, Gu Y, Han C, et al: O-GlcNAcylation
is a novel regulator of lung and colon cancer malignancy. Biochim
Biophys Acta. 1812:514–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gu Y, Mi W, Ge Y, et al: GlcNAcylation
plays an essential role in breast cancer metastasis. Cancer Res.
70:6344–6351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lynch TP, Ferrer CM, Jackson SR, Shahriari
KS, Vosseller K and Reginato MJ: Critical role of O-linked
β-N-acetylglucosamine transferase in prostate cancer invasion,
angiogenesis, and metastasis. J Biol Chem. 287:11070–11081.
2012.
|
|
14
|
Caldwell SA, Jackson SR, Shahriari KS, et
al: Nutrient sensor O-GlcNAc transferase regulates breast cancer
tumorigenesis through targeting of the oncogenic transcription
factor FoxM1. Oncogene. 29:2831–2842. 2010. View Article : Google Scholar
|
|
15
|
Ma Z, Vocadlo DJ and Vosseller K:
Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive
NF-κB activity in pancreatic cancer cells. J Biol Chem.
288:15121–15130. 2013.PubMed/NCBI
|
|
16
|
Fromowitz FB, Viola MV, Chao S, et al: ras
p21 expression in the progression of breast cancer. Hum Pathol.
18:1268–1275. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qin JM, Fu XY, Li SJ, et al: Gene and
protein expressions of p28GANK in rat with liver regeneration.
World J Gastroenterol. 9:2523–2527. 2003.PubMed/NCBI
|
|
18
|
Yuzwa SA, Macauley MS, Heinonen JE, et al:
A potent mechanism-inspired O-GlcNAcase inhibitor that blocks
phosphorylation of tau in vivo. Nat Chem Biol. 4:483–490. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Zhang J, Mi W, et al: Silencing of
GM3 synthase suppresses lung metastasis of murine breast cancer
cells. Breast Cancer Res. 10:R12008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Leber B and Andrews DW: Cytoplasmic
O-glycosylation prevents cell surface transport of E-cadherin
during apoptosis. Embo J. 20:5999–6007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Love DC, Kochan J, Cathey RL, Shin SH and
Hanover JA: Mitochondrial and nucleocytoplasmic targeting of
O-linked GlcNAc transferase. J Cell Sci. 116:647–654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itkonen HM, Minner S, Guldvik IJ, et al:
O-GlcNAc transferase integrates metabolic pathways to regulate the
stability of c-MYC in human prostate cancer cells. Cancer Res.
73:5277–5287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin SH, Love DC and Hanover JA: Elevated
O-GlcNAc-dependent signaling through inducible mOGT expression
selectively triggers apoptosis. Amino Acids. 40:885–893. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirohashi S: Inactivation of the
E-cadherin-mediated cell adhesion system in human cancers. Am J
Pathol. 153:333–339. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SY, Kim HS, Kim NH, et al: Snail1 is
stabilized by O-GlcNAc modification in hyperglycaemic condition.
EMBO J. 29:3787–3796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pokutta S and Weis WI: Structure and
mechanism of cadherins and catenins in cell-cell contacts. Annu Rev
Cell Dev Biol. 23:237–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y, Tomic J, Wen F, et al: Aberrant
O-GlcNAcylation characterizes chronic lymphocytic leukemia.
Leukemia. 24:1588–1598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen DL, Gloster TM, Yuzwa SA and Vocadlo
DJ: Insights into O-linked N-acetylglucosamine ([0-9]O-GlcNAc)
processing and dynamics through kinetic analysis of O-GlcNAc
transferase and O-GlcNAcase activity on protein substrates. J Biol
Chem. 287:15395–15408. 2012.
|
|
29
|
Zhu Q, Zhou L, Yang Z, et al:
O-GlcNAcylation plays a role in tumor recurrence of hepatocellular
carcinoma following liver transplantation. Med Oncol. 29:985–993.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayward SW, Dahiya R, Cunha GR, Bartek J,
Deshpande N and Narayan P: Establishment and characterization of an
immortalized but non-transformed human prostate epithelial cell
line: BPH-1. In Vitro Cell Dev Biol Anim. 31:14–24. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hayward SW, Wang Y, Cao M, et al:
Malignant transformation in a nontumorigenic human prostatic
epithelial cell line. Cancer Res. 61:8135–8142. 2001.PubMed/NCBI
|
|
32
|
Webber MM, Quader ST, Kleinman HK, et al:
Human cell lines as an in vitro/in vivo model for prostate
carcinogenesis and progression. Prostate. 47:1–13. 2001. View Article : Google Scholar : PubMed/NCBI
|