Introduction
It is generally accepted that hepatic ischemia
reperfusion injury (HI/R) is an important non-immunologic injury
that may occur during circulatory shock, hepatic trauma, liver
transplantation and elective liver resection (1). Severe HI/R contributes to liver
failure, remote organ failure and even mortality (2,3).
Therefore, HI/R has always been a key concern in the development of
liver surgery techniques. Several mechanisms appear to be involved
in the pathophysiology of HI/R injury. It is well established that
reactive oxygen species (ROS) are conceived to be a critical factor
in the pathogenesis of HI/R injury. Recent studies have illustrated
that excessive formation of ROS during ischemic insult not only
causes the destruction of cellular structures, but also results in
mitochondrial dysfunction, finally activating apoptotic cascades
(4). Chandra et al
(5) found that increased
H2O2 levels in tissue may lead to apoptotic
damage by upregulating the Fas-FasL system.
H2O2 may have damaged the mitochondrial
membrane, thus contributing to the release of pro-apoptotic
components located in the mitochondria. The injured mitochondria
activated a number of transcription factors and promoted their
translocation into the nucleus, including p53 and nuclear factor
(NF)-κB. Additionally, the expression of pro-apoptotic genes may be
facilitated by the suppression of ROS and survival-associated
genes. By contrast, natural anti-oxidants may attenuate I/R injury.
Superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH)
reductase treatment have effectively alleviated I/R injury in
animals (6). Taking into account
the fact that ischemic injury is associated with oxidation, it is
important to examine the hepatoprotective agents that may
ameliorate the damage of ROS in HI/R injury. Apoptosis is another
important mechanism involved in HI/R injury. Kohli et al
(7) identified that 50–70% of
sinusoidal liver endothelial cells and 40–60% of hepatocytes
underwent apoptosis.
Huperzine A (HupA), a novel alkaloid extracted from
the Chinese traditional medicine, Huperzia serrate, is
considered to be a drug with high clinical prospects. Previous
studies have demonstrated that it has several beneficial effects
for patients with Alzheimer’s disease (AD) (8) and, in China it is one of the most
commonly prescribed drugs for various types of dementia, including
AD (9), as a result of its
inhibitory effect on acetylcholinesterase (AchE). Ruan et al
(10) reported that HupA markedly
decreased ROS generation and oxidative damage in
D-galactose-treated rats. Following renal I/R injury, HupA was also
found to inhibit cellular apoptosis (11). These studies confirmed that HupA
possessed anti-oxidative and anti-apoptotic properties. However, it
remains unclear whether HupA may alleviate HI/R in rats. Therefore,
the present study was conducted to assess the hepatoprotective
effects against H/IR and further examine the potential mechanisms
underlying these effects.
Materials and methods
Animals and induction of HI/R
Male Wistar rats (weight, 240±40 g) were housed in
individual cages under a controlled environment (12:12 h light/dark
cycle, 50–70% humidity, 24°C) and provided with free access to
water and food. All of the experimental procedures were approved by
the animal ethics committee of Maanshan Municipal Health Hospital
For Women and Children in China (Maanshan, China). All experimental
procedures were performed in a manner that minimized suffering and
reduced the number of animals used.
HI/R was induced according to the method described
previously with minor modifications (11–13).
Under the chloral hydrate (200 mg/kg) and ether anesthesia, rats
underwent a median laparotomy. The hepatoportal vein, hepatic
arterial and hepatic duct were separated, which were clamped for 30
min followed by a 6 h reperfusion with an atraumatic vascular clamp
(Hengao Company of Beijing, Beijing, China). The body temperature
of the animals was maintained constantly using a heating blanket
during the reperfusion period. The sham group underwent all surgery
with the exception of the occlusion of the hepatoportal vein,
hepatic artery and hepatic duct.
Drug administration
HupA (purity, >95%; Sigma, St. Louis, MO, USA)
was dissolved in physiological saline and was injected into the
tail vein 5 min prior to the induction of HI/R. The chemical
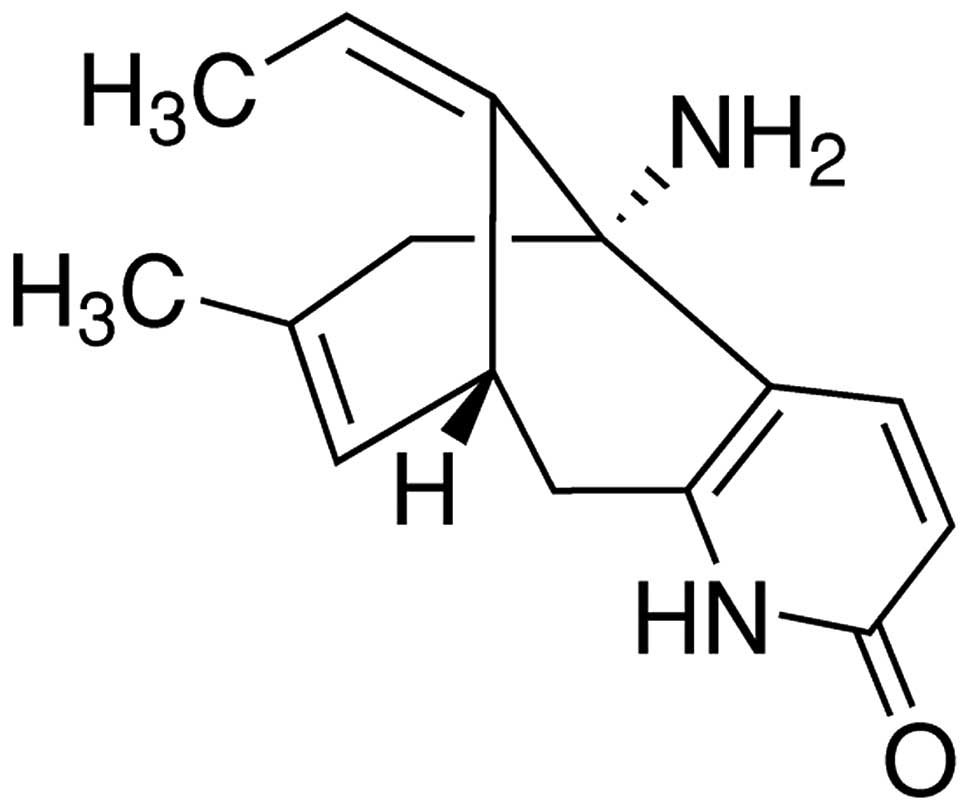
structure of HupA was indicated in Fig. 1. A total of 24 rats were randomly
divided into the following four groups (n=6 per group): Sham,
Vehicle and HupA treatment (varietal does of HupA: 167 μg/kg and
500 μg/kg) groups. The vehicle and HupA groups underwent the HI/R
procedure prior to injections with the same volume of physiological
saline or HupA, respectively, through the tail veil. At the end of
the reperfusion period, the rats were sacrificed by spinal
dislocation and the blood and liver samples were collected.
Separate tissue samples were quantified with microscopic scoring
under light microscope (Nikon Corporation, Nikon, Tokyo, Japan)
following hematoxylin and eosin (H&E) staining for the
histological analysis. Blood samples were drawn from the
supra-hepatic vena cava by a fine needle (Trade of Antai Company,
Suzhou, China) and then centrifuged at 3,000 × g for 5 min to
collect the serum for the determination of the alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) levels.
The liver tissue samples from each animal were stored at −80°C for
the measurement of hepatic tissue SOD, CAT, GSH and malondiadehyde
(MDA) levels, together with evaluating the activity of caspase-3
and the protein expression of caspase-3, Bcl-2 and Bax.
Histological examination
The liver tissue samples were fixed in 4%
paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) for
12 h, followed by two days in 30% sucrose buffer at room
temperature. The serial coronal sections (6 μm-thick) were obtained
using a microtome and stained with H&E and examined by light
microscopy. The liver histopathological evaluation was performed in
a blinded manner.
Measurement of serum ALT and AST
levels
An automated autobiochemical analyzer (Toshiba,
Tokyo, Japan) was employed to determine serum ALT and AST levels as
described previously (12–14).
Measurement of SOD, CAT, GSH and MDA
activities
The enzymatic activity of SOD, GSH, GSH-peroxidase
(PX) and MDA was measured according to the manufacturer’s
instructions in different commercial assay kits (Nanjing Jian Cheng
Bioengineering Institute, Nanjing, China).
The SOD activity in the hepatic tissue homogenate
was estimated by calculating the rate of inhibition of nucleotide
oxidation. The results are expressed as the U/mg protein. The CAT
was assayed by quantifying flaxen complex compound, configured by
ammonium molybdate and the reminder peroxide, at the wavelength of
405 nm. The result are provided as U/mg protein. The content of GSH
was assayed by quantifying the rate of oxidation of the reduced
glutathione to the oxidized glutathione by
H2O2. The results are indicated in mg GSH/g
protein. The content of MDA was assayed for the products of lipid
peroxidation by monitoring thiobarbituric acid reacting substances
at a wavelength of 532 nm. The level of MDA was expressed as nmol
MDA/mg protein.
Western blot assay
Western blot analysis was performed on the hepatic
samples. Briefly, the samples were homogenized in an ice-cold lysis
buffer [10 mM Tris (pH 8.0), 150 mM NaCl, 10% glycerol, 1% NP-40, 5
mM EDTA and protease inhibitor cocktail]. Following centrifugation
at 13200 × g for 20 min at 4°C, the supernatant was collected and
the total protein levels were quantified by a bicinchoninic protein
assay kit (Beyotime Institute of Biotechnology, Shanghai, China).
An equal quantity of protein (50 μg) was separated by means of
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred onto nitrocellulose membranes
(Millipore, Billerica, MA, USA). The membranes were blocked with 5%
skimmed milk for 1 h at room temperature and then probed,
respectively, with the following primary antibodies: Anti-caspase-3
polyclonal rabbit antibody (1:300; Cell Signaling Technology, Inc.,
Beverly, MA, USA), anti-Bcl-2 monoclonal rabbit antibody (1:200;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), anti-Bax
monoclonal rabbit antibody (1:200; Santa Cruz Biotechnology, Inc.)
and anti-β-actin monoclonal rabbit antibody (1:2,000; Santa Cruz
Biotechnology, Inc.), respectively, at 4°C overnight. After the
membranes were washed with three changes of Tris-buffered saline
with Tween-20, they were incubated for 2 h with peroxidase-labeled
goat anti-rabbit IgG (1:5,000; Santa Cruz Biotechnology, Inc.).
Immunodetection was conducted with enhanced chemiluminesecence
(Applygen, Beijing, China) and exposed on an X-ray film. β-actin
was used as an internal reference for relative quantification. The
films were digitized by a scanner (Hewlett-Packard Development
Company, Beijing, China) and the grey value of the protein bands
was analyzed using Quantity One software (Bio-Rad, Hercules, CA,
USA).
Assay of caspase-3 activity
The reduction in the chromogenic caspase-3 substrate
acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) was used to
assess the activity of caspase-3. The quantity of caspase-3 was
measured using a colorimetric approach with a commercial kit
(Beyotime Institute of Biotechnology). The protein samples of the
hepatic tissues were acquired as indicated in the western blot
analysis. Approximately 50 μg protein was added to a reaction
buffer involvement Ac-DEVD-pNA (2 mM), incubated at 37°C for 4 h
and the absorbance of yellow pNA was calculated by a spectrometer
(Shanghai CSOIF Company, Shanghai, China) at a wavelength of 405
nm. The specific activity of caspase-3, which was normalized for
the total protein in the liver was then expressed as the fold
change of the baseline caspase-3 activity of the control group.
Statistical analysis
The results were expressed as the mean ± standard
deviation. Comparisons between the groups were performed by one-way
analysis of variance with Dunnett’s test using SPSS 13.0 software
(SPPS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Histopathological examination
As demonstrated in Fig.
2A and B, the sham group exhibited normal liver cellular
structure. As observed in Fig. 2C and
D, the vehicle group exhibited a mass of hepatocytes
cytoplasmic color fading and nuclear condensation. When the
ischemic rats were treated with HupA at the doses of 167 and 500
μg/kg, it was noted that the cytoplasmic color fading and nuclear
condensation of the hepatocytes were significantly diminished, as
illustrated in Fig. 2E–H.
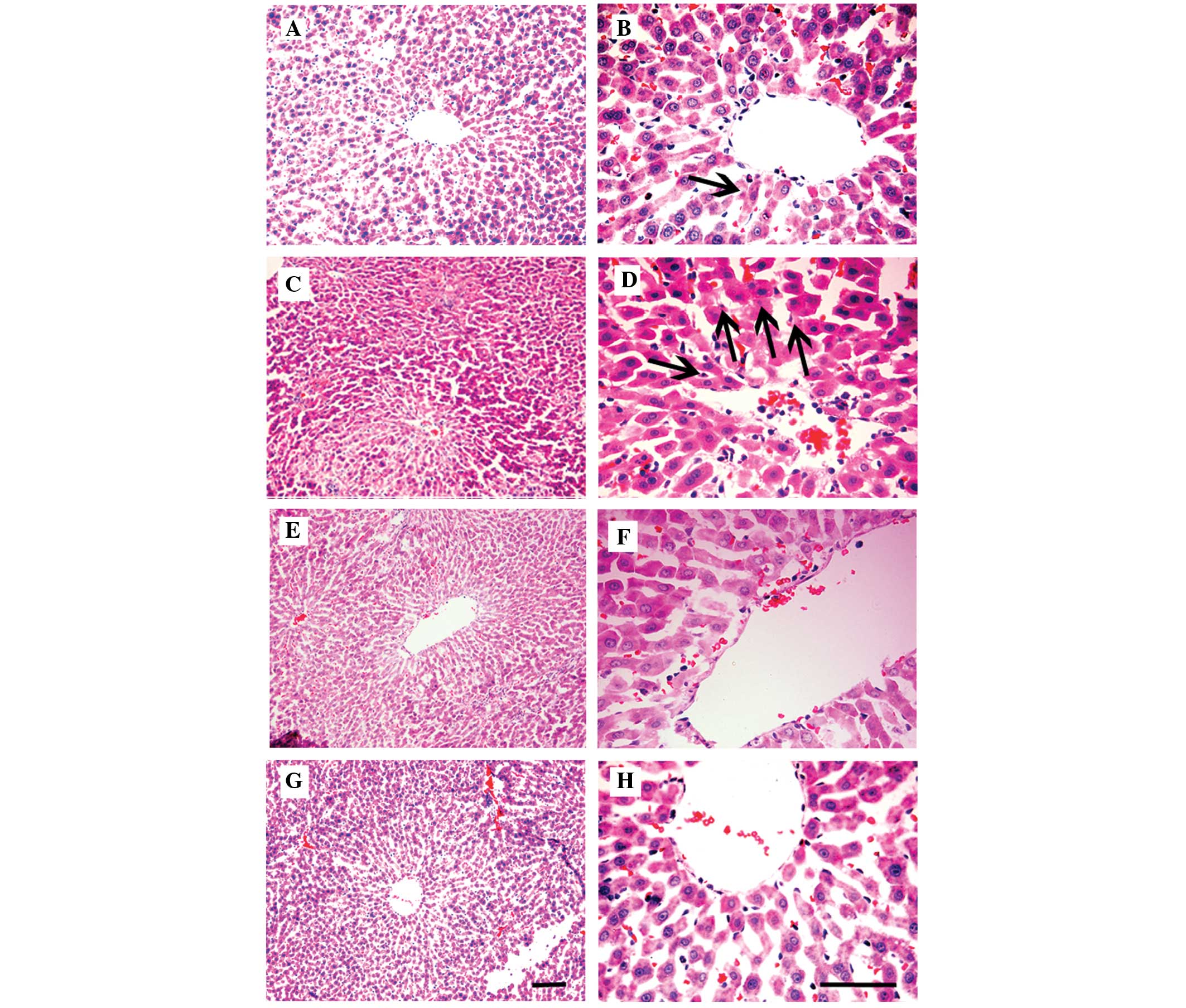 | Figure 2Effects of HupA on hepatic tissue 6 h
following HI/R with hematoxylin and eosin staining. The
representative micrographs of hepatic tissues from (A and B),
sham-operated; (C and D), vehicle-treated; (E and F), HupA (167
μg/kg)-treated and (G and H), HupA (500 μg/kg)-treated groups were
established. Magnification, (A, C, E and G) ×200; (B, D, F and H)
×400. Pyknosis in the sham operated and hepatic ischemia groups is
marked with arrows. Scale bar, 10 μm. HupA, huperzine A; HI/R,
hepatic ischemia reperfusion. |
Serum ALT and AST levels
In the physiological saline-treated HI/R group, the
levels of serum ALT, which was the marker of hepatic damage, were
significantly increased (Fig. 3A)
from 36.10±8.37 to 2034.77±45.84 U/l (P<0.01, n=6) compared with
the sham group. However, the HupA groups (167 and 500 μg/kg)
markedly reduced the ALT level from 2034.77±45.84 to 342.92±38.64
(P<0.01, n=6) and 319.53±50.05 U/l (P<0.01, n=6),
respectively, compared with the HI/R group. Similarly, the levels
of serum AST of the vehicle group were notably enhanced compared
with the sham group (Fig. 3B) from
72.77±11.83 to 2738.10±43.23 U/l (P<0.01, n=6). However the AST
levels in the HupA groups (167 and 500 μg/kg) were markedly
decreased from 2738.10±43.23 to 507.92±23.40 (P<0.01, n=6) and
422.86±38.71 U/l (P<0.01, n=6), respectively compared with the
vehicle group.
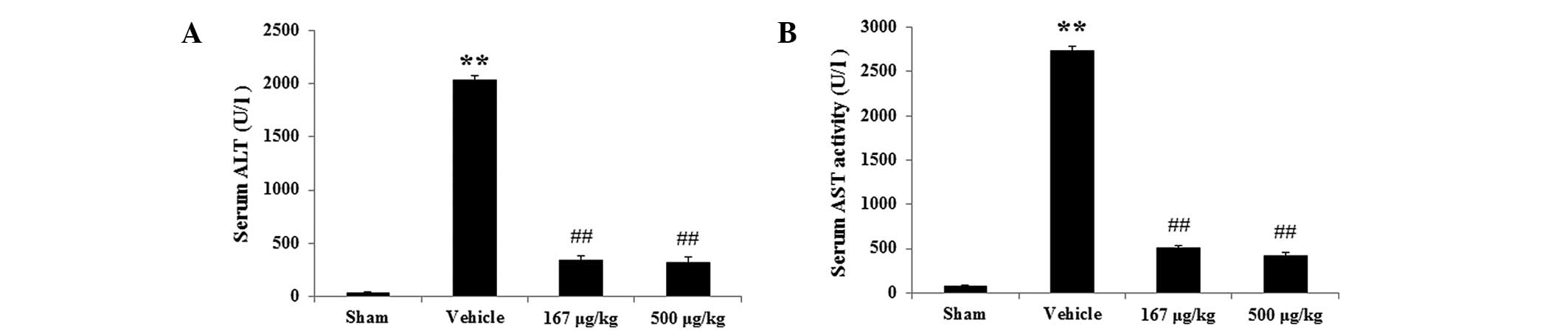 | Figure 3Effects of HupA on the levels of serum
ALT and AST 6 h following HI/R (mean ± standard deviation, n=6).
Levels of serum (A) ALT and (B) AST, respectively, in the different
groups. **P<0.01 vs. sham-operated group;
##P<0.01 vs. vehicle-treated group. Sham,
sham-operated; Vehicle, vehicle-treated; 167 μg/kg, HupA (167
μg/kg)-treated; 500 μg/kg, HupA (167 μg/kg)-treated; HupA,
huperzine A; ALT, alanine aminotransferase; AST, aspartate
aminotransferase. |
Activity of anti-oxidative enzymes (SOD
and CAT) and the levels of MDA and GSH in hepatic tissue
In order to examine the effects of HupA on oxidative
stress during HI/R injury in rats, the activity of anti-oxidative
enzymes (SOD and CAT) and the levels of GSH and MDA in hepatic
tissue were investigated in the present study. Fig. 4A demonstrates that the activity of
SOD, one of the most important anti-oxidative enzymes, was
significantly reduced in the vehicle group from 344.44±17.41 to
113.10±14.14 U/mg protein compared with the sham group (P<0.01,
n=6). Following administration of HupA (167 and 500 μg/kg), the
activity of SOD was significantly enhanced from 113.10±14.14 in the
HI/R group to 256.25±15.19 (P<0.01, n=6) and 297.86±12.14 U/mg
protein (P<0.01, n=6) in the 167 and 500 μg/kg HupA groups,
respectively. Similarly, the activity of CAT in the vehicle group
was also decreased compared with the sham group from 39.10±7.58
(P<0.01, n=6) to 15.77±5.87 U/mg protein (P<0.01, n=6).
Notably, following treatment with HupA at the doses of 167 and 500
μg/kg, the activity of CAT was enhanced from 15.77±5.87 in the
ischemic group to 30.75±6.31 (P<0.01, n=6) and 37.03±6.82 U/mg
protein (P<0.01, n=6) respectively (Fig. 4B). As revealed in Fig. 4C, the quantity of GSH in the
vehicle group markedly reduced to 1.60±0.43 mg/g protein compared
with the sham group (4.27±0.76, P<0.01, n=6). Following
treatment with HupA at doses of 167 and 500 μg/kg, the content of
GSH was increased to 2.92±0.53 (P<0.01, n=6) and 3.53±0.53
(P<0.01, n=6), respectively. Additionally, the content of MDA
(Fig. 4D), a marker of lipid
peroxidation, in the vehicle group was significantly increased in
the hepatic tissue from 6.10±0.76 to 12.93±1.58 nmol/mg protein
(P<0.01, n=6), compared with the sham group. A marked reduction
in the MDA level was observed in the HupA-treated (167 and 500
μg/kg) rats from 12.93±1.58 in the vehicle-treated ischemic rats to
8.42±0.78 and 6.70±0.98 nmol/mg protein (P<0.01, n=6),
respectively.
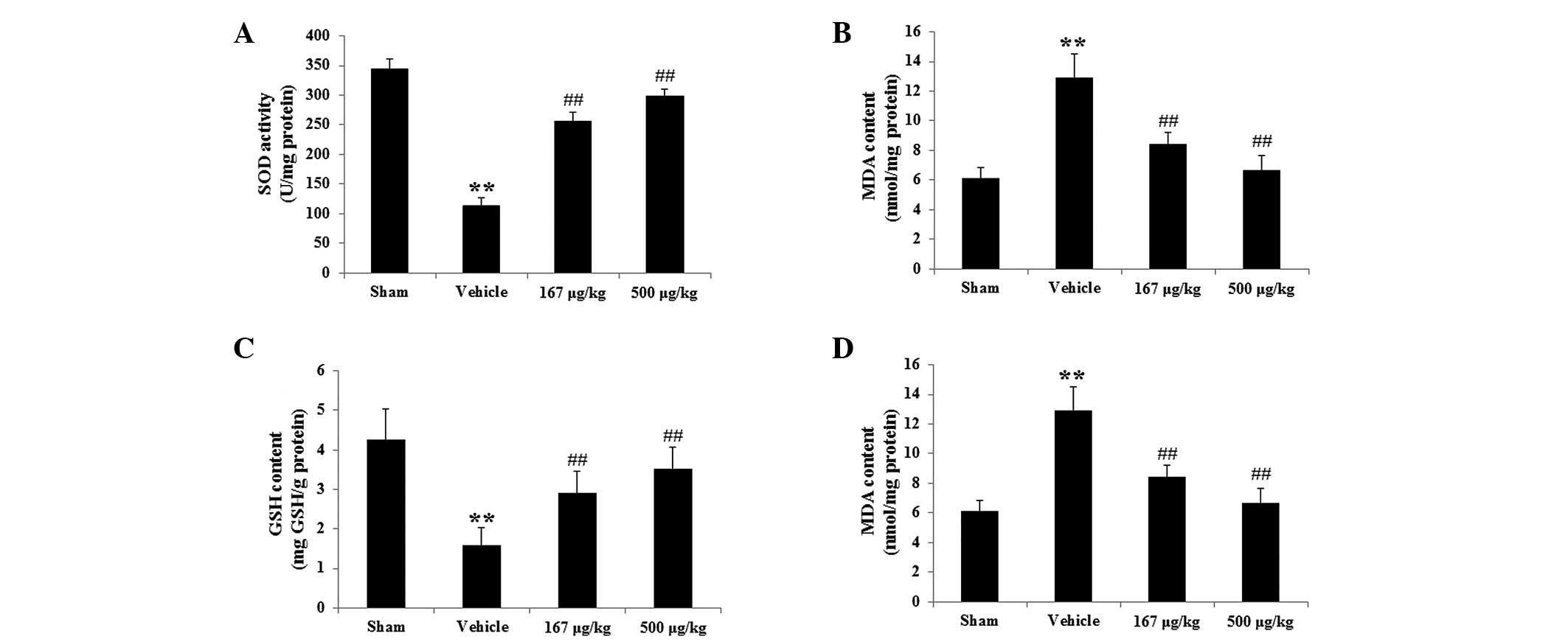 | Figure 4Effects of HupA on the activity of
antioxidant enzymes (SOD, CAT) and the contents of GSH and MDA in
hepatic tissues 6 h following HI/R (mean ± standard deviation,
n=6). The graphs show the activity of (A) SOD, (B) CAT and the
contents of (C) GSH and (D) MDA, respectively, in the different
groups. **P<0.01 vs. the sham-operated group;
##P<0.01 vs. vehicle-treated group. Sham,
sham-operated; Vehicle, vehicle-treated; 167 μg/kg, HupA (167
μg/kg)-treated; 500 μg/kg, HupA (167 μg/kg)-treated; HupA,
huperzine A; SOD, superoxide dismutase; CAT, catalase; GSH,
glutathione; MDA, malondiadehyde. |
Protein expression of Bcl-2, Bax and
caspase-3
Western blot analysis was further performed to
examine the effect of HupA on the expression of
apoptosis-regulatory proteins, including caspase-3, Bcl-2 and Bax
in hepatic tissues. Fig. 5A
demonstrates the western blotting results with antibodies specific
to caspase-3, Bcl-2 and Bax. The protein expression of caspase-3 in
ischemic rats hepatic tissues was significantly elevated from
0.60±0.15 to 1.43±0.27 (P<0.01, n=6) compared with that in the
sham group. However, when treated with HupA (167 and 500 μg/kg),
the caspase-3 protein level was markedly reduced to 0.82±0.08
(P<0.01, n=6) and 0.80±0.05 (P<0.01, n=6), respectively,
compared with the vehicle-treated group, as demonstrated in
Fig. 5B. The protein expression of
Bcl-2 in the hepatic tissue of the vehicle group was markedly
reduced from 1.70±0.08 to 0.73±0.10 (P<0.01, n=6) compared with
the sham group. HupA treatment at doses of 167 and 500 μg/kg caused
a marked elevation in the Bcl-2 protein expression level from
0.73±0.10 to 1.33±0.15 (P<0.01, n=6) and 1.51±0.09 (P<0.01,
n=6) compared with the vehicle group (Fig. 5C). Additionally, in the vehicle
group, the protein expression of Bax was significantly increased
from 0.85±0.09 to 1.73±0.39 (P<0.01, n=6) compared with the sham
group. However, the protein level of Bax was markedly decreased to
0.97±0.07 (P<0.01, n=6) and 0.94±0.03 (P<0.01, n=6),
respectively, following treatment with HupA at doses of 167 and 500
μg/kg, compared with the vehicle group (Fig. 5D).
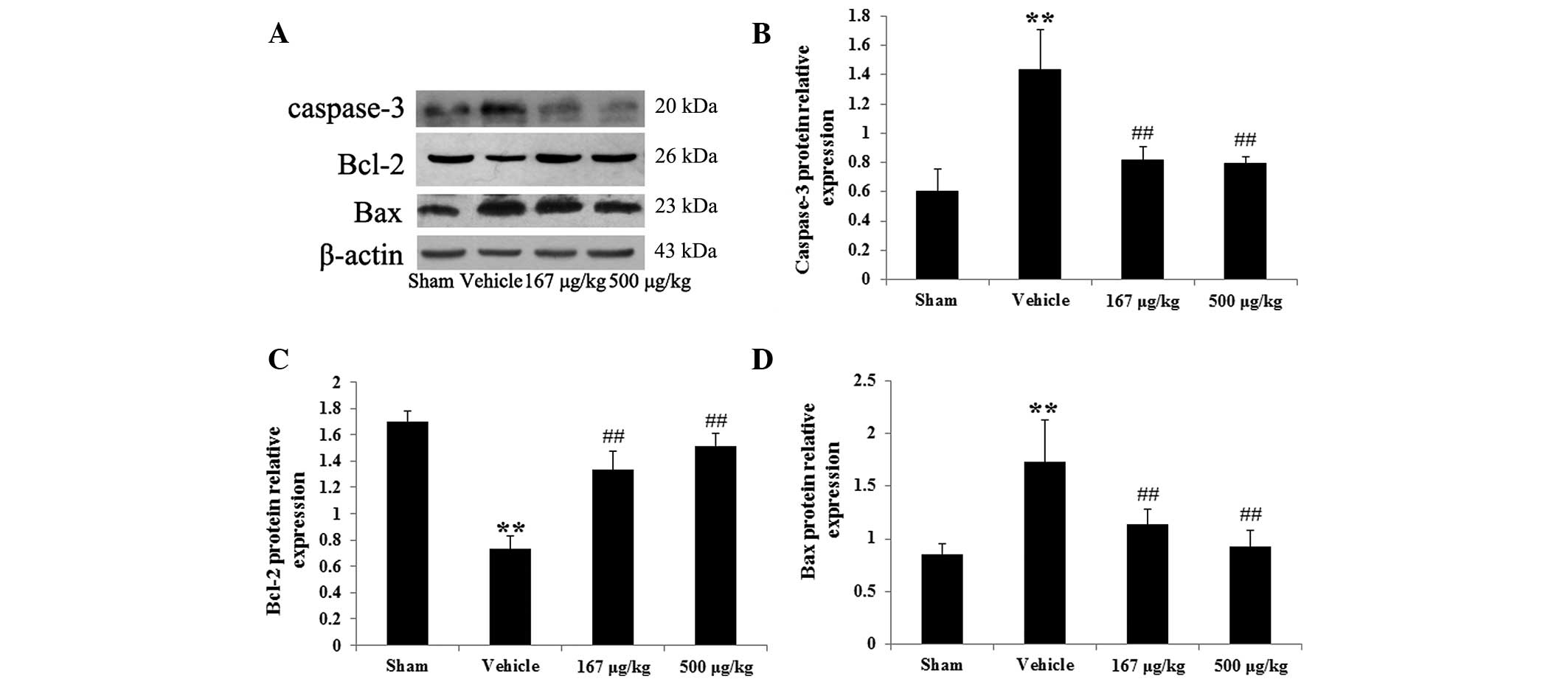 | Figure 5Effects of HupA on the protein
expression of caspase-3, Bcl-2 and Bax in hepatic tissues 6 h
following HI/R (mean ± standard deviation, n=6). (A) Representative
images of immunoblots for caspase-3, Bcl-2 and Bax in the different
groups. The quantitative analysis of the protein levels of (B)
caspase-3, (C) Bcl-2 and (D) Bax in the different groups,
respectively. The data were normalized to the loading control
β-actin. **P<0.01 vs. the sham-operated group;
##P<0.01 vs. the vehicle-treated group. Sham,
sham-operated; Vehicle, vehicle-treated; 167 μg/kg, HupA (167
μg/kg)-treated; 500 μg/kg, HupA (167 μg/kg)-treated; HupA,
huperzine A; HI/R, hepatic ischemia reperfusion. |
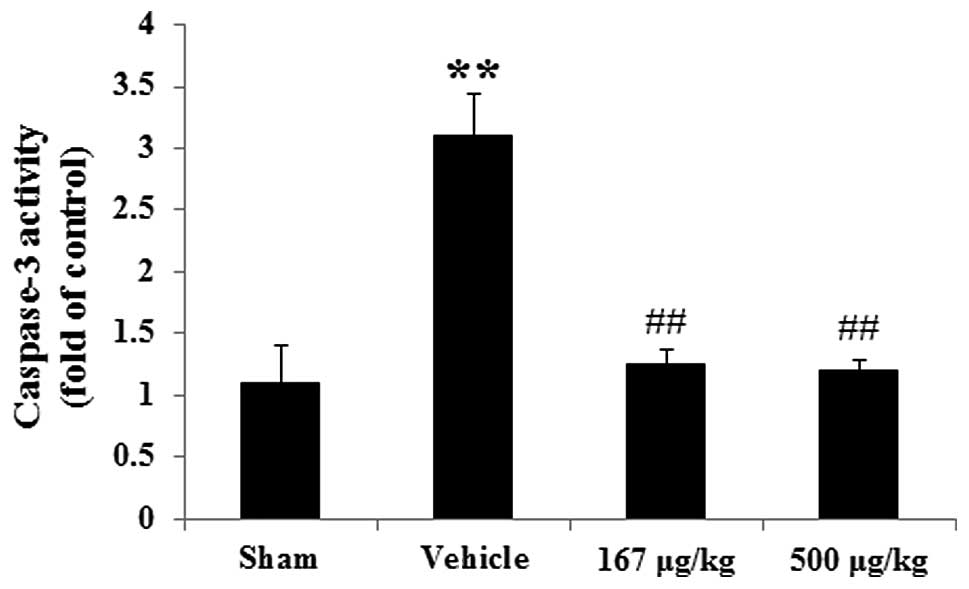
Caspase-3 activity
To identify whether HupA was able to suppress
caspase-3 activity, a colorimetric analysis was performed. As
revealed in Fig. 6, caspase-3
activity in the vehicle group was markedly enhanced by 181.82%
(P<0.01, n=6), compared with the sham group. In the HupA
treatment (167 and 500 μg/kg) groups, there was an evident
reduction in caspase-3 activity by 59.68% (P<0.01, n=6) and
61.29% (P<0.01, n=6), respectively, compared with that in the
vehicle group.
Discussion
HupA is an alkaloid isolated from the Chinese herb
Huperzia serrate, and has been widely used as a selective
inhibitor of AchE to treat AD and vascular dementia in China. As
well as inhibiting AchE, HupA was also reported to have
neuroprotective effects against cerebral ischemic injury (15). Recently, Wang et al
(16). demonstrated that HupA
inhibited the overexpression of proinflammatory enzymes induced by
oxygen-glucose deprivation in C6 rat glioma cells, partly through
activation of a cholinergic anti-inflammatory pathway In addition,
a previous investigation demonstrated that HupA was able to
diminish the excessive production of ROS following middle cerebral
artery occlusion in rats (4).
However, to the best of our knowledge, there is no evidence of the
protective effects of HupA against hepatic warm I/R injury. It was
hypothesized that the administration of HupA may reduce HI/R. To
the best of our knowledge, the present study demonstrated for the
first time, that HupA exerted protection from HI/R injury and this
hepatoprotective effect may be associated with its anti-oxidative
and anti-apoptotic properties.
It is well established that the accumulation of ROS
is closely correlated with the pathogenesis of HI/R injury
(17,18). Enhanced hepatic anti-oxidative
ability reduces the damage induced by ischemia reperfusion. A
previous study demonstrated that mice overexpressing SOD and CAT
exhibited significant improvements following HI/R injury compared
with the normal mice (6). In
another study, intravenous administration of GSH protected
hepatocytes and improved animal survival following HI/R (19). The MDA level, a biomarker for
evaluating the severity of reperfusion injury, is evidently
increased during ischemia reperfusion. Under physiological
condition, ROS levels are rapidly detoxified by endogenous
anti-oxidative enzymes and low-molecular weight anti-oxidants,
including SOD, CAT and GSH. In the present study, the SOD and CAT
activity as well as the GSH content were markedly higher following
the treatment with HupA compared with that in the ischemic rats,
but the content of MDA was significantly lower. The present results
indicated that HupA alleviated HI/R injury, at least, partly
through its anti-oxidative activity.
Hepatic damage following ischemic injury occurs via
oxidative stress and/or mitochondrial dysfunction, and ultimately
activates an apoptotic cascade. It is well established that
caspases are a family of cystein-dependent proteases with a
critical role in the initiation and execution of cellular
apoptosis. Caspases are specifically activated by apoptotic stimuli
and caspase-3 is conceived as an executioner of apoptosis (20). Cumulative evidence has supported
the hypothesis that caspase-3 expression is upregulated following
hepatic ischemia. In addition to caspases, Bcl-2 family proteins
have also been demonstrated to exhibit a critical role in the
modulation of neuronal apoptosis. Bcl-2 itself acts as an
anti-apoptotic protein, whereas another member of the family, Bax,
functions as a pro-apoptotic molecule (21). The present study demonstrated that
HupA markedly decreased the protein expression levels of caspase-3
and Bax, and elevated Bcl-2 in rats induced by HI/R injury.
Consistent with these data, HupA was also found to inhibit cellular
apoptosis following renal I/R injury (11), suggesting that enhanced the
therapeutic effect of HupA may also be associated with its
anti-apoptotic action in ischemic rats.
In conclusion, the present study demonstrated that
HupA attenuated HI/R injury by minimizing oxidative stress and
decreasing the expression of apoptosis-associated proteins,
including caspase-3, Bcl-2 and Bax. Therefore it was concluded that
the hepatoprotective effect of HupA may be associated with its
anti-oxidative and anti-apoptotic properties in HI/R injury in
rats.
References
|
1
|
Bayramoglu G, Bayramoglu A, Engur S,
Senturk H, Ozturk N and Colak S: The hepatoprotective effects of
Hypericum perforatum L. on hepatic ischemia/reperfusion
injury in rats. Cytotechnology. Jun 23–2013.(Epub ahead of
print).
|
|
2
|
Liu DL, Jeppsson B, Hakansson CH and
Odselius R: Multiple-system organ damage resulting from prolonged
hepatic inflow interruption. Arch Surg. 131:442–447. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Çekın AH, Gür G, Türkoğlu S, et al: The
protective effect of L-carnitine on hepatic ischemia-reperfusion
injury in rats. Turk J Gastroenterol. 24:51–56. 2013.
|
|
4
|
Chan PH: Mitochondria and neuronal
death/survival signaling pathways in cerebral ischemia. Neurochem
Res. 29:1943–1949. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandra J, Samali A and Orrenius S:
Triggering and modulation of apoptosis by oxidative stress. Free
Radic Biol Med. 29:323–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He SQ, Zhang YH, Venugopal SK, et al:
Delivery of antioxidative enzyme genes protects against
ischemia/reperfusion-induced liver injury in mice. Liver Transpl.
12:1869–1879. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohli V, Selzner M, Madden JF, Bentley RC
and Clavien PA: Endothelial cell and hepatocyte deaths occur by
apoptosis after ischemia-reperfusion injury in the rat liver.
Transplantation. 67:1099–1105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang R, Yan H and Tang XC: Progress in
studies of huperzine A, a natural cholinesterase inhibitor from
Chinese herbal medicine. Acta Pharmacol Sin. 27:1–26. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang HY, Zheng CY, Yan H, et al:
Potential therapeutic targets of huperzine A for Alzheimer’s
disease and vascular dementia. Chem Biol Interact. 175:396–402.
2008.PubMed/NCBI
|
|
10
|
Ruan Q, Liu F, Gao Z, et al: The
anti-inflamm-aging and hepatoprotective effects of huperzine A in
D-galactose-treated rats. Mech Ageing Dev. 134:89–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye W, Gong X, Xie J, et al: AChE
deficiency or inhibition decreases apoptosis and p53 expression and
protects renal function after ischemia/reperfusion. Apoptosis.
15:474–487. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang F, Mao Y, Qiao H, et al: Protective
effects of taurine against endotoxin-induced acute liver injury
after hepatic ischemia reperfusion. Amino Acids. 38:237–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang H, Meng F, Li W, Tong L, Qiao H and
Sun X: Splenectomy ameliorates acute multiple organ damage induced
by liver warm ischemia reperfusion in rats. Surgery. 141:32–40.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gillissen A, Bartling A, Schoen S and
Schultze-Werninghaus G: Antioxidant function of ambroxol in
mononuclear and polymorphonuclear cells in vitro. Lung.
175:235–242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Zhang HY and Tang XC: Huperzine A
attenuates cognitive deficits and hippocampal neuronal damage after
transient global ischemia in gerbils. Neurosci Lett. 313:137–140.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang ZF and Tang XC: Huperzine A protects
C6 rat glioma cells against oxygen-glucose deprivation-induced
injury. FEBS Lett. 581:596–602. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang KJ: Mechanism of hepatic
ischemia/reperfusion injury and protection against reperfusion
injury. Transplant Proc. 34:2659–2661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Teoh NC and Farrell GC: Hepatic ischemia
reperfusion injury: pathogenic mechanisms and basis for
hepatoprotection. J Gastroenterol Hepatol. 18:891–902. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schauer RJ, Gerbes AL, Vonier D, et al:
Glutathione protects the rat liver against reperfusion injury after
prolonged warm ischemia. Ann Surg. 239:220–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perry DK, Smyth MJ, Stennicke HR, et al:
Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A
novel target for zinc in the inhibition of apoptosis. J Biol Chem.
272:18530–18533. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin S and Dai CL: Attenuation of
reperfusion-induced hepatocyte apoptosis is associated with
reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal
occlusion. J Surg Res. 174:298–304. 2012. View Article : Google Scholar : PubMed/NCBI
|