Introduction
Inflammation has a crucial role in the progression
of numerous acute and chronic renal injuries. The persistence of an
inflammatory response may lead to renal fibrosis, and inhibiting
such inflammatory reactions is effective in delaying the
progression of fibrosis (1,2). The
unilateral ureteral obstruction (UUO) model is a classic animal
model that is used to study obstructed renal interstitial
inflammation and fibrosis (3). The
renal damaging effects caused by UUO mainly include interstitial
inflammatory response, apoptosis and progressive interstitial
fibrosis (4). Among these,
inflammatory responses are characterized by an excessive generation
of cytokines, including interstitial pro-inflammatory cytokines and
growth factors, as well as reactions involving inflammatory cell
packages, actuate renal tubular atrophy and interstitial fibrosis.
Therefore, inhibiting the inflammatory response may facilitate
attenuating renal tubular epithelial cell apoptosis and
interstitial fibrosis (5).
Ulinastatin (UTI) is a glycoprotein separated and
purified from human urine. As a typical Kunitz-type wide spectral
and highly effective protease inhibitor (6), it is able to eradicate oxygen free
radicals and inhibit the release of inflammatory mediators and
package inflammatory cells (7,8). Its
clinical applications include treatment of acute pancreatitis,
shock and extracorporeal circulation injury. Several recent studies
have indicated that UTI has protective effects on numerous types of
acute renal injury (9,10). In one study by Katoh et al
(11), it was confirmed that
ulinastatin effectively reduced the TGF-β1 content in the lung
tissues of rats with radioactive lung injury, and prevented
radioactive lung injury and fibrosis. However, to the best of our
knowledge, there have been no investigations on the effectiveness
of UTI in reducing renal interstitial inflammatory responses and
fibrosis in UUO-inflicted rats. Therefore, the present study aimed
to examine the effects of UTI on renal injury of UUO-inflicted
rats, to further investigate its potential clinical utility as a
renal protective strategy.
Materials and methods
Reagents and antibodies
The chemicals and reagents of analytical grade were
purchased from ZSGB-BIO (Beijing, China) unless otherwise stated.
UTI was purchased from Techpool Bio-Pharma Co., Ltd. (Guangdong,
China) and the Sircol Collagen Assay kit was purchased from
Biocolor Ltd. (Carrickfergus, Antrim, UK). The following primary
antibodies were used for immunohistochemistry: Rabbit polyclonal
antibody against transforming growth factor-β1 (TGF-β1) was
obtained from Abcam (Cambridge, UK); mouse monoclonal antibody to
CD68 and rabbit polyclonal antibody against nuclear factor
(NF)-κBp65 were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA); rabbit polyclonal antibody against type I collagen,
rabbit monoclonal antibodies against tumor necrosis factor-α
(TNF-α) and interleukin 1β (IL-1β) were purchased from Boster
Biological Technology Co., Ltd. (Wuhan, China); malondialdehyde
(MDA), superoxide dismutase (SOD) and the Coomassie Brilliant Blue
Assay kit were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China).
Experimentation animals
A total of 24 male Wistar rats, 8–12 weeks old and
weighing 180–220 g, were purchased from Vital River Laboratories
Co., Ltd. (Beijing, China). The present study was conducted in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal experimental procedure was reviewed and approved
by the Institutional Animal Care and Use Committee (IACUC) of the
Second Affiliated Hospital Harbin Medical University (Harbin,
Heilongjiang, China). All of the rats were bred and maintained in
accordance with the Care and Use of Laboratory Animals guidelines
published by the China National Institute of Health.
Grouping
The 24 Wistar rats were randomly divided into three
groups: The sham operation group (SOR group, n=8), the UUO control
group (UUO group, n=8) and the UTI treatment group (UTI group,
n=8). The UUO surgery followed the established procedure (12). Under intraperitoneal anesthesia,
the left abdominal cavity of the rats was accessed through left
lateral incision. The left ureter was separated and exposed, and
thereafter ligated at two locations using size 5-0 sutures. The
ureter was cut in between the two ligation points and layered
suture was performed. For the SOR group, the left ureter was
separated following abdominal incision, but no ligation was
performed. The UTI group was injected with 40 kU kg−1
d−1 UTI into the abdomen following surgery, while the
SOR and UUO groups were injected the same amount of 1 ml
kg−1 d−1 normal saline in the abdomen for
comparison. All of the rats were sacrificed in each group on the
7th day following surgery. The samples were harvested and frozen at
−80°C. Blood was obtained from abdominal cardinal veins. The serum
from the centrifugation was refrigerated at −20°C.
Biochemical indicator measurement
BUN and Scr levels between the different groups of
rats were measured using a Roche Automatic Biochemical
Analyzer.
Histopathological examination
Paraffin-embedded sections of nephridial tissues
were stained with hematoxylin and eosin (H&E) and Masson
trichrome for the morphological studies. A total of 20 consecutive
high power fields of each renal cortex section were examined under
light microscopy. H&E staining provided semi-quantitative
scoring on the degree of interstitial injury that assigned points
(0 to 3) for the extent of interstitial fibrosis, tubular atrophy
(defined as luminal dilation and flattened tubular epithelial
cells) and interstitial inflammatory cell infiltration, while
Masson staining calculated the renal interstitial fibrosis area
(the percentage of blue staining in the whole area). The analysis
was conducted by two independent observers and the average of all
values was obtained.
Sircol collagen assay
Analysis of total soluble collagen concentration
within each renal cortical tissue sample was performed using the
Sircol Collagen Assay according to the manufacturer’s instructions
(Biocolor Ltd.) The tissue samples were dissolved in 0.5 M acetic
acid (wet weight, 20 ml/g) and heated for 120 min at 60°C. The
tissue suspensions were centrifuged, supernatants collected and the
total collagen concentration measured at 540 nm. Soluble collagen
content was expressed as the tissue wet weight (mg/g).
Immunohistochemical analysis
Streptavidin horseradish peroxidase biotin staining
(streptavidin-peroxidase method) was employed to measure CD68+
macrophage count and TNF-α, IL-1β, NF-κB, TGF-β1 and
Col-I expression. In the negative control group, the primary
antibody was replaced with phosphate-buffered saline (PBS; pH 7.4).
Paraffin-embedded tissue sections were sliced at 4-μm intervals
following formalin fixation, and thereafter deparaffinized in
xylene and rehydrated through a graded ethanol series. Antigen was
retrieved with boiling under high pressure and endogenous
peroxidase activity was suppressed by placing the slide-mounted
tissues in H2O2. Following natural cooling,
the sections were rinsed in PBS and then incubated with polyclonal
antibodies overnight at 4°C: CD68 (diluted 1:100), TNF-α (diluted
1:100), IL-1β (diluted 1:100), NF-κB (diluted 1:150),
TGF-β1 (diluted 1:100) and Col I (diluted 1:200).
Following rinsing, the section slides were incubated at room
temperature with biotinylated goat anti-rabbit immunoglobulin G
secondary antibody for 20 min. The immune complexes were detected
using a diaminobenzidine (DAB) substrate. The sections were
subjected to a final 40 sec counterstaining of H&E and PBS
rinsing, before they were mounted onto transparent neutral balsam.
Red-stained areas were positive. The percentage of positive stained
areas in the tubule-interstitum on the section slides was measured
with an image analyzer (Lumina Vision, Version 2.0)
Assessment of oxidative stress [lipid
peroxidation (MDA) and antioxidant enzyme activities (SOD)]
estimation
The rat tissues were homogenized in 1.15% potassium
chloride (KCl) buffer (1:9, w/v) using a manual glass homogenizer
(Tempest Virtishear, model 278069; VirTis, Gardiner, NY, USA) for
~5 min. The supernatant was used for analysis of the MDA and the
content of homogenates was determined spectrophotometrically by
measuring the presence of thiobarbituric acid-reactive substances
(13). A total of 3 ml of 1%
phosphoric acid and 1 ml of 0.6% thiobarbituric acid solution were
added to 0.5 ml of plasma and pipetted into a tube. The mixture was
heated in boiling water for 45 min. Following cooling, the color
was extracted into 4 ml of n-butanol. Absorbance was
measured in spectrophotometer (Ultraspec Plus; Biochrom, Ltd.,
Cambridge, UK) at a 532 nm wavelength. The quantities of lipid
peroxides were calculated as thiobarbituric acid-reactive
substances of lipid peroxidation and were provided in units of nM/g
tissue.
Total (Cu-Zn and Mn) SOD (EC 1.15.1.1) activity was
determined according to the method described by Shin et al
(14). The principle of the method
is based on the inhibition of nitroblue tetrazolium (NBT) reduction
by the xanthine-xanthine oxidase system as a superoxide generator.
One unit of SOD is defined as the enzyme amount causing 50%
inhibition in the NBT reduction rate. SOD activity was expressed as
the U/mg protein.
The activity of SOD was measured using a SOD assay
kit (Trevigen, Gaithersburg, MD, USA). This method is based on the
reduction of nitro blue tetrazolium by SOD. Superoxide ions convert
nitro blue tetrazolium into blue formazan, which absorbs light at a
wavelength of 550 nm. SOD reduces the superoxide ion concentration
and thereby lowers blue formazan formation. The extent of reduction
in terms of appearance of blue formazan reflects the amount of SOD
activity in a sample.
Western blot analysis
The protein levels were assessed by western blot
analysis. Rat nephridial tissue total protein was extracted to
measure its protein content using the Bicinchoninic Acid (BCA)
method. A total of 50 μg of the protein was sampled, separated on
12% SDS-PAGE and transferred onto a nitrocellulose membrane with a
100 mA current. The membrane was then blocked for 1 h in 5% skimmed
milk powder in Tris-buffered saline with Tween-20 (TBS-T) at room
temperature and incubated overnight at 4°C with primary antibodies
(rabbit anti-rat NF-κB/p65 antibody diluted at 1:1,000). Following
washing the membrane three times in 0.05% Tween-20/PBS for 60 min,
it was incubated in HRP-anti rabbit IgG (diluted 1:10,000)
secondary antibody for 1 h at room temperature and then washed in
0.05% Tween-20/PBS again. Enhanced chemiluminescence reagent was
added and incubated with the membrane for one minute before it was
exposed to an X-ray film, developed and fixed. Using GAPDH as the
internal control, the relative ratios in all groups were measured
using National Institutes of Health Image software (NIH, Bethesda,
MD, USA; version 1.6).
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for analysis. Statistical data are presented as
the mean ± standard deviation. The results were assessed by
analysis of variance between the groups for comparison among
groups. A 95% confidence level was used for statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
UUO and UTI do not affect rat renal
function
There was no statistically significant difference
(P>0.05) between the levels of blood urea nitrogen (BUN) and
serum creatinine (Scr) among the rats in the different groups
(Table I).
 | Table IBiochemical test results among SOR,
UUO and UTI groups (mean ± standard deviation). |
Table I
Biochemical test results among SOR,
UUO and UTI groups (mean ± standard deviation).
| Group | Sample size (n) | BUN (mmol
l−1) | Scr (μmol
l−1) |
|---|
| SOR | 8 | 5.12±0.65 | 40.30±5.54 |
| UUO | 8 | 6.18±1.15 | 42.35±5.19 |
| UUO+UTI | 8 | 6.05±1.74 | 42.10±5.37 |
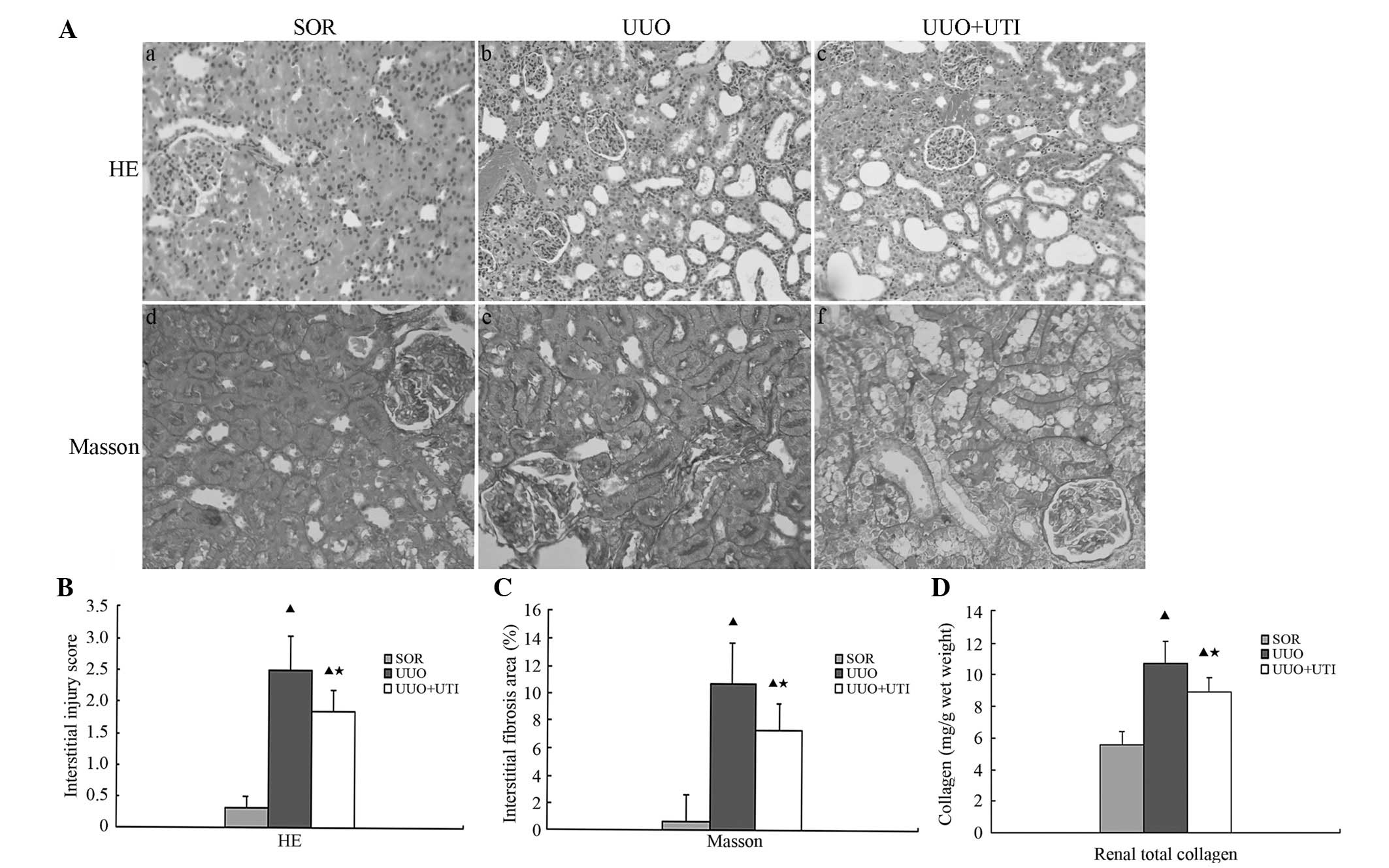
Ulinastatin improves renal interstitial
pathological injury in UUO
No apparent interstitial damage was observed in the
SOR group at either observation point. Interstitial inflammatory
cell infiltration, partial renal tubular expansion, loose
arrangement, swelling and vacuolar degeneration were observed in
the epithelial cells seven days following UUO; at 14 days after the
surgery, the above changes became significant. In addition, partial
epithelial cell exfoliation and partial renal tubular atrophy were
observed at this time-point. As compared with the UUO group, the
UTI group exhibited an attenuated inflammatory cellular
infiltration and markedly reduced tubular expansion and atrophy at
the two observation points (Fig.
1A). The renal interstitial injury score was significantly
higher in each UUO group as compared with that in the SOR groups
(P<0.01). The score was significantly lower in the UTI group
than that in the UUO group at the respective observation point
(P<0.05), although it remained higher than that of the SOR group
(P<0.01; Fig. 1B).
There was no evidence of interstitial fibrosis in
the rats’ kidneys following SOR as determined by Masson’s trichrome
staining for the degree of fibrosis. The interstitial fibrosis area
was significantly increased in each UUO group, particularly on day
14. The administration of UTI markedly decreased the area of
interstitial fibrosis in the UUO group (Fig. 1A). The percentage of renal
interstitial fibrosis area was significantly higher in every UUO
group, as compared with the SOR groups (P<0.01). It was
significantly lower in the UTI group than that in the UUO group at
the respective observation point (P<0.05), although it remained
higher than that in the SOR group (P<0.01; Fig. 1C). The renal total soluble collagen
(I–IV), which was measured with the Sircol collagen assay kit, in
the UTI group was significantly lower than that in the UUO group
(P<0.05; Fig. 1D).
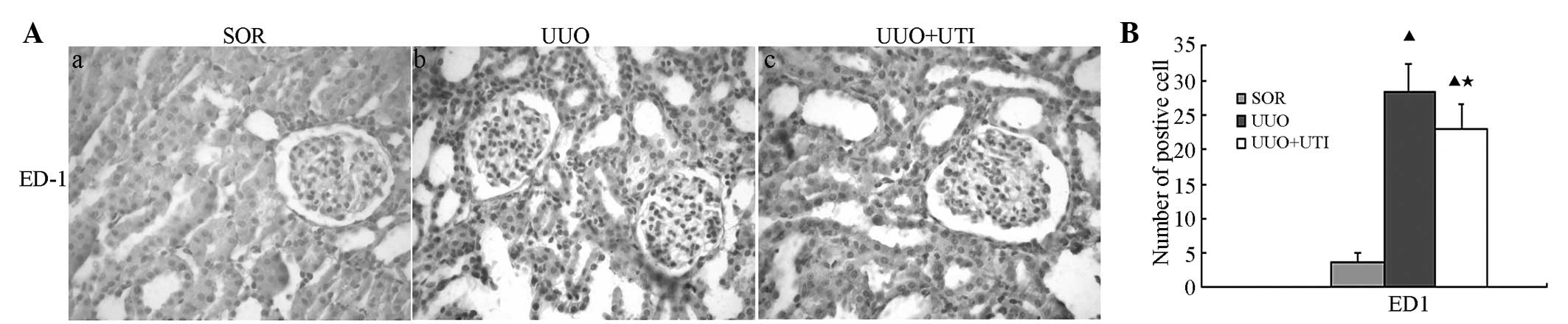
Ulinastatin reduces the expression of
CD68+ macrophages, TNF-α, IL-1β and NF-κB in the obstructed rat
kidney
The present study then examined whether UTI
treatment inhibited renal interstitial inflammation. For this, the
expression of indicative pro-inflammatory and cytotoxic mediators,
namely CD68+ macrophages, IL-1β and TNF-α, were assessed in the
whole kidney by immunohistochemistry. CD68+ macrophages were
detected on day seven following UUO in the cortical and medullar
areas of the kidney. Treatment with UTI led to a clear reduction in
the interstitial monocyte/macrophage levels (Fig. 2). In the UUO group, the tissue
expression levels of IL-1β and TNF-α were significantly higher than
those in the SOR group. Of note, the levels of these mediators in
the UUO rats treated with UTI were significantly lower than those
in the obstructed kidneys (Fig.
3). Semi-quantitative analyses using immunohistochemistry
demonstrated significantly enhanced IL-1β and TNF-α expression in
the UUO group compared with the SOR group (P<0.05). In
comparison with the UUO group, the UTI group exhibited
significantly reduced IL-1β and TNF-α expression (P<0.05;
Fig. 3B).
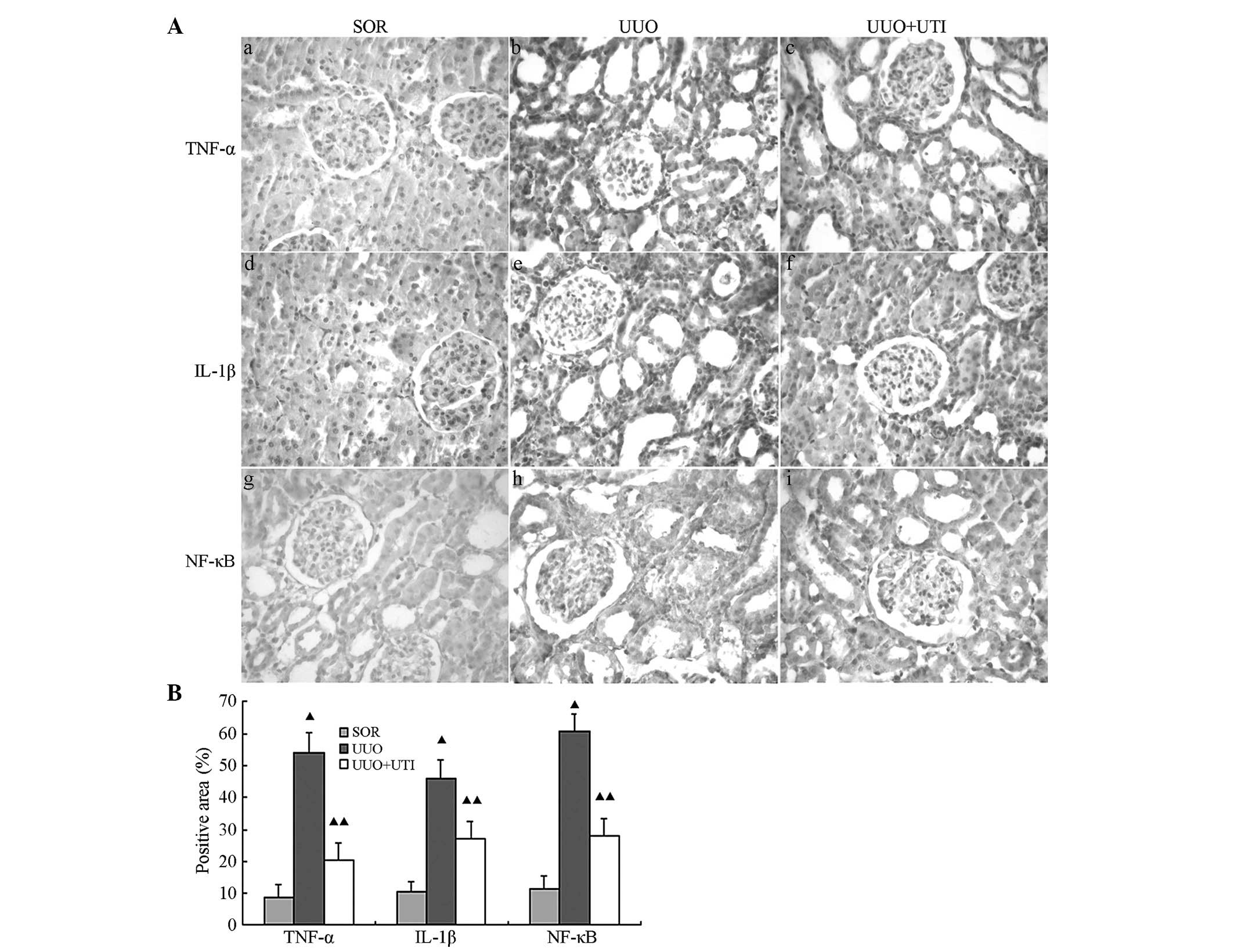 | Figure 3UTI suppresses TNF-α, IL-1β and NF-κB
expression in the UUO model at day seven. (A) Immunohistochemical
detection of TNF-α, IL-1β and NF-κB in kidney tissue following SOR,
UUO, UUO+UTI (magnification, ×400). (B) Histogram of the
semiquantitative analysis results of TNF-α, IL-1β, NF-κB deposition
in the kidneys. All data are represented as the mean ± standard
deviation. The number of rats in each group was 8.
▲P<0.01, UUO and UTI vs. SOR and for UTI vs UUO. UTI,
ulinastatin; TNF-α, tumor necrosis factor-α; IL-1β interleukin 1β;
NF-κB, nuclear factor-κB; UUO, unilateral ureteral obstruction;
IHC, immunohistochemistry; SOR, sham operation. |
NF-κβ is a ubiquitous transcription factor involved
in the upregulation of numerous pro-inflammatory genes (15). NF-κβ binding sites in the promoter
region have been demonstrated in a variety of inducible cell
adhesion molecules and cytokines, including TNF (15). At least five genes belong to the
NF-κβ family, but the most common dimers are composed of the Rel A
(p65) and NF-κβ1 (p50) or NF-κβ2 (p52) subunits (15). In the majority of cell types, NF-κβ
dimers are sequestered in an inactive cytoplasmic complex by
binding to its inhibitory subunit, inhibitor-κB (I-κB). Upon
stimulation, I-κB undergoes phosphorylation, followed by
ubiquitination and rapid degradation through a proteasome-dependent
pathway (16). This pathway allows
translocation of free active NF-κβ complexes (including p65 and p50
subunits) into the nucleus, where they bind to specific DNA motifs
in the promoter/enhancer regions of target genes and activate
transcription (16).
NF-κB expression is located in the cell nucleus. The
SOR group showed low amounts of NF-κB protein expression in renal
glomerular endothelial and tubular epithelial cell nuclei. However,
the UUO group exhibited a highly positive expression for NF-κB,
while the expression following UTI treatment was notably lower
(Fig. 3Ag–l). Semi-quantitative
analyses using immunohistochemistry demonstrated significantly
enhanced NF-κB expression in the UUO group, as compared with the
SOR group (P<0.05), whereas in comparison with the UUO group,
the UTI group showed significantly reduced NF-κB expression
(P<0.05; Fig. 3B).
Ulinastatin reduces expression of TGF-β1
and Col-I in obstructed kidneys
TGF-β1 is an important profibrotic growth factor in
the progression of interstitial fibrosis. The activation of TGF-β1
has an important role in epithelial mesenchymal transition (EMT)
and renal fibrosis. The protein deposition of active TGF-β1 was
measured by immunohistochemistry. As revealed in Fig. 4A, the SOR group showed low amounts
of TGF-β1 protein expression in the tubular epithelial cell
cytoplasm of rat nephridial tissues and almost no expression in the
renal corpuscles. The post-operative UUO group exhibited TGF-β1
expression distributed throughout the renal tubule with cortical
and medullar damages, and the expression levels were significantly
augmented with increments in the tubular damage range and
deterioration of interstitial fibrosis. Following UTI treatment,
the expression of TGF-β1 and the degree of interstitial injury were
markedly reduced. At each observation point, the UUO group showed
notably higher TGF-β1 expression levels, as compared with the SOR
group (P<0.01). By contrast, the UTI treatment group exhibited a
notably lower TGF-β1 expression than the UUO group (P<0.01;
Fig. 4B).
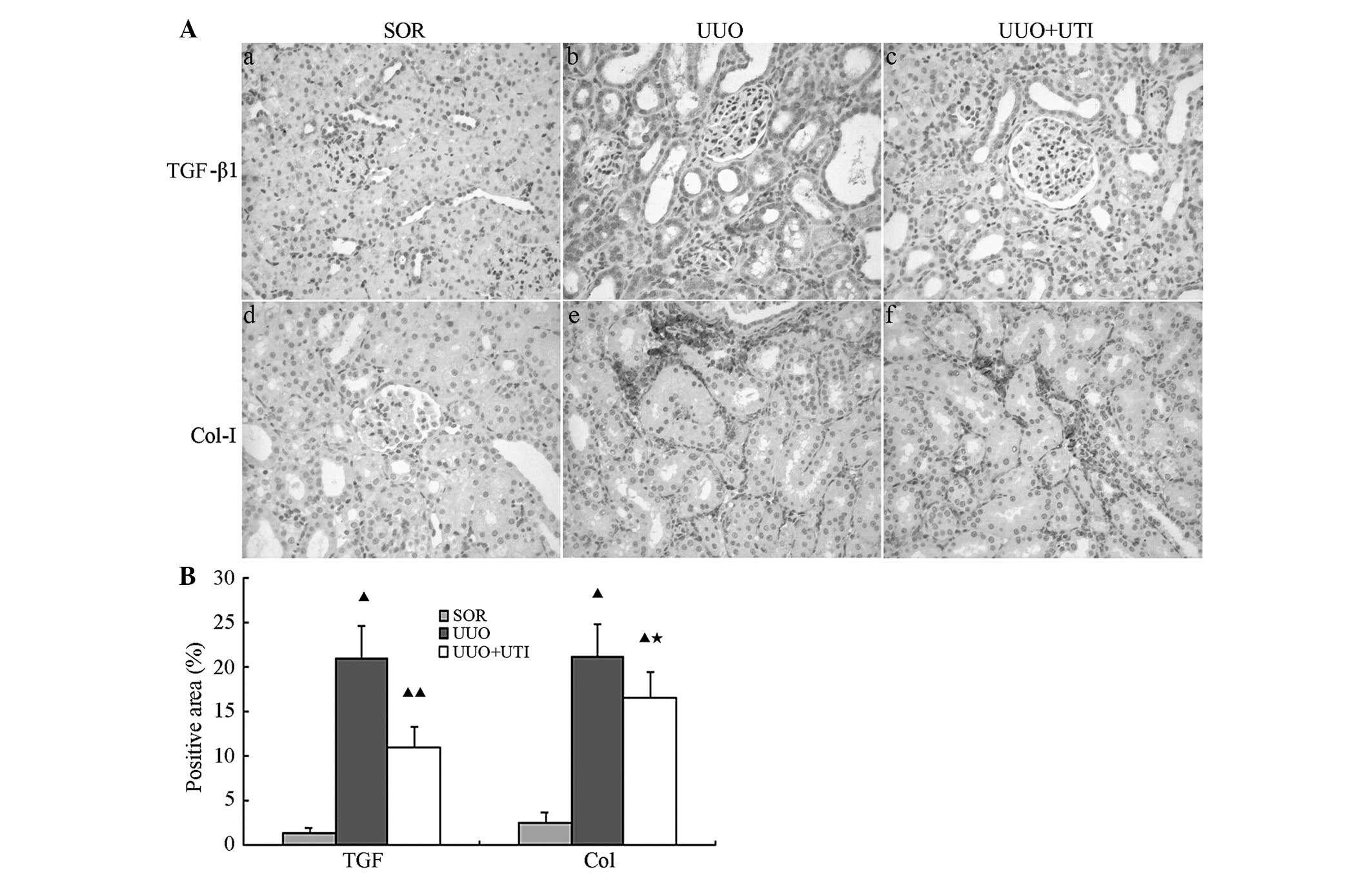 | Figure 4UTI suppresses TGF-β1 and Col-I
expression in the UUO model. (A) Immunohistochemical assessment of
TGF-β1 and Col-I in kidneys following SOR, UUO and UUO+UTI
(magnification, ×200). (B) Histogram of the semiquantitative
analysis results of TGF-β1 and Col-I deposition in the kidneys. All
data were presented as the mean ± standard deviation. The number of
rats in each group is 8. ▲P<0.01, UUO & UTI vs.
SOR; ★P<0.05, UTI vs. UUO; ▲P<0.01, UTI
vs. UUO. UTI, ulinastatin; TGF-β1, transforming growth factor-β1;
Col-I, type I collagen; UUO, unilateral ureteral obstruction; IHC,
immunohistochemistry; SOR, sham operation. |
Col-I is one of the extracellular matrix (ECM)
proteins and is most commonly expressed in the renal arterioles.
However, it is rarely present in the renal interstitium. There was
almost no Col-I protein expression in the renal interstitium
following SOR, whereas large quantities of interstitial Col-I
deposition were observed in the UUO group, the expression of which
increased with time. The Col-I levels were higher in the UUO group
as compared with those in the SOR group at each observation point.
Following UTI treatment, Col-I expression was reduced (Fig. 4Ad–f). Semiquantitative analyses of
the immunohistochemistry of Col-I in the obstructed renal tissues
revealed significantly higher Col-I expression levels in the UUO
group than those in the SOR group (P<0.01). Although the UTI
treatment group exhibited notably lower levels of Col-I expression
(P<0.01) in comparison with those in the UUO group, they
remained higher than those in the SOR group (P<0.01; Fig. 4B).
UTI suppresses oxidative stress in
UUO
To confirm whether UTI reduced renal tissue oxidant
enzyme levels and lipid peroxidation, MDA levels were measured in
the kidney tissue. In the UUO group, the average MDA levels were
significantly higher and the SOD levels were lower than those in
the SOR group (Table II).
Furthermore, the average levels of MDA in the UTI-treated
obstructed kidneys were significantly lower than those in the
obstructed kidneys, whereas SOD levels were significantly higher.
This indicated that UTI treatment was partially but significantly
capable of preventing oxidative stress.
 | Table IIResults of MDA and SOD among SOR, UUO
and UTI groups (mean ± standard deviation). |
Table II
Results of MDA and SOD among SOR, UUO
and UTI groups (mean ± standard deviation).
| Group | Sample size (n) | MDA (mmol/mg) | SOD (U/mg) |
|---|
| SOR | 8 | 2.89±0.33 | 278.00±25.31 |
| UUO | 8 | 5.59±1.25a | 161.70±21.77a |
| UUO+UTI | 8 | 3.83±0.97a,b | 207.20±44.16a,b |
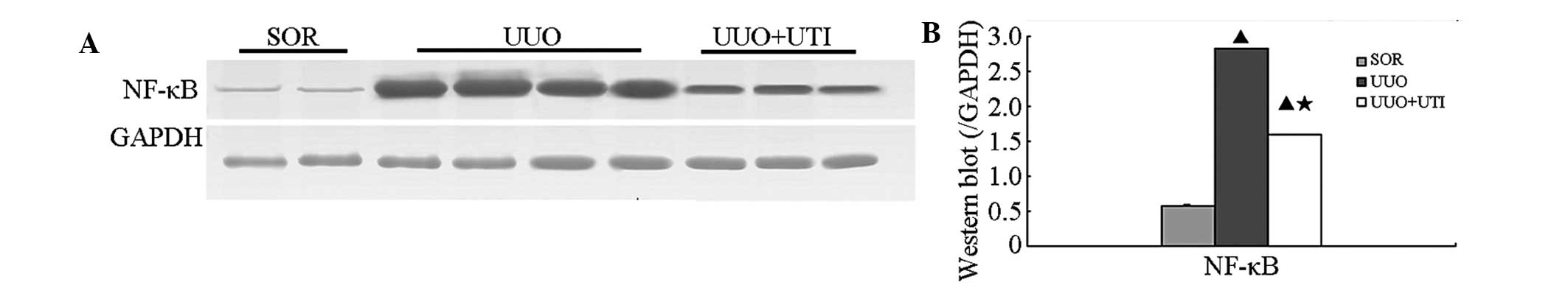
Ulinastatin reduces expression of NF-κB
by western blot analysis
To elucidate whether UTI is able to suppress NF-κB
expression in the obstructed kidney, NF-κB expression was examined
by western blot analysis. At each observation point, the SOR group
of the rats exhibited lower NF-κB protein levels. The UUO group of
rats exhibited significantly elevated NF-κB expression, while the
expression levels of the above proteins following UTI treatment
were notably lower (Fig. 5A).
Semiquantitative analyses of the western blots of
protein from the obstructed nephridial tissues of rats in the three
groups revealed that NF-κB expression levels were significantly
higher in the kidneys of rats in the UUO group than those in the
SOR group at each observation point (P<0.05). In comparison with
the UUO group, the UTI group showed notably lower NF-κB protein
expression levels (P<0.05; day seven; Fig. 5B).
Discussion
Among all causes of kidney damage, the lack of blood
or oxygen in renal tissue and subsequent activation of kidney
intrinsic cells releases a great amount of chemokines. Inflammatory
cell infiltration in the circulation is localized to the damaged
tissue, inducing a renal inflammatory response, which in turn leads
to the generation and secretion of inflammatory mediators and
profibrotic cytokines. Inflammatory mediators mediate the cascade
amplification and sustenance of inflammatory responses, and cause
apoptosis of renal tubular cells and kidney fibrosis. As such, the
renal inflammatory response is one of the actuating links in the
pathogenesis of kidney fibrosis (1,2).
Numerous studies have demonstrated that inhibition of UUO renal
interstitial inflammatory cell infiltration (12), as well as a reduction in the
expression of certain cytokines, including TNF-α, IL-1, NF-κB and
TGF-β1 (5), alleviate apoptosis of
renal tubular epithelial cells and the subsequent development of
interstitial fibrosis.
UTI has inhibitory effects on a number of enzymes,
including serine protease-like trypsin, α-chymotrypsin, neutrophil
elastase, hyaluronidase, sulfhydral enzymes and plasmin (6). Its functions include eradication of
oxygen free radicals, inhibition of inflammatory mediator
production, neutrophil activation and microvascular permeability
(7). It has numerous clinical
applications ranging from the treatment of acute pancreatitis,
shock, ischemia reperfusion injury, multiple organ dysfunction
(MODS) and acute respiratory distress syndrome (ARDS) to
administration post organ transplantation and extra-corporal
circulation operation, to inhibit inflammation and apoptosis,
protect cells, and improve circulation and blood coagulation
disturbance. In addition, UTI has immunomodulatory effects. It has
also been identified that by attenuating excessive inflammatory
reactions, ulinastatin protects lipopolysaccharide (LPS) and severe
burn-induced lung injury (7,17).
In another study, it was also able inhibit NF-κB activity and
alleviate live ischemia reperfusion injury (18). Furthermore, it was demonstrated to
decrease the expression of TNF-α, IL-6, caspase-3 and thus reduced
lipopolysaccharide/d-gal-induced acute liver damage (13) and myocardial ischemia reperfusion
injury (14). A previous study by
our group demonstrated that UTI improves renal fibrosis (19); however, the mechanism underlying
this effect remained elusive.
Animal studies have indicated that UTI significantly
increases superoxide dismutase (SOD) levels in the kidney tissue of
rats with kidney damage and lowers its serum creatinine (Scr) and
BUN (9) levels. It may also reduce
renal tubular epithelial cell necrosis, inflammatory cell
filtration and protect kidney function in rats with zymosan-induced
MODS (10). Biochemical
examination in the present study revealed no significant impact of
UUO or UTI on the kidney functions of the experimental rats. HE and
Masson staining results indicated that following UTI treatment,
UUO-inflicted rats had a significantly lower renal interstitial
injury index with significantly reduced amounts of renal
interstitial inflammation infiltration and area of fibrosis. This
indicated that UTI had protective effects on injuries in the rat
obstructed kidney tissues.
As one of the actuating links of obstructed kidney
injury, the inflammatory response involves the infiltration of
inflammatory corpuscles (primarily monokaryons/macrophages)
(20) and the release of
inflammatory mediators. Active radical oxygen species are important
inflammatory mediators. MDA is a product of the ROS lipid
peroxidation reaction, whose amount directly reflects that of ROS.
The main function of SOD is anti-oxidative activity. It indirectly
reflects the organism’s ability to eradicate ROS. The present study
revealed that UTI reduces the amount of renal interstitial
infiltration of CD68+ macrophages in rats with UUO, while
simultaneously lowering the expression of the primary inflammatory
factors IL-1β and TNF-α (P<0.05). With UTI intervention, the MDA
content was reduced and the SOD content increased again (P<0.05)
in the renal tissues, as compared with that in the UUO group. These
data demonstrated that UTI inhibited the generation of ROS and
hence it is concluded that ulinastatin is able to significantly
inhibit inflammatory responses in obstructed rat kidneys.
NF-κB, as a general nuclear transcription factor, is
a homo- or hetro-dimer consisting of two glutenin subunits, p65 and
p50, that resides in the center of inflammatory response. NF-κB
controls the gene expression of numerous inflammation-associated
substances, including downstream inflammatory cytokines (IL-1α,
IL-1β, IL-2, IL-6, IL-8, TNF-α, nitric oxide), inflammatory
chemokines (monocyte chemotactic protein-1 and regulated on
activation, normal T cell expressed and secreted), cell adhesion
molecules (intercellular adhesion molecule 1, vascular cell
adhesion molecule 1, E-selectin), a number of inducible enzymes
(cyclooxygenase-2, nitric oxide synthase), growth factors and
certain acute reactive proteins (21,22).
At the same time, it is regulated by the positive feedback of
certain cytokines (IL-1β, TNF-α), ROS as well as other molecules,
such as angiotensin II (16). As
such, NF-κB and its regulatory molecules together form the NF-κB
signaling pathway.
NF-κB is activated at an early stage of UUO and
participates in the pathological formation and progression of
kidney obstruction (22). By
inhibiting NF-κB activity, mononuclear cell infiltration and
inflammatory gene over-expression are reduced, and concurrently,
apoptosis and interstitial fibrosis are attenuated (23). In the present study,
immunohistochemistry and western blot analysis confirmed
significantly elevated levels of NF-κB deposition and protein
expression in the obstructed kidney tissues post UUO operation, as
well as reduced NF-κB expression with UTI intervention. It is
therefore concluded that UTI reduces the UUO-induced kidney
interstitial inflammatory response by inhibiting the NF-κB
signaling pathway. This also proves the existence of a positive
feedback regulation of IL-1β, TNF-α, ROS and NF-κB.
The ECM includes numerous components, including
collagen I, III, fibronectin and others. Under normal physiological
conditions, the generation and degradation of the ECM is balanced.
Excessive deposition of the ECM is one of the main pathological
changes in renal interstitial fibrosis. Currently, TGF-β1 is
recognized as one of the key factors in the formation and
progression of interstitial fibrosis. It may induce renal tubular
epithelial cell apoptosis, transdifferentiation of tubular
epithelial cells into myofibroblasts (MFB), as well as excessive
concentration of the ECM proteins, and eventually lead to
interstitial fibrosis (24–26).
The present study observed a significant increase in TGF-β1, Col-I
and α-SMA expression levels in nephridial tissue, as well as
interstitial fibrosis in the UUO group of rats. In comparison, the
UTI treatment group showed a significantly reduced TGF-β1 and ECM
concentration and ameliorated renal interstitial fibrosis.
In conclusion, UTI is able to inhibit the
inflammatory response and fibrosis in rats with UUO and appears to
have certain protective effects on the injury of the
obstruction-side kidney. As an endogenous protease inhibitor, UTI
has complex biological actions and thus, further studies are
required to elucidate the detailed mechanism underlying its renal
protective effects.
Acknowledgements
The present study was supported by the Doctoral
Research Fund of the Second Affiliated Hospital of Harbin Medical
University (BS2010-10) and the Harbin Science and Technology Bureau
(2006RFXS073).
References
|
1
|
Noronha IL, Fujihara CK and Zatz R: The
inflammatory component in Progressive renal disease - are
interventions possible? Nephrol Dial Transplant. 17:363–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Filiopoulos V and Vlassopoulos D:
Inflammatory syndrome in chronic kidney disease: pathogenesis and
influence on outcomes. Inflamm Allergy Drug Targets. 8:369–382.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chevalier RL, Thornhill BA, Forbes MS and
Kiley SC: Mechanisms of renal injury and progression of renal
disease in congenital obstructive nephropathy. Pediatr Nephrol.
25:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grande MT, Pérez-Barriocanal F and
López-Novoa JM: Role of inflammation in túbulo-interstitial damage
associated to obstructive nephropathy. J Inflamm (Lond).
7:192010.
|
|
6
|
Fries E and Blom AM: Bikunin - not just a
plasma proteinase inhibitor. Int J Biochem Cell Biol. 32:125–137.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang Y, Xu P, Gu C, et al: Ulinastatin
improves pulmonary function in severe burn-induced acute lung
injury by attenuating inflammatory response. J Trauma.
71:1297–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu M, Wen XH, Chen SP, An XX and Xu HY:
Addition of ulinastatin to preservation solution promotes
protection against ischemia-reperfusion injury in rabbit lung. Chin
Med J (Engl). 124:2179–2183. 2011.PubMed/NCBI
|
|
9
|
Gao C, Huan J, Li W and Tang J: Protective
effects of Ulinastatin on pancreatic and renal damage in rats
following early scald injury. Burns. 35:547–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Q, Liu X, Liu M, Zhang L and Guan Y:
Ulinastatin-mediated protection against zymosan-induced multiple
organ dysfunction in rats. Biologicals. 38:552–556. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Katoh H, Ishikawa H, Hasegawa M, et al:
Protective effect of urinary trypsin inhibitor on the development
of radiation-induced lung fibrosis in mice. J Radiat Res.
51:325–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lange-Sperandio B, Trautmann A, Eickelberg
O, et al: Leukocytes induce epithelial to mesenchymal transition
after unilateral ureteral obstruction in neonatal mice. Am J
Pathol. 171:861–871. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Chen YP, Wan R, Guo CY and Wang XP:
Protective effects of ulinastatin on acute liver failure induced by
lipopolysaccharide/D-galactosamine. Dig Dis Sci. 57:399–404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shin IW, Jang IS, Lee SM, et al:
Myocardial protective effect by ulinastatin via an
anti-inflammatory response after regional ischemia/reperfusion
injury in an in vivo rat heart model. Korean J Anesthesiol.
61:499–505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam NH: Naturally occurring NF-kappaB
inhibitors. Mini Rev Med Chem. 6:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baugé C, Beauchef G, Leclercq S, et al:
NFkappaB mediates IL-1beta-induced down-regulation of TbetaRII
through the modulation of Sp3 expression. J Cell Mol Med.
12:1754–1766. 2008.PubMed/NCBI
|
|
17
|
Gao C, Li R and Wang S: Ulinastatin
protects pulmonary tissues from lipopolysaccharide-induced injury
as an immunomodulator. J Trauma Acute Care Surg. 72:169–176.
2012.PubMed/NCBI
|
|
18
|
Wu YJ, Ling Q, Zhou XH, et al: Urinary
trypsin inhibitor attenuates hepatic ischemia-reperfusion injury by
reducing nuclear factor-kappa B activation. Hepatobiliary Pancreat
Dis Int. 8:53–58. 2009.
|
|
19
|
Ning XH, Ge XF, Cui Y and An HX:
Ulinastatin inhibits unilateral ureteral obstruction-induced renal
interstitial fibrosis in rats via transforming growth factor β
(TGF-β)/Smad signalling pathways. Int Immunopharmacol. 15:406–413.
2013.PubMed/NCBI
|
|
20
|
Ferenbach D, Kluth DC and Hughes J:
Inflammatory cells in renal injury and repair. Semin Nephrol.
27:250–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spann KM, Tran KC and Collins PL: Effects
of nonstructural proteins NS1 and NS2 of human respiratory
syncytial virus on interferon regulatory factor 3, NF-kappaB, and
proinflammatory cytokines. J Virol. 7:5353–5362. 2005. View Article : Google Scholar
|
|
22
|
Esteban V, Lorenzo O, Rupérez M, et al:
Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway,
regulates the inflammatory response in unilateral ureteral
obstruction. J Am Soc Nephrol. 15:1514–1529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyajima A, Kosaka T, Seta K, et al: Novel
nuclear factor kappa B activation inhibitor prevents inflammatory
injury in unilateral ureteral obstruction. J Urol. 169:1559–1563.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh YC, Wei WC, Wang YK, et al:
Transforming growth factor-{beta}1 induces Smad3-dependent {beta}1
integrin gene expression in epithelial-to-mesenchymal transition
during chronic tubulointerstitial fibrosis. Am J Pathol.
177:1743–1754. 2010. View Article : Google Scholar
|
|
25
|
Shirakihara T, Horiguchi K, Miyazawa K, et
al: TGF-β regulates isoform switching of FGF receptors and
epithelial-mesenchymal transition. EMBO J. 30:783–795. 2011.
|
|
26
|
Phanish MK, Wahab NA, Colville-Nash P,
Hendry BM and Dockrell ME: The differential role of Smad2 and Smad3
in the regulation of pro-fibrotic TGFbetal responses in human
proximal-tubule epithelial cells. Biochem J. 393:601–607. 2006.
View Article : Google Scholar : PubMed/NCBI
|