Introduction
Severe acute pancreatitis (SAP) is an acute
abdominal disease with high prevalence, severe symptoms,
complicated pathogenesis and mortality as high as 20–30% (1,2).
Although the mechanisms underlying SAP have not been fully
elucidated, changes in secretion patterns of pancreatic acinar
cells, intracellular activation of proteases and generation of
inflammatory mediators may be linked to SAP pathogenesis (1). Approximately 10% of patients with
acute pancreatitis show necrosis of the pancreatic and
peripancreatic tissues, leading to infection of the necrotic
tissue, multiple organ failure, and mortality (2). A few studies emphasized that the
major damage occurring in SAP patients is not necrosis of the
pancreas, but intestinal bacterial translocation, enterogenic
endotoxemia and secondary pancreatic infection (3,4). The
small intestine may become damaged during SAP due to alterations in
microcirculation associated with fluid loss, hypovolemia,
splanchnic vasoconstriction and ischemia-reperfusion injury, and
failure of the small intestine tends to aggravate the course of SAP
(5). Intestinal permeability,
which develops as a result of intestinal barrier damage early in
acute pancreatitis, is directly associated with endotoxemia
(6). Furthermore, increased gut
permeability has been suggested to be the initial event in the
bacterial contamination of pancreatic necrosis in SAP (4). Samel et al (3) focused on bacterial translocation
across the gut as a functional aspect of mucosal barrier function
during SAP in rats. The investigators directly observed the
translocation of fluorescent bacteria from the small bowel to the
pancreas, providing evidence supporting the gut origin of
microorganisms responsible for the infectious complications in SAP
(3). Lu et al (7) reported an increase in small
intestinal capillary leakage in a rat model of SAP; they concluded
that various inflammatory mediators and cytokines released during
SAP may directly attack capillary endothelial cells, resulting in
cell apoptosis and necrosis, and increasing intestinal barrier
permeability. Although disruption of the intestinal barrier
function appears to be a key step and possibly a turning point in
the progression of SAP, the patients with highest mortality risk
are those whose inflammatory response to pancreatic injury leads to
organ failure (5). Through the
action of a number of cascades, systemic inflammatory response
syndrome can eventually lead to multiple organ dysfunction syndrome
(8).
Mesenchymal stem cells (MSCs) isolated from various
tissues, including the stroma of bone marrow and adipose tissue,
have been demonstrated to exert therapeutic effects on intestinal
injury as well as inflammatory, cardiovascular, degenerative and
skeletal diseases (9–14). MSCs have the potential for
proliferation and multipotent differentiation into cells of
mesodermal, ectodermal and endodermal lineages (15–17).
Viable strategies to foster lineage-specific differentiation of
MSCs have been proposed, rendering feasible novel applications
(10). MSCs possess
immunoregulatory properties and have great potential for the
treatment of inflammatory response; they respond to inflammation by
homing to the inflamed tissues, which provides local control of
inflammation and facilitates tissue repair (14). During SAP, where translocation of
bacteria and toxins promotes the development of inflammation, human
bone marrow-derived MSCs were shown to reduce inflammation and
pancreatic tissue damage in a rat model of SAP, reducing the levels
of cytokines and suppressing rat T-cell proliferation (18). Considering the unpredictable course
of SAP and the absence of effective therapies, a cell-based
therapeutic strategy may be promising for SAP treatment. Additional
studies are needed to better understand the potential of MSCs to
limit pancreatic damage in SAP, which may possibly rely on
restoring the structure and function of pancreatic acinar cells
(18).
In the present study, MSCs obtained by multiple
digestions and passages of cells isolated from rat bone marrow were
injected into male Sprague Dawley (SD) rats with
taurocholate-induced SAP, in order to investigate the effects of
MSC transplantation on intestinal barrier function and bacterial
translocation. Our study aimed to investigate the mechanism
underlying MSC-induced repair of tissue injury. Results of this
study provide evidence for effective treatment of SAP with stem
cell transplantation.
Materials and methods
Materials
A total of 54 specific-pathogen-free adult male SD
rats, weighing 300±30 g, were purchased from the Shanghai
Laboratory Animal Co. (SLAC), Ltd. (animal license no. SCXK [Hu]
2007–0005; Shanghai, China). Animals were grown at 20–28°C in an
environment with 40–70% humidity. The animals were allowed to
accommodate to the environment for one week prior to the
experiments. This study used sodium taurocholate (Sigma-Aldrich,
St. Louis, MO, USA), CM-DiI (Thermo Fisher Scientific Inc.,
Waltham, MA, USA), enzyme-linked immunosorbent assay (ELISA) kits
for detection of tumor necrosis factor (TNF)-α and diamine oxidase
(DAO) (eBioscience Inc., San Diego, CA, USA), rabbit anti-rat
monoclonal antibody targeting aquaporin (AQP)-1 (Cell Signaling
Technology, Danvers, MA, USA) at a 1:2,000 dilution and
biotin-conjugated rabbit anti-rat secondary polyclonal antibody at
a 1:100 dilution (Cell Signaling Technology, Danvers, MA, USA). The
amylase detection kit was purchased from Meikang Biotechnology
(Ningbo, Jiangsu, China).
Preparation of CM-Dil-conjugated
MSCs
MSCs were isolated from aseptically collected and
cultured bone marrow from sacrificed male rats by multiple
digestions and passages as follows: First, MSCs were digested with
0.25% trypsin (Hyclone, Logan, UT, USA) in 0.1% EDTA (Gibco-BRL,
Hercules, CA, USA), and then serum containing medium was used to
terminate the digestion reaction. Cells were harvested by
centrifugation at 139.875 × g for 10 min. The supernatant was
removed, and cells were washed in phosphate-buffered saline (PBS)
once. The cell suspension (106 cells/ml) was prepared
with serum-free medium, and then CM-DiI labeling solution, a
fluorescent dye that covalently conjugates to the thiol group in
the cells, was added at 5 μl/ml of medium. The cells were
resuspended and incubated at 37°C for 20 min. After centrifugation
at 139.875 × g for 5 min, the supernatant was removed and cells
were washed in PBS twice. Following trypan blue staining, viable
cells were counted. CM-DiI-conjugated MSCs were injected into the
rats via the dorsal penile vein. The rat intestine was collected,
freeze-sectioned (5-μm sections) and observed by fluorescence
microscopy (IX51 Biological Inverted Microscope, Olympus, Tokyo,
Japan).
Establishment of the SAP rat model
Animals were deprived of food for 12 h, but given
access to water ad libitum. Following intraperitoneal
anesthesia with 10% chloral hydrate at 3.0 ml/kg, the rat abdomen
was sterilized and a midline incision was performed at 3 cm from
the lower part of the xiphoid. The pancreas was exposed, and the
bile duct was clamped with a non-invasive clamp at the porta
hepatic. Then, a 24-G trocar was inserted via the contralateral
intestine, and the stylet was withdrawn. The outer cannula was
inserted forward to the main pancreatic duct (0.5-cm) and fixed.
Next, 4% sodium taurocholate (0.1 ml/100 g) was injected by a
micropump at a rate of 0.1 ml/min. The cannula was left in the
pancreatic duct for 5 min and the injection pressure was
maintained. Pancreatic edema and congestion were observed, and then
the clamps and cannula were removed. The intestine was sutured and
returned to the abdominal cavity, followed by wound closure. After
surgery, 2 ml of normal saline were subcutaneously injected for
fluid supplementation.
Study design and procedures
Animal groups
SD rats were randomly assigned to 3 groups (n=24
each): SAP, SAP + MSCs and sham-operated (SO). Rats in the SAP and
SAP + MSCs group received retrograde injection of 4% sodium
taurocholate via the biliopancreatic duct to induce SAP, as
described above. MSCs (2×106 cells/100 g) were injected
into half (n=24) of the SAP-induced rats via the dorsal penile vein
1 h later (SAP + MSCs group). Rats in the SO group received
laparotomy, and the pancreas and intestine were massaged, followed
by wound closure. The SAP and SAP + MSCs group animals were
sacrificed at 6, 12, 24 and 48 h after SAP induction. The SO group
animals were sacrificed 6, 12, 24 and 48 h after surgery.
Detection of α-amylase
The temporal changes in the level of amylase were
measured in the 3 groups at 6, 12, 24 and 48 h after SAP induction.
The enzymatic activity of serum amylase was detected using
ethylidene-4-nitrophenyl-α-D-maltoheptaoside as a substrate, and
were quantified using a spectrophotometer (7150; Hitachi Ltd.,
Tokyo, Japan). The amylase activity was calculated using the
equation: Amylase activity (U/L) =
(DAsample/min-DAcontrol/min) * F, where F =
VT/VS/mɛ * P. VT, total volume;
VS, sample volume; P=1 cm, mɛ (molar extinction
coefficient) for p-nitrophenylcarbimide (pNP) = 10.567, and F=4826
in the test.
Detection of TNF-α and DAO
Blood was collected from the rat abdominal aorta and
the plasma was stored at −70°C. The TNF-α level and the DAP
activity were detected by ELISA kits, following the manufacturer’s
recommendations.
Intestinal histopathology and
immunohistochemical detection of AQP-1
The terminal ileum (~5 cm) was collected and
processed in paraffin-embedded sections (4 μm). Then, hematoxylin
and eosin (H&E) staining was performed, and sections were
observed under a light microscope (Leica DM 2000, Leica
Microsystems, Wetzlar, Germany). The intestine was collected and
fixed in 4% paraformaldehyde. Following dehydration, tissues were
embedded in paraffin, followed by sectioning (4 μm). After
deparaffinization and hydration, antigen retrieval was performed in
citrate acid (pH 6.0). Endogenous peroxidase was inactivated with
3% H2O2. Following incubation with the
primary anti-AQP-1 antibody at room temperature, sections were
treated with biotin-conjugated rabbit anti-rat secondary antibody,
followed by visualization with 3,3′-diaminobenzidine (DAB).
Counterstaining was done with H&E, followed by dehydration,
transparentization and drying. After mounting in neutral gum, the
sections were observed under a light microscope.
Pathological scoring of intestinal
tissue
Intestinal tissue samples were fixed in 10%
formaldehyde and processed in paraffin-embedded sections. Two
sections were selected from each rat. H&E staining was
performed, and the sections were observed under a light microscope.
For each section, 50 fields (×200) were randomly selected and
scored using the scoredescribed by Chilu et al (19).
Statistical analysis
Data were expressed as mean with standard deviation
(SD), and presented in bar charts. Comparisons were performed with
analysis of variance (ANOVA), and resulting p-values were adjusted
for multiple testing with the Bonferroni method (20). Data were analyzed using the SPSS
15.0 statistical software (IBM, Armonk, NY, USA). P<0.05 were
considered to indicate statistically significant differences.
Results
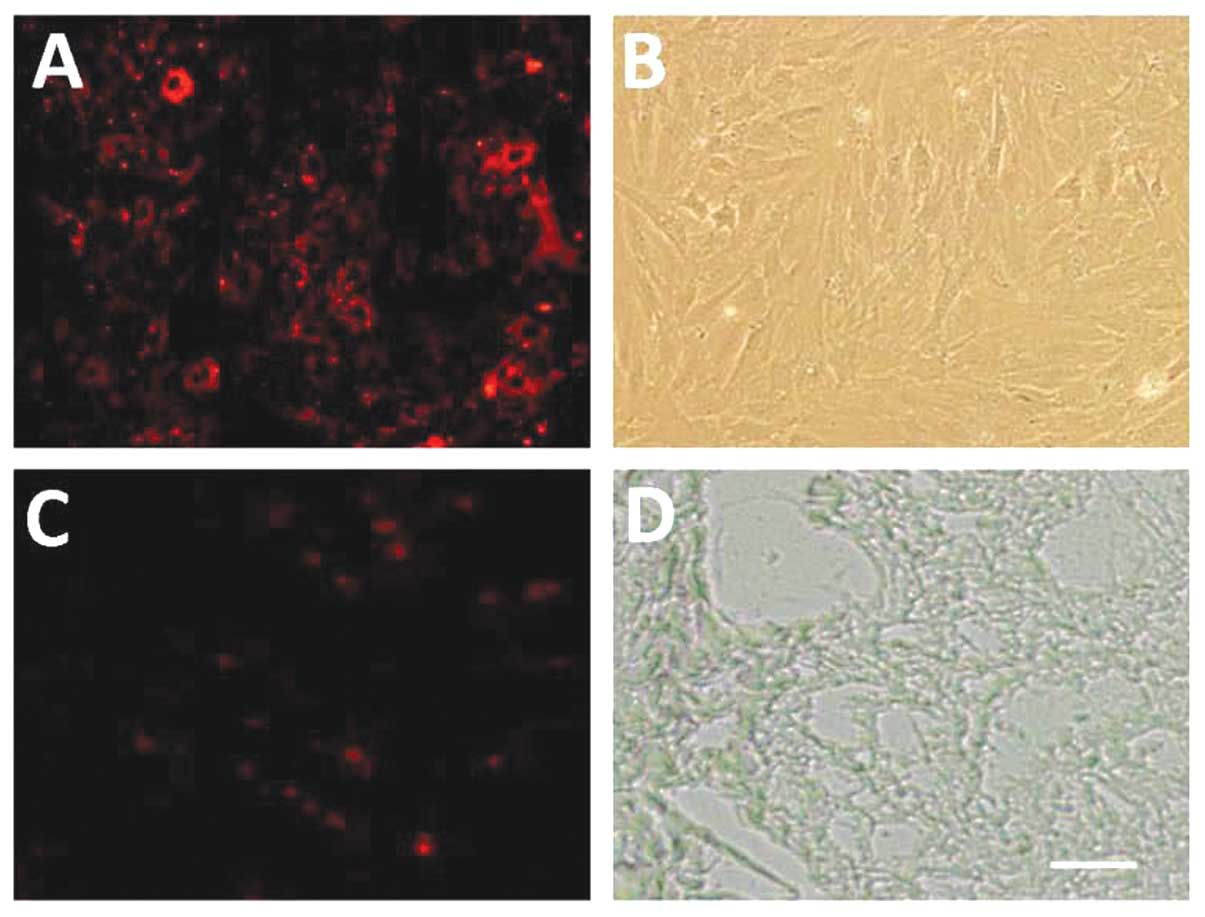
In vitro tracing and location of
CM-DiI-labeled MSCs in the intestinal tissue
Following CM-DiI labeling, cells were observed under
a fluorescence microscope. CM-DiI-labeled MSCs displayed red
fluorescence. The adherent cells were spindle-like. Passage 0 MSCs
were circular, with numerous granules. The fluorescence intensity
was high, but not detected in the nucleus (Fig. 1A and B). Fig. 1C shows the fluorescence of frozen
sections at the blue channel, revealing CM-DiI-positive cells in
the intestine. Fig. 1D shows a
frozen observed under a phase contrast light microscope.
Detection of markers for decreased
intestinal barrier integrity
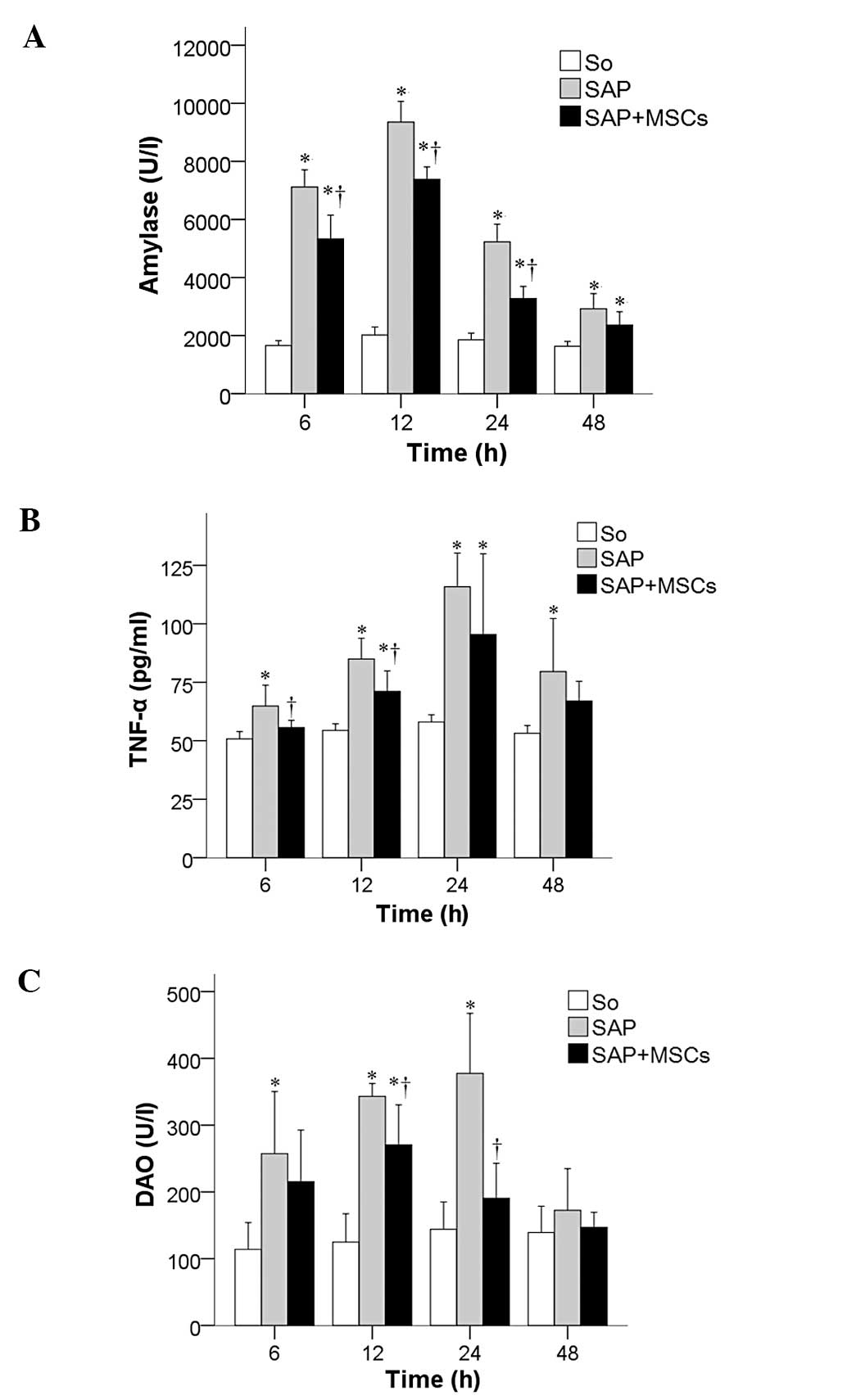
The temporal changes in the levels of intestinal
barrier integrity biomarkers amylase, TNF-α and DAO were measured
in the SAP, SAP + MSCs and SO groups at 6, 12, 24, and 48 h after
SAP induction. Amylase (Fig. 2A),
TNF-α (Fig. 2B) and DAO values
(Fig. 2C) were significantly
increased in the SAP group compared to the SO group, and were
decreased in the SAP + MSCs group compared to the SAP group (all
P<0.05).
Pathological analysis of pancreatic
tissues
The structure of the pancreatic lobules as observed
under the light microscope was normal. Interstitial edema and
infiltration were detected in a few inflammatory cells, and the
alveoli were normal at 6 h after SAP induction in the SAP group
(Fig. 3A). At 12 h post-SAP,
infiltration of inflammatory cells was detected in the pancreatic
interstitial tissue, accompanied by hemorrhage, which was more
severe compared to that observed 6 h post-SAP induction. Vacuolar
degeneration was observed in the pancreatic lobules, the alveoli
were largely disrupted, and hemorrhage and necrosis were detected
in the adipose tissues (Fig. 3C).
At 24 h post-SAP, massive necrosis was observed in the pancreas,
blood vessels in the interstitium were markedly enlarged and
hemorrhage occurred, infiltration of inflammatory cells was
obvious, and alveoli were severely disrupted (Fig. 3E). At 48 h post-SAP, necrosis of
the pancreas was aggravated, and vacuolar degeneration, congestion
of blood vessels in the interstitium, infiltration of numerous
inflammatory cells, patchy necrosis of adipose tissues, hemorrhage
and saponification were observed (Fig.
3G). Injection of MSCs had protective effects on rats with
taurocholate-induced SAP, overall reducing the damage in the
pancreas (Fig. 3B, D, F and
H).
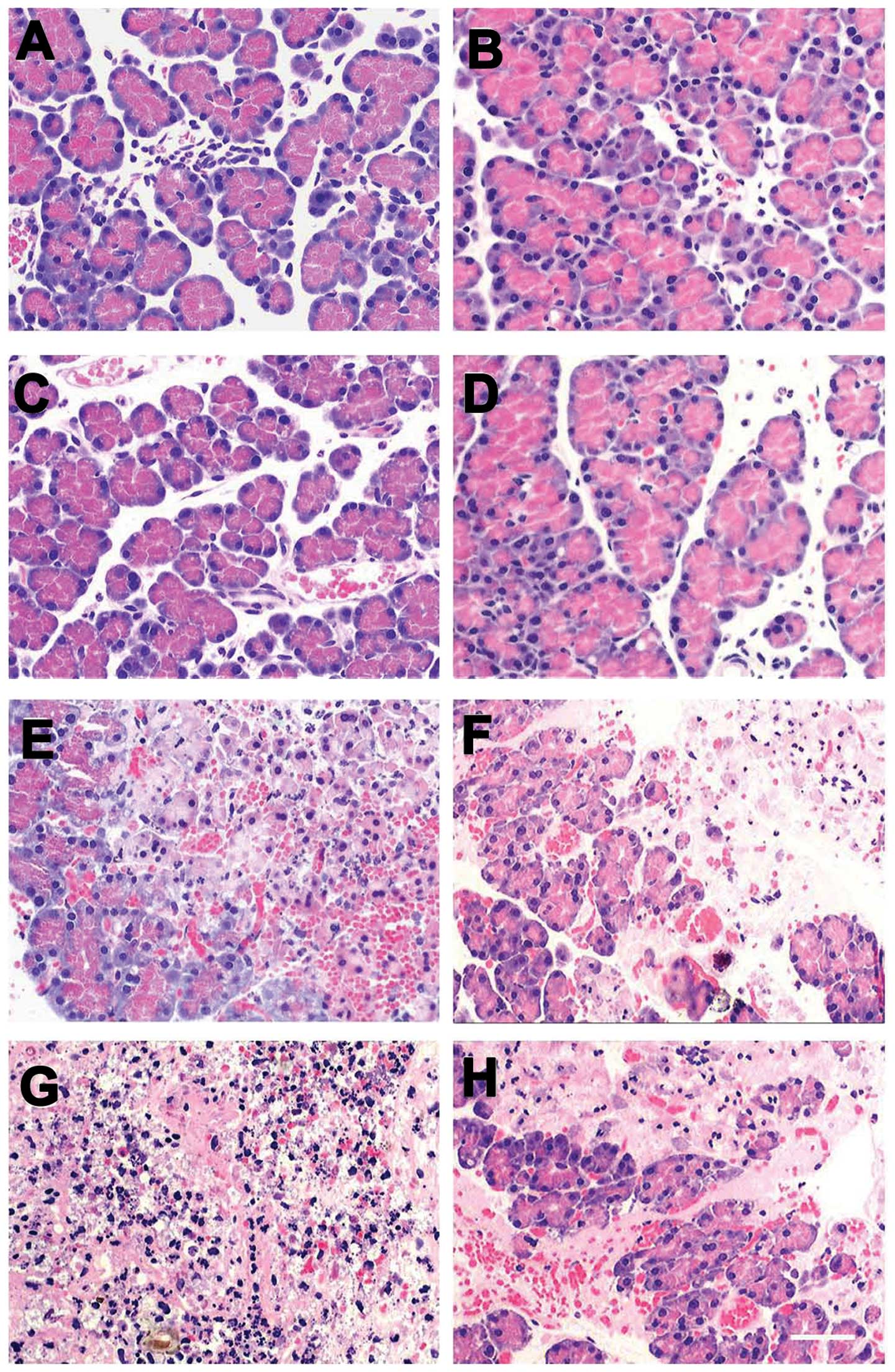 | Figure 3Temporal changes in histology of the
affected pancreas. Pancreatic sections stained with hematoxylin and
eosin (H&E) are from either SAP-(A, C, E and G) or SAP +
MSCs-treated (B, D, F and H) rats at different time points (6 h, A
and B; 12 h, C and D; 24 h, E and F; 48 h, G and H). Scale bar, 100
μm. SAP, severe acute pancreatitis; MSCs, mesenchymal stem
cells. |
Pathological analysis of intestinal
tissues
Under a light microscope, mild edema was observed in
the ileal interstium, accompanied by spotty hemorrhage. In
addition, the epithelium and lamina propria were mildly separated
at 6 h post-SAP induction in the SAP group (Fig. 4A). At 12 h, the mucosal injury of
the ileum deteriorated as compared to that observed at 6 h post-SAP
induction, and interstitial edema and infiltration of a few
inflammatory cells were detected (Fig.
4C). At 24 h post-SAP, the villous edema of the ileum was
obvious, and erosion, necrosis and shedding of intestinal villi
were observed, accompanied by infiltration of numerous inflammatory
cells (Fig. 4E). At 48 h post-SAP,
the mucosal injury of the intestine was alleviated, and some repair
of the intestinal mucosa was noted (Fig. 4G). At 6 h after treatment with
MSCs, disruption at the top of the intestinal villi, interstitial
edema and infiltration of neutrophils were attenuated when compared
to those observed in the SAP group at corresponding time points
(Fig. 4B). At 12 h after MSC
transplantation, necrosis and shedding of the intestinal villi, and
infiltration of inflammatory cells were improved compared to the
SAP group at corresponding time points (Fig. 4D). At 24 h, lodging, shortening and
loosening of intestinal villi were also observed, but the extent of
the damage and the infiltration of inflammation were alleviated
when compared to that observed in the SAP group at corresponding
time points (Fig. 4F). At 48 h
post-MSC transplantation, pathological changes were improved in the
SAP + MSCs compared to the SAP group at corresponding time points
(Fig. 4H).
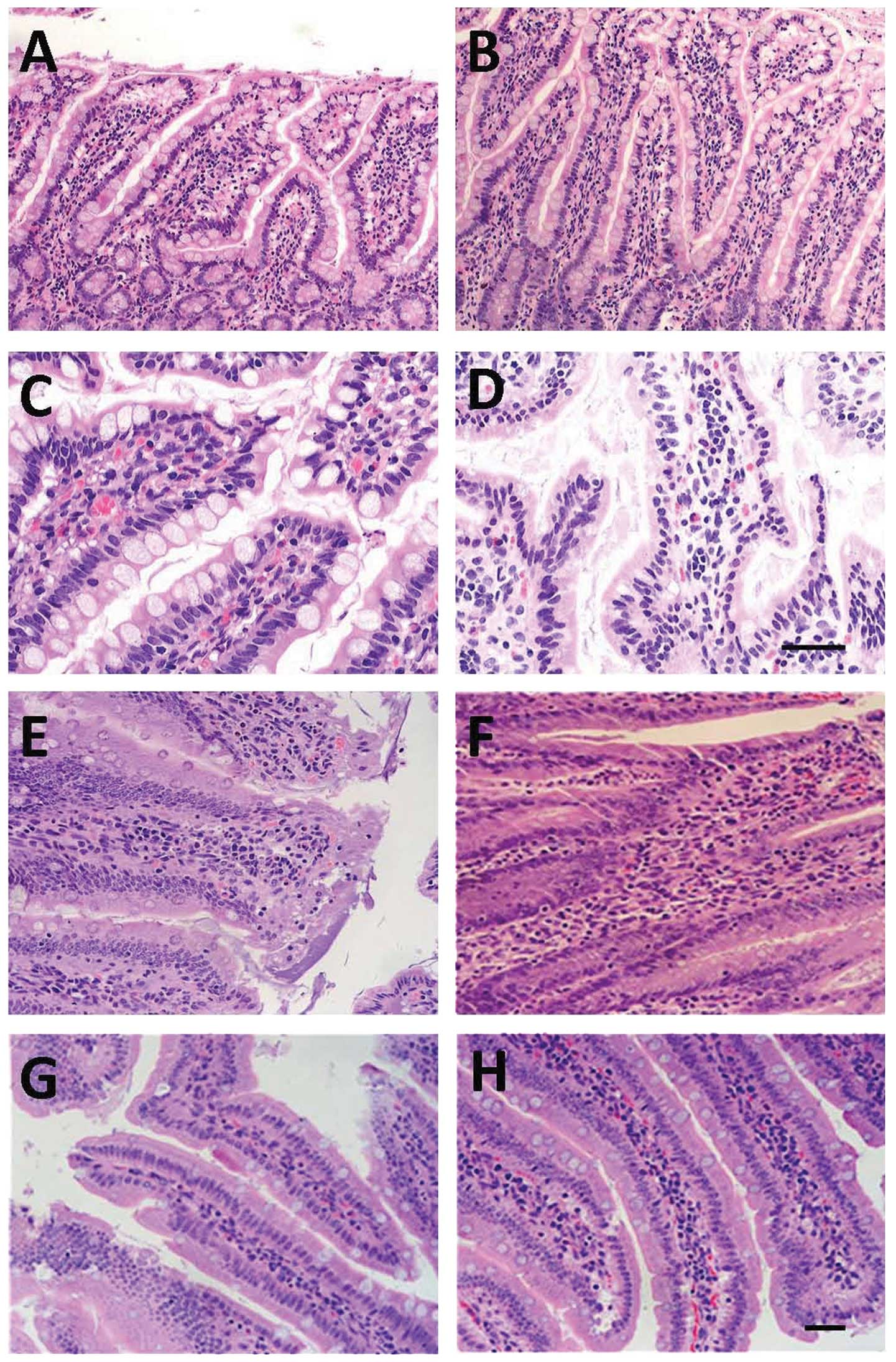 | Figure 4Temporal changes in histology of the
affected intestine. Intestinal sections stained with hematoxylin
and eosin (H&E) are from either SAP-(A, C, E and G) or SAP +
MSCs-treated (B, D, F and H) rats at different time points (6 h, A
and B; 12 h, C and D; 24 h, E and F; 48 h, G and H). Scale bar, 100
μm for panels A, B, E, F, G and H, and 50 μm for panels C and D.
SAP, severe acute pancreatitis; MSCs, mesenchymal stem cells. |
Immunohistochemical detection of
AQP-1
Immunohistochemistry analysis showed that AQP-1 is
highly expressed in the capillaries, small blood vessels and
endothelial cells of the central chyle duct of the intestine (brown
cells; Fig. 5A). At 6 h after SAP,
AQP-1 expression was markedly reduced compared to the SO group at
different time points (Fig. 5B, C, E
and G), with the lowest level detected at 24 h after SAP
induction (Fig. 5E). At 12 h after
MSC transplantation, AQP-1 expression in the intestine was markedly
increased when compared to the SAP group at different time points
(Fig. 5D, F and H).
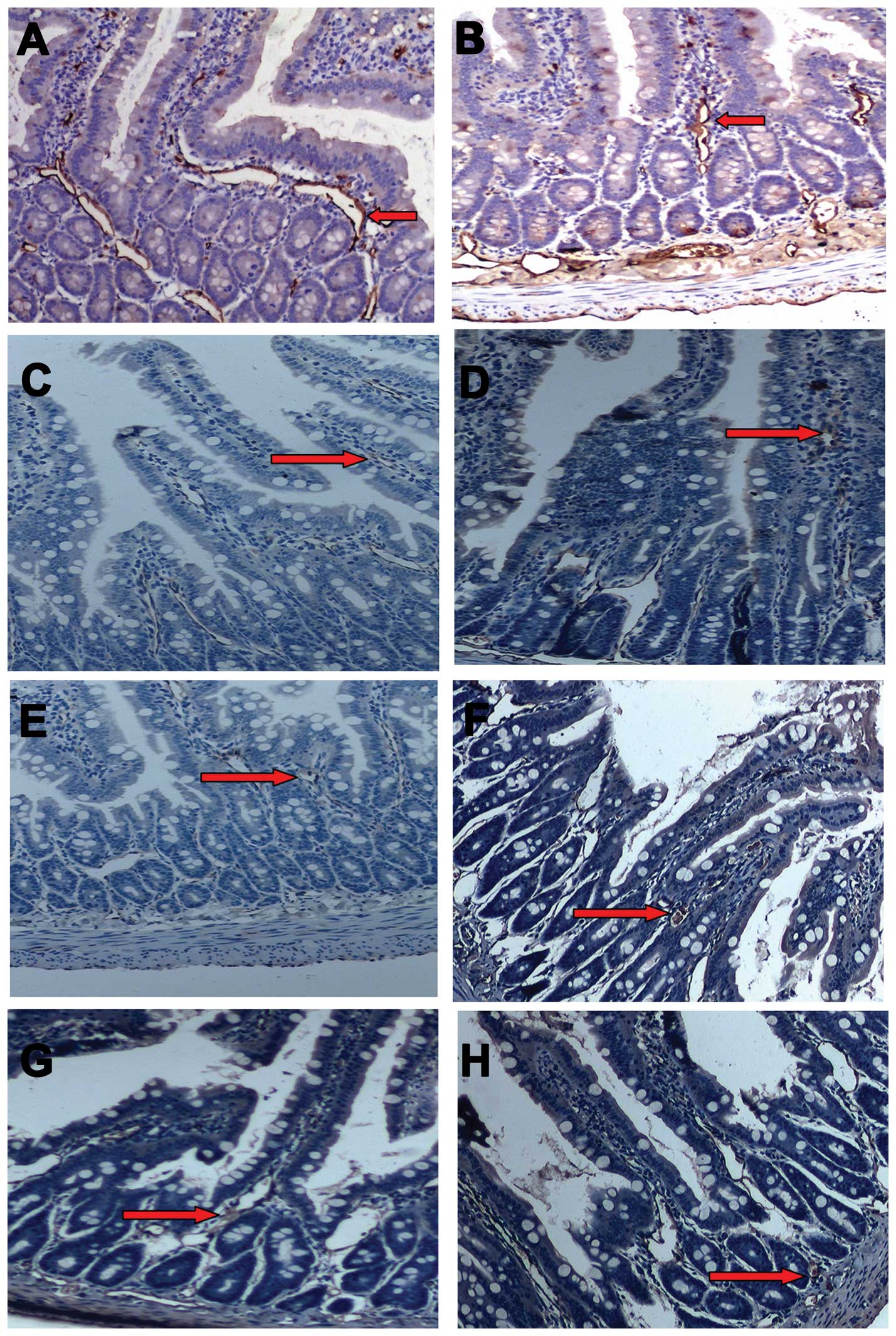 | Figure 5Immunohistochemical detection of
aquaporin (AQP)-1 in affected intestines. Intestinal sections from
the SO (A) and SAP (B) groups at 6 h, and from the SAP (C, E and G)
and SAP + MSCs (D, F and H) groups at three time points (12 h, C
and D; 24 h, E and F; 48 h, G and H) were immunostained with an
antibody targeting AQP-1. Scale bar, 50 μm. SO, sham-operated
group; SAP, severe acute pancreatitis; MSCs, mesenchymal stem
cells. Red arrows, positive staining for AQP-1. |
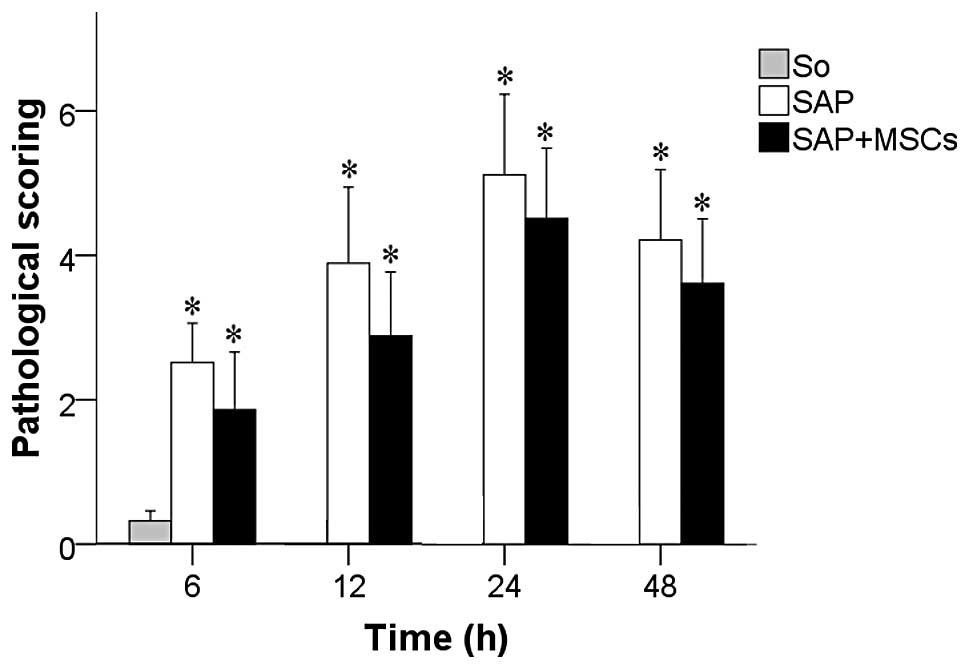
Pathological scoring of intestinal
tissue
The comparison among the 3 groups with regards to
the intestinal tissue pathological scores, obtained by applying the
Chiu scoring method (19), is
shown in Fig. 6. The H&E
staining levels in both the SAP and the SAP + MSCs group
continuously increased within 6–24 h, and then decreased at 48 h,
and were significantly higher compared to the SO group at all time
points (6, 12, 24 and 48 h). Although rats of the SAP + MSCs group
showed a higher staining level compared to those of the SAP group
at all time points, these differences were not statistically
significant (Fig. 6).
Discussion
In the present study, rats with taurocholate-induced
SAP were treated with MSCs, and results demonstrated that
intestinal injury was prominently improved compared to rats that
were not treated with MSCs. The transplantation of MSCs reduced the
levels of the intestinal enzyme amylase and the production of the
inflammatory cytokine TNF-α. In addition, the DAO level reached a
peak at 12 h after SAP induction and then was gradually reduced,
indicating that a reduced level of the enzyme was being released
from mucosal epithelial cells in the injured tissue after MSC
transplantation. These findings, together with those of
pathological examination and microscopy, suggest that treatment
with MSC contributes to the prevention or attenuation of intestinal
injury during SAP, including improvement of the capillary
permeability and of intestinal microcirculation. In addition, we
observed that AQP-1 expression was lower in the SAP compared to the
SO group starting from 6 h after SAP, reaching the lowest
expression level at 24 h post-SAP induction. However, following MSC
treatment, AQP-1 expression was significantly increased in the
intestine as compared to the SO group from the 12-h time point
onwards. Thus, we hypothesize that MSCs may inhibit systemic
inflammation and reduce TNF-α release, which enhances the activity
of the AQP-1 promoter, and upregulates mRNA and protein
expression of AQP-1, leading to the attenuation of the intestinal
barrier dysfunction.
Under normal conditions, the intestinal barrier is
composed of mechanical, immune, biological and chemical barriers,
which can potently inhibit the translocation of intestinal bacteria
and toxins into extraintestinal tissues and organs, and protect
from injury induced by endogenous microorganisms and toxins.
However, at the early stage of SAP, the white blood cells in the
pancreas and intestinal mucosa are overactivated and produce
numerous inflammatory cytokines [e.g., interleukin (IL)-1, IL-6,
IL-8, TNF-α and endotoxin (ET)] that mediate mucosal inflammation,
resulting in damage of the intestinal barrier. A functional
intestinal barrier does not allow endotoxins and DAO from the
mucosal epithelial cells to enter the circulation. However, when
the integrity of intestinal epithelial cells is altered and gut
permeability increases, DAO may be released into the circulation.
The translocation of intestinal bacteria (21) and endotoxemia induced by intestinal
endotoxins may cause a ‘second strike’ (8,22),
resulting in secondary pancreatic infection and a cascade of
inflammatory responses. Two studies by Towne et al (23,24)
confirmed that TNF-α can activate the nuclear factor (NF)-κB
pathway via the TNF-α receptor, which then downregulates AQP-5
expression. AQP-1 and AQP-5 are homologous. Thus, we hypothesize
that TNF-α can regulate AQP-1 via a similar mechanism in
SAP-induced intestinal barrier dysfunction. It has been shown that
AQP-1 is widely expressed in the capillaries, small blood vessels
and endothelial cells of the central chyle duct in the
gastrointestinal system, and that it mediates the transmembrane
transport of water in the gastrointestinal tract (25). Intestinal edema manifests at the
early stage of SAP. It may affect cellular viability, aggravate
ischemia/hypoxia-induced injury and result in diffusion of
intestinal bacteria and endotoxins into other organs and tissues,
which may finally lead to systemic infection and multiple organ
dysfunction (8). Thus, maintaining
the intestinal barrier and preventing the translocation of bacteria
and endotoxins may protect from the above-described ‘second strike’
following SAP.
MSCs are a group of non-hematopoietic stem cells
having the potential for self-renewal and multilineage
differentiation. MSCs have been used in autologous transplantation,
transfection of exogenous genes and regulation of gene expression
(9,11,12,13).
In addition, MSCs have the unique property of specifically homing
to damaged tissues. This is especially valuable in SAP, since the
MSCs respond to inflammation by homing to the inflamed tissues,
providing local control of the inflammation and facilitating tissue
repair (11,14). MSCs may exert a protective effect
on the intestinal barrier of animals with SAP via the following
mechanisms: Numerous injury-induced cytokines in the
microenvironment promote the differentiation of MSCs into specific
tissues. MSCs also secrete numerous cytokines and chemokines at the
injured site (25). MSCs enter the
circulation at the injured sites, which improves the focal
circulation and blood supply (26). This also improves the nutritional
status, which is beneficial for the recovery of the cells and
subsequently, of the tissues. We suggest that this may be one of
the mechanisms underlying MSC-induced repair of injured tissues. In
addition, MSCs have potent ability to regulate the immune system.
Previous studies showed that MSCs can downregulate the expression
of pro-inflammatory cytokines such as IL-1 and TNF-α, and that of
inducible nitric oxide synthase (iNOS), but upregulate the
expression of anti-inflammatory cytokines such as IL-1 and
transforming growth factor (TGF), which attenuate inflammation and
improve tissue injury (14,27).
Jung et al (18) showed
that the migration of MSCs into the inflammatory site induces the
expression of forkhead box P3 (Foxp3), inhibits T cell
proliferation, attenuates inflammation and facilitates tissue
repair. Finally, the transplanted MSCs localize in the submucosal
layer of the injured intestine and then differentiate into
epithelial cells, which promotes mucosal recovery (28,29).
These cells can also differentiate into intestinal subepithelial
myofibroblasts (ISEMFs), which may improve the microenvironment for
stem cells and indirectly promote intestinal epithelial cell repair
and angiogenesis in the intestine following injury (30).
Although previous studies have reported the
effectiveness and safety of using bone marrow stem cells in the
treatment of intestinal ischemia/reperfusion, the use of MSCs still
has limitations: i) in vitro-transplanted MSCs tend to
rapidly proliferate. Thus, following transplantation into animals,
they may transform into malignant cells (potential tumorigenesis)
(31); ii) MSCs have a large
volume and potent adherent ability. The transplantation of MSCs via
the tail vein usually has poor efficacy, since the majority of MSCs
stay in the lung and only a few MSCs reach the injured site to
exert therapeutic effects. Thus, it is difficult to optimize the
number of MSCs and the frequency of transplantation; iii) although
a few MSCs locate in the intestine, whether or not these cells may
differentiate into intestinal epithelial cells for further repair
of tissue is still unclear. These limitations in MSC
transplantation may further limit the interpretation of our
results.
Additional studies are needed to confirm our
findings on the anti-inflammatory and immunomodulatory properties
of MSCs. Our previous study (32)
reported that MSCs can relieve injury of pancreatic acinar cells in
a rat model of SAP, attenuate inflammation and injury in the
epithelium of the small intestine, promote proliferation of the
enteric epithelium and mucosal repair, thereby helping to maintain
the integrity of the intestinal barrier function.
In conclusion, this study showed that SAP induces
systemic inflammation in rats. Transplanted MSCs may migrate into
the injured intestine, inhibiting the release of inflammatory
mediators, increasing AQP-1 expression, reducing mucosal
permeability of the intestine, promoting the recovery of intestinal
epithelial cells and maintaining the integrity of the intestinal
mucosal barrier. In the present study, transplanted MSCs inhibited
systemic inflammation, reduced necrosis of intestinal epithelial
cells and reduced TNF-α release in a rat model of SAP-induced
intestinal injury, suggesting that MSCs exert a protective effect
on the intestinal barrier during SAP. Our findings may provide
evidence for the prevention of SAP-induced intestinal barrier
dysfunction.
Acknowledgements
This study was supported by the Health and Medicine
Scientific Research Foundation of Nanjing Military Area Command
(no. 08Z029).
References
|
1
|
Gaisano HY and Gorelick FS: New insights
into the mechanisms of pancreatitis. Gastroenterology.
136:2040–2044. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Warshaw AL: Improving the treatment of
necrotizing pancreatitis - a step up. N Engl J Med. 362:1535–1537.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samel S, Lanig S, Lux A, et al: The gut
origin of bacterial pancreatic infection during acute experimental
pancreatitis in rats. Pancreatology. 2:449–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Juvonen PO, Alhava EM and Takala JA: Gut
permeability in patients with acute pancreatitis. Scand J
Gastroenterol. 35:1314–1318. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Capurso G, Zerboni G, Signoretti M,
Valente R, Stigliano S, Piciucchi M and Delle Fave G: Role of the
gut barrier in acute pancreatitis. J Clin Gastroenterol. 46(Suppl):
S46–S51. 2012. View Article : Google Scholar
|
|
6
|
Koh YY, Jeon WK, Cho YK, et al: The effect
of intestinal permeability and endotoxemia on the prognosis of
acute pancreatitis. Gut Liver. 6:505–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu F, Huang H, Wang F and Chen Y:
Intestinal capillary endothelial barrier changes in severe acute
pancreatitis. Hepatogastroenterology. 58:1009–1017. 2011.PubMed/NCBI
|
|
8
|
Hassoun HT, Kone BC, Mercer DW, Moody FG,
Weisbrodt NW and Moore FA: Post-injury multiple organ failure: the
role of the gut. Shock. 15:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venkataramana NK, Kumar SK, Balaraju S, et
al: Open-labeled study of unilateral autologous bone-marrow-derived
mesenchymal stem cell transplantation in Parkinson’s disease.
Transl Res. 155:62–70. 2010.PubMed/NCBI
|
|
10
|
Tay CY, Yu H, Pal M, et al: Micropatterned
matrix directs differentiation of human mesenchymal stem cells
towards myocardial lineage. Exp Cell Res. 316:1159–1168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang YJ, Qian HY, Huang J, et al: Combined
therapy with simvastatin and bone marrow-derived mesenchymal stem
cells increases benefits in infarcted swine hearts. Arterioscler
Thromb Vasc Biol. 29:2076–2082. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdallah BM and Kassem M: The use of
mesenchymal (skeletal) stem cells for treatment of degenerative
diseases: current status and future perspectives. J Cell Physiol.
218:9–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang F, Leung VY, Luk KD, Chan D and
Cheung KM: Mesenchymal stem cells arrest intervertebral disc
degeneration through chondrocytic differentiation and stimulation
of endogenous cells. Mol Ther. 17:1959–1966. 2009. View Article : Google Scholar
|
|
14
|
Newman RE, Yoo D, LeRoux MA and
Danilkovitch-Miagkova A: Treatment of inflammatory diseases with
mesenchymal stem cells. Inflamm Allergy Drug Targets. 8:110–123.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pittenger MF and Martin BJ: Mesenchymal
stem cells and their potential as cardiac therapeutics. Circ Res.
95:9–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petersen BE, Bowen WC, Patrene KD, et al:
Bone marrow as a potential source of hepatic oval cells. Science.
284:1168–1170. 1999.PubMed/NCBI
|
|
18
|
Jung KH, Song SU, Yi T, et al: Human bone
marrow-derived clonal mesenchymal stem cells inhibit inflammation
and reduce acute pancreatitis in rats. Gastroenterology.
140:998–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiu CJ, McArdle AH, Brown R, Scott H and
Gurd FN: Intestinal mucosal lesion in low-flow states. I. A
morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benjamini YD and Yekutieli D: The control
of the false discovery rate in multiple testing under dependency.
Ann Statist. 29:1165–1188. 2001.
|
|
21
|
Gong Q and Li YY: The mechanism of severe
acute pancreatitis combined with gastrointestinal disturbance. Int
J Surg. 33:213–216. 2006.(In Chinese).
|
|
22
|
Ogawa M: Acute pancreatitis and cytokines:
‘second attack’ by septic complication lead to organ failure.
Pancreas. 16:312–315. 1998.
|
|
23
|
Towne JE, Harrod KS, Krane CM and Menon
AG: Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung
after acute viral infection. Am J Respir Cell Mol Biol. 22:34–44.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Towne JE, Krane CM, Bachurski CJ and Menon
AG: Tumor necrosis factor-alpha inhibits aquaporin 5 expression in
mouse lung epithelial cells. J Biol Chem. 276:18657–18664. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brittan M, Chance V, Elia G, Poulsom R,
Alison MR, MacDonald TT and Wright NA: A regenerative role for bone
marrow following experimental colitis: contribution to
neovasculogenesis and myofibroblasts. Gastroenterology.
128:1984–1995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Zhang ZG, Li Y, et al: Intravenous
administration of human bone marrow stromal cells induces
angiogenesis in the ischemic boundary zone after stroke in rats.
Circ Res. 92:692–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tögel F, Hu Z, Weiss K, Isaac J, Lange C
and Westenfelder C: Administered mesenchymal stem cells protect
against ischemic acute renal failure through
differentiation-independent mechanisms. Am J Physiol Renal Physiol.
289:F31–F42. 2005.
|
|
28
|
Tanaka F, Tominaga K, Ochi M, et al:
Exogenous administration of mesenchymal stem cells ameliorates
dextran sulfate sodium-induced colitis via anti-inflammatory action
in damaged tissue in rats. Life Sci. 83:771–779. 2008. View Article : Google Scholar
|
|
29
|
Yabana T, Arimura Y, Tanaka H, et al:
Enhancing epithelial engraftment of rat mesenchymal stem cells
restores epithelial barrier integrity. J Pathol. 218:350–359. 2009.
View Article : Google Scholar
|
|
30
|
Gong Z and Niklason LE: Small-diameter
human vessel wall engineered from bone marrow-derived mesenchymal
stem cells (hMSCs). FASEB J. 22:1635–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou YF, Bosch-Marce M, Okuyama H, et al:
Spontaneous transplantation of cultured mouse bone-narrow derived
stromal cells. Cancer Res. 66:10849–10854. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tu XH, Song JX, Xue XJ, et al: Role of
bone marrow-derived mesenchymal stem cells in a rat model of severe
acute pancreatitis. World J Gastroenterol. 18:2270–2279. 2012.
View Article : Google Scholar : PubMed/NCBI
|