Introduction
The increasing number and prevalence of neoplastic
diseases, as well as their associated high mortality rates, have
stimulated an unprecedented level of studies directed towards the
development of novel antitumor drugs (1). Due to their compromised immunity,
cancer patients during chemotherapy are highly susceptible to
microbial infections. Co-administration of multiple drugs in the
treatment of patients with neoplasms accompanied with low immunity
may induce additional health problems, particularly in those whose
kidney function is impaired. Furthermore, numerous chemotherapeutic
agents have a number of unpleasant side effects, which may severely
worsen the quality of life for the patient (2). Therefore, treatment with a single
drug with low cytotoxicity as a monotherapy may be beneficial both
therapeutically and economically.
Pyrimidine-containing compounds have been recently
reported to possess a variety of anticancer effects (3,4).
1-calcium phosphate-uracil (1-CP-U) is a synthetic uracil
derivative, which is a pyrimidine-containing compound and has been
suggested to demonstrate a wide range of highly selective functions
(5). It was hypothesized in the
current study that 1-CP-U may enhance human immunity, regulate
renal function and possess several pharmacological properties,
including analgesic and antipyretic activities. In light of the
above hypothesis, it was considered important to elucidate the
anticancer effects of 1-CP-U, which, to the best of our knowledge,
has not been studied previously, with the aim of identifying a more
active and selective anticancer agent for the treatment of
malignant neoplasms.
Apoptosis, or programmed cell death, is attenuated
in numerous types of tumors that succeed in progressing to states
of high-grade malignancy and resistance to therapy (6). A number of preclinical and clinical
studies have demonstrated that the magnitude of induction of
apoptosis is associated with tumor response to therapy (7) and that the disruption of the apotosis
program may decrease treatment sensitivity (8). Loss of pro-apoptotic protein B-cell
lymphoma (Bcl-2)-associated X protein (Bax) or overexpression of
the anti-apoptotic protein Bcl-2 is frequently observed in tumor
cells resistant to cancer therapy (9). Metastasis is a prominent limitation
in the treatment of cancer, as the majority of the associated
mortality is correlated with the disseminated disease rather than
the primary tumor (10). During
the process of metastasis formation, the degradation of the
extracellular matrix (ECM) by proteases, including matrix
metalloproteinases (MMPs), has an important role. The expression
and activity of MMPs are enhanced in almost all types of human
cancer, and this correlates with advanced tumor stage and shortened
survival (11).
The present study has revealed for the first time,
to the best of our knowledge, a broad spectrum of anti-cancer
activities of 1-CP-U against a number of different human tumor cell
lines. The obtained data indicate that 1-CP-U possesses a variety
of effects on cancer cell proliferation, apoptosis, migration and
invasion in vitro.
Materials and methods
Cell lines and culture
Human cervical cancer cell lines HeLa and CaSki,
human ovarian cancer cell line SKOV3, human hepatocellular
carcinoma cell line SMMC-7721, human lung adenocarcinoma cell line
A549, human colorectal carcinoma cell line LS174T, normal lung
fibroblasts MRC-5 and human embryonic kidney (HEK-293) cells were
obtained from the American Type Culture Collection (Manassas, VA,
USA). SKOV3, Hela, SMMC-7721, A549, LS174T and HEK-293 cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (FBS; HyClone, Logan, UT, USA) and 100 U/ml penicillin
and streptomycin (Wuhan Boster Biological Technology., Ltd., Wuhan,
China). CaSki cells were cultured in RPMI-1640 medium (HyClone)
supplemented with 10% FBS, and 100 U/ml penicillin and
streptomycin. The MRC-5 cell line was maintained in DMEM
supplemented with 15% FBS and 100 U/ml penicillin and streptomycin.
All of the cells were cultured at 37°C in a 5% CO2
incubator.
Chemotherapeutic drug and antibodies
1-CP-U, synthesized according to the reported
procedure (5), was generously
provided by Ms. Ning Qizhi, who originally synthesized the agent.
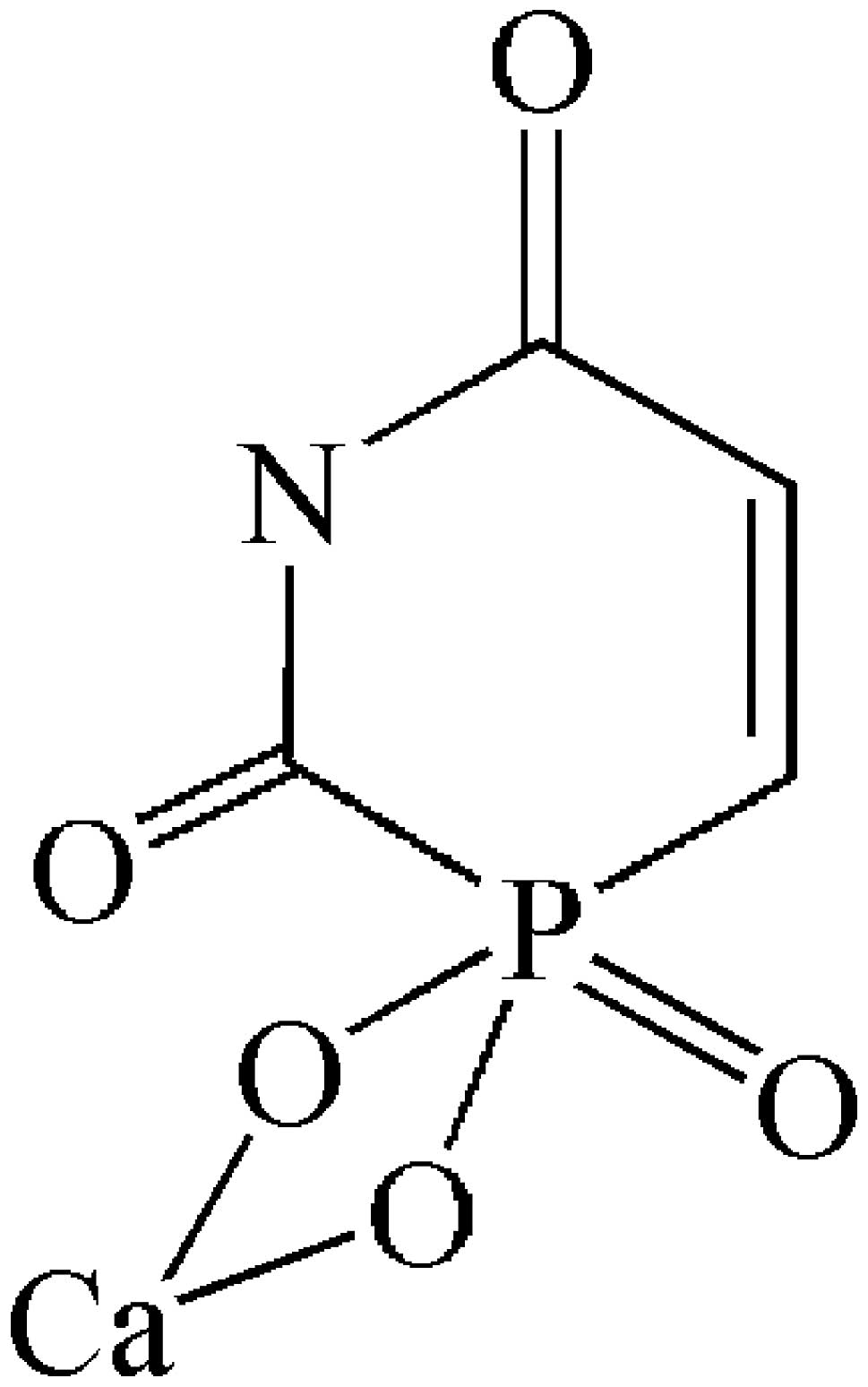
The chemical structure of 1-CP-U is demonstrated in Fig. 1. A total of 1.0 g of 1-CP-U crystal
was weighed, totally dissolved it in ultrapure water facilitated by
a 0.25 mol/l hydrochloric acid solution, and then the pH was
adjusted to 4.0 by adding 0.25 mol/l sodium hydroxide solution.
Calculating the concentration of the stock solution as 63.39 mM, it
was diluted to the required concentrations in conditional medium
and then stored at 4°C. The antibodies were as follows: Polyclonal
rabbit anti-human Bax, MMP-2 and MMP-9, monoclonal mouse anti-human
Bcl-2, (Wuhan Boster Biological Technology., Ltd.; A0315-2, BA0569,
BA0573 and BM0200, respectively), and β-actin was purchased from
Zhongshan Golden Bridge Biotechnology Co., Ltd., (Beijing, China;
TA-09). The goat anti-mouse or anti-rabbit secondary antibodies
were from Zhongshan Golden Bridge Biotechnology (ZB-2305 and
ZB-2301).
Cell proliferation assay
The effects of 1-CP-U on the different cell lines
were determined by the MTT assay as described previously (12). Briefly, 5,000 cells were incubated
in triplicate on a 96-well plate under normal culture conditions
overnight. MRC-5, HEK-293, LS174T and CaSki cell lines were then
treated with 1.0 μmol/l of 1-CP-U while SKOV3, HeLa, SMMC-7721 and
A549 were treated with different concentrations of 1-CP-U (0.7, 1.0
and 1.4 μmol/l). The control group was incubated with an identical
volume of drug-free dilute solution. Following this, 5 mg/ml MTT
solution (Beyotime Institute of Biotechnology, Haimen, China) was
added every 24 h to examine the cell viability on each day.
Following 4 h incubation with MTT at 37°C, 150 μl dimethyl
sulfoxide (DMSO) was added into each well to dissolve the formed
crystals. Using the DMSO without tumor cells as a blank control,
the optical density (OD) at 595 nm was measured by a 96-well
microplate reader (Model-680; Bio-Rad, Hercules, CA, USA). The cell
viability was expressed as a percentage according to the following
equation: OD of the experiment samples / OD of the control × 100%.
From these results, a dose- and time-dependent curve of the
1-CP-U-treated tumor cell lines was generated. The cytotoxic
effects of 1-CP-U were expressed as the 50% inhibitory
concentration (IC50) calculated by SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA).
Apoptosis analysis
The rate of apoptosis was analyzed by two different
methods. Firstly, chromatin staining with Hoechst 33342 was
performed to detect nuclear condensation. Briefly, tumor cell
cultures were seeded in six-well plates and treated with different
concentrations of 1-CP-U for 48 h. The apoptotic morphology of the
cells was evaluated by Hoechst 33342 (C1025; Beyotime Institute of
Biotechnology) following the manufacturer’s instructions. Secondly,
the Annexin V-fluorescein isothiocyanate (FITC) assay was employed
to measure the percentage of the apoptotic cells under 1-CP-U
treatment. Following treatment with 1-CP-U, the cells were
trypsinized, collected and washed with phosphate-buffered saline
(PBS). After using the Annexin V-FITC Apoptosis Detection kit
(KGA107; KeyGen Biotech Co., Ltd., Nanjing, China) which contained
propidium iodide (PI), the stained cells were analyzed using a flow
cytometer (BD FACS AriaII; BD Biosciences; Franklin Lakes, NJ,
USA).
Wound healing migration assay
The cell motility was measured by a wound healing
assay as described previously (10). Initially, equal numbers of tumor
cells were allowed to grow in six-well plates overnight. The next
day, the cells were scraped with pipette tips and washed with PBS.
The cells were then treated with or without the 1-CP-U under
starvation to inactivate cell proliferation. The cells migrated
into the wound surface and the average distance of migrating cells
was determined under an Eclipse TS100 inverted microscope (Nikon
Corporation, Tokyo, Japan) at the designated time-points.
Cell invasion assay
The invasion assay was performed using Boyden
chambers consisting of a 24-well Millicell inserts and a membrane
filter (8 μm pore size) (Merck Millipore, Chengdu, China) as
described previously (13).
Briefly, the upper chamber of the Boyden chamber was coated with 40
μl Matrigel (1 mg/ml), and left to solidify at 37°C for 30 min. The
bottom chambers were filled with 600 μl DMEM containing 20% FBS.
The top chambers were seeded with 2×105 cells in the
absence or presence of 0.7 μmol/l 1-CP-U. Following 24 h of
incubation, the cells on the top surface of the filter were
scraped, and those spread on the bottom sides (invasive cells) were
fixed with cold 4% paraformaldehyde and stained with eosin
(AR1180-2; Wuhan Boster Biological Technology., Ltd.) alone. Images
of the cells were captured with a the inverted microscope and then
quantified.
Immunoblot analyses
The HeLa cells were treated with different
concentrations of 1-CP-U for 48 h. Whole cellular protein was
extracted with radioimmunoprecipitation assay buffer (P0013B;
Beyotime) containing 1 mM phenylmethanesulfonylfluoride (ST506;
Beyotime). The mix was centrifuged at 12,000 × g for 5 min at 4°C.
Following this, the protein concentration was determined by the
bicinchoninic acid method (P0012S; Beyotime) according to the
manufacturer’s instructions using a Bio-Rad assay. After heating to
95°C for 5 min, a total of 25 μg protein was separated by 8%
Tris-HCl SDS-PAGE, and then transferred to an polyvinylidene
fluoride membrane (Bio-Rad; 162-0177) using wet transfer apparatus
(Bio-Rad; MiniProtean Tetra). The membrane was incubated in
blocking solution [5% non-fat milk in Tris-buffered saline
containing 0.1% Tween-20 (TBS/T)] for 1 h with gentle rocking at
room temperature. The primary antibody was diluted and the membrane
was incubated at 4°C overnight with the following antibodies: Bax
(1:600), Bcl-2 (1:600), MMP-2 (1:400) and MMP-9 (1:400). Following
washing for 15 min in TBS/T three times, the membranes were
incubated with the secondary antibodies (1:5,000) for 1 h. After
washing, visual detection was performed using the enhanced
chemiluminescence method (WBKLS0100; Merck Millipore).
Statistical analysis
The results are expressed as the mean ± standard
deviation and statistically compared by one-way analysis of
variance test or unpaired Student’s t-test in different
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
1-CP-U treatment inhibits the growth of
human tumor cell lines
To determine whether 1-CP-U was able to exert
cytotoxic effects on human tumor cells, a panel of human tumor cell
lines, including LS174T, CaSki, SKOV3, HeLa, SMMC-7721 and A549,
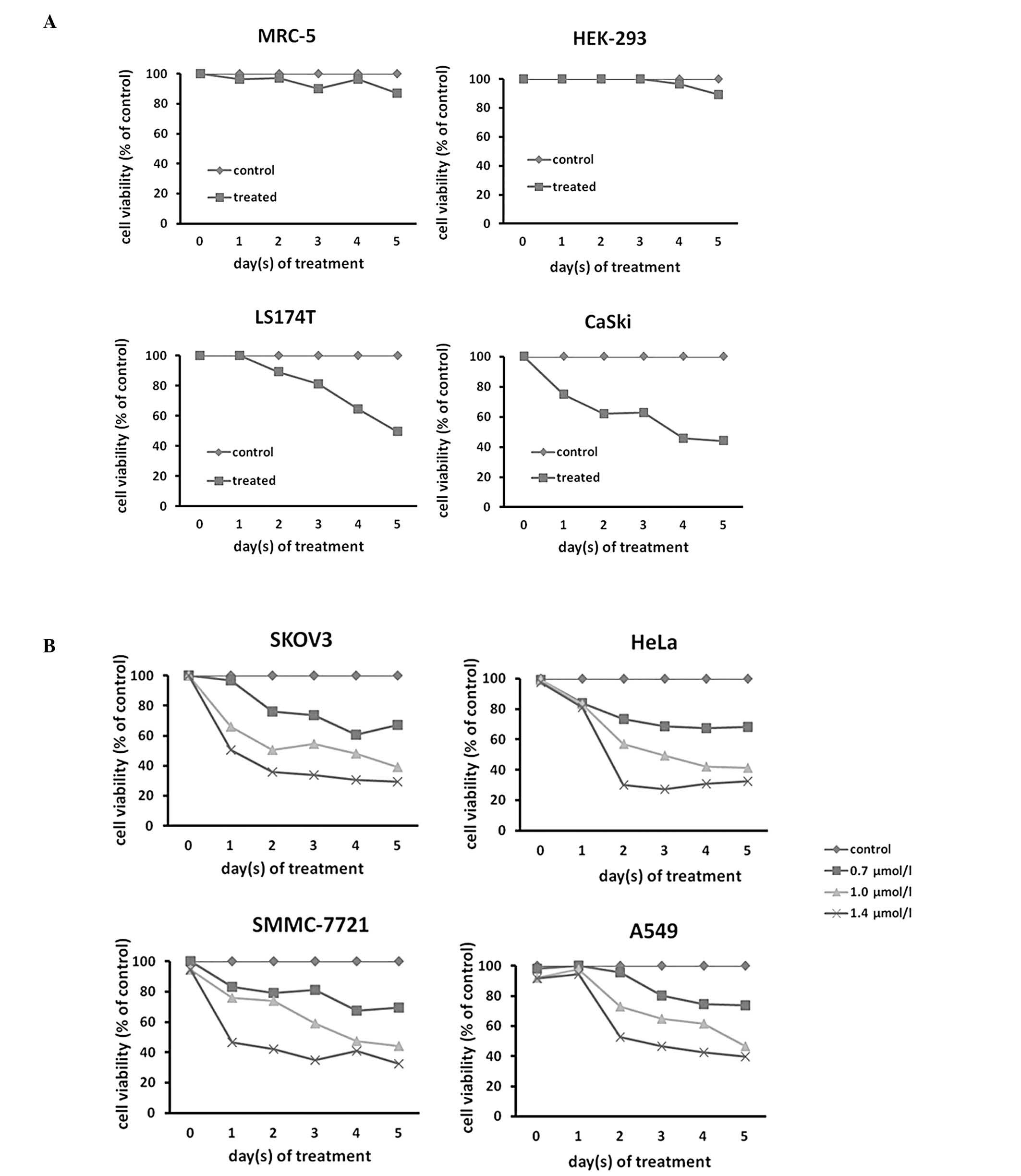
were treated with 1-CP-U. The results demonstrated that treatment
with 1.0 μmol/l of 1-CP-U resulted in >50% cell death following
five-day treatment in the LS174T and CaSki populations (Fig. 2A). Next, the impact of 1-CP-U
treatment (0.7, 1.0 and 1.4 μmol/l for five days) on SKOV3, HeLa,
SMMC-7721 and A549 cell lines was investigated and it was
identified that 1-CP-U concentration-dependently suppressed
proliferation in the four examined tumor cell lines (Fig. 2B). The IC50 of 1-CP-U
for the SKOV3, HeLa, SMMC-7721 and A549 cell lines was 0.909,
0.941, 1.068 and 0.971 μmol/l following five days of exposure,
respectively. There was a significant difference between the
viability of the treated cell lines and that of the control
population following an incubation period of at least 24 h
(P<0.05). To determine the selectivity of 1-CP-U to tumor cells,
the normal MRC-5 and HEK-293 cells were treated with 1.0 μmol/l
1-CP-U, which resulted in only a marginal toxic effect (Fig. 2A), indicating that this effect was
likely specific to cancer cells.
1-CP-U induces apoptosis in tumor
cells
Following observing the decline in cell viability
caused by 1-CP-U, particularly at higher concentrations, the
induction of apoptosis by 1-CP-U was assessed. Firstly, tumor cells
were cultured for 48 h with or without 1-CP-U, and
apoptosis-specific morphology was visualized by Hoechst 33342
staining. Condensed chromatin and apoptotic bodies were identified
in the 1-CP-U-treated cells, as determiend by fluorescence
microscopy (Fig. 3A). Secondly,
flow cytometric analysis was performed following staining of the
cells with Annexin-V-FITC plus PI (Fig. 3B). The apoptotic rates in the 1.0
μmol/l 1-CP-U group were 25.50±4.33, 31.00±12.04, 22.87±8.57 and
6.03±1.76 for SKOV3, HeLa, SMMC-7721 and A549 cell lines,
respectively. The apoptotic rates in the 1.4 μmol/l 1-CP-U groups
were 37.17±5.13, 69.57±12.63, 46.07±11.01 and 7.97±1.61%,
respectively. The apoptotic rates in the two treated groups were
significantly higher than those in the control group (15.93±5.18,
7.60±4.31, 5.43±1.40% and 3.23±0.47%, respectively; P<0.05),
enhanced with increasing doses of 1-CP-U (Fig. 3C). Among the four cell lines
exposed to 1-CP-U, the number of apoptotic cells in the SKOV3, HeLa
and SMMC-7721 populations increased markedly, particularly in the
HeLa cells (9.15-fold at 1.4 μmol/l 1-CP-U treatment as compared
with the corresponding control group), while a comparably modest
increase was detected in the A549 cells (2.47-fold at 1.4 μmol/l
1-CP-U treatment as compared with the corresponding control group),
suggesting that different cell lines varied in their sensitivity to
1-CP-U.
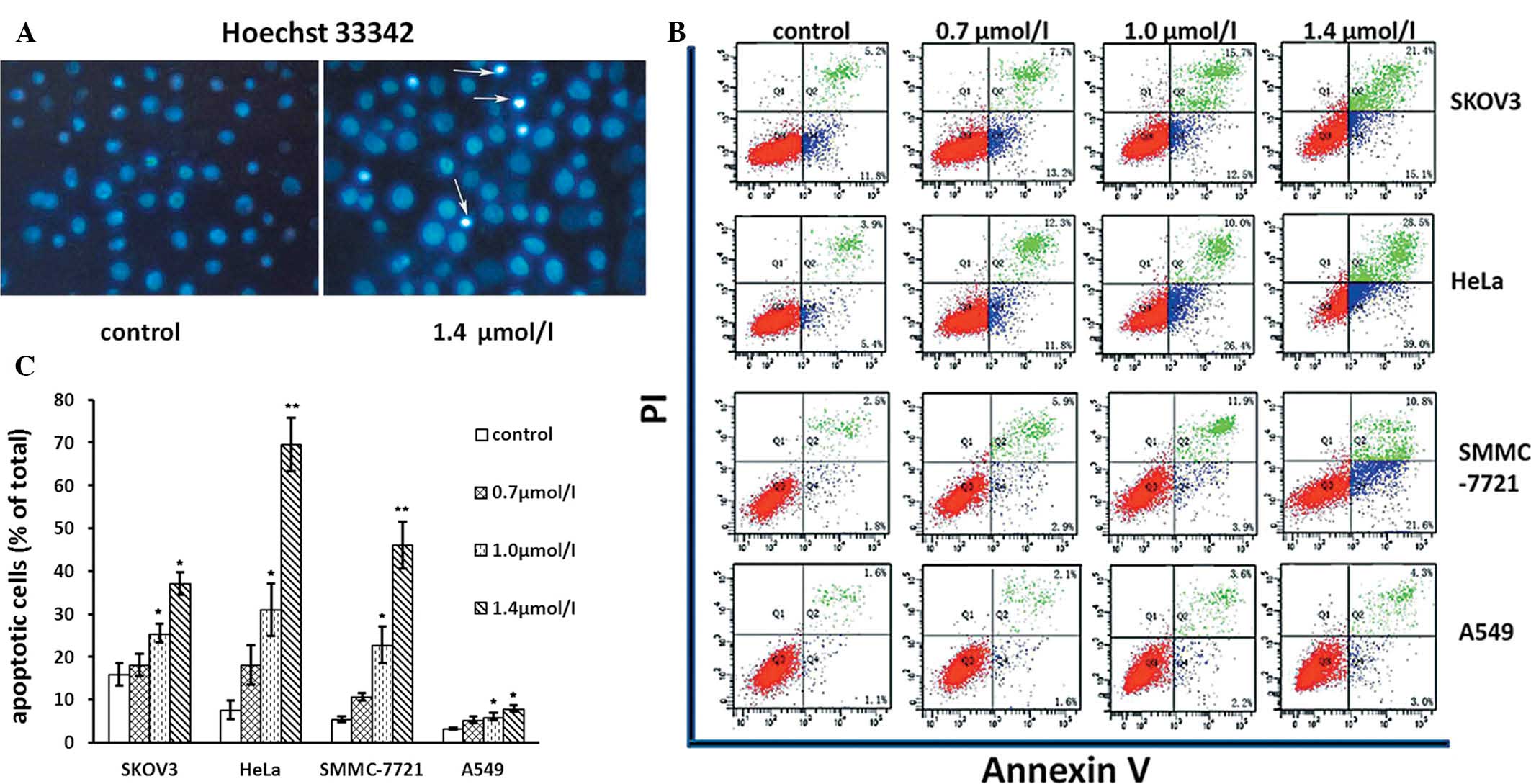 | Figure 3Effects of 1-CP-U on apoptosis. (A)
Tumor cells were treated with various concentrations of 1-CP-U
(0.7, 1.0 and 1.4 μmol/l) for 48 h, and then stained with Hoechst
33342 dye. The strong blue signals indicate apoptotic cells of
SMMC-7721 with nuclear chromatin condensation and the formation of
nuclear fragments and apoptotic bodies (arrows; magnification,
×40). (B) Tumor cells were stained with Annexin V/PI dye at the
indicated time. Analyses were conducted on 10,000 cells in each
trail. The cells in the early stages of apoptosis were only Annexin
V-FITC positive. The cells in the late stage of apoptosis and those
moving toward secondary necrosis stained positive with both Annexin
V-FITC and PI (Q3, live cells; Q4, early stages of apoptosis; Q2,
late stage of apoptosis and secondary necrosis); (C) The percentage
of Annexin V positive cells representing apoptosis in (B) was
quantified and statistically analyzed as the mean ± standard
deviation. *P<0.05; **P<0.001 vs.
controls. 1-CP-U, 1-calcium phosphate-uracil; PI, propidium iodide;
FITC, fluorescein isothiocyanate. |
1-CP-U inhibits tumor cell migration and
invasion in vitro
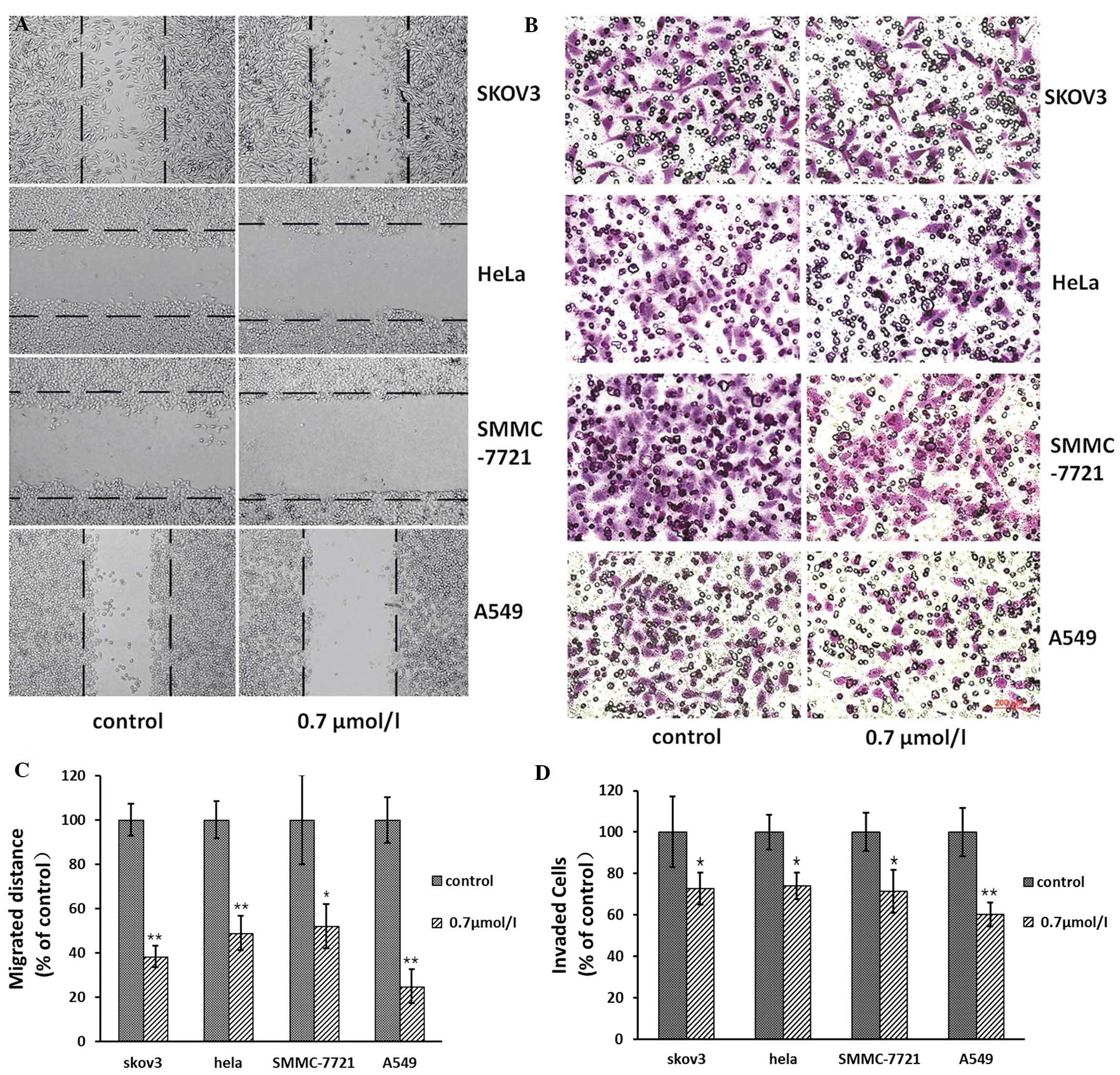
The wound healing assay was performed in the
presence or absence of 1-CP-U in the SKOV3, HeLa, SMMC-7721 and
A549 cells. The tumor cells were imaged following treatment for 0
and 24 h at the same marked site. The migration distance, which was
the difference in wound width at both time-points (0 and 24 h
following treatment), was measured (Fig. 4A). The effect of 1-CP-U on the
invasion of tumor cells was measured by Boyden chamber invasion
assay (Fig. 4B). The ratio of the
migration distance in the treatment group to that in the
corresponding control group was 38.26, 48.95, 52.08 and 24.84% for
SKOV3, HeLa, SMMC-7721 and A549 cells, respectively (Fig. 4C). Of note, 1-CP-U markedly
inhibited tumor cell migration. The number of SKOV3, HeLa,
SMMC-7721 and A549 cells passing through the Matrigel in the
negative control group (35±12, 30±5, 32±6 and 39±9, respectively)
was significantly higher than that in the 0.7 μmol/l 1-CP-U group
(13±4, 10±2, 14±3 and 17±5, respectively). The ratio of the number
of invaded tumor cells in the treatment group to that in the
corresponding control group was calculated and presented in a bar
chart (Fig. 4D). The results
indicated that 1-CP-U markedly suppressed the cell invasion through
the Matrigel.
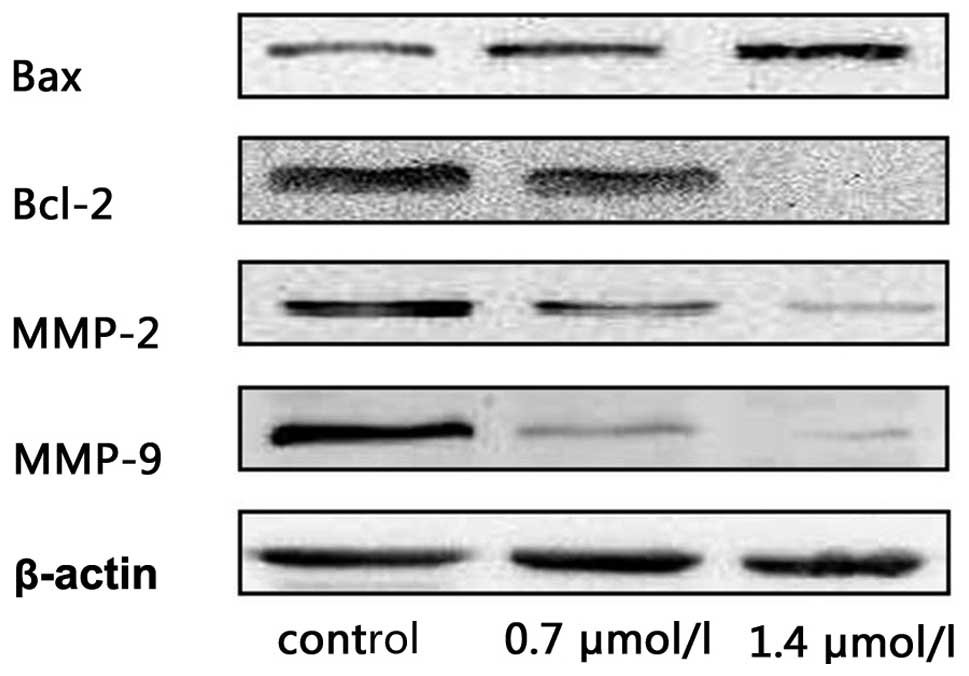
1-CP-U affected expression of apoptotic
and MMP proteins
The finding that 1-CP-U induced apoptosis and
inhibited the invasion of tumor cells prompted us to examine its
effect on apoptotic and MMP proteins using western blotting
analysis. HeLa cells were treated with 1-CP-U for 48 h at different
concentrations (0, 0.7 and 1.0 μmol/l). Increased expression levels
of the pro-apoptotic protein Bax and a marked reduction of Bcl-2
levels were also associated with increased 1-CP-U concentrations.
Meanwhile, 1-CP-U suppressed the secretion of MMP-2 and MMP-9 in a
dose-dependent manner (Fig.
5).
Discussion
A number of recent studies have revealed that
pyrimidine derivatives demonstrated efficient anticancer activities
(14). For example, pyrrole and
pyrrolo[2,3-d]pyrimidine derivatives have aroused notable attention
as potent anticancer agents (15).
Pyrimidine-2,4,6-triones have been identified as a new effective
and selective class of MMP inhibitors (16). 1-CP-U is a synthetic pyrimidine
derivative (5). Compared with
polysubstituted pyrimidines mentioned previously, 1-CP-U has a
simpler chemical structure, which increases its cost-effectiveness
as well as its ease of production and administration. However, the
structure of 1-CP-U remains to be confirmed by electrospray
ionization-mass spectrometry, and in addition, 1H and
13C nuclear magnetic resonance spectral analysis and the
purity determination should be investigated in future studies. Thus
far, few of its bioactivities against human tumor types have been
reported.
In the present study, a variety of biological
responses initiated by 1-CP-U in vitro were investigated for
the first time to the best of our knowledge. Initially, the effects
of 1-CP-U on tumor cell proliferation were investigated. 1-CP-U
effectively induced growth inhibition in cultured SKOV3, HeLa,
SMMC-7721 and A549 cells, with IC50 values of ~1.0
μmol/l (Fig. 2B). Additionally,
whether 1-CP-U may affect the viability of non-cancerous cells was
examined. The data obtained demonstrated that 1-CP-U exhibited low
cytotoxicity on the healthy MRC-5 and HEK-293 cell lines at the
concentration of 1.0 μmol/l (Fig.
2A), suggesting that cell proliferation inhibition caused by
1-CP-U is an effect specific to cancer cells.
It is well established that the majority of
anticancer agents induce apoptosis (7). Therefore, following detecting a
decline in cell viability caused by 1-CP-U, the apoptosis induced
by 1-CP-U was assessed using Hoechst 33342 staining and flow
cytometric analysis (Fig. 3A and
B). It was noted that 1-CP-U at 1.0 and 1.4 μmol/l induced
significant levels of apoptosis in SKOV3, HeLa, SMMC-7721 and A549
cell lines (Fig. 3C).
Additionally, 1-CP-U initiated only a modest increase in the
apoptotic rate in A549 cells compared with that in the SKOV3, HeLa
and SMMC-7721 cell lines. Possibly heterogeneous tumor cell
populations exhibit different drug sensitivities and are also
susceptible to more than one type of cell death (8). The activation of the pro-apoptotic
proteins Bax and Bcl-2 homologous antagonist killer (Bak) results
in the translocation of Bax/Bak from the mitochondria to the
cytoplasm, thereby promoting Bax/Bak oligomerization, which leads
to the release of a number of small molecules (17). This is inhibited by the
anti-apoptotic proteins Bcl-2 and Bcl-2 extra large protein
(Bcl-xL), which are major inhibitors of apoptotic cell death
(18). In the present study,
1-CP-U increased the expression levels of Bax while suppressing the
levels of Bcl-2 in a dose-dependent manner (Fig. 5).
Migration and invasion of cancer cells are key steps
in tumor metastasis (19). The
results revealed that 0.7 μmol/l 1-CP-U significantly inhibited
both the migration and invasion of the SKOV3, HeLa, SMMC-7721 and
A549 cell lines (Fig. 4). MMPs are
a family of zinc-dependent endopeptidases first described almost
half a century ago (20). They
have a crucial role in ECM degradation, associated with tissue
repair, cancer cell invasion, metastasis and angiogenesis (21,22).
Among several MMPs, MMP-2 and -9 have been demonstrated to be
critical factors in tumor invasion (23), which is secreted by tumor cells as
a pro-enzyme (pro-MMP-2) and activated in the extracellular milieu
to execute their proteolytic activity, then accordingly enables
cells to invade into the target organ and develop tumor metastasis
(24,25). A previous study demonstrated that
increased expression of MMPs (26)
is linked with lymphatic invasion and lymph node metastases.
Inhibition of MMPs attenuated angiogenesis and lymphangiogenesis,
and reduced lymph node metastasis (27). In the present study, western blot
analysis identified that treatment with 1-CP-U inhibited the
expression of MMP proteins in a dose-dependent manner in the HeLa
cells (Fig. 5). The results
indicated that MMP-2 may be a downstream target of 1-CP-U.
Of note, it was observed that 1-CP-U significantly
inhibited the migration and invasion at a lower concentration (0.7
μmol/l) compared with the dosage of 1-CP-U required to achieve
significant inhibition of apoptosis (1.0 and 1.4 μmol/l). These
results revealed that 1-CP-U appeared to be more effective at
inhibiting cell migration and invasion than inducing apoptosis,
suggesting that the anti-migration and anti-invasion functions of
1-CP-U may have more clinical potential over its pro-apoptotic
activity.
In conclusion, the present study demonstrated that
1-CP-U exhibited anti-cancer activity on a panel of SKOV3, HeLa,
SMMC-7721 and A549 cell lines, comprising cell proliferation,
apoptosis, migration and invasion. The antitumor effects suggested
that 1-CP-U may be considered as a candidate anticancer agent
against a broad spectrum of tumor types, which deserves further
investigation in order to examine its biological activities and
molecular mechanisms in a clinical setting.
Acknowledgements
1-CP-U was generously provided by Ms. Ning Qizhi,
who developed this agent. This study was supported by the Science
Grant from Science and Technology Department of Sichuan Province,
China (no. 2012SZ0136) to Shanling Liu, the Science Grant from
National Natural Science Foundation of China (no. 81270660) to He
Wang and the Young Scientific Innovation Team in Neurological
Disorders grant (no. 2011JTD0005) from the Department of Science
and Technology of Sichuan Province, China.
References
|
1
|
Eckhardt S: Recent progress in the
development of anticancer agents. Curr Med Chem Anticancer Agents.
2:419–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu Y, Xin Y, Diao Y, Lu C, Fu J, Luo L and
Yin Z: Synergistic effects of apigenin and paclitaxel on apoptosis
of cancer cells. PLoS One. 6:e291692011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Zhao YF, Zhao XL, Yuan XY and Gong
P: Synthesis and anti-tumor activities of novel
pyrazolo[1,5-a]pyrimidines. Arch Pharm (Weinheim). 339:593–597.
2006.
|
|
4
|
Manetti F, Brullo C, Magnani M, Mosci F,
Chelli B, Crespan E, Schenone S, Naldini A, Bruno O, Trincavelli
ML, Maga G, Carraro F, Martini C, Bondavalli F and Botta M:
Structure-based optimization of pyrazolo[3,4-d]pyrimidines as Abl
inhibitors and antiproliferative agents toward human leukemia cell
lines. J Med Chem. 51:1252–1259. 2008.
|
|
5
|
Ning QZ: 1-Calcium phosphate-uracil and
method for preparing thereof. US patent 20050277622. Filed June 10,
2004; Issued December 15, 2005.
|
|
6
|
Lowe SW, Cepero E and Evan G: Intrinsic
tumour suppression. Nature. 432:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
8
|
Kim R, Emi M and Tanabe K: The role of
apoptosis in cancer cell survival and therapeutic outcome. Cancer
Biol Ther. 5:1429–1442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hasenjager A, Gillissen B, Muller A,
Normand G, Hemmati PG, Schuler M, Dorken B and Daniel PT: Smac
induces cytochrome c release and apoptosis independently from
Bax/Bcl-xL in a strictly caspase-3-dependent manner in human
carcinoma cells. Oncogene. 23:4523–4535. 2004. View Article : Google Scholar
|
|
10
|
Tang F, Zhang R, He Y, Zou M, Guo L and Xi
T: MicroRNA-125b induces metastasis by targeting STARD13 in MCF-7
and MDA-MB-231 breast cancer cells. PLoS One. 7:e354352012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geng W, Ng KT, Sun CK, Yau WL, Liu XB,
Cheng Q, Poon RT, Lo CM, Man K and Fan ST: The role of proline rich
tyrosine kinase 2 (Pyk2) on cisplatin resistance in hepatocellular
carcinoma. PLoS One. 6:e273622011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Kuang L, Pan X, Liu J, Wang Q, Du
B, Li D, Luo J, Liu M, Hou A and Qian M: Isoalvaxanthone inhibits
colon cancer cell proliferation, migration and invasion through
inactivating Rac1 and AP-1. Int J Cancer. 127:1220–1229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amr AG, Mohamed AM, Mohamed SF,
Abdel-Hafez NA and Hammam Ael-F: Anticancer activities of some
newly synthesized pyridine, pyrane, and pyrimidine derivatives.
Bioorg Med Chem. 14:5481–5488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moriarty KJ, Koblish HK, Garrabrant T,
Maisuria J, Khalil E, Ali F, Petrounia IP, Crysler CS, Maroney AC,
Johnson DL and Galemmo RA Jr: The synthesis and SAR of
2-amino-pyrrolo[2,3-d]pyrimidines: a new class of Aurora-A kinase
inhibitors. Bioorg Med Chem Lett. 16:5778–5783. 2006.PubMed/NCBI
|
|
16
|
Maquoi E, Sounni NE, Devy L, Olivier F,
Frankenne F, Krell HW, Grams F, Foidart JM and Noël A:
Anti-invasive, antitumoral, and antiangiogenic efficacy of a
pyrimidine-2,4,6-trione derivative, an orally active and selective
matrix metalloproteinases inhibitor. Clin Cancer Res. 10:4038–4047.
2004. View Article : Google Scholar
|
|
17
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: a requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verrier F, Deniaud A, Lebras M, Métivier
D, Kroemer G, Mignotte B, Jan G and Brenner C: Dynamic evolution of
the adenine nucleotide translocase interactome during
chemotherapy-induced apoptosis. Oncogene. 23:8049–8064. 2004.
View Article : Google Scholar
|
|
19
|
Kim YM, Kim IH and Nam TJ: Inhibition of
AGS human gastric cancer cell invasion and proliferation by
Capsosiphon fulvescens glycoprotein. Mol Med Rep. 8:11–16.
2013.PubMed/NCBI
|
|
20
|
Gross J and Lapiere CM: Collagenolytic
activity in amphibian tissues: a tissue culture assay. Proc Natl
Acad Sci USA. 48:1014–1022. 1962. View Article : Google Scholar
|
|
21
|
Singh RP, Raina K, Sharma G and Agarwal R:
Silibinin inhibits established prostate tumor growth, progression,
invasion, and metastasis and suppresses tumor angiogenesis and
epithelial-mesenchymal transition in transgenic adenocarcinoma of
the mouse prostate model mice. Clin Cancer Res. 14:7773–7780. 2008.
View Article : Google Scholar
|
|
22
|
Levy AT, Cioce V, Sobel ME, Garbisa S,
Grigioni WF, Liotta LA and Stetler-Stevenson WG: Increased
expression of the Mr 72,000 type IV collagenase in human colonic
adenocarcinoma. Cancer Res. 51:439–444. 1991.PubMed/NCBI
|
|
23
|
Dashevsky O, Varon D and Brill A:
Platelet-derived microparticles promote invasiveness of prostate
cancer cells via upregulation of MMP-2 production. Int J Cancer.
124:1773–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kitamura T and Taketo MM: Keeping out the
bad guys: gateway to cellular target therapy. Cancer Res.
67:10099–10102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Björklund M and Koivunen E:
Gelatinase-mediated migration and invasion of cancer cells. Biochim
Biophys Acta. 1755:37–69. 2005.PubMed/NCBI
|
|
26
|
Langenskiöld M, Holmdahl L, Falk P and
Ivarsson ML: Increased plasma MMP-2 protein expression in lymph
node-positive patients with colorectal cancer. Int J Colorectal
Dis. 20:245–252. 2005.PubMed/NCBI
|
|
27
|
Nakamura ES, Koizumi K, Kobayashi M and
Saiki I: Inhibition of lymphangiogenesis-related properties of
murine lymphatic endothelial cells and lymph node metastasis of
lung cancer by the matrix metalloproteinase inhibitor MMI270.
Cancer Sci. 95:25–31. 2004. View Article : Google Scholar
|