Introduction
Ovarian cancer is a gynecological malignancy with
the highest known risk of mortality (1). It has been reported that ~70% of
patients, at initial presentation, are diagnosed with advanced
ovarian cancer (International Federation of Gynecology and
Obstetrics stage III-IV) (2). The
standard treatment for ovarian cancer is up-front cytoreductive
surgery, followed by platinum and paclitaxel chemotherapy every
three weeks (3).
Chemotherapy-resistant advanced ovarian cancer is associated with a
five year survival rate of <30% (4). Disease recurrence within six months
is classed as resistance to treatment (5), with relapse occurring in nearly all
of the patients tolerant to chemotherapy. There is currently no
clinically effective treatment for recurrent ovarian cancer. It is
therefore important to find a novel strategy to reverse
chemoresistance and improve the survival rate of patients with
ovarian cancer.
Metformin is an oral anti-diabetic drug in the
biguanide class that is used in the treatment of type II diabetes.
Previously, two large epidemiological studies demonstrated that
long-term use of metformin reduced the risk of ovarian cancer
occurrence (6). Furthermore, in
patients with ovarian cancer combined with diabetes, metformin
treatment was shown to significantly increase progression-free
survival, as compared with those who were not administered with
metformin (7). Previously,
metformin was found to inhibit cell growth in ovarian cancer in
vitro (8), although the
mechanism for this is unclear. Furthermore, metformin significantly
enhanced the effects of cisplatin, paclitaxel and doxorubicin in
the inhibition of tumor cell growth, reduced the required dose of
doxorubicin, and prolonged disease remission in lung, breast and
prostate cancer nude mice models (9–11).
These results identify metformin as a potential regulator of tumor
cell sensitivity to chemotherapeutic drugs. However, the mechanism
remains unclear.
Cell damage and chemoresistance are mediated
primarily through the mitogen-activated protein kinase (MAPK) and
phosphoinositide kinase-3-threonine protein kinase B signaling
pathways (8). The signaling
pathways mediated by the MAPK family include p38 MAPK,
extracellular signal-regulated kinase (ERK), c-jun N-terminal
kinase and other subfamilies; of these pathways the p38 MAPK and
ERK1/2 are considered to be the most important. Phosphorylated MAPK
subsequently phosphorylates the B cell lymphoma-2 (Bcl-2) and
Bcl-2-associated death proteins, which have been shown to weaken
the effects of platinum and taxane in tumor cell apoptosis and
increase cancer resistance to chemotherapeutic drugs (12,13).
The MAPK signaling pathway has an important role in cell
proliferation, apoptosis and chemoresistance in a variety of
malignant tumors, including ovarian cancer (14,15).
In the present study, MAPK pathway activation was
investigated in paclitaxel and platinum-resistant ovarian carcinoma
specimens. The cell proliferation of SKOV3/DDP cisplatin-resistant
ovarian cancer cells was determined using a bromodeoxyuridine
(BrdU) ELISA kit. The effects of metformin on cell proliferation,
irrespective of the presence of a p38 MAPK signaling pathway
inhibitor, were confirmed in the SKOV3/DDP cell line. The
expression of phosphorylated p38 MAPK (P-p MAPK) was determined in
both drug-resistant and primary ovarian cancer tissues. The effects
of metformin, both alone and in combination with a p38 MAPK
inhibitor, were observed on the reversal of ovarian cancer
cisplatin-resistance in SKOV3/DDP cells. In addition, the present
study investigated the therapeutic mechanisms of metformin in
drug-resistant ovarian cancer, in an effort to develop novel
clinical strategies against recurrent ovarian cancer.
Materials and Methods
Materials
A total of 20 pairs of epithelial ovarian cancer
(EOC) tissue samples were collected from the archives of the
Department of Gynecology of the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China), between July 2012 and May
2013. The tissue samples were obtained from patients who had been
treated with cytoreductive surgery and standard chemotherapy, but
had relapsed following treatment. The criteria for enrollment to
the study were as follows: Complete medical records, confirmed
pathological diagnosis, and disease recurrence following standard
chemotherapy treatment. The tissue samples of both the primary and
recurrent cancers were collected. The tissue samples of the control
group were collected from patients with ovarian cancer, following
cytoreductive surgery, but not chemotherapy. All specimens were
collected within 30 min of excision from the patient, and stored at
−80°C until further use. The specimens were collected after
obtaining the informed consent from the patients. The study was
approved by the Ethics Committee of the First Affiliated Hospital
of Zhengzhou University.
Cell lines and reagents
SKOV3/DDP, adherent and moderately/well
differentiated, cisplatin-resistant cells of human ovarian serous
cystadenocarcinoma, were maintained in phenol red RPMI-1640 medium,
supplemented with 10% fetal bovine serum (FBS) at 37°C in 5%
CO2. The cell cultures were routinely passaged every 3–5
days. The rabbit anti-human polyclonal antibodies: p38 MAPK, P-p38
MAPK, and GAPDH were purchased from Cell Signaling Technology Inc.
(Danvers, MA, USA); metformin and the p38 MAPK inhibitor SB203580
were purchased from Sigma-Aldrich (St. Louis, MO, USA); RPMI-1640
culture medium and FBS were purchased from Gibco-BRL (Carlsbad, CA,
USA); and the BrdU ELISA kit was purchased from Roche Diagnostics
GmbH (Mannheim, Germany).
Immunohistochemical staining
The paraffin-embedded blocks of primary and
recurrent ovarian cancer specimens were sectioned at 4 μm thickness
and mounted onto slides. The sections were fixed with 10%
paraformaldehyde, and the immunohistochemical streptavidin
peroxidase-conjugated method was adopted. The tissue sections were
processed in strict accordance with the manufacturer’s
instructions. Phosphate-buffered saline (PBS; Thermo Fisher
Scientific, Boston, MT, USA) was used, instead of primary antibody,
as a negative control and a known positive plate was used as a
positive control. The distribution of P-p38 MAPK in the cancer
tissue was visualized and quantified to the average gray value.
Gray-scale images were examined, depending on the size and shape of
the tissue.
Western blotting
Protein was extracted from the ovarian cancer
tissues using radioimmunoprecipitation assay buffer, containing 1%
nonyl phenoxypolyethoxylethanol-40, 0.5 sodium deoxycholate and
0.1% sodium dodecyl sulfate. A total of 20 μg protein extract was
separated by 10% SDS-PAGE, and electrotransferred onto a
nitrocellulose membrane. Subsequently, the membrane was blocked
with 5% nonfat dry milk and 0.1% Tween® 20 for 1 h at
room temperature with constant agitation, followed by incubation
with a primary antibody (1:1,000 dilution) overnight at 4°C. The
membranes were washed three times each, for 5 min, with PBST (PBS
with Tween), and the membrane was incubated with a secondary
horseradish peroxidase-linked antibody (1:2,000 dilution) for 2 h.
The membrane was then washed a further three times with PBST, and
the bands were visualized by enhanced chemiluminescence, according
to the manufacturer’s instructions (Pierce Biotechnology Inc.,
Rockford, IL, USA). The relative protein expression was normalized
to GAPDH (1:1,000 dilution), and expressed as a ratio to the
control subjects. The protein bands, including GAPDH, were
quantified by densitometry using the Quantity One®
imaging program (Bio-Rad, Hercules, CA, USA).
Cell proliferation studies
Cell proliferation assays were performed using the
BrdU ELISA kit (Roche Diagnostics GmbH). The SKOV3/DDP cells were
plated into 96-well plates at a concentration of 1×104
cells/well for 24 h. The cells were subsequently serum-starved for
an additional 24 h, and treated with different concentrations of
metformin and 5 μM SB203580 for 72 h. The effects of metformin and
SB203580 were calculated as a percentage of the control cell
growth, obtained from PBS or dimethyl sulfoxide-treated cells grown
in the same 96-well plates. The assays were performed under
serum-free conditions. DNA synthesis was monitored based on the
incorporation of BrdU into the DNA, which was detected by
immunoassay, according to the manufacturer’s instructions.
Following incubation, the cells were re-incubated with 10 μl/well
BrdU labeling solution for an additional 2 h at 37°C. The labeling
medium was removed, 200 μl/well FixDenat (Selleck, Houston, TX,
USA) was added, and the cells were incubated for 30 min at 20°C.
The FixDenat solution was subsequently removed. The cells were
incubated with 100 μl/well anti-BrdU peroxidase working solution
for 90 min at 20°C. The antibody conjugate was removed and the
cells were rinsed three times with washing solution. Following the
removal of the washing solution, and the addition of 100 μl/well
substrate solution, the cells were incubated at 20°C for 20 min,
followed by the addition of 25 μl of 1 M
H2SO4. The cells were incubated for 1 min
with agitation at 300 rpm, and the absorbance of the samples was
measured in an ELISA reader (Thermo Fisher Scientific) at 450 nm,
with a reference wavelength of 690 nm. Each experiment was
performed in triplicate, and repeated three times in order to
assess the consistency of the results. The results were also
compared using the BrdU technique with an MTT assay, which
confirmed the validity of the findings (data not shown).
Statistical analyses
All statistical analyses were performed using SPSS
version 13.0 software (SPSS Inc., Chicago, IL, USA). The data
between the two groups were analyzed by a Student’s t-test. The
data between multiple groups were analyzed by a one-way analysis of
variance. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of P-p38 MAPK protein in
drug-resistant and primary ovarian cancer tissues
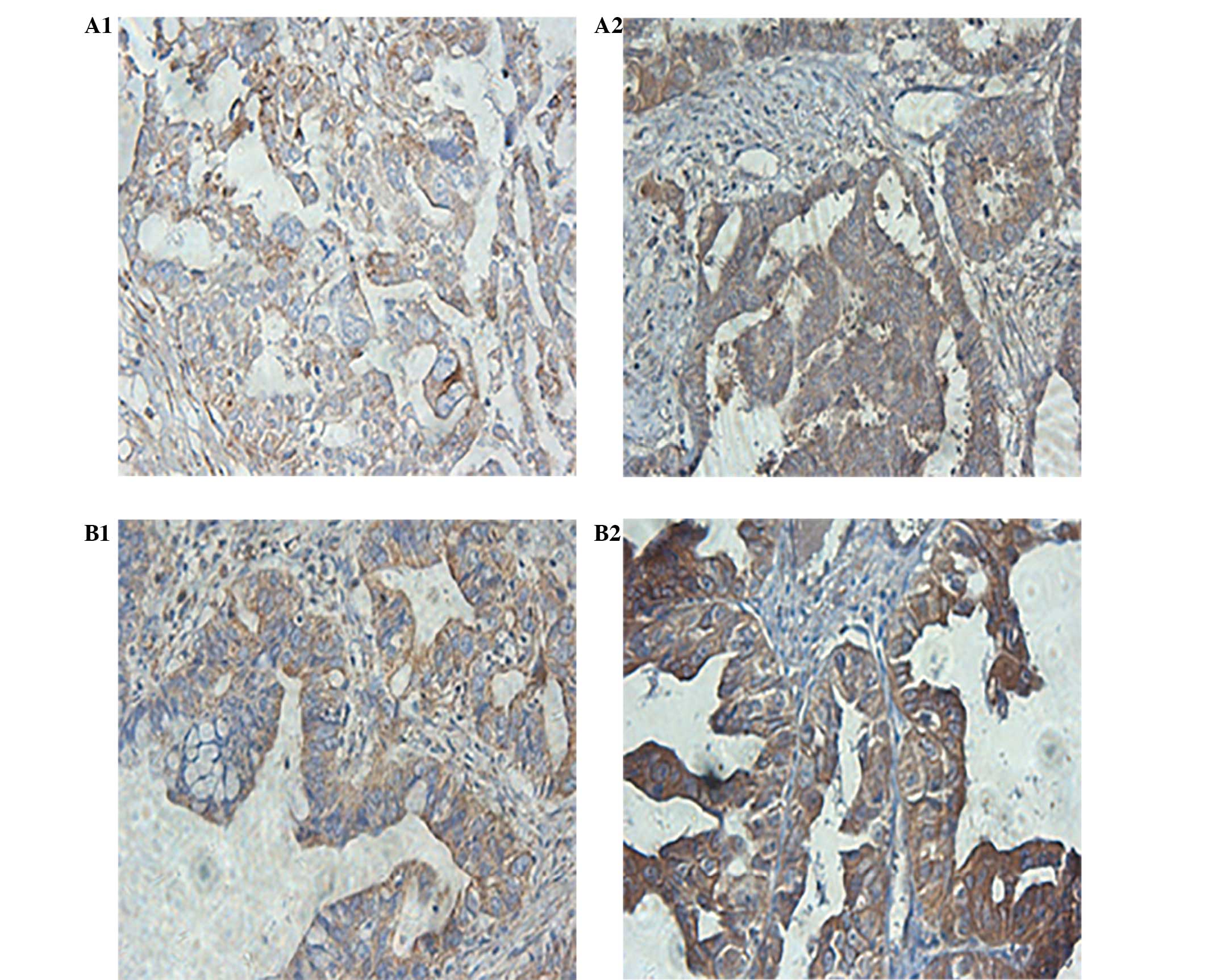
Immunohistochemistry
P-p38 MAPK protein was observed to be mainly
distributed in the cytoplasm, and the nuclei of the cells of both
the drug-resistant, and primary ovarian cancer tissues (Fig. 1). The relative expression in the
drug-resistant ovarian cancer tissues was significantly increased,
as compared with the primary ovarian cancer tissues (P<0.05).
The difference in the average gray value of the P-p38 MAPK
expression was significantly different between the drug-resistant
and the primary ovarian cancer tissues (P<0.05).
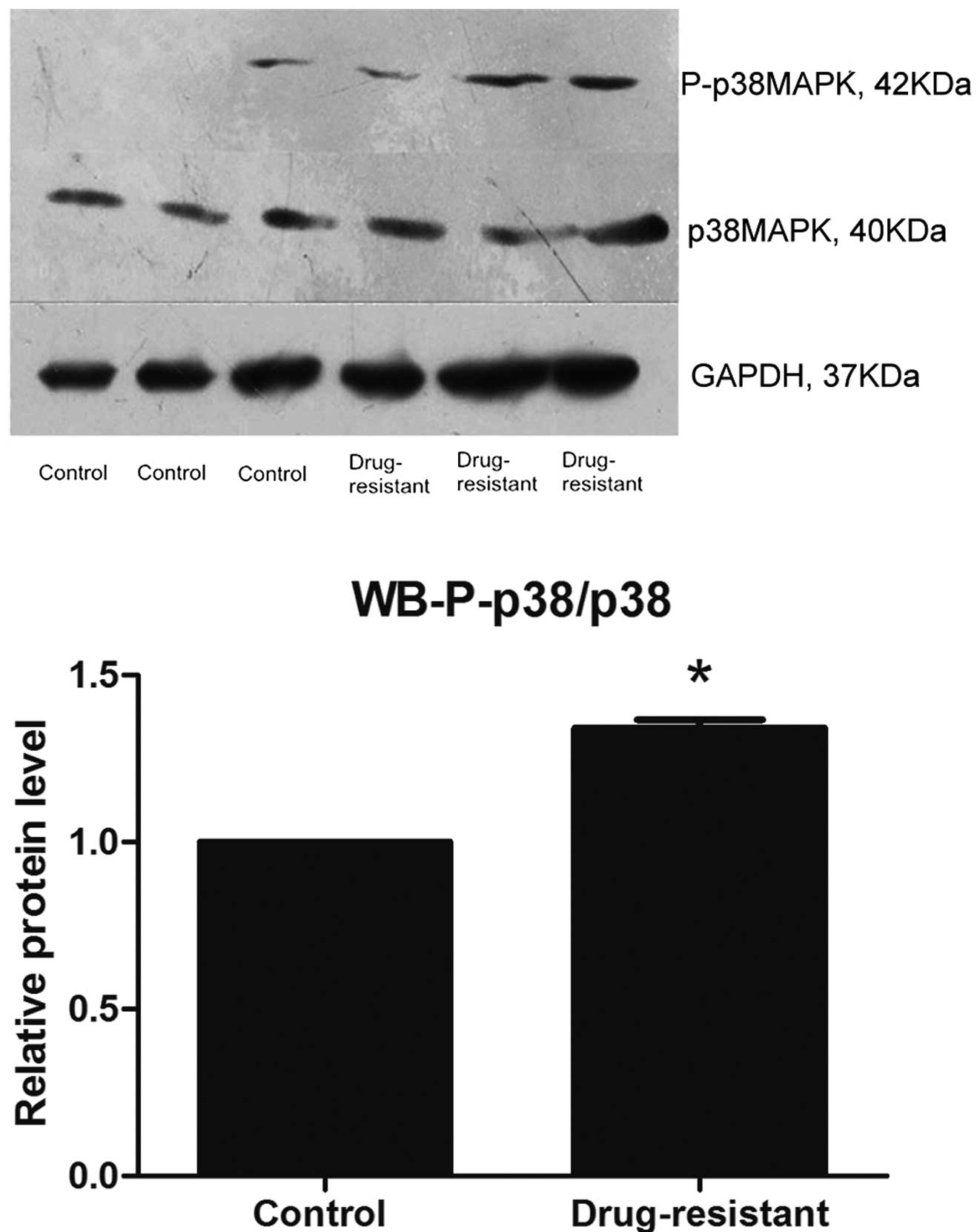
Western blotting
The relative expression of P-p38 MAPK protein in
chemotherapy-resistant EOC tissues was significantly increased, as
compared with the primary ovarian cancer tissues (Fig. 2), as determined by western
blotting.
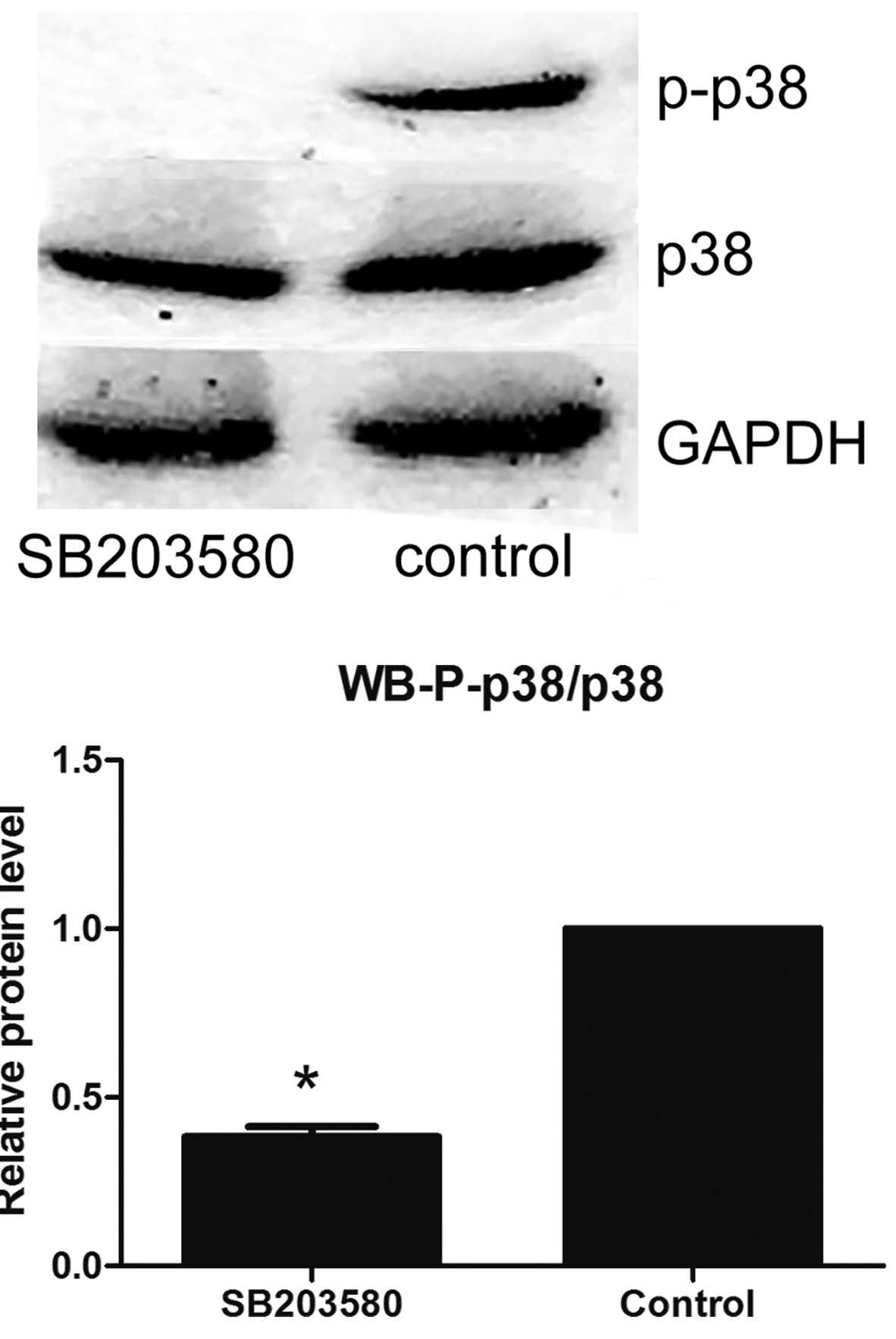
Effects of p38 MAPK inhibitor on the
expression of p38 MAPK and P-p38 MAPK protein in SKOV3/DDP
cells
Following treatment with the p38 MAPK inhibitor
SB203580, the relative expression of P-p38 MAPK protein in
SKOV3/DDP cells was shown to be significantly reduced, as compared
with the control (Fig. 3).
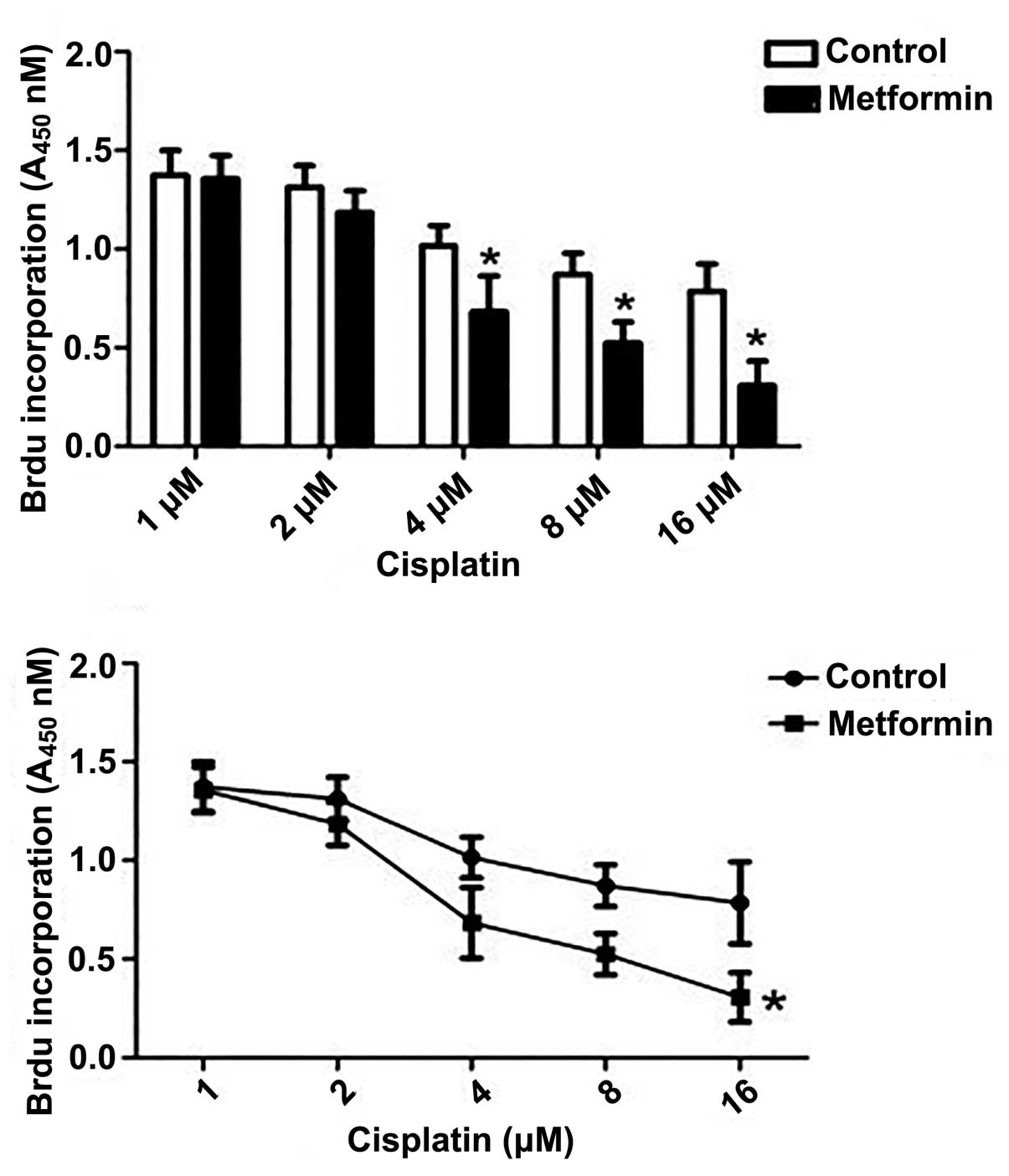
Effect of metformin on the
proliferation of endometrial cancer cells
Cisplatin was shown to markedly inhibit the
proliferation of SKOV3/DDP cells, as determined by the BrdU ELISA
assay. The most significant effect was observed when the cells were
treated with 1 mM metformin (P<0.05) (Fig. 4).
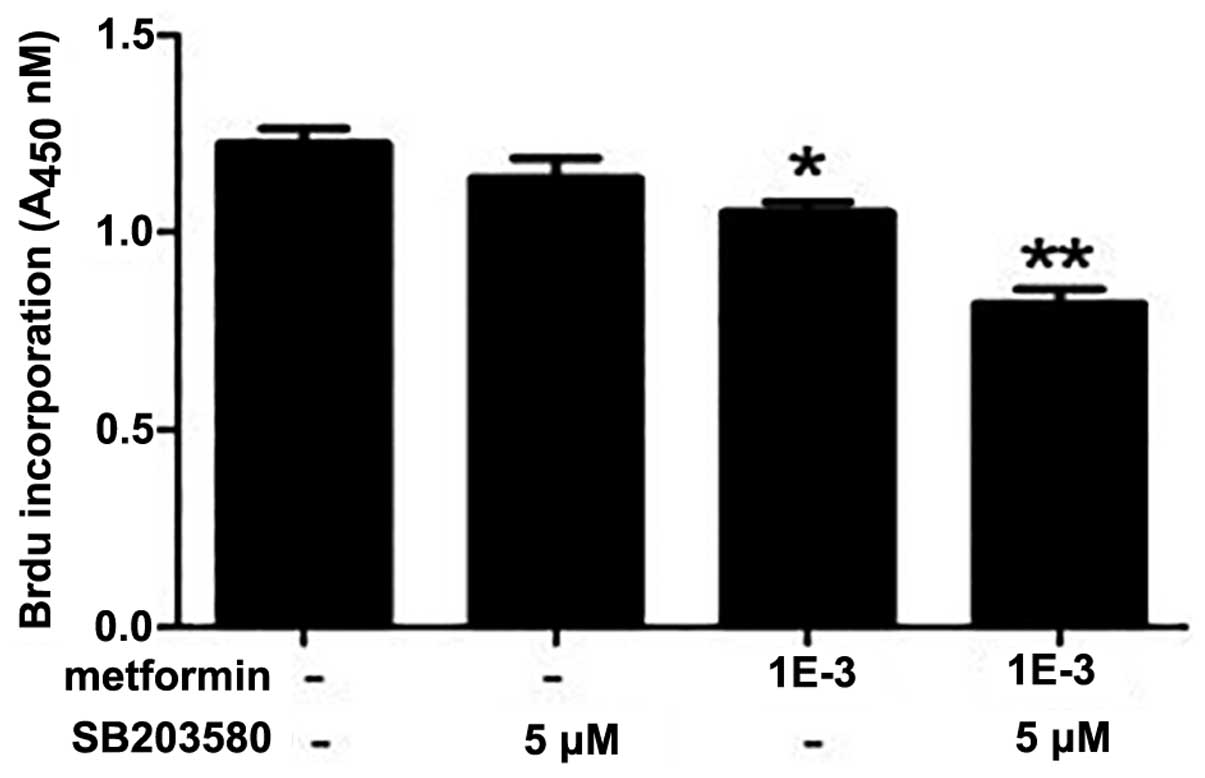
Metformin combined with SB203580
significantly improves the sensitivity of SKOV3/DDP cells to
cisplatin treatment
Treatment with metformin and SB203580 alone
significantly inhibited the proliferation of SKOV3/DDP cells, as
compared with the control (P<0.05). Treatment of the cells with
metformin combined with SB203580, synergistically inhibited cell
proliferation, resulting in a statistically significant reduction
in proliferation, as compared with both the control and the
separate treatment strategies (P<0.01) (Fig. 5).
Discussion
Epithelial ovarian cancer (EOC) is associated with
the highest mortality rate of all known gynecological cancers, and
surgery and chemotherapy are considered the gold standard of
advanced EOC treatment. The occurrence of resistance to
chemotherapy has a serious effect on the prognosis of ovarian
cancer (1). The present study
identified that the p38 MAPK signaling pathway was abnormally
activated in ovarian cancer tissues that were resistant to platinum
combined with paclitaxel, thus indicating that the MAPK signaling
pathway may have an important role in chemoresistance. It was also
observed that treatment with metformin inhibited the growth of
drug-resistant ovarian cancer cells, and reversed drug resistance
in cisplatin-resistant ovarian cancer cell lines. Previous studies
have found that metformin inhibited the growth of ovarian cancer
cells in vitro (8,9,16),
which is consistent with the present findings.
Previous studies have shown that inhibitors of the
mammalian target of rapamycin (mTOR) or the MAPK signaling pathways
enhanced the anti-tumor effects of metformin. A suggested mechanism
for this may be metformin enhancing the paclitaxel-induced
cytotoxicity, as previously observed in non-small cell lung cancer
cells (17). The present study
found that metformin, in combination with the p38 MAPK inhibitor
SB203580, improved cisplatin sensitivity in drug-resistant ovarian
cancer cells. These results demonstrated that cisplatin
chemoresistance may be associated with the MAPK signaling pathway
in ovarian cancer. Tseng et al (17) found that metformin reduced the
expression of the excision repair gene-1. The change in gene
expression was shown to be mediated by phosphorylated MAPK
pathways, which were being activated by paclitaxel. These findings
suggest that inhibitors of p38 MAPK or the p42 MAPK signaling
pathways, may increase the anti-tumor effects of metformin and
paclitaxel.
Liu et al (18) found that a combination of the mTOR
inhibitor RAD001 and metformin enhanced the cytotoxicity of
chemotherapeutic drugs in breast cancer. Monteagudo et al
(19) found that decreasing the
expression of p42 MAPK through small interfering RNAs,
significantly enhanced the anti-tumor effects of metformin in
prostate cancer cells. These results suggest that metformin, in
combination with signaling pathway inhibitors, may enhance the
efficacy of platinum and paclitaxel in chemotherapy-resistant
ovarian cancer (20). However, the
precise mechanism is currently unclear. A previous study showed
that metformin treatment reversed chemoresistance in breast cancer,
and significantly inhibited multidrug resistance 1 (MDR1) gene
transcription, which eventually significantly reduced the amount of
the intracellular P-glycoprotein. It was demonstrated that
metformin may reverse breast cancer chemoresistance by activating
5′ AMP-activated kinase, resulting in the suppression of MDR1
expression (21). The specific
mechanisms underlying metformin reversal cisplatin-chemoresistance
in ovarian cancer cells is a future research goal.
In conclusion, the MAPK signaling pathway in
advanced EOC was shown to be abnormally activated in drug-resistant
ovarian cancer tissues, as compared with primary ovarian cancer
tissues. Metformin treatment improved the sensitivity of SKOV3/DDP
cisplatin-resistant ovarian cancer cells, to cisplatin. There was a
marked improvement in SKOV3/DDP cisplatin sensitivity, when the
cells were treated with metformin in combination with the p38 MAPK
inhibitor SB203580. The results of the present study support a
potential clinical trial of metformin combined with MAPK signaling
pathway inhibitors, in the treatment of chemotherapy-resistant
ovarian cancers.
References
|
1
|
Morrison J, Haldar K, Kehoe S and Lawrie
TA: Chemotherapy versus surgery for initial treatment in advanced
ovarian epithelial cancer. Cochrane Database Syst Rev.
8:CD0053432012.
|
|
2
|
Ozols RF: Treatment goals in ovarian
cancer. Int J Gynecol Cancer. 15:3–11. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McLemore MR, Miaskowski C, Aouizerat BE,
Chen LM and Dodd MJ: Epidemiological and genetic factors associated
with ovarian cancer. Cancer Nurs. 32:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Comprehensive Cancer Network.
Clinical Practice Guidelines™ in Oncology (version
1.2011). 2011
|
|
6
|
Bodmer M, Becker C, Meier C, Jick SS and
Meier CR: Use of metformin and the risk of ovarian cancer: a
case-control analysis. Gynecol Oncol. 123:200–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romero IL, McCormick A, McEwen KA, et al:
Relationship of type II diabetes and metformin use to ovarian
cancer progression, survival, and chemosensitivity. Obstet Gynecol.
119:61–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gotlieb WH, Saumet J, Beauchamp MC, et al:
In vitro metformin anti-neoplastic activity in epithelial ovarian
cancer. Gynecol Oncol. 110:246–250. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011.PubMed/NCBI
|
|
10
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar
|
|
11
|
Rocha GZ, Dias MM, Ropelle ER, et al:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing D and Orsulic S: Modeling resistance
to pathway-targeted therapy in ovarian cancer. Cell Cycle.
4:1004–1006. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohta T, Ohmichi M, Hayasaka T, et al:
Inhibition of phosphatidylinositol 3-kinase increases efficacy of
cisplatin in in vivo ovarian cancer models. Endocrinology.
147:1761–1769. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee S, Choi EJ, Jin C and Kim DH:
Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA
amplification contributes to cisplatin resistance in an ovarian
cancer cell line. Gynecol Oncol. 97:26–34. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo MT, Liu Z, Wei Y, et al: Induction of
human MDR1 gene expression by 2-acetylaminofluorene is mediated by
effectors of the phosphoinositide 3-kinase pathway that activate
NF-kappaB signaling. Oncogene. 21:1945–1954. 2002. View Article : Google Scholar
|
|
16
|
Rattan R, Giri S, Hartmann LC and Shridhar
V: Metformin attenuates ovarian cancer cell growth in an AMP-kinase
dispensable manner. J Cell Mol Med. 15:166–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie Y, Wang YL, Yu L, et al: Metformin
promotes progesterone receptor expression via inhibition of
mammalian target of rapamycin (mTOR) in endometrial cancer cells. J
Steroid Biochem Mol Biol. 126:113–120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tseng SC, Huang YC, Chen HJ, et al:
Metformin-mediated downregulation of p38 mitogen-activated protein
kinase-dependent excision repair cross-complementing 1 decreases
DNA repair capacity and sensitizes human lung cancer cells to
paclitaxel. Biochem Pharmacol. 85:583–594. 2013. View Article : Google Scholar
|
|
19
|
Liu H, Scholz C, Zang C, et al: Metformin
and the mTOR inhibitor everolimus (RAD001) sensitize breast cancer
cells to the cytotoxic effect of chemotherapeutic drugs in vitro.
Anticancer Res. 32:1627–1637. 2012.
|
|
20
|
Monteagudo S, Pérez-Martinez FC,
Pérez-Carrión MD, et al: Inhibition of p42 MAPK using a nonviral
vector-delivered siRNA potentiates the anti-tumor effect of
metformin in prostate cancer cells. Nanomedicine (Lond.).
7:493–506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HG, Hien TT, Han EH, et al: Metformin
inhibits P-glycoprotein expression via the NF-κB pathway and CRE
transcriptional activity through AMPK activation. Br J Pharmacol.
162:1096–1108. 2011.PubMed/NCBI
|