Introduction
Hepatocellular carcinoma (HCC) is a global health
problem, ranking as the fifth most common cancer worldwide and the
third leading cause of cancer-related mortality (1). It is the second cause of
cancer-related mortality in China, and its prevalence is
increasing, probably due to a high prevalence of hepatitis B
(2,3). Surgical resection and liver
transplantation are effective forms of therapy (4), but most patients have limited options
and cannot afford these treatments. Therefore, improving our
understanding of the HCC pathogenesis is especially important,
since it may allow identifying effective, novel targets for
therapy.
The signal transducer and activator of transcription
3 (STAT3) protein is a member of the STAT transcription factor
family. STAT proteins mediate signal transduction induced by
cytokines, growth factors, and oncogenes (5). In healthy cells, activation of the
STAT3 protein is tightly controlled, in order to prevent
deregulated gene transcription. STAT3 is constitutively activated
in certain transformed cells (3).
STAT3 activation is induced via phosphorylation of tyrosine
(Tyr705), which allows STAT3 dimerization. The dimer translocates
to the nucleus and directly regulates gene expression (5). Phosphorylated STAT3 (p-STAT3)
contributes to malignant progression in various types of cancer,
including carcinomas of the lung (6), breast (7), prostate (8) and melanoma (9). Oncogenesis, invasion, and metastasis
of HCC are associated with activation of STAT3 (1,10).
Cellular immune responses play a critical role in
the surveillance of malignancy and the control of HCC progression,
with CD4+ and CD8+ T cells being the primary
antitumor immune cells (11).It
was previously shown that STAT3 is constitutively activated not
only in tumor cells, but also in tumor endothelial and myeloid
cells, tumor-associated macrophages and dendritic cells (12). Other studies have suggested that
active STAT3 is upregulated in tumor-infiltrating immune cells
including dendritic cells, natural killer cells, and granulocytes
(13,14). STAT3 signaling restrains natural
tumor immune surveillance, and inhibition of hematopoietic STAT3
activity in tumor-bearing hosts elicits multicomponent therapeutic
antitumor immunity (13). In
addition, the expression of p-STAT3 in peripheral CD4+
and CD8+ T cells is increased in patients with multiple
sclerosis (15), and p-STAT3
expression is induced by IL-10 in CD4+ and
CD8+ T cells isolated from tumor-draining lymph nodes
and tumors of mice bearing squamous carcinoma (16). However, whether the aberrant
expression of p-STAT3 in peripheral CD4+ T and
CD8+ T cells of HCC patients may affect their immune
surveillance and immune tolerance and thus, contribute to HCC
pathogenesis, remains unclear.
The T helper cells (Th) are a sub-group of
lymphocytes that play a central role in immune protection (17). Th1 cells mediate antitumor
reactivity through secretion of cytokines, including IFN-γ and
tumor necrosis factor-α (TNF-α). IL-4, IL-6 and IL-10 are secreted
by Th2 cells, and downregulate antitumor immunity (18,19).
The Th1/Th2 balance is altered in HCC patients, and this event can
lead to tumorigenesis (20).
However, whether the Th1/Th2 imbalance is related to the abnormal
expression of p-STAT3 in peripheral CD4+ and
CD8+ T cells of HCC patients remains to be
investigated.
STAT3 mediates cytokine signaling (21). IL-6 is a pro-inflammatory cytokine,
playing an important role in regulating the immune response and
other processes involved in the inflammatory response (10,22).
IL-6 is one of the most important cytokines for STAT3 activation,
and leads to STAT3 activation via the Janus-activated kinase (JAK)
(22,23). IL-6 levels appear to be higher in
HCC patients compared to healthy individuals (10). IL-10 is a potent immunosuppressive
cytokine that downregulates the expression of Th1 cytokines and
co-stimulatory molecules (24,25).
IL-10 can also induce phosphorylation of STAT3 through activation
of the JAK pathway (26). IL-10
expression is upregulated in HCC patients (27). IL-10 induces STAT3 phosphorylation
in CD4+ and CD8+ T cells isolated from
tumor-draining lymph nodes of mice bearing squamous carcinomas
(16). However, whether IL-6 and
IL-10 induce p-STAT3 expression in peripheral CD4+ and
CD8+ T cells in HCC is still unclear.
In this study, we analyzed peripheral blood of HCC
patients and healthy volunteers for the expression of p-STAT3. We
also co-cultured healthy human peripheral blood mononucleated cells
(PBMCs) with human hepatoma cells to investigate the expression of
p-STAT3 in CD4+ and CD8+ T cells. Our data
show that p-STAT3 is aberrantly expressed in CD4+ and
CD8+ T cells of the peripheral blood in HCC patients,
suggesting that activation of this transcription factor may
contribute to the development of hepatocellular carcinoma.
Materials and methods
Study population
Patients with HCC and healthy controls were
recruited from the Yuhuangding Hospital (Yantai, Shandong, China),
from April, 2013 through August, 2013. Sample collection procedures
for this study were approved by the University of Binzhou Medical
College Ethics Committee, and informed consent was obtained from
all patients. The study subjects included patients with HCC (n=10)
and healthy controls (n=10). HCC was diagnosed on the basis of
image findings [sonography, computed tomography (CT) scans, or
magnetic resonance imaging (MRI) scans], biochemical tests
[α-fetoprotein (AFP) levels ≥400 ng/ml], and histopathology,
according to the guidelines of the American Association for the
Study of Liver Diseases (28).
Venous blood (4 ml) was collected from all subjects into two
heparin tubes. Two ml of venous blood were used to isolate the
serum. The serum was isolated by centrifugation at 1,734 g for 10
min at 4°C, and was then stored at −80°C. The remaining 2 ml of
venous blood were lysed and fixed prior to flow cytometry. The
erythrocytes were lysed and the leucocytes were fixed by
immediately adding 30 ml of 1× pre-warmed Lyse/Fix Buffer (BD
Biosciences, San Jose, CA, USA) and incubating in 37°C for 10
min.
Co-culture
PBMCs were isolated from venous blood by
Ficoll-Paque density gradient (Amersham Pharmacia Biotech,
Piscataway, NJ, USA) centrifugation at 771 × g, for 30 min. PBMCs
were removed from the Ficoll/serum interface, washed twice in
phosphate buffered saline (PBS), supplemented with 2% bovine serum
albumin (BSA; Beyotime, Songjiang, Shanghai, China), and counted
using an inverted microscope (OLYMPUS, Shinjuku, Tokyo, Japan).
The human hepatoma cell line Huh7 was purchased from
the Cell Station of Shanghai Institute of Chinese Academy of
Sciences. Cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 20% fetal bovine serum (FBS), 2 mM HEPES,
2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin
(all from Hyclone, Logan, UT, USA) in 5% CO2 at
37°C.
Huh7 cells were seeded into 12-well Corning
Costar® plates (Corning, NY, USA) at densities of
1.25×105, 2.5×105 and 5×105
cells/well. Samples were incubated for 4 h, and 0.5 ml of PBMCs
(5×105/ml) were added to each well. After 24, 36, 48 and
60 h, PBMCs were harvested by centrifugation at 193 g for 5 min
used 5 ml centrifuge tubes (Corning, NY, USA), washed in PBS
supplemented with 2% BSA (Beyotime), and fixed prior to flow
cytometry. The PBMCs were fixed by immediately adding pre-warmed
Cytofix™ Fixation Buffer (BD Biosciences), and incubating at 37°C
for 10 min. In addition, the supernatants were collected,
centrifuged at 1,000 × g for 5 min, and then stored at −80°C.
Flow cytometry
Fixed cells were washed in Stain Buffer (BD
Biosciences), vortexed and permeabilized by adding 1 ml of chilled
Phosflow™ Perm Buffer III (BD Biosciences) and incubating for 30
min on ice. Samples were then washed and incubated with the mouse
anti-human antibodies (all from BD Biosciences) anti-CD3-PE-Cy5
(clone UCHT1;), -CD4-FITC (clone RPA-T4), -CD8-FITC (clone RPA-T8),
and -phosphorylated (p)-STAT3-PE (Phosflow™) at room temperature
for 60 min. Then, the cells were washed and resuspended in Stain
Buffer (BD Biosciences), and were subjected to flow cytometry
analysis on a FACSCalibur instrument (BD Biosciences). To analyze
the mean fluorescence intensity (MFI) of p-STAT3 in peripheral
CD4+ and CD8+ T cells, cells were gated in
CD3+ T/CD4+ T and CD3+
T/CD8+ T regions. The results were analyzed with the
CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Cytokine measurements
The production of IFN-γ, IL-4, IL-6 and IL-10 was
measured in the supernatant and the serum samples by enzyme-linked
immunosorbent assay (ELISA) using commercial kits (R&D Systems,
Minneapolis, MN, USA) and following the manufacturer’s
instructions. All samples were assayed in triplicate.
Statistical analyses
Data are presented as mean ± standard error of the
mean (SEM). Data were processed with the SPSS 17.0 statistical
software (IBM, Armonk, NY, USA). A one-way analysis of variance
(ANOVA) was used to analyze the differences in the p-STAT3 mean
fluorescence intensity (MIF) in CD4+ and CD8+
T cells, and in the cytokine levels between HCC patients or PBMCs
co-cultured with Huh7 cells and their controls. To analyze the
correlation between the levels of IFN-γ, IL-4, IL-6, IL-10 and
p-STAT3, we performed a Spearman’s correlation analysis. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
The p-STAT3 level is higher in peripheral
CD4+ and CD8+ T cells of HCC patients
compared to healthy controls
The p-STAT3 level in peripheral CD4+ and
CD8+ T cells of HCC patients and healthy subjects was
measured using flow cytometry. The results showed that the
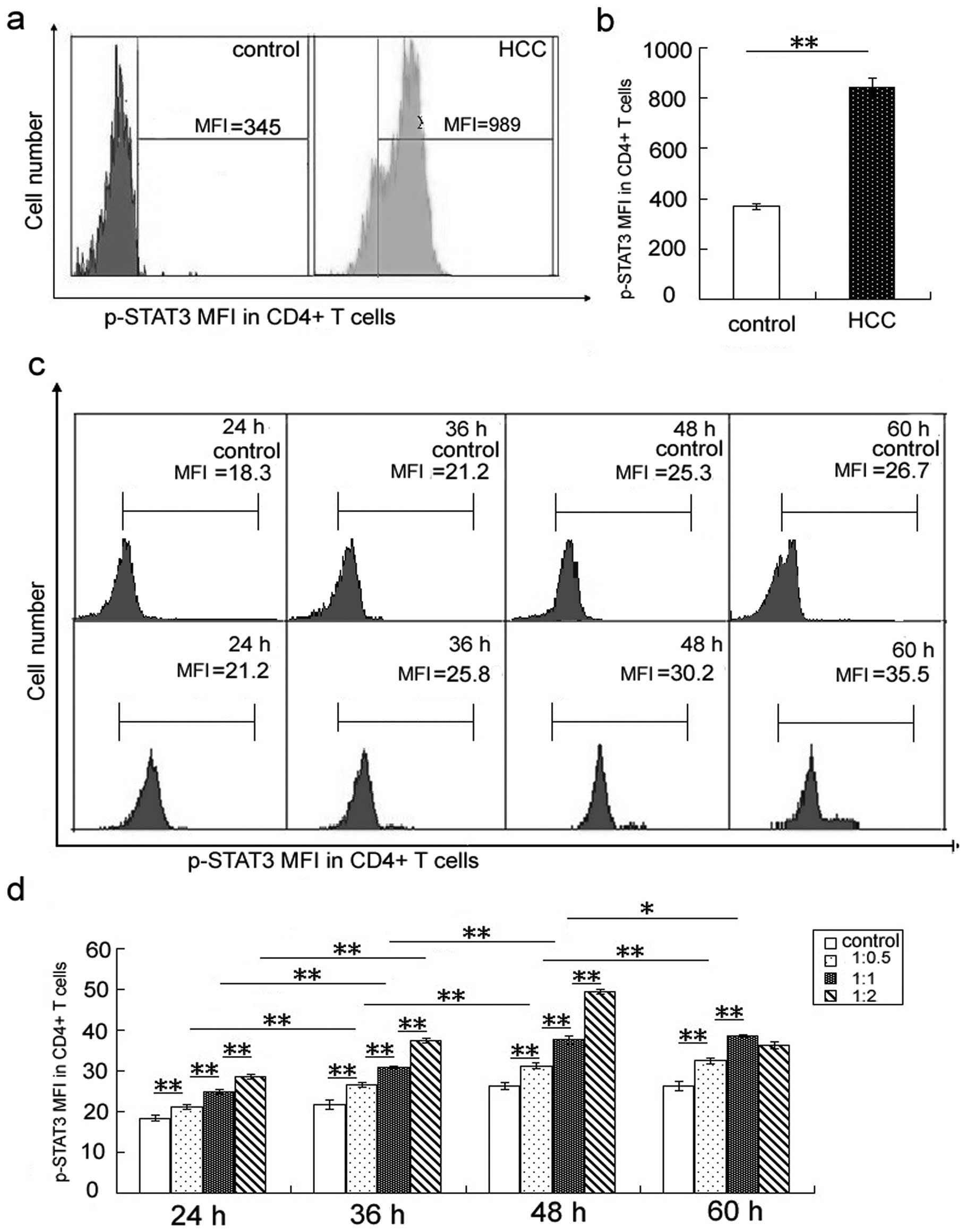
expression of p-STAT3 in CD4+ T cells (Fig. 1a and b) and CD8+ T cells
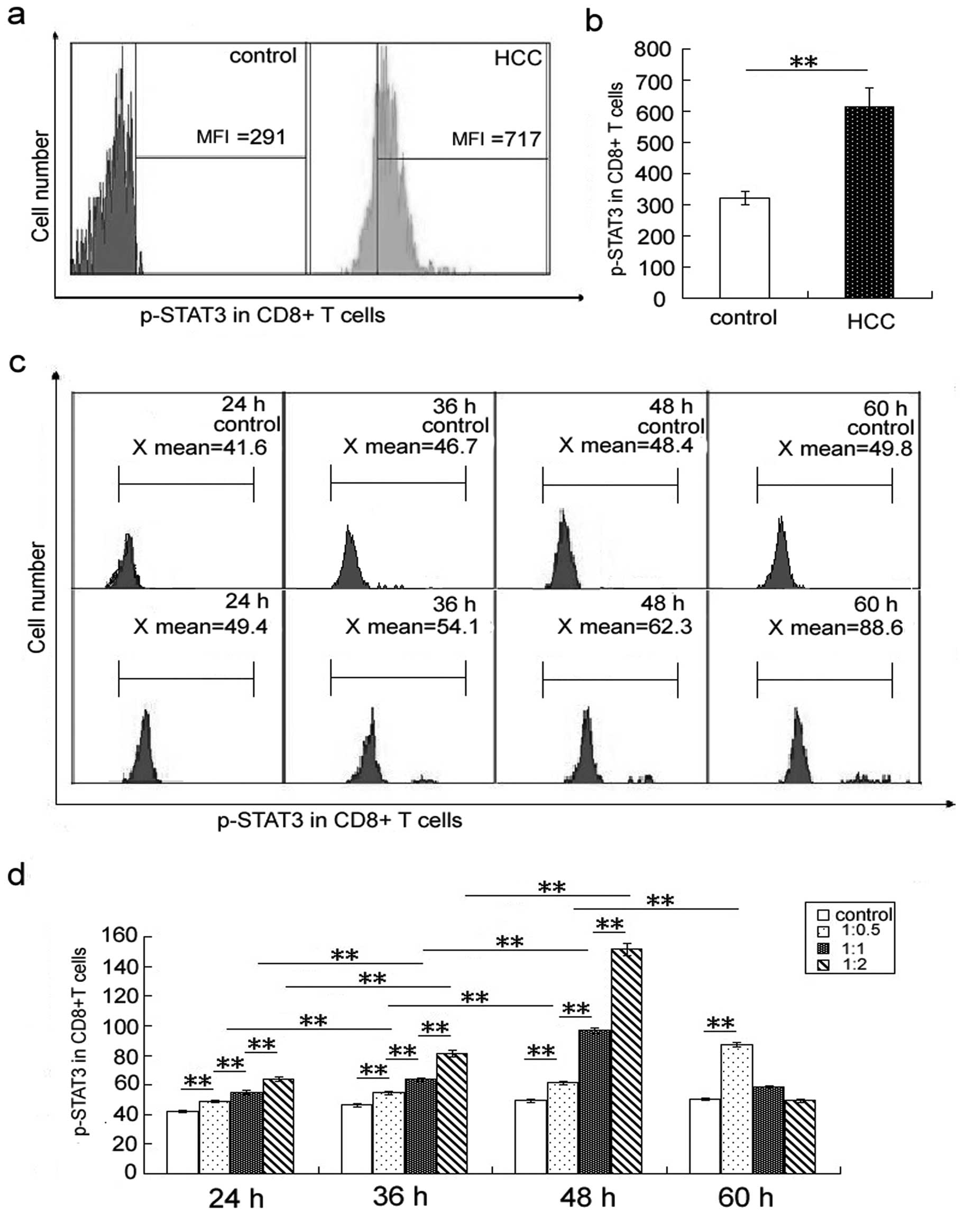
(Fig. 2a and b) is higher in HCC
patients than in healthy controls.
Furthermore, the p-STAT3 level in CD4+
and CD8+ T cells from PBMCs co-cultured with Huh7 cells
was higher than that of PBMCs cultured with medium alone (Figs. 1d and 2d). Expression of p-STAT3 in
CD4+ and CD8+ T cells increased with the time
of co-culture (Figs. 1c and d,
2c and d).
The IFN-γ level is decreased and the
IL-4, IL-6 and IL-10 levels are increased in the serum of HCC
patients
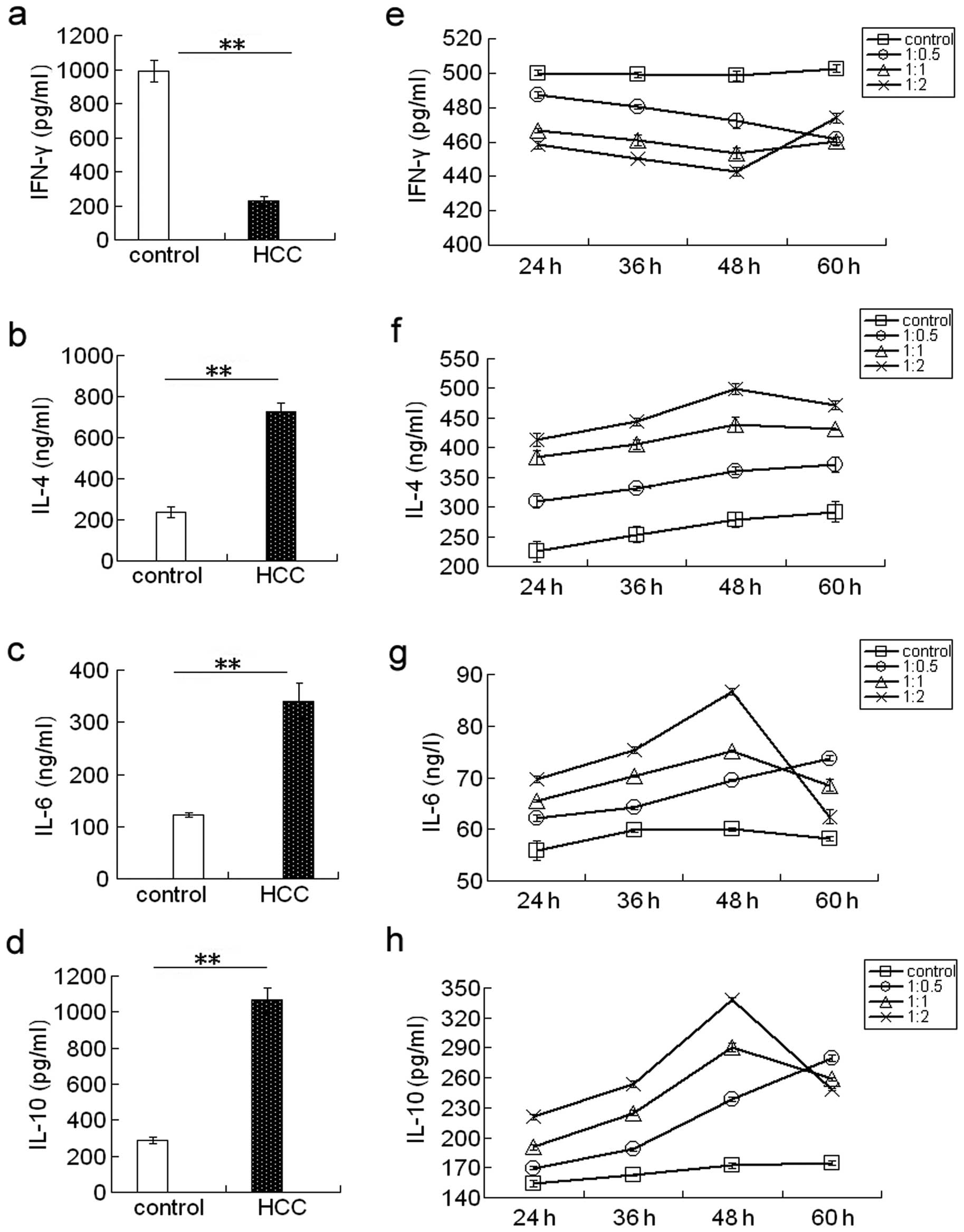
ELISA results showed that the IFN-γ levels are lower
(Fig. 3a), whereas the IL-4
(Fig. 3b), IL-6 (Fig. 3c), and IL-10 (Fig. 3d) levels are higher in the serum of
HCC patients than in controls.
Furthermore, the IFN-γ level was decreased (Fig. 3a and e), whereas the IL-4 (Fig. 3b and f), IL-6 (Fig. 3c and g), and IL-10 (Fig. 3d and h) levels were increased in
the supernatants of Huh7 cells co-cultured with PBMCs compared to
the controls. The IFN-γ level was decreased, while the IL-4, IL-6
and IL-10 levels increased with the time of co-culture and with the
increasing ratio of PBMCs to Huh7 cells.
Correlation analysis between the the
p-STAT3 level and the IFN-γ/IL-4 ratio, the IFN-γ, IL-4, IL-6 and
IL-10 levels in peripheral CD4+ and CD8+ T
cells from HCC patients
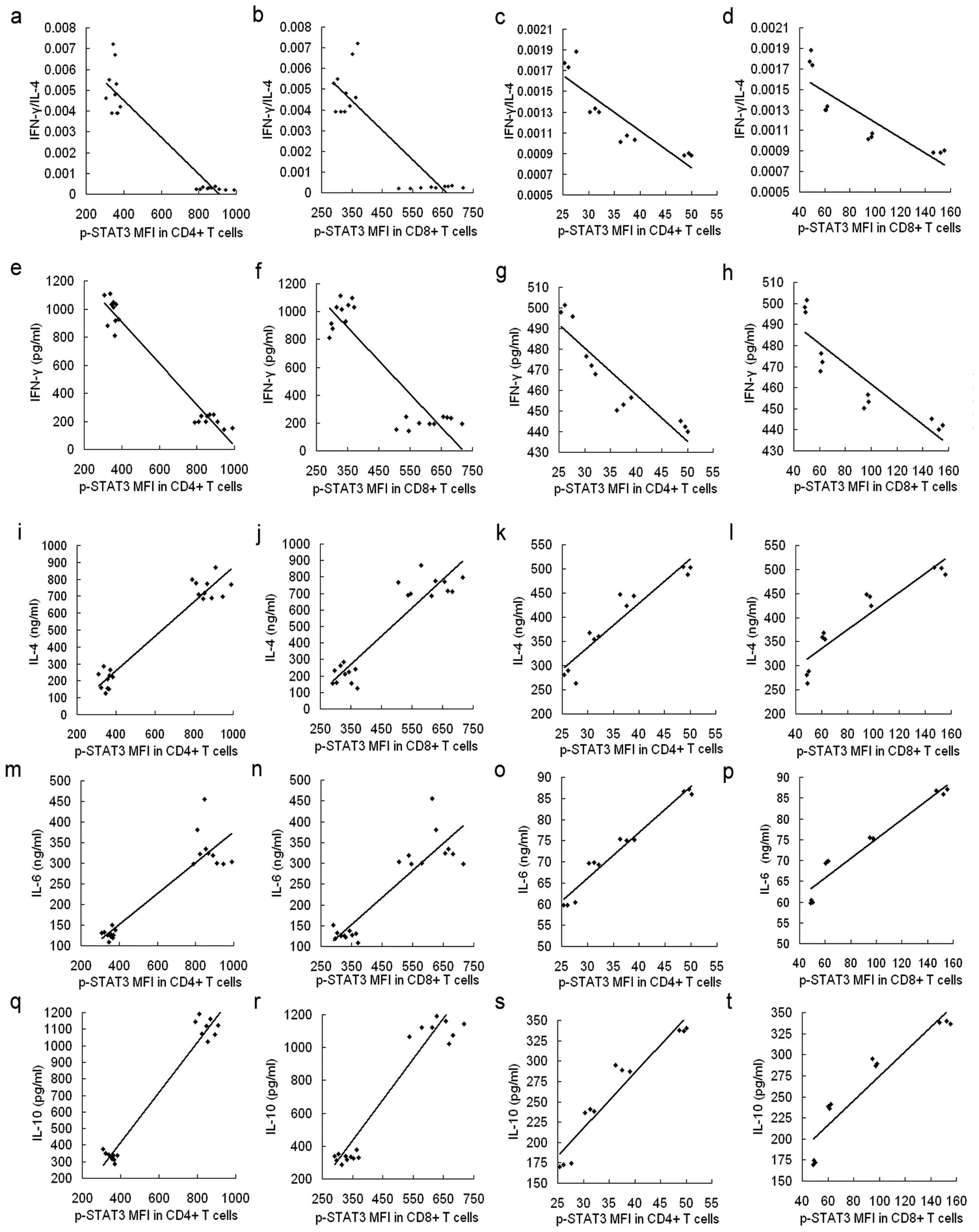
Spearman’s correlation analysis was used to
determine the correlation between the ratio of IFN-γ/IL-4, the
levels of IFN-γ, IL-4, IL-6 and IL-10 and the p-STAT3 level in
CD4+ and CD8+ T cells in patient samples and
in the co-culture system. The ratio of IFN-γ/IL-4 and the level of
IFN-γ negatively correlated to the level of p-STAT3 in peripheral
CD4+ and CD8+ T cells (R=−0.923, P<0.001;
R=−0.853, P<0.001; R=−0.926, P<0.001; R=−0.827 and
P<0.001, respectively) (Fig. 4a, b,
e and f). Similar correlations were found in data collected
from CD4+ and CD8+ T cells from PBMCs
co-cultured with Huh7 cells for 48 h (R=−0.870, P=0.001; R=−0.829,
P=0.003; R=−0.916, P<0.001; R=−0.890, and P<0.001,
respectively) (Fig. 4c, d, g and
h). In addition, the IL-4, IL-6 and IL-10 levels positively
correlated to the p-STAT3 level in peripheral CD4+ and
CD8+ T cells from HCC patients (R=0.733, P<0.001;
R=0.632, P=0.002; R=0.919, P<0.001; R=0.903, P<0.001;
R=0.985, P<0.001; R=0.921 and P<0.001, respectively)
(Fig. 4i, j, m, n, q and r) and
from the co-culture system (R=0.947, P<0.001; R=0.934,
P<0.001; R=0.980, P<0.001; R=0.966, P<0.001; R=0.955,
P<0.001; R=0.938 and P<0.001, respectively) (Fig. 4k, l, o, p, s and t).
Discussion
HCC is one of the most common cancers worldwide;
approximately 600,000 patients die of this disease each year in the
world (29). Constitutively
activated STAT3 has been shown to strongly correlate to the
development and progression of a number of cancers, including HCC
(30–34). A previous study suggested that the
phosphorylation of STAT3 is upregulated in tumor-infiltrating
immune cells, including dendritic cells, natural killer cells, and
granulocytes, and that inhibiting STAT3 activity in hematopoietic
cells triggers an intrinsic immune-surveillance system that
inhibits tumor growth and metastasis (13). The expression of p-STAT3 in
peripheral CD4+ and CD8+ T cells is increased
in active multiple sclerosis patients compared to healthy subjects,
and the level of p-STAT3 is associated with the function of T cell
responses in multiple sclerosis relapse cases (15). It is well established that cellular
immune responses, especially those mediated by CD4+ and
CD8+ T cells, play a critical role in the surveillance
of malignancy and the control of HCC progression (35). In this study, we found that the
p-STAT3 level in CD4+ and CD8+ T cells from
peripheral blood of HCC patients is higher compared to that of
healthy controls, and that the p-STAT3 level in CD4+ and
CD8+ T cells from PBMCs co-cultured with Huh7 cells is
higher than that from PBMCs cultured with medium alone. In
addition, we found that the expression of p-STAT3 increased with
the time in co-culture and with the increasing ratio of Huh7 cells
to PBMCs. These results suggest that the HCC microenvironment
induces p-STAT3 expression in peripheral CD4+ and
CD8+ T cells. High levels of p-STAT3 in peripheral
CD4+ and CD8+ T cells may result in an
abnormal immune response in HCC cells, or may decrease the levels
of immune surveillance and induce immune tolerance to HCC.
Therefore, these findings may enhance our understanding of the
immunologic role of p-STAT3 in HCC progression.
Cytokines mediate numerous innate and adaptive
immunity responses. We observed that the IFN-γ level is decreased
and the IL-4 level is increased in the serum of HCC patients
compared to healthy controls, in agreement with a previous study
(36). Cytokine profiles
indicating a deregulation of both Th1- and Th2-type cells have been
previously associated with the development of HCC (18). Th1 cytokines (IFN-γ and IL-2) are
related to cell-mediated immune responses in HCC (19). Our results showed that the ratio of
IFN-γ/IL-4 and the IFN-γ level negatively correlate, while the
level of IL-4 positively correlate to the p-STAT3 level in
peripheral CD4+ and CD8+ T cells in patient
samples and co-culture samples. These results indicate that the HCC
microenvironment may induce the aberrant expression of p-STAT3 in
peripheral CD4+ and CD8+ T cells, resulting
in abnormal cytokine secretion and thereby, downregulating
cell-mediated immune responses, which may overall contribute to the
progression of HCC.
Cytokine signaling pathways involving transcription
factors of the STAT family, and especially STAT3, play a key role
in the pathogenesis of diseases. IL-6 was previously shown to be
involved in STAT3 activation in HCC (22). In addition, STAT3 is constitutively
activated in HCC (23). The serum
level of IL-6 was also found to be elevated in HCC, and a higher
serum level of IL-6 was associated with HCC progression (37). Here, we found that IL-6 expression
is increased and positively correlates to the p-STAT3 level in the
serum of HCC patients and in the supernatant of co-cultured PBMCs
and Huh7 cells. This result indicates that IL-6 may activate STAT3
in CD4+ and CD8+ T cells of HCC patients. In
addition, a previous study demonstrated that IL-10 is another
cytokine that promotes STAT3 activation, the level of which is
continuously increased in HCC (27). Another study showed that IL-10
induces p-STAT3 in PBMCs of healthy volunteers (38). In our study, the IL-10 level was
also increased and positively correlated to the p-STAT3 level in
the serum of HCC patients and in the supernatant of PBMCs
co-cultured with Huh7 cells. Similar to IL-6, IL-10 may activate
STAT3 in CD4+ and CD8+ T cells of the
peripheral blood in HCC patients. Both cytokines may contribute to
the immune tolerance observed in HCC patients.
The aberrant expression p-STAT3 in CD4+
and CD8+ T cells of the peripheral blood of HCC patients
suggests that other factors in pathways that lie upstream of this
transcription factor may result in the abnormal cell-mediated
immune response to HCC cells and to their immune tolerance.
Overall, our study may help to broaden the current view on the
relationship between p-STAT3 expression in CD4+ and
CD8+ T cells and HCC pathogenesis. It may also provide
valuable data for the development of targets for therapeutic agents
in the clinical treatment of hepatocellular carcinoma.
Acknowledgements
We thank Dr Jiang Wenjin, at the Yuhuangding
Hospital, (Yantai, Shandong, China), for providing blood samples.
This study was supported by funds from the Nature Science
Foundation of Shandong Province (ZR2013HM050), the Foundation
Project in Shandong Province Department of Education (J02K12), and
the Science and Technology Planning Project of Binzhou City
(2011ZC0917).
References
|
1
|
Tai WT, Shiau CW, Chen HL, Liu CY, Lin CS,
Cheng AL, Chen PJ and Chen KF: Mcl-1-dependent activation of Beclin
1 mediates autophagic cell death induced by sorafenib and SC-59 in
hepatocellular carcinoma cells. Cell Death Dis. 4:e4852013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li W, Huang X, Tong H, Wang Y, Zhang T,
Wang W, Dai L, Li T, Lin S and Wu H: Comparison of the regulation
of β-catenin signaling by type I, type II and type III interferons
in hepatocellular carcinoma cells. PLoS One. 7:e470402012.
|
|
3
|
Liao J, Xu T, Zheng JX, Lin JM, Cai QY, Yu
DB and Peng J: Nitidine chloride inhibits hepatocellular carcinoma
cell growth in vivo through the suppression of the
JAK1/STAT3 signaling pathway. Int J Mol Med. 32:79–84.
2013.PubMed/NCBI
|
|
4
|
Cao M, Cabrera R, Xu Y, Firpi R, Zhu H,
Liu C and Nelson DR: Hepatocellular carcinoma cell supernatants
increase expansion and function of CD4+ CD25+
regulatory T cells. Lab Invest. 87:582–590. 2007.PubMed/NCBI
|
|
5
|
Subramaniam A, Shanmugam MK, Perumal E, Li
F, Nachiyappan A, Dai X, Swamy SN, Ahn KS, Kumar AP, Tan BK, Hui KM
and Sethi G: Potential role of signal transducer and activator of
transcription (STAT)3 signaling pathway in inflammation, survival,
proliferation and invasion of hepatocellular carcinoma. Biochim
Biophys Acta. 1835:46–60. 2013.
|
|
6
|
Lin HY, Chiang CH and Hung WC: STAT3
upregulates miR-92a to inhibit RECK expression and to promote
invasiveness of lung cancer cells. Br J Cancer. 109:731–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ren L, Wang X, Dong Z, Liu J and Zhang S:
Bone metastasis from breast cancer involves elevated IL-11
expression and the gp130/STAT3 pathway. Med Oncol. 30:6342013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho KH, Jeong KJ, Shin SC, Kang J, Park CG
and Lee HY: STAT3 mediates TGF-β1-induced TWIST1 expression and
prostate cancer invasion. Cancer Lett. 336:167–173. 2013.
|
|
9
|
Lesinski GB: The potential for targeting
the STAT3 pathway as a novel therapy for melanoma. Future Oncol.
9:925–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Fuchs J, Li C and Lin J: IL-6, a
risk factor for hepatocellular carcinoma: FLLL32 inhibits
IL-6-induced STAT3 phosphorylation in human hepatocellular cancer
cells. Cell Cycle. 9:3423–3427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt N, Neumann-Haefelin C and Thimme
R: Cellular immune responses to hepatocellular carcinoma: lessons
for immunotherapy. Dig Dis. 30:483–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xin H, Zhang C, Herrmann A, Du Y, Figlin R
and Yu H: Sunitinib inhibition of Stat3 induces renal cell
carcinoma tumor cell apoptosis and reduces immunosuppressive cells.
Cancer Res. 69:2506–2513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kortylewski M, Kujawski M, Wang T, Wei S,
Zhang S, Pilon-Thomas S, Niu G, Kay H, Mulé J, Kerr WG, Jove R,
Pardoll D and Yu H: Inhibiting Stat3 signaling in the hematopoietic
system elicits multicomponent antitumor immunity. Nat Med.
11:1314–1321. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang T, Niu G, Kortylewski M, Burdelya L,
Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola
D, Dalton W, Jove R, Pardoll D and Yu H: Regulation of the innate
and adaptive immune responses by Stat-3 signaling in tumor cells.
Nat Med. 10:48–54. 2004. View
Article : Google Scholar
|
|
15
|
Iorio R, Frisullo G, Nociti V, Patanella
KA, Bianco A, Marti A, Mirabella M, Tonali PA and Batocchi AP:
T-bet, pSTAT1 and pSTAT3 expression in peripheral blood mononuclear
cells during pregnancy correlates with post-partum activation of
multiple sclerosis. Clin Immunol. 131:70–83. 2009. View Article : Google Scholar
|
|
16
|
Emmerich J, Mumm JB, Chan IH, LaFace D,
Truong H, McClanahan T, Gorman DM and Oft M: IL-10 directly
activates and expands tumor-resident CD8+ T cells
without de novo infiltration from secondary lymphoid organs. Cancer
Res. 72:3570–3581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J and Paul WE: CD4 T cells: fates,
functions, and faults. Blood. 112:1557–1569. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ognjanovic S, Yuan JM, Chaptman AK, Fan Y
and Yu MC: Genetic polymorphisms in the cytokine genes and risk of
hepatocellular carcinoma in low-risk non-Asians of USA.
Carcinogenesis. 30:758–762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nieters A, Yuan JM, Sun CL, Zhang ZQ,
Stoehlmacher J, Govindarajan S and Yu MC: Effect of cytokine
genotypes on the hepatitis B virus-hepatocellular carcinoma
association. Cancer. 103:740–748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou D, Gu FM, Gao Q, Li QL, Zhou J and
Miao CH: Effects of anesthetic methods on preserving anti-tumor
T-helper polarization following hepatectomy. World J Gastroenterol.
18:3089–3098. 2012. View Article : Google Scholar
|
|
21
|
Willson TA, Jurickova I, Collins M and
Denson LA: Deletion of intestinal epithelial cell STAT3 promotes
T-lymphocyte STAT3 activation and chronic colitis following acute
dextran sodium sulfate injury in mice. Inflamm Bowel Dis.
19:512–525. 2013. View Article : Google Scholar
|
|
22
|
Lepiller Q, Abbas W, Kumar A, Tripathy MK
and Herbein G: HCMV activates the IL-6-JAK-STAT3 axis in HepG2
cells and primary human hepatocytes. PLoS One. 8:e595912013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Liu A, Li H, Li C and Lin J:
Celecoxib inhibits interleukin-6/interleukin-6 receptor-induced
JAK2/STAT3 phosphorylation in human hepatocellular carcinoma cells.
Cancer Prev Res (Phila). 4:1296–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaudhry A, Samstein RM, Treuting P, Liang
Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller
W and Rudensky AY: Interleukin-10 signaling in regulatory T cells
is required for suppression of Th17 cell-mediated inflammation.
Immunity. 34:566–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin HD, Park BL, Kim LH, Jung JH, Kim JY,
Yoon JH, Kim YJ and Lee HS: Interleukin 10 haplotype associated
with increased risk of hepatocellular carcinoma. Hum Mol Genet.
12:901–906. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nieminen JK, Niemi M, Sipponen T, Salo HM,
Klemetti P, Färkkilä M, Vakkila J and Vaarala O: Dendritic cells
from Crohn’s disease patients show aberrant STAT1 and STAT3
signaling. PLoS One. 8:e707382013.
|
|
27
|
Hsia CY, Huo TI, Chiang SY, Lu MF, Sun CL,
Wu JC, Lee PC, Chi CW, Lui WY and Lee SD: Evaluation of
interleukin-6, interleukin-10 and human hepatocyte growth factor as
tumor markers for hepatocellular carcinoma. Eur J Surg Oncol.
33:208–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruix J and Sherman M: Practice Guidelines
Committee, American Association for the Study of Liver Diseases:
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar
|
|
29
|
Li F, Ren W, Zhao Y, Fu Z, Ji Y, Zhu Y and
Qin C: Downregulation of GRIM-19 is associated with hyperactivation
of p-STAT3 in hepatocellular carcinoma. Med Oncol. 29:3046–3054.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bandyopadhyay D, Cruz J, Morales LD, Arman
HD, Cuate E, Lee YS, Banik BK and Kim DJ: A green approach toward
quinoxalines and bis-quinoxalines and their biological evaluation
against A431, human skin cancer cell lines. Future Med Chem.
5:1377–1390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tremblay ML: On the role of tyrosine
phosphatases as negative regulators of STAT signaling in breast
cancers: new findings and future perspectives. Breast Cancer Res.
15:3122013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garner JM, Fan M, Yang CH, Du Z, Sims M,
Davidoff AM and Pfeffer LM: Constitutive activation of signal
transducer and activator of transcription 3 (STAT3) and nuclear
factor-κB signaling in glioblastoma cancer stem cells regulates the
Notch pathway. J Biol Chem. 288:26167–26176. 2013.
|
|
33
|
Nishimoto A, Kugimiya N, Hosoyama T, Enoki
T, Li TS and Hamano K: JAB1 regulates unphosphorylated STAT3
DNA-binding activity through protein-protein interaction in human
colon cancer cells. Biochem Biophys Res Commun. 438:513–518. 2013.
View Article : Google Scholar
|
|
34
|
Mano Y, Aishima S, Fujita N, Tanaka Y,
Kubo Y, Motomura T, Taketomi A, Shirabe K, Maehara Y and Oda Y:
Tumor-associated macrophage promotes tumor progression via STAT3
signaling in hepatocellular carcinoma. Pathobiology. 80:146–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Behboudi S, Boswell S and Williams R:
Cell-mediated immune responses to alpha-fetoprotein and other
antigens in hepatocellular carcinoma. Liver Int. 30:521–526. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuan JM, Fan Y, Ognjanovic S, Wang R, Van
Den Berg D, Govindarajan S and Yu MC: Genetic polymorphisms of
epidermal growth factor in relation to risk of hepatocellular
carcinoma: two case-control studies. BMC Gastroenterol. 13:322013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liang H, Block TM, Wang M, Nefsky B, Long
R, Hafner J, Mehta AS, Marrero J, Gish R and Norton PA:
Interleukin-6 and oncostatin M are elevated in liver disease in
conjunction with candidate hepatocellular carcinoma biomarker GP73.
Cancer Biomark. 11:161–171. 2012.PubMed/NCBI
|
|
38
|
Cui HD, Qi ZM, Yang LL, Qi L, Zhang N,
Zhang XL, Du SY and Jiang Y: Interleukin-10 receptor expression and
signalling were down-regulated in CD4+ T cells of lupus
nephritis patients. Clin Exp Immunol. 165:163–171. 2011. View Article : Google Scholar
|