Introduction
Osteosarcoma is a common clinical malignant bone
tumor, representing ~35% of cases and constituting ~0.07% of all
the neoplasms (1), and it mainly
targets the adolescent age group (2). There has been great progress in the
treatment of osteosarcoma, with almost 80% of patients being
treated with limb-salvage and the 5-year survival rate for patients
with osteosarcoma has increased from 20% to about 80%; however,
more than half of all patients succumbed to metastasis and
recurrence of osteosarcoma (3).
With increased knowledge of tumor molecular biology, the concept of
gene therapy for tumors is proposed and the experimental results
show potential for clinical application. Several cytogenetic and
molecular studies have recently been undertaken in osteosarcoma,
and a number of genes (tumor suppressors, oncogenes and genes
coding for growth factors) have been identified to be abnormally
expressed. These genes may be used as diagnostic cytogenetic or
molecular markers for osteosarcoma. For example, the retinoblastoma
(RB) gene, the association of which has been well recognized
between osteosarcoma and RB. It is reported that patients with
hereditary RB have up to 1,000 times the incidence of osteosarcoma
compared with the general population (4,5), and
sporadic osteosarcoma revealed alterations of the RB gene in ~70%
of cases (6). p53, a tumor
suppressor gene, is also thought to be significant in the
development of osteosarcoma. The p53 gene product can induce the
transcription of numerous genes that are involved in the cell cycle
control and apoptosis (7), and
numerous reports have identified abnormalities of the p53 gene in
osteosarcoma in up to 50% of cases. Additionally, the MDM2 gene has
also been identified to be overexpressed in osteosarcoma, which may
provide an alternative mechanism for the disruption of the normal
p53 pathway (6,8). In addition, MDM2 is reported to be
amplified in 36% of osteosarcomas and 100% of parosteal
osteosarcomas (9).
Several alterations of genes and gene pathways
associated with osteosarcoma have been identified in the past few
years, which enhance the knowledge of the pathological mechanisms
of osteosarcomas. However, there remains no specific diagnostic
biomarker and further strides are urgently required to identify
abnormalities that have prognostic and therapeutic implications,
which may be useful in guiding the diagnosis and therapy for
osteosarcomas.
In the present study, the expression profiling data
between samples from human osteosarcoma and normal individuals were
compared in order to identify significant tissue-specific genes. In
addition, their function was investigated with the aim of
identifying noticeable diagnostic markers for osteosarcoma.
Materials and methods
Osteosarcoma related gene expression
profiles
The gene expression data GSE16088 were obtained from
the Gene Expression Omnibus database (10). A total of 20 samples were examined
in the present study, among which 14 patients were diagnosed with
osteosarcoma while the 6 normal samples served as negative
controls.
Preprocessing of expression data
At first, the raw data downloaded were transformed
into the expression data format by Affy package in R language
(11), and then the missing data
was imputed (12). At last, the
expression profiling data were standardized using the median
standardization method.
Analysis of differential expression
Three statistical tests in the multtest package in R
language (13), including the
t-test and Wilcox and Fisher’s exact tests were used to conduct the
differential-expression analysis between the osteosarcoma and
normal groups. The P-value obtained from each test was corrected by
the multiple testing Benjamini and Hochberg method (14), and the log FC (fold change) of
every gene expression value was also inspected. The genes that
could be screened by a variety of test methods simultaneously were
regarded as differentially-expressed genes with high degree of
confidence. These differentially-expressed genes were used to
identify the abnormal genes between the osteosarcoma and normal
control samples according to the threshold of P<0.05 and
|logFC|>1.
Construction of interaction network
After the differentially-expressed genes were
selected with high confidence through rigorous testing methods,
Search Tool for the Retrieval of Interacting Genes (STRING)
(15) was utilized to search for
the interaction objects of differentially-expressed gene products
and a protein interaction network was constructed.
Analysis of network module
The module of the whole network obtained previously
was decomposed and the function modules with modular nature,
including the genes identified through analysis, were found. Gene
Otology annotations were performed using Cytoscape (16), Mcode (17) and Bingo (18) software based on the hypergeometric
distribution and function enrichment threshold of adjp<0.05.
Results
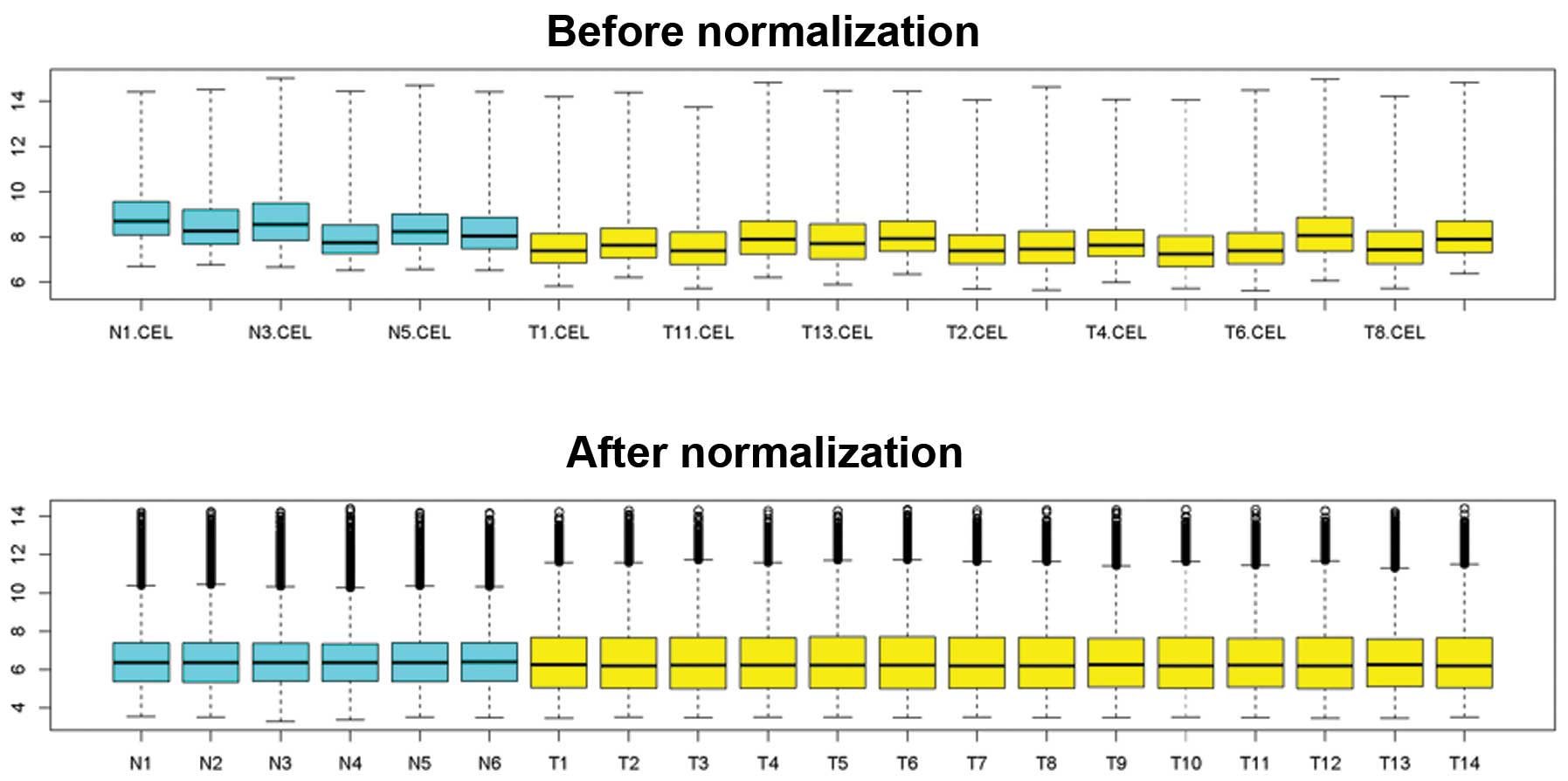
Results of data preprocessing
There were various factors, including background and
probe design that could cause a difference in the original chip
data. Therefore, standardization of the data was required prior to
the analysis. The difference between data prior to and following
standardization was significant (Fig.
1). It was observed that fluctuation of the data following
standardization was significantly less compared with that without
standardization.
Analysis of differential expression
Three statistical testing methods were used to
analyze the gene expression data and the data were further
corrected by multiple methods. Genes with P<0.05 and with |log
FC|>1 obtained in three different manners were selected
(Table I). Three
differentially-expressed genes met the strict requirements of high
degree confidence, and these genes were analyzed further.
 | Table IList of differentially-expressed
genes. |
Table I
List of differentially-expressed
genes.
| ID-REF | Gene symbol | T test-adj | Wilcox-adj | Fisher-adj | logFC |
|---|
| 201667-at | GJA1 | 1.11E-11 | 0.00054674 | 0.02724097 | 1.18443996 |
| 202404-s-at | COL1A2 | 6.05E-13 | 0.00137961 | 0.02724097 | 1.20747741 |
| 221729-at | COL5A2 | 1.23E-12 | 0.00137961 | 0.02724097 | 1.24898200 |
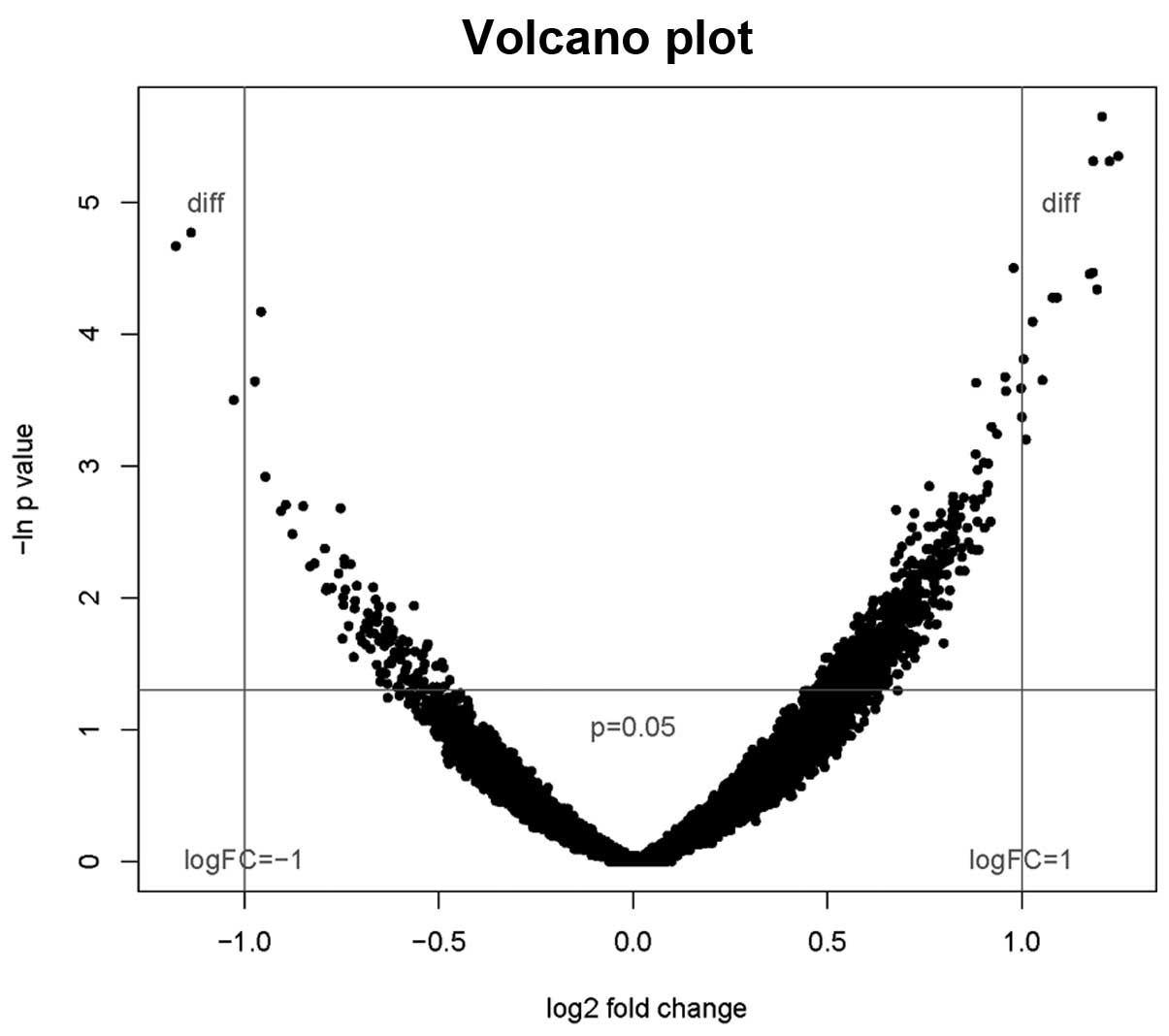
A volcanic plot provides a simultaneous
representation of P-values and log2 fold change for the gene
expression data. The smaller the P-value, the higher the
corresponding fold change (Fig.
2)
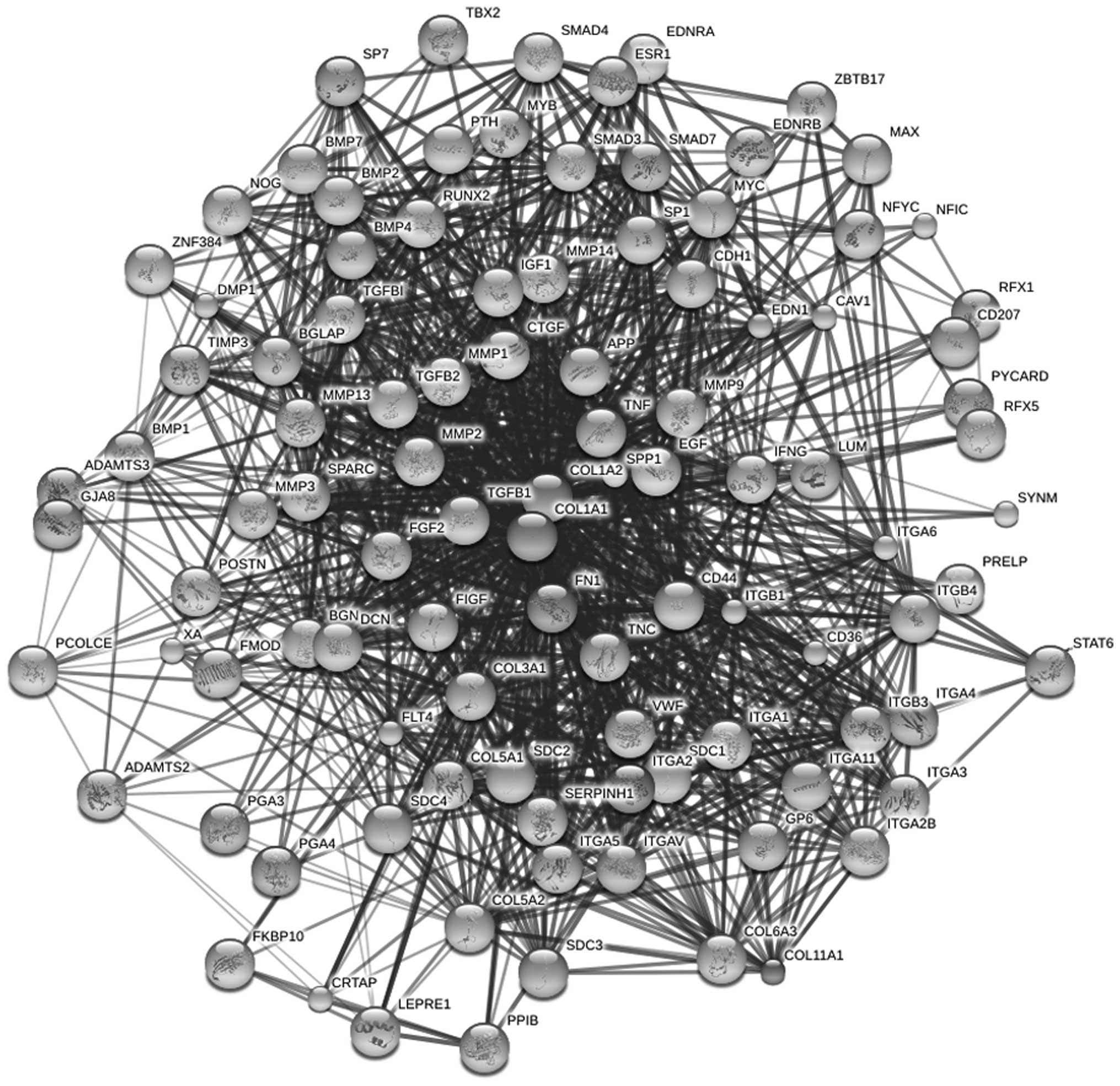
Results of interaction network
construction
The three genes screened as the core were selected
and combined with their possible interaction protein predicted from
the STRING database. Next, the interaction network was built
(Fig. 3).
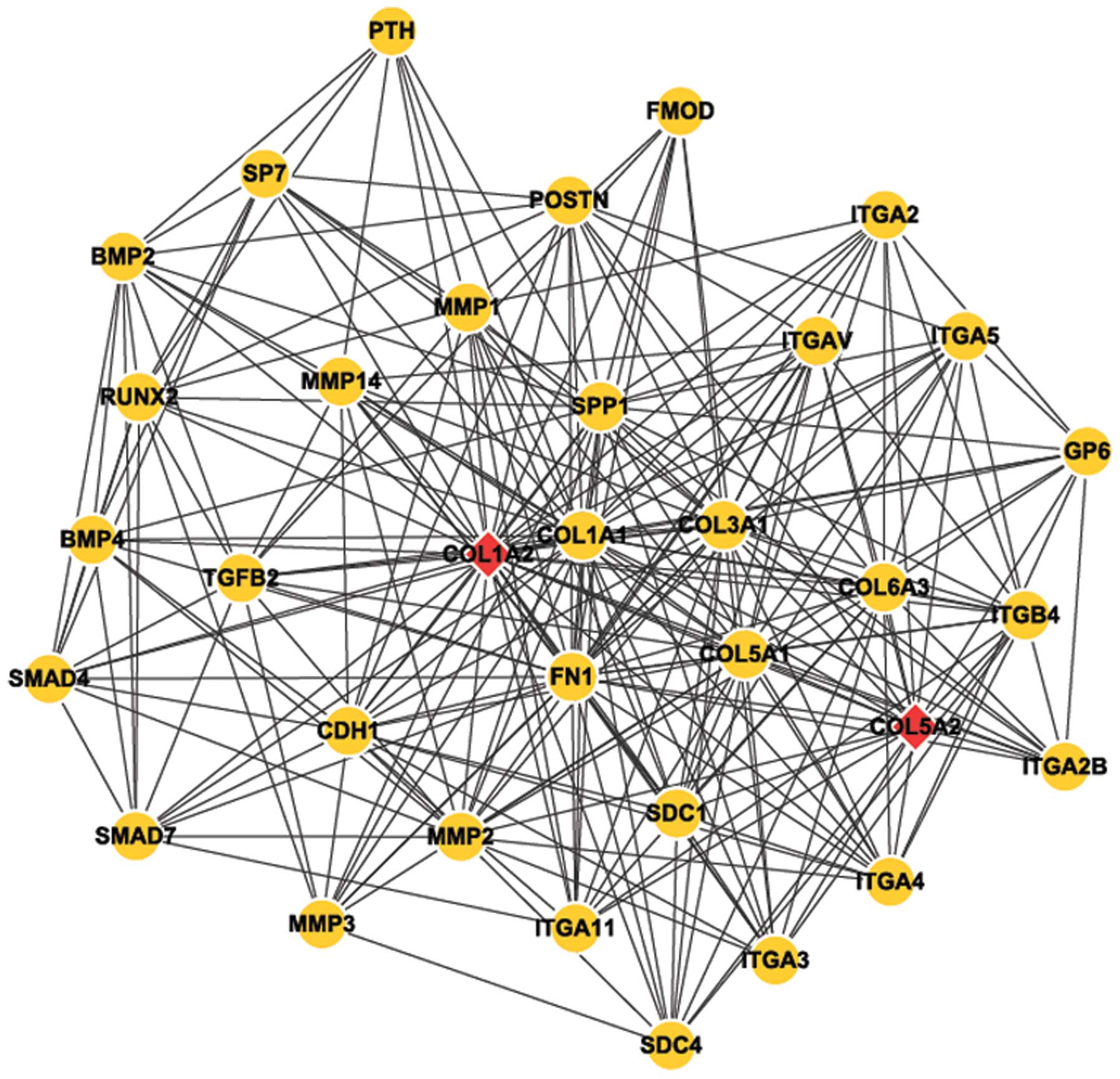
Analysis results of network module
Cytoscape software was used to perform module
analysis of the network constructed (Fig. 4). Next, Mcode was applied to
identify the common module in the two genes, and the annotation of
module function was undertaken by Bingo (Table II).
 | Table IIList of GO functional annotation. |
Table II
List of GO functional annotation.
| GO-ID | Function | Corr P-value |
|---|
| 7229 | Integrin-mediated
signaling | 1.18E-12 |
| 48513 | Organ
development | 1.18E-12 |
| 48731 | System
development | 3.69E-10 |
| 22610 | Biological
adhesion | 3.69E-10 |
| 7155 | Cell adhesion | 3.69E-10 |
| 48856 | Anatomical structure
development | 3.24E-09 |
| 9888 | Tissue
development | 5.69E-08 |
| 32501 | Multicellular
organismal process | 7.84E-08 |
| 7275 | Multicellular
organismal development | 7.84E-08 |
| 7166 | Cell surface receptor
linked signal transduction | 1.35E-07 |
| 7167 | Enzyme-linked
receptor protein signaling pathway | 1.68E-07 |
| 7178 | Transmembrane
receptor protein serine/threonine kinase signaling pathway | 1.94E-07 |
Discussion
In the present study, through comparing the
expression data from normal and osteosarcoma tissues, three
differentially-expressed genes were identified. Among which, the
COL1A2 and COL5A2 genes were selected as the core in order to
construct a network and to analyze the interaction between genes
correlated with osteosarcoma in order to understand the role of the
collagen gene (COL) family and proteins in osteosarcoma.
Collagens are the main fibrous proteins of
connective tissue and are the most abundant proteins of the
extracellular matrix (ECM). Thus, mutations in different COL genes
may disrupt the same collagen fibril. In humans, mutations in 13
different COL genes have been associated with disease phenotypes
(19,20). Mutations in the same COL gene can
also give rise to different human diseases. For example, mutations
in the COL1A2 gene result in at least five different forms of
chondrodysplasia and cartilage degeneration (21). There are 19 to 20 types of collagen
that have been identified to date, and type I COL, the major
component of ECM in skin, bone and ligaments is composed of
glycine- and proline rich two-α1 (I) and one-α2 (I) chains
(22). In the present study, COL
was found to be significantly upregulated and the established
network module with COL1A2 and COL5A2 as core revealed that
numerous other genes participated in this network and interacted
with COL1A2 and COL5A2, such as TGFβ, MMP, SMAD and RUNX2. These
genes have all been demonstrated to to be important in the
pathogenesis of osteosarcoma. A number of studies have been
performed on the correlation between these genes and COL1A2 and
COL5A2 and their interaction on the pathogenesis of osteosarcoma.
For example, TGFβ2 is a member of the transforming growth factor
(TGF)-subfamily, TGFβ induces the synthesis of numerous ECM
proteins, such as COL, fibronectin, laminin and tenascin, and
inhibits the matrix degrading enzymes. Therefore, TGFβ2 is involved
in wound healing, fibrosis, embryogenesis and tumorigenesis
(23,24). A previous study provides evidence
that TGFβ stimulates human COL1A2 promoter activity through Smad
signaling molecules (25). The
Smad family contains TGF-β receptor-dependent R-Smads (such as
Smad2/3), Co-Smads (such as Smad4) and anti-Smads (such as
Smad6/7). It is reported that transient expression of Smad3 or
Smad4 in human skin fibroblasts leads to stimulation of COL1A2
promoter activity. In addition, overexpression of anti-Smad and
Smad7 represses basal as well as TGFβ-stimulated COL1A2 promoter
activity, indicating its antagonistic effect on TGFβ signaling in
human dermal fibroblasts (25).
MMP, which is a hallmark of invasive cancers,
including osteosarcoma (26), is
capable of cleaving type I COL triple helices. Additionally,
membrane-bound MMP-14 may be particularly important, as MMP-14
knockout mice exhibit severe COL turnover deficiencies (27).
Collagen fibers, as well as fibers produced by
cancer-associated fibroblasts, may form an invasion barrier
(28). However, MMPs are essential
for maintaining the invasive phenotype and supporting migration and
proliferation of cancer cells (29,30).
Moreover, the critical function of collagenolytic MMPs in cancer is
clearing an invasion path through the barrier of type I collagen
fibers in the stroma and blood vessel walls (31,32).
Therefore, massive production of MMPs that degrade collagen may
solve this problem of invasion barriers. As a consequence, the
COL1A2 gene may be used as a diagnostic marker with a higher
expression level in cancer cells (33) and restoring type I collagen may be
used as a therapeutic target for cancer.
Additionally, the Runt-related transcription factor
Runx2, which also interacts with COL1A2, has a well-defined role in
mediating the final stages of osteoblast maturation and is required
for normal osteogenesis. Runx2 deficiency or mutations affecting
the function of Runx2 protein result in severe bone abnormalities
in mice and humans. Runx2 is implicated in early osteoblast
differentiation, and is involved in the later stages of chondrocyte
differentiation, maturation and possibly, endochondral ossification
(34). Runx2 expression is
essential for the commitment of preosteoblasts to the osteoblast
lineage. The cooperative interaction between Runx2 and the RB tumor
suppressor protein leads to progressive growth arrest and increases
expression of the mature osteoblast phenotype (35), which is reconciled with the
molecular pathogenesis of osteosarcoma (36). As the genes mentioned above are all
osteosarcoma related and co-interact with COL1A2 and COL5A2, a
small mutation in the COL1A2 and COL5A2 genes may affect these
other genes, resulting in pathological changes in osteoblasts which
lead to osteosarcoma development.
Osteosarcoma, has been a key focus of research as it
is a disease that effects adolescents. Jeon et al (37) investigated 25 cases of patients
with primary malignant curettage, the local recurrence rate was 18%
and the overall survival rate was 65%. The average 5- and 10-year
survival rates of these patients were reduced due to local
recurrence (38). Bramer et
al (39) observed 89 cases of
adult osteosarcoma following chemotherapy and observed that their
alkaline phosphatase (AP) levels correlated with response to
chemotherapy and survival. The results revealed that the prognosis
was poor when the AP level was elevated 2-fold compared with the
normal level.
In conclusion, in the present study, it was observed
that the selected COL gene was significantly upregulated, and this
gene was involved in the interleukin conducted signaling pathway.
Thus, the upregulation of the COL gene may be considered as a
diagnostic marker for osteosarcoma. However, further studies are
required to understand the role of collagen in osteosarcoma
development in detail.
References
|
1
|
Fletcher CD, Unni KK and Mertens F; WHO;
IARC. WHO classification of tumours: Pathology and genetics of
tumours of soft tissue and bone. 4th edition. IARC Press; Lyon,
France: 2002
|
|
2
|
Bielack SS, Kempf-Bielack B, Delling G, et
al: Prognostic factors in high-grade osteosarcoma of the
extremities or trunk: an analysis of 1,702 patients treated on
neoadjuvant cooperative osteosarcoma study group protocols. J Clin
Oncol. 20:776–790. 2002. View Article : Google Scholar
|
|
3
|
Ferrari S, Smeland S, Mercuri M, et al:
Neoadjuvant chemotherapy with high-dose Ifosfamide, high-dose
methotrexate, cisplatin, and doxorubicin for patients with
localized osteosarcoma of the extremity: a joint study by the
Italian and Scandinavian Sarcoma Groups. J Clin Oncol.
23:8845–8852. 2005. View Article : Google Scholar
|
|
4
|
Abramson DH, Ellsworth RM, Kitchin FD and
Tung G: Second nonocular tumors in retinoblastoma survivors. Are
they radiation-induced? Ophthalmology. 91:1351–1355. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kitchin FD and Ellsworth RM: Pleiotropic
effects of the gene for retinoblastoma. J Med Genet. 11:244–246.
1974. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ladanyi M and Gorlick R: Molecular
pathology and molecular pharmacology of osteosarcoma. Pediatr
Pathol Mol Med. 19:391–413. 2000. View Article : Google Scholar
|
|
7
|
Hung J and Anderson R: p53: functions,
mutations and sarcomas. Acta Orthop Scand Suppl. 273:68–73.
1997.PubMed/NCBI
|
|
8
|
Miller CW, Aslo A, Won A, Tan M, Lampkin B
and Koefflar HP: Alterations of the p53, Rb and MDM2 genes in
osteosarcoma. J Cancer Res Clin Oncol. 122:559–565. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Noble-Topham SE, Burrow SR, Kandel RA, et
al: SAS is amplified predominantly in surface osteosarcoma. J
Orthop Res. 14:700–705. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paoloni M, Davis S, Lana S, et al: Canine
tumor cross-species genomics uncovers targets linked to
osteosarcoma progression. BMC Genomics. 10:6252009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujita A, Sato JR, de Rodrigues LO,
Ferreira CE and Sogayar MC: Evaluating different methods of
microarray data normalization. BMC Bioinformatics. 7:4692006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Troyanskaya O, Cantor M, Sherlock G, et
al: Missing value estimation methods for DNA microarrays.
Bioinformatics. 17:520–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pollard KS, Dudoit S and van der Laan MJ:
Multiple testing procedures: R multtest package and applications to
genomics. Bioinformatics and Computaional Biology Solutions using R
and Bioconductor. Statistics for Biology and Health 2005. 249–271.
2005.
|
|
14
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: a practical and powerful approach to
multiple testing. J R Statist Soc. B. 289–300. 1995.
|
|
15
|
Szklarczyk D, Franceschini A, Kuhn M, et
al: The STRING database in 2011: functional interaction networks of
proteins, globally integrated and scored. Nucleic Acids Res.
39:D561–D568. 2011. View Article : Google Scholar
|
|
16
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: new features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rivera CG, Vakil R and Bader JS: NeMo:
network module identification in Cytoscape. BMC Bioinformatics.
11:S612010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maere S, Heymans K and Kuiper M: BiNGO: a
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar
|
|
19
|
Olsen BR: Mutations in collagen genes
resulting in metaphyseal and epiphyseal dysplasias. Bone.
17:S45–S49. 1995. View Article : Google Scholar
|
|
20
|
Prockop DJ and Kivirikko KI: Collagens:
molecular biology, diseases, and potentials for therapy. Ann Rev
Biochem. 64:403–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vikkula M, Metsäranta M and Ala-Kokko L:
Type II collagen mutations in rare and common cartilage diseases.
Ann Med. 26:107–114. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh AK: Factors involved in the
regulation of type I collagen gene expression: implication in
fibrosis. Exp Biol Med. 227:301–314. 2002.PubMed/NCBI
|
|
23
|
Branton MH and Kopp JB: TGF-β and
fibrosis. Microbes Infect. 1:1349–1365. 1999.
|
|
24
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor β in human disease. N Engl J Med.
342:1350–1358. 2000.
|
|
25
|
Chen SJ, Yuan W, Mori Y, Levenson A,
Trojanowska M and Varga J: Stimulation of type I collagen
transcription in human skin fibroblasts by TGF-β: involvement of
Smad 3. J Invest Dermatol. 112:49–57. 1999.
|
|
26
|
Mignatti P and Rifkin DB: Biology and
biochemistry of proteinases in tumor invasion. Physiol Rev.
73:161–195. 1993.PubMed/NCBI
|
|
27
|
Holmbeck K, Bianco P, Caterina J, et al:
MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and
connective tissue disease due to inadequate collagen turnover.
Cell. 99:81–92. 1999. View Article : Google Scholar
|
|
28
|
Makareeva E, Han S, Vera JC, et al:
Carcinomas contain a matrix metalloproteinase-resistant isoform of
type I collagen exerting selective support to invasion. Cancer Res.
70:4366–4374. 2010. View Article : Google Scholar
|
|
29
|
Yong HY and Moon A: Roles of
calcium-binding proteins, S100A8 and S100A9, in invasive phenotype
of human gastric cancer cells. Arch Pharm Res. 30:75–81. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen PN, Kuo WH, Chiang CL, et al: Black
rice anthocyanins inhibit cancer cells invasion via repressions of
MMPs and u-PA expression. Chem Biol Interact. 163:218–229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Itoh Y and Seiki M: MT1-MMP: a potent
modifier of pericellular microenvironment. J Cell Physiol. 206:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nabha SM, dos Santos EB, Yamamoto HA, et
al: Bone marrow stromal cells enhance prostate cancer cell invasion
through type I collagen in an MMP-12 dependent manner. Int J
Cancer. 122:2482–2490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mori K, Enokida H, Kagara I, et al: CpG
hypermethylation of collagen type I alpha 2 contributes to
proliferation and migration activity of human bladder cancer. Int J
Oncol. 34:1593–1602. 2009.PubMed/NCBI
|
|
34
|
Pratap J, Galindo M, Zaidi SK, et al: Cell
growth regulatory role of Runx2 during proliferative expansion of
preosteoblasts. Cancer Res. 63:5357–5362. 2003.PubMed/NCBI
|
|
35
|
Thomas DM, et al: Terminal osteoblast
differentiation, mediated by runx2 and p27KIP1, is disrupted in
osteosarcoma. J Cell Biology. 167:925–934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thomas DM, Carty SA, Piscopo DM, et al:
The retinoblastoma protein acts as a transcriptional coactivator
required for osteogenic differentiation. Mol Cell. 8:303–316. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeon DG, Lee SY and Kim JW: Bone primary
sarcomas undergone unplanned intralesional procedures-the
possibility of limb salvage and their oncologic results. J Surg
Oncol. 94:592–598. 2006. View Article : Google Scholar
|
|
38
|
Ayerza MA, Muscolo DL, Aponte-Tinao LA and
Farfalli G: Effect of erroneous surgical procedures on recurrence
and survival rates for patients with osteosarcoma. Clin Orthop
Relat Res. 452:231–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bramer JA, Abudu AA, Tillman RM, Carter
SR, Sumathi VP and Grimer RJ: Pre-and post-chemotherapy alkaline
phosphatase levels as prognostic indicators in adults with
localised osteosarcoma. Eur J Cancer. 41:2846–2852. 2005.
View Article : Google Scholar : PubMed/NCBI
|