Introduction
Perinatal hypoxic-ischemic brain injury (HIBI) is a
major cause of mortality and neurodevelopmental impairment in
newborn infants (1). The increased
risk of neurodevelopmental impairment may be due to the rate of
survival of premature infants, with survivors displaying
neurological sequelae (2,3). Thus, investigations are required to
develop effective drugs to protect against neurodevelopmental
impairment in newborn infants with HIBI. Polydatin (Fig. 1) is one of the primary bioactive
components of Polygonum cuspidatum and has been reported to
exhibit a neuroprotective effect in the rat brain, as well as
protect against learning and memory impairment in a rat model of
vascular dementia, alleviate oxygen and glucose deprivation-induced
myocardial cell injury and attenuate ischemia-reperfusion-induced
cardiac myocyte damage (4–8). However, the protective effect and
mechanism of polydatin on perinatal rats with HIBI has yet to be
elucidated.
Neonatal hypoxic-ischemic model rats exhibit
predictable brain injuries similar to those observed clinically in
humans (9–12). In the present study, a neonatal rat
model of HIBI was generated using a unilateral carotid artery
ligation method on postnatal seven-day-old rats, comparable to the
34-week-old human fetus (13). The
protective effect of polydatin was then investigated on
neurodevelopmental impairment in the neonatal rats with HIBI. The
effect of polydatin on learning and memory in neonatal rats with
HIBI was assessed using a behavioral test. Furthermore, the
expression of brain-derived neurotrophic factor (BDNF) was assessed
in the rat hippocampus following polydatin treatment. The findings
of the present study may be useful to determine the potential
neuroprotective effect of polydatin in neonatal rats with HIBI and
to develop a potential clinical treatment of neonatal
hypoxic-ischemic encephalopathy.
Materials and methods
Polydatin and experimental animals
Polydatin dry powder (Yousi Biotechnology Inc.,
Shanghai, China) was purified, analyzed using high-performance
liquid chromatography and the purity was determined to be >99%.
A total of 156 seven-day-old, healthy Sprague-Dawley rats, weighing
between 12 and 19 g, were selected regardless of gender from the
Animal Experimental Center of Dalian Medical University (Animal
license no: SYXK20080002; Dalian, China). Rats were maintained
under a 12-h light/dark cycle at 22°C with free access to food and
water.
Neonatal rats with HIBI, grouping and
treatment design
The animal model of HIBI was generated using Rice’s
method (9). In brief, a midline
incision was made at the rat neck and the subcutaneous fat was
separated. The left carotid artery was exposed and permanently
ligated. The rats were maintained in nitrogen gas containing 8%
oxygen in closed containers for 2 h. Following 2 h hypoxia, the
rats were allowed to recover. Polydatin dry powder was dissolved in
physiological saline and intraperitoneally injected into the rats
(10 mg/kg body weight) once a day for 10 consecutive days. A total
of 156 experimental Sprague-Dawley rats were randomly divided into
the following three groups: Sham-operated (SO; n=40), model (rats
with HIBI treated with saline; n=58) and polydatin (PD; rats with
HIBI treated with polydatin; n=58). The study was approved by the
ethics committee of the Hospital of Maternal and Child Health of
Dalian, Dalian, China.
Y-maze learning test
The Y-maze learning test was used to assess the
learning and memory of the rats. Ten days following
hypoxic-ischemic damage, rats with HIBI were tested using the
stochastic restless method. The rats underwent 20 trials every 24 h
with the Y-maze parameters (60V; 5 sec duration). The error
reaction number (ERN), total reaction time (TRT) and correct
avoidance rate (CAR) were measured to assess the learning ability
of the rats.
Immunohistochemical staining
Rats were sacrificed using an intracardiac perfusion
fixation method. Brains were removed and fixed using 4%
paraformaldehyde in phosphate-buffered saline for 48 h. The tissues
were dehydrated and embedded in paraffin for immunohistochemistry.
The embedded brain tissues were sliced to a thickness of 4 μm.
Rabbit anti-mouse BDNF antibodies (H-117; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and a streptavidin-peroxidase
immunohistochemistry kit (Zhongshan Jinqiao Biotechnology Co.,
Ltd., Beijing, China) were used for the staining. Image-Pro Plus 6
software (Media Cybernetics, Inc., Rockville, MD, USA) was used to
calculate the average optical density of the staining.
Western blot analysis
Rats were sacrificed using decapitation. The left
hippocampal tissues were isolated from the fresh brain tissue and
stored in liquid nitrogen for western blot analysis. Total protein
was extracted from the hippocampal tissues and measured. Equal
aliquots of protein were used for western blot analysis using
rabbit anti-mouse BDNF and β-actin antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Gray value analysis
software (Quantity One; Bio-Rad Inc., Hercules, CA, USA) was used
to analyze the gray value of the protein bands. Relative BDNF
protein expression was calculated using the ratio of BDNF gray
value to β-actin gray value.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analyses. All experimental data are
presented as the mean ± standard deviation for each group.
Differences among the groups were compared using one-way analysis
of variance (ANOVA), or multiple ANOVA followed by least
significant difference tests. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of polydatin on memory impairment
in rats with HIBI
On days one and two, Y-maze testing revealed that
the TRT and ERN of the rats in the model group were significantly
increased (P<0.01), while the CAR was significantly reduced
(P<0.01) compared with the rats in SO group (Tables I and II). Compared with the rats in model
group, the TRT of the rats in the PD and SO groups was observed to
be significantly decreased (P<0.01), while the CAR was found to
be significantly increased (P<0.01; Tables I and II). These findings suggested that rat
memory was significantly impaired by hypoxia and ischemia and that
polydatin ameliorated the HIBI-induced memory impairment.
 | Table IEffect of polydatin on memory
impairment in HIBI rats on day one assessed using the Y-maze
test. |
Table I
Effect of polydatin on memory
impairment in HIBI rats on day one assessed using the Y-maze
test.
| Groups | Samples | TRT (%) | ERN (%) | CAR (%) |
|---|
| SO | 10 | 157.92±10.31 | 2.80±1.23 | 43.50±5.80 |
| Model | 10 | 201.47±21.96a | 10.10±0.74 | 21.00±6.99a |
| PD | 10 | 179.80±11.23b | 5.60±1.26 | 29.50±4.38b |
 | Table IIEffect of polydatin on memory
impairment in HIBI rats on day two assessed using the Y-maze
test. |
Table II
Effect of polydatin on memory
impairment in HIBI rats on day two assessed using the Y-maze
test.
| Groups | Samples | TRT (%) | ERN (%) | CAR (%) |
|---|
| SO | 10 | 148.94±12.60 | 2.40±0.52 | 45.00±7.45 |
| Model | 10 | 198.40±26.69a | 9.80±1.40 | 18.00±7.15a |
| PD | 10 | 173.84±9.72b | 5.00±0.94 | 31.00±8.76b |
Polydatin increases BDNF expression in
the rat hippocampus
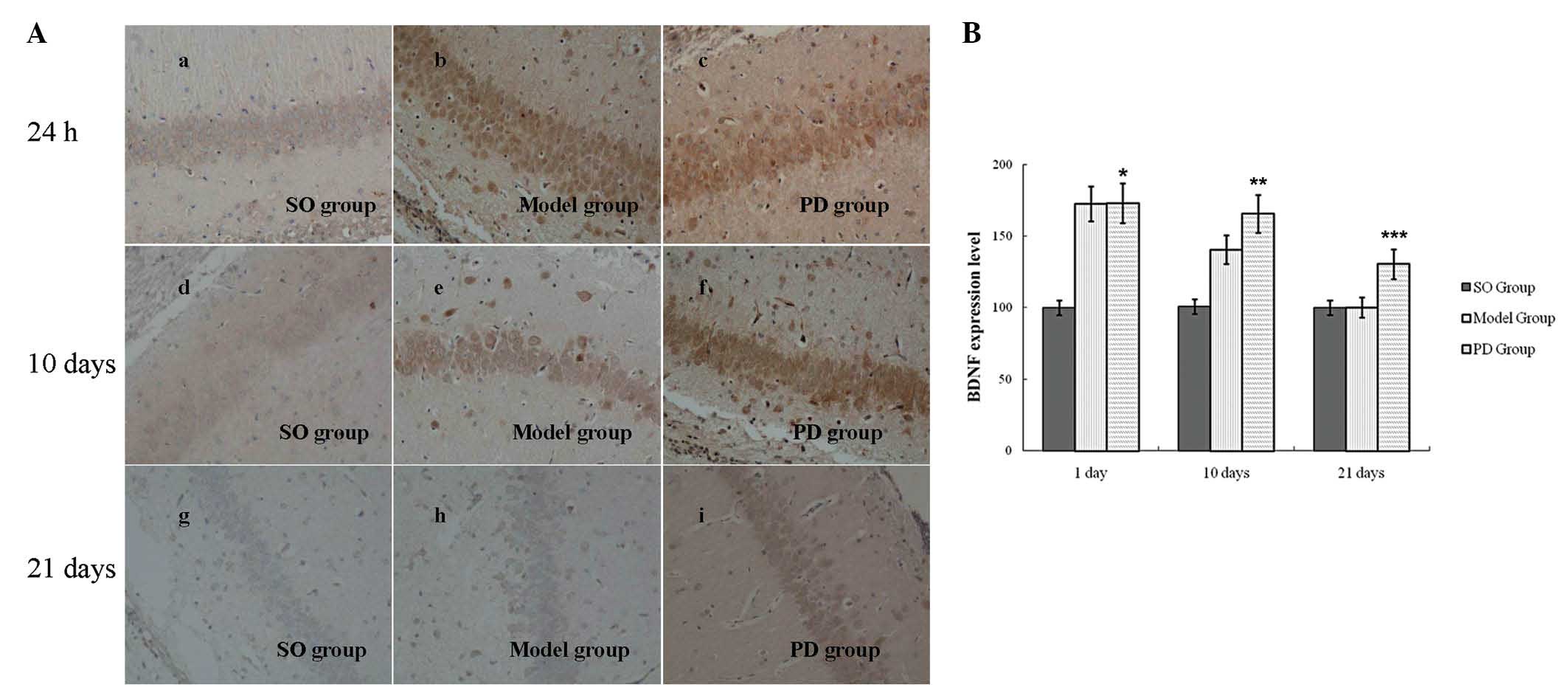
BDNF expression in the rat hippocampus was assessed
using immunohistochemistry and western blot analyses. In the rats
in the SO group, BDNF was observed to be expressed in the
hippocampal CA1 and CA3 regions. At 24 h after hypoxic-ischemic
damage, BDNF expression in the left hippocampal CA1 region was
found to be significantly increased in the rats in the model group
compared with the rats in the SO group (P<0.01; Fig. 2). There was no significant
difference in BDNF expression in the rats in the model group
compared with those in the PD group (P>0.05; Fig. 2). On days 10 and 21 following
hypoxic-ischemic damage, BDNF expression in the hippocampal CA1
region in the rats in the model group was observed to be
significantly increased compared with those in the SO group
(P<0.01; Fig. 2). Furthermore,
BDNF expression in the hippocampal CA1 region of the rats in the PD
group was found to be significantly increased compared with the
rats in the model group (P<0.05; Fig. 2). BDNF expression in the
hippocampal CA3 region exhibited a similar pattern of expression to
that in the hippocampal CA1 area (data not shown).
Western blot analysis revealed that at 24 h after
hypoxic-ischemic damage, BDNF expression was increased in the
hippocampus of the rats in the model group compared with those in
the SO group (Fig. 3).
Furthermore, BDNF expression was observed to be increased in the
rats in the PD group compared with those in the SO and model
groups. On day 10 following hypoxic-ischemic damage, BDNF
expression in hippocampus of the rats in the model group was found
to be reduced compared with that on day one. On day 21 after
hypoxic-ischemic damage, there was no significant difference in
BDNF expression in the hippocampus of the rats in the SO group
compared with the model group; however, BDNF expression in the rats
in the PD group remained high (Fig.
3). These findings suggested that polydatin induced BDNF
expression in the hippocampus of rats and sustained the expression
of BDNF in rats with HIBI.
Discussion
Polydatin has been reported to have an important
role in neuroprotection against cerebral cell injury induced by
focal ischemic-reperfusion (8). In
the present study, a neonatal HIBI rat model was used to
investigate the effect of polydatin on HIBI-induced learning and
memory impairment.
Y-maze tests were used to detect the spatial
learning and memory of the rats (14,15).
Rats in the maze exhibited a passive avoidance reaction to
electrical stimulation. Generally, subsequent to training, rats
learn and remember the spatial location of the safe zone within the
maze. However, hippocampal damage impairs memory and spatial
orientation (12). In the present
study, the Y-maze test results reflected HIBI-induced hippocampal
neuron damage and memory impairment in the rats. Following
polydatin treatment, the Y-maze test revealed that TRT and ERN were
reduced, while CAR was increased, compared with the rats in the
model group. These findings suggested that hypoxia and ischemia
impaired learning and memory in neonatal rats and that polydatin
enhanced spatial orientation and memory in rats with
hypoxic-ischemic injury, as well as alleviated neurologic sequelae
in rats with HIBI.
BDNF is widely distributed throughout the brain to
protect against brain injury. Moreover, BDNF expression is
increased in cerebral ischemia (16–18).
BDNF reduces neuronal damage following ischemia, as well as
promotes nerve repair (19–23).
In the present study, in the rat model of HIBI, cerebral ischemia
and reperfusion injury caused high BDNF expression, which is
associated with local neuronal resistance to injury. Of note, BDNF
exerted protective effects on spatial memory deficits following
neonatal hypoxic-ischemic injury.
The present study showed that hippocampal BDNF
expression was increased following HIBI, but gradually decreased to
normal levels within 21 days. Furthermore, polydatin was found to
have a protective effect against memory impairment in rats with
HIBI and significantly increased the expression of BDNF. Thus,
while the neuroprotective mechanism of polydatin in HIBI treatment
requires further investigation, the high levels and duration of
BDNF expression induced by polydatin may have a protective role in
neurons against brain damage induced by hypoxia-ischemia. In
conclusion, the present study showed that polydatin may have an
important role in promoting neuronal survival and may contribute to
neuron recovery following injury, as well as HIBI-induced memory
impairment. Polydatin was found to increase the expression of
hippocampal BDNF, which may be the long-term mechanism underlying
the improvements in learning and memory induced by polydatin in
rats with HIBI.
Acknowledgements
This study was supported by the Program for Liaoning
Excellent Talent in University to Dr Weifeng Mao, Liaoning Science
and Technology Project (nos. 2013225086 and 2011225013) and the
Liaoning Medicine Engineering Project and the Dalian Science and
Technology Project (no. 2010E15SF158).
References
|
1
|
Vannucci RC: Experimental biology of
cerebral hypoxia-ischemia: relation to perinatal brain damage.
Pediatr Res. 27:317–326. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willson-Costello D, Friedman H, Minich N,
Fanaroff AA and Hack M: Improved survival rates with increased
neurodevelopmental disability for extremely low birth weight
infants in the 1990s. Pediatrics. 115:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Handel M, Swaab H, de Vries LS and
Jongmans MJ: Long-term cognitive and behavioral consequences of
neonatal encephalopathy following perinatal asphyxia: a review. Eur
J Pediatr. 166:645–654. 2007.PubMed/NCBI
|
|
4
|
Zhao KS, Jin C, Huang X, Liu J, Yan WS,
Huang Q and Kan W: The mechanism of Polydatin in shock treatment.
Clin Hemorheol Microcirc. 29:211–217. 2003.PubMed/NCBI
|
|
5
|
Cheng Y, Zhang HT, Sun L, Guo S, Ouyang S,
Zhang Y and Xu J: Involvement of cell adhesion molecules in
polydatin protection of brain tissues from ischemia-reperfusion
injury. Brain Res. 1110:193–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miao Q, Wang S, Miao S, Wang J, Xie Y and
Yang Q: Cardioprotective effect of polydatin against
ischemia/reperfusion injury: roles of protein kinase C and mito
K(ATP) activation. Phytomedicine. 19:8–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ji H, Zhang X, Du Y, Liu H, Li S and Li L:
Polydatin modulates inflammation by decreasing NF-kappaB activation
and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and
ameliorates blood-brain barrier permeability for its
neuroprotective effect in pMCAO rat brain. Brain Res Bull.
87:50–59. 2012. View Article : Google Scholar
|
|
8
|
Li RP, Wang ZZ, Sun MX, Hou XL, Sun Y,
Deng ZF and Xiao K: Polydatin protects learning and memory
impairments in a rat model of vascular dementia. Phytomedicine.
19:677–681. 2012. View Article : Google Scholar
|
|
9
|
Rice JE, Vannucci RC and Brierley JB: The
influence of immaturity on hypoxic-ischemic brain damage in the
rat. Ann Neurol. 9:131–141. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vannucci RC, Connor JR, Mauger DT, Palmer
C, Smith MB, Towfighi J and Vannucci SJ: Rat model of perinatal
hypoxic-ischemic brain damage. J Neurosci Res. 55:158–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yager JY: Animal models of
hypoxic-ischemic brain damage in the newborn. Semin Pediatr Neurol.
11:31–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Golan H and Huleihel M: The effect of
prenatal hypoxia on brain development: short- and long-term
consequences demonstrated in rodent models. Dev Sci. 9:338–349.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hagberg H, Bona E, Gilland E and
Puka-Sundvall M: Hypoxia-ischaemia model in the 7-day-old rat:
possibilities and shortcomings. Acta Paediatr Suppl. 422:85–88.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul CM, Magda G and Abel S: Spatial
memory: Theoretical basis and comparative review on experimental
methods in rodents. Behav Brain Res. 203:151–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng S, Zhang Y, Zhang J, Wang H and Ren
B: Effect of ketamine on ERK expression in hippocampal neural cell
and the ability of learning behavior in minor rats. Mol Biol Rep.
37:3137–3142. 2010. View Article : Google Scholar
|
|
16
|
Yamada K, Mizuno M and Nabeshima T: Role
for brain-derived neurotrophic factor in learning and memory. Life
Sci. 70:735–744. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng F, Zhou X, Moon C and Wang H:
Regulation of brain-derived neurotrophic factor expression in
neurons. Int J Physiol Pathophysiol Pharmacol. 4:188–200.
2012.PubMed/NCBI
|
|
18
|
Wang Y, Cao M, Liu A, Di W, Zhao F, Tian Y
and Jia J: Changes of inflammatory cytokines and neurotrophins
emphasized their roles in hypoxic-ischemic brain damage. Int J
Neurosci. 123:191–195. 2013. View Article : Google Scholar
|
|
19
|
Galvin KA and Oorschot DE: Continuous
low-dose treatment with brain-derived neurotrophic factor or
neurotrophin-3 protects striatal medium spiny neurons from mild
neonatal hypoxia/ischemia: a stereological study. Neuroscience.
118:1023–1032. 2003. View Article : Google Scholar
|
|
20
|
Kurozumi K, Nakamura K, Tamiya T, Kawano
Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O,
Houkin K, Date I and Hamada H: BDNF gene-modified mesenchymal stem
cells promote functional recovery and reduce infarct size in the
rat middle cerebral artery occlusion model. Mol Ther. 9:189–197.
2004. View Article : Google Scholar
|
|
21
|
Marini AM, Jiang X, Wu X, Tian F, Zhu D,
Okagaki P and Lipsky RH: Role of brain-derived neurotrophic factor
and NF-kappaB in neuronal plasticity and survival: From genes to
phenotype. Restor Neurol Neurosci. 22:121–130. 2004.
|
|
22
|
Liu L, Zhang X, Wang L, Yang R, Cui L, Li
M, Du W and Wang S: The neuroprotective effects of Tanshinone IIA
are associated with induced nuclear translocation of TORC1 and
upregulated expression of TORC1, pCREB and BDNF in the acute stage
of ischemic stroke. Brain Res Bull. 82:228–233. 2010. View Article : Google Scholar
|
|
23
|
Im SH, Yu JH, Park ES, Lee JE, Kim HO,
Park KI, Kim GW, Park CI and Cho SR: Induction of striatal
neurogenesis enhances functional recovery in an adult animal model
of neonatal hypoxic-ischemic brain injury. Neuroscience.
169:259–268. 2010. View Article : Google Scholar
|