Introduction
Medicinal herbs, which have been used for thousands
of years in traditional Oriental medicine, are attractive sources
of novel therapeutics or preventives (1,2). The
herbs have been pre-validated for effectiveness and are expected to
have fewer safety issues than chemically synthesized drugs
(3).
Asiasari radix (A. radix) has been used as a
flavoring substance in a wide variety of dietary products, and as
an ingredient in drinks, cosmetics, soaps, shampoos, fragrances and
herbal products, without reported adverse effects (4). A. radix is termed seshin in
Korean, xì xīn in Chinese and saishin in Japanese,
and is used for treating pain, allergies and inflammatory disorders
in traditional Oriental medicine (3,5,6). A.
radix is primarily derived from either Asiasarum
heterotropoides or Asiasarum sieboldii (6). Studies has been performed regarding
the anti-inflammatory and anti-allergy effects of A. radix
(7,8).
A. radix has been suggested for use in the treatment
of oral diseases, including toothache and aphthous stomatitis
(9,10), as this therapy been suggested to
exert antibacterial and anti-inflammatory effects (3,6).
However, only limited information is currently available regarding
the effects of A. radix on dental tissue (11) and no information is available on
the effects on mesenchymal stem cells derived from the gingiva.
The aim of the present study was to evaluate the
effects of Asiasarum heterotropoides extract on the
morphology and viability of the human stem cells derived from the
gingiva.
Materials and methods
Preparation
The dry roots of Asiasarum heterotropoides
(400 g) were immersed in distilled water and boiled under reflux
for 150 min. The resulting extract was centrifuged at 5,000 × g for
10 min, and the supernatant was concentrated to 300 ml using a
rotary evaporator under reduced pressure (Eyela NE-1001, Tokya
Rikakikai Co. Ltd, Tokyo, Japan). The concentrates were then
freeze-dried in a lyophilizer (Labconco, Kansas, MO, USA) to obtain
65 g solid residue (yield 16%, w/w).
Isolation and culture of stem cells
derived from the gingiva
Healthy gingival tissue samples were collected from
healthy patients undergoing clinical crown lengthening procedures.
The design of this study was reviewed and approved by the
Institutional Review Board of Seoul St. Mary’s Hospital, College of
Medicine, the Catholic University of Korea, Seoul, Republic of
Korea (KC11SISI0348) and informed consent was obtained from the
patients.
The gingival tissue was de-epithelialized, minced
and digested with collagenase IV (Sigma-Aldrich, St. Louis, MO,
USA). The cells were incubated at 37°C in a humidified incubator
with 5% CO2 and 95% O2. After 24 h, any
non-adherent cells were washed with phosphate-buffered saline
(Welgene, Daegu, Korea) and fresh medium was added. The media was
changed every 2–3 days.
Determination of cell viability
The cells were plated at a density of
2.0×103 cells/well in 96-well plates. The cells were
incubated in minimum essential medium-α containing 15% fetal bovine
serum (Gibco-BRL, Carlsbad, CA, USA), 100 U/ml penicillin, 100
μg/ml streptomycin (Sigma-Aldrich), 200 mm L-Glutamine
(Sigma-Aldrich) and 10 mm ascorbic acid 2-phosphate (Sigma-Aldrich)
in the presence of A. radix at concentrations of: 0 (untreated
control), 0, 0.1, 1, 10, 100 and 1,000 μg/ml, respectively.
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium, monosodium salt (WST-8; Cell Counting kit-8 CCK-8;
Dojindo, Tokyo, Japan) was then added and the cells were incubated
for 1 h at 37°C. The analysis was performed on days 2, 3, 5 and 7.
Viable cells were identified using the CCK-8 assay, which relies on
the ability of mitochondrial dehydrogenases to oxidize WST-8 to a
formazan product. The spectrophotometric absorbance at 450 nm was
measured using a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA). The experiments were performed in
triplicate.
Evaluation of cellular morphology
The morphology of the cells was viewed under an
inverted microscope (Leica DM IRM; Leica Microsystems, Wetzlar,
Germany) on days 1, 3, 5 and 7. The images were saved as JPEGs.
Statistical analysis
The data are presented as the mean ± standard
deviation. A one-way analysis of variance with post hoc test was
performed to determine the differences among the groups using a
commercially available program (SPSS 12 for Windows; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell morphology
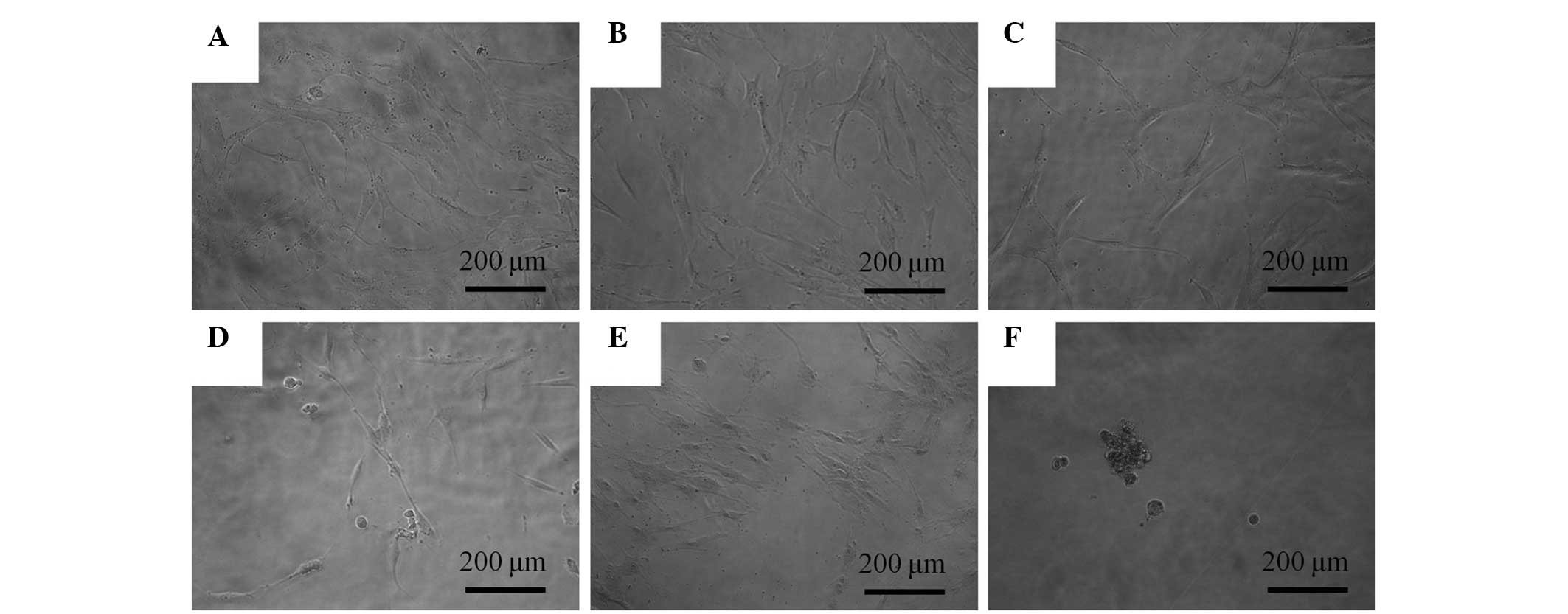
The control group exhibited normal fibroblast
morphology on day 1 (Fig. 1A). The
shapes of the cells following 0.1, 1, 10 and 100 μg/ml A. radix
treatment were similar to those of the control group (Fig. 1B–E). A significant alteration was
noted in the 1,000 μg/ml group when compared with the untreated
group. The shapes of the cells in 1,000 g/ml group were rounder and
fewer cells were present (Fig.
1F).
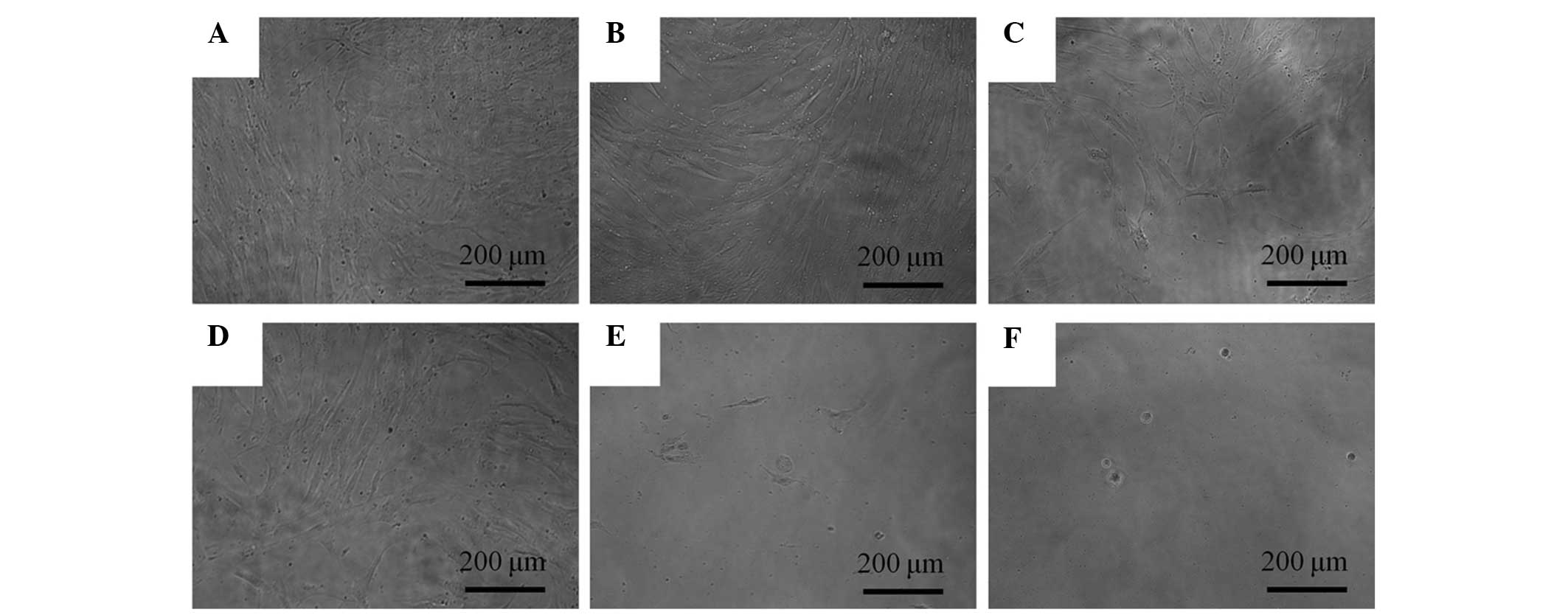
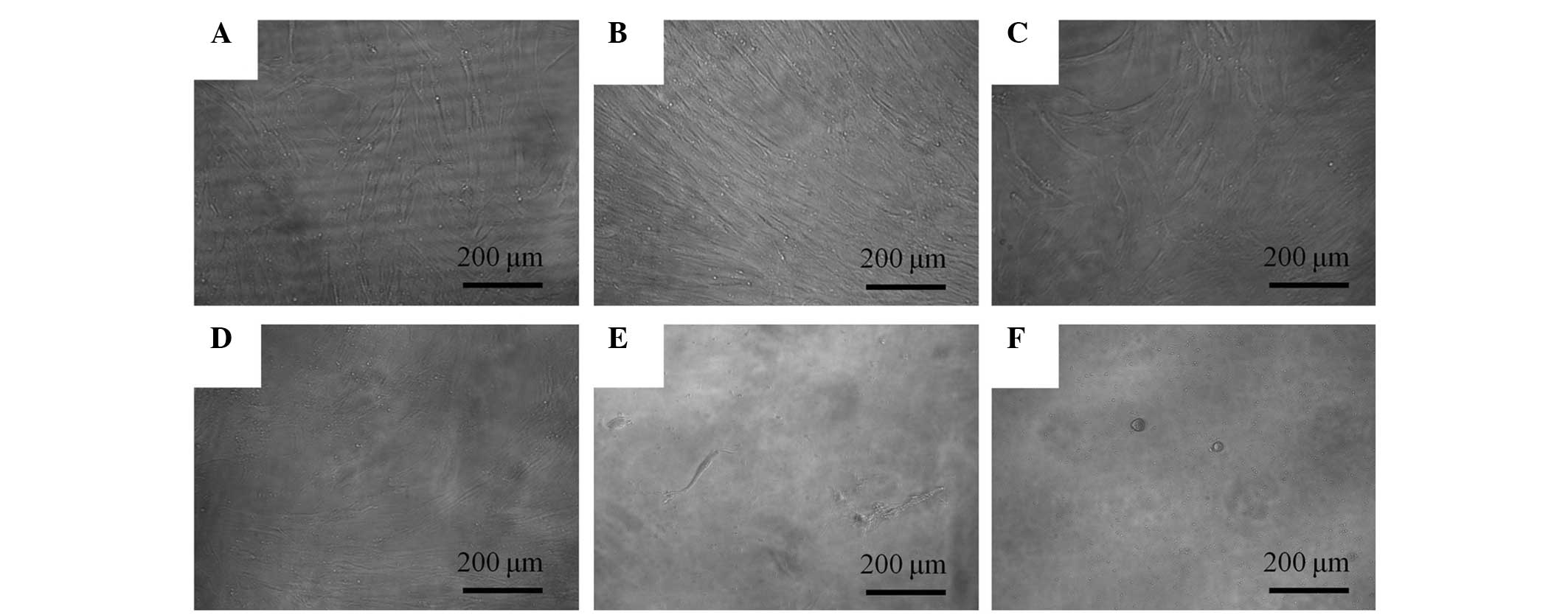
The morphology of the cells on day 3 is shown in
Fig. 2. The shapes of the cells
subsequent to 0.1, 1, 10 and 100 μg/ml A. radix treatement remained
similar to those of the control group. The morphologies of the
cells on days 5 and 7 are shown in Figs. 3 and 4, respectively. Marked alterations in
cytoskeletal organization were noticed in the 100 and 1,000 μg/ml
A. radix groups. The shapes of these cells were rounder, and fewer
cells were present, when compared with those of the control
group.
Cellular viability
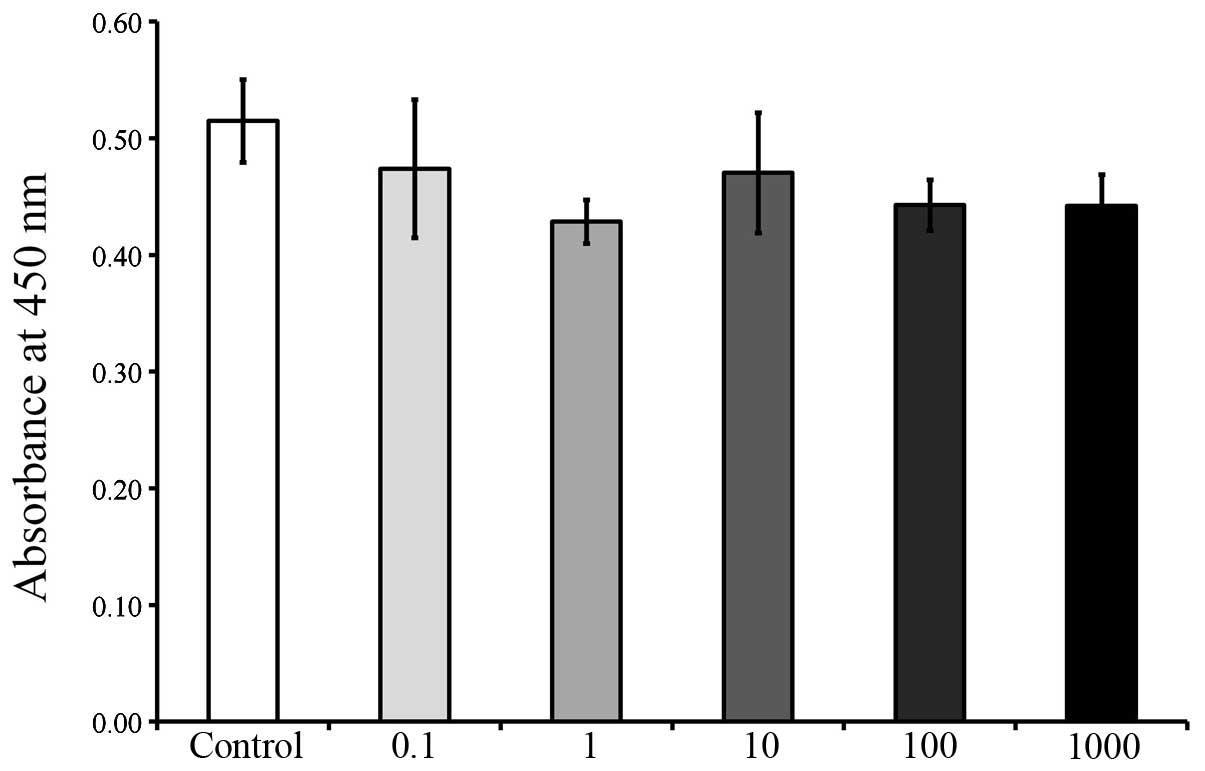
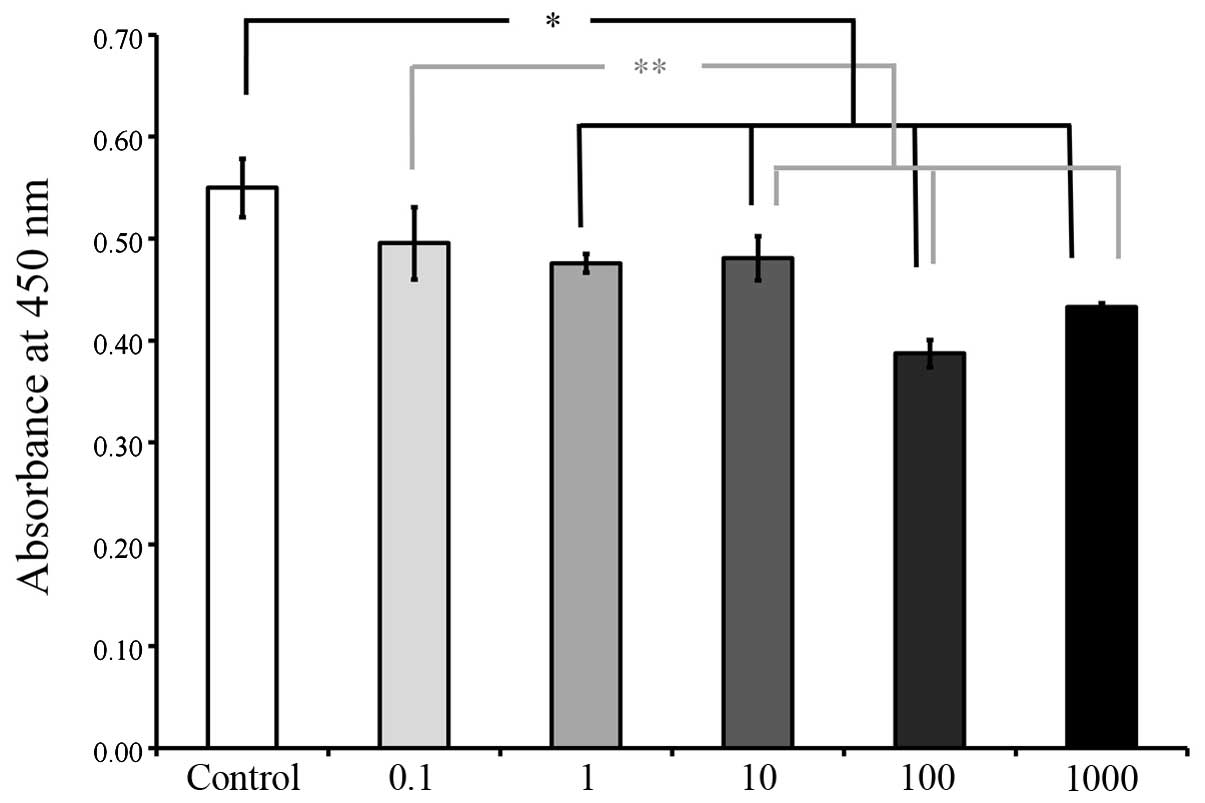
The CCK-8 results on days 2, 3, 5 and 7 are shown in
Figs. 5, 6, 7 and
8, respectively. The cultures
growing in the presence of A. radix did not exhibit any changes in
the CCK-8 assays on day 2 and no significant differences were
identified among the six groups (Fig.
5). However, on day 3, a significant reduction in cell
viability was observed in the groups with higher concentrations A.
radix treatment (1, 10, 100 and 1,000 μg/ml), when compared with
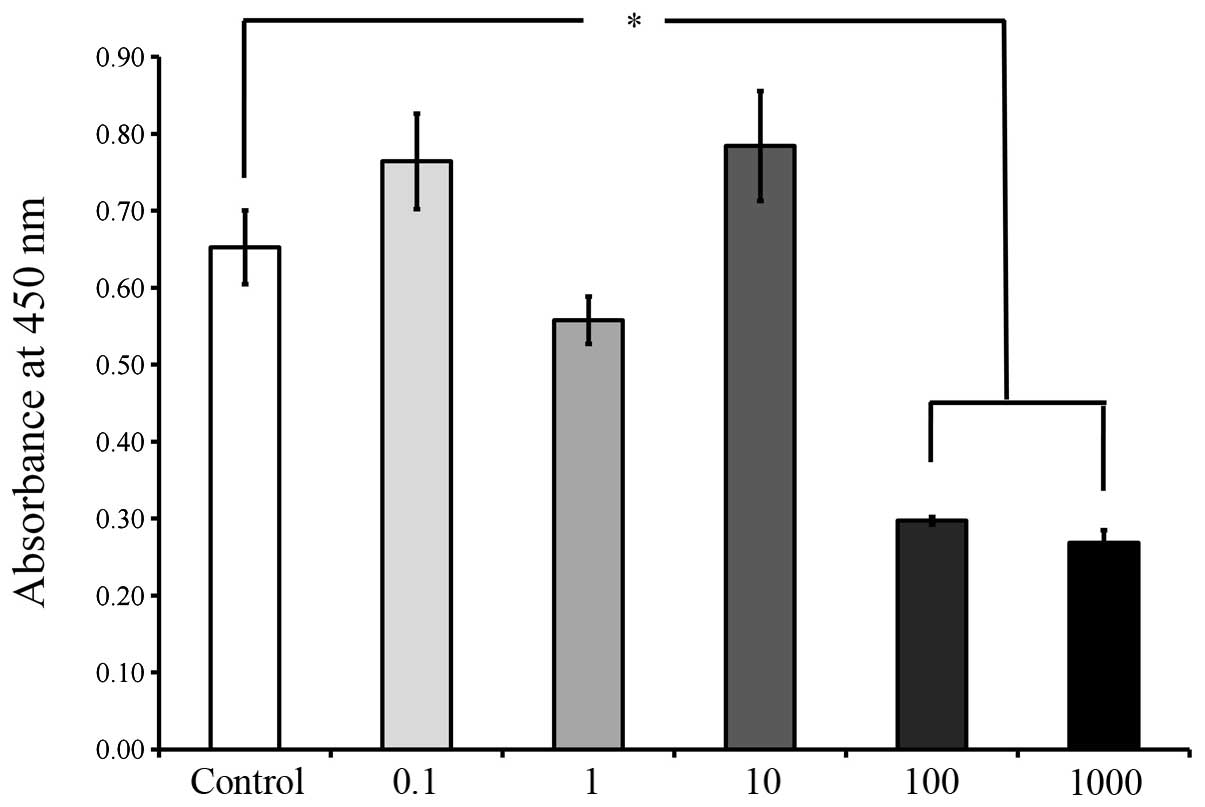
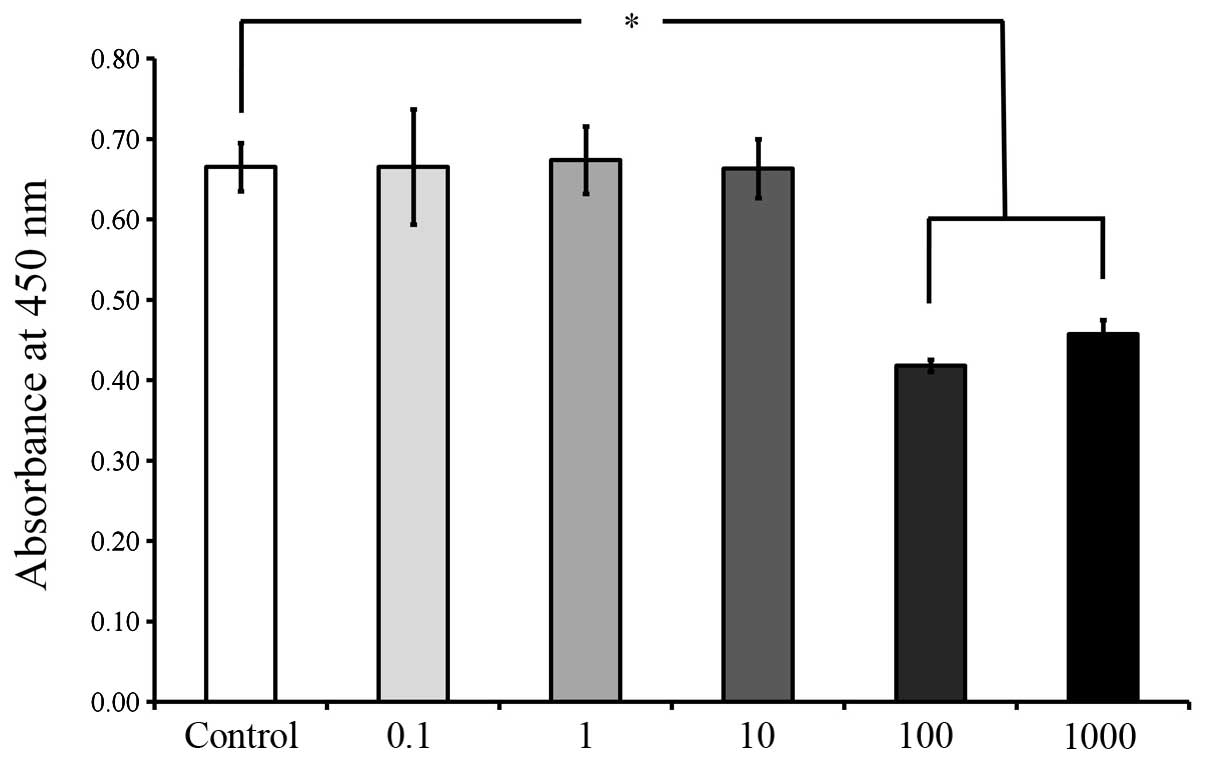
the cells in the control group(P<0.05; Fig. 6). The viability results on days 5
and 7, as shown in Figs. 7 and
8, respectively, revealed
significant reductions in cell viability following 100 and 1,000
μg/ml A. radix treatment (P<0.05; Figs. 7 and 8), when compared with the control
cells.
Discussion
In this report, the effects of A. radix on the
morphology and cell viability of stem cells derived from the
gingiva under predetermined concentrations were examined. High
concentrations of A. radix exerted adverse effects on the gingival
stem cells, and a significant reduction in cellular viability was
observed at 100 and 1,000 μg/ml A. radix concentrations.
The effects of A. radix have been previously
analyzed in in vitro and in vivo experiments
(4,12,13).
One study revealed no significant effect on the growth of HeLa
cells following A. radix treatment for 72 h at a range of
concentrations between 0.0001 and 1,000 μg/ml (12). The safety of an A. radix methanol
extract was investigated in an oral sub-acute 28-day toxicity study
in Sprague-Dawley rats at doses of 50, 250 and 500 mg/kg/day, and
the authors concluded that the methanolic extract of A. radix
appeared to be safe and nontoxic within the experimental conditions
(4). The acute oral toxicity of A.
radix methanol extract was evaluated in ICR mice; the results
revealed no cases of mortality, signs of toxicity or abnormalities
in the gross findings (13). The
authors concluded that the methanol extract of A. radix was
toxicologically safe for oral administration. However, the results
of the present study demonstrated that gingival cells treated with
100 and 1,000 μg/ml A. radix exhibited cell damage with significant
reductions in cell viability on days 3, 5 and 7. The conflicting
results regarding the different responses to A. radix may, in part,
be attributed to the type of cells, culturing period or culturing
conditions (14).
In the present study, a CCK-8 assay utilizing
water-soluble tetrazolium salt-8 was used to evaluate the viability
of the gingival cells. MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is
reported to be a more sensitive assay than trypan blue (15), which is determined by the principle
that viable cells have intact cell membranes that exclude the
trypan blue dye (16). Dead cells
absorb trypan blue and appear blue as a consequence, as the cell
membranes are unable to control the passage of macromolecules. The
MTT assay determines cellular viability by determining
mitochondrial dehydrogenase activity; however, further treatment is
required to solubilize the formazan crystals and the MTT agent may
be toxic to the cells (17). CCK-8
assay has been reported to be more sensitive than the MTT assay and
less toxic to cells (17).
Increasing interest has been generated in the
potential of stem cells, which are promising candidates for the
regeneration of tissues and the treatment of diseases (18). Mesenchymal stem cells (MSCs) may be
isolated from various tissues, including bone marrow (19), adipose tissue (20) and muscle (21). Recently, MSCs have been identified
in various oral tissues, including dental pulp (22), the lamina propria of the oral
mucosa (23) and the periodontal
ligaments (24). These tissues may
not be easily accessible or obtainable (25); however, the gingiva is easily
accessible in dental clinics and, as this tissue is discarded in
routine periodontal surgery, may be utilized for the isolation of
human MSCs. Thus, stem cells derived from the gingiva may be useful
in the investigation and treatment of disease.
A. radix, a traditional herbal medicine commonly
used to treat various diseases, has been reported to be safe and
nontoxic in previous studies (4,13).
A. radix has been shown to treat dental diseases (9,10,26);
however, limited information is available regarding the optimal
dosage. The present study provided clarification with regard to
these issues. A. radix was shown to influence the viability of stem
cells derived from the gingiva, with reduced viability at higher
concentrations. Therefore, the direct application of A. radix to
oral tissues may produce adverse effects at high doses. Thus, the
concentration and application time of A. radix requires meticulous
control to obtain optimal results.
Acknowledgements
The present study was supported by the Basic Science
Research Program, through the National Research Foundation of Korea
funded by the Ministry of Science, ICT & Future Planning
(NRF-2014R1A1A1003106).
References
|
1
|
Lee KH: Research and future trends in the
pharmaceutical development of medicinal herbs from Chinese
medicine. Public Health Nutr. 3:515–522. 2000.PubMed/NCBI
|
|
2
|
Feng Y, Wu Z, Zhou X, Zhou Z and Fan W:
Knowledge discovery in traditional Chinese medicine: state of the
art and perspectives. Artif Intell Med. 38:219–236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh SM, Kim J, Lee J, et al: Anticancer
potential of an ethanol extract of Asiasari radix against
HCT-116 human colon cancer cells in vitro. Oncol Lett. 5:305–310.
2013.PubMed/NCBI
|
|
4
|
Ramesh T, Lee K, Lee HW and Kim SJ:
Subacute toxicological evaluation of Asiasari radix methanol
extract. Drug Chem Toxicol. 32:243–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeon HC, Rho EJ, Kim HR and Yun YG: A
study on application of Radix Asari main blended prescription from
Dongeubogam. Korean J Oreint Med Prescription. 12:57–76. 2004.(In
Korean).
|
|
6
|
Jang JY, Lee JH, Shin HK, et al: Partially
purified Asiasari radix inhibits melanogenesis through
extracellular signal-regulated kinase signaling in B16F10 cells.
Int J Mol Med. 25:287–292. 2010.
|
|
7
|
Kamei T, Kondoh T, Nagura S, et al:
Improvement of C-reactive protein levels and body temperature of an
elderly patient infected with Pseudomonas aeruginosa on
treatment with Mao-bushi-saishin-to. J Altern Complement Med.
6:235–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HM and Moon YS: Asiasari radix
inhibits immunoglobulin E production on experimental models in
vitro and in vivo. Immunopharmacol Immunotoxicol. 21:469–481. 1999.
View Article : Google Scholar
|
|
9
|
Han Y and Kim SJ: Memory enhancing actions
of Asiasari radix extracts via activation of insulin
receptor and extracellular signal regulated kinase (ERK) I/II in
rat hippocampus. Brain Res. 974:193–201. 2003.PubMed/NCBI
|
|
10
|
Kim KS, Kim NS, Kim SD, et al: Regulatory
effect of inflammatory reaction by Asiasari radix. Korean J
Orient Physiol Pathol. 19:779–784. 2005.
|
|
11
|
Zhou RH: Resource Science of Chinese
Medicinal Materials. China Medical and Pharmaceutical Sciences
Press; Beijing: 1993
|
|
12
|
Takara K, Horibe S, Obata Y, et al:
Effects of 19 herbal extracts on the sensitivity to paclitaxel or
5-fluorouracil in HeLa cells. Biol Pharm Bull. 28:138–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramesh T, Lee K, Lee HW and Kim SJ: Acute
oral toxicity study of Asiasari radix extract in mice. Int J
Toxicol. 26:247–251. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Wang WL, Xie WL, et al: Puerarin
stimulates proliferation and differentiation and protects against
cell death in human osteoblastic MG-63 cells via ER-dependent
MEK/ERK and PI3K/Akt activation. Phytomedicine. 20:787–796. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meleti Z, Shapiro IM and Adams CS:
Inorganic phosphate induces apoptosis of osteoblast-like cells in
culture. Bone. 27:359–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stoddart MJ: Cell viability assays:
introduction. Methods Mol Biol. 740:1–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Almazin SM, Dziak R, Andreana S and
Ciancio SG: The effect of doxycycline hyclate, chlorhexidine
gluconate, and minocycline hydrochloride on osteoblastic
proliferation and differentiation in vitro. J Periodontol.
80:999–1005. 2009. View Article : Google Scholar
|
|
18
|
Sekiya I, Larson BL, Smith JR, et al:
Expansion of human adult stem cells from bone marrow stroma:
conditions that maximize the yields of early progenitors and
evaluate their quality. Stem Cells. 20:530–541. 2002. View Article : Google Scholar
|
|
19
|
Kuznetsov SA, Friedenstein AJ and Robey
PG: Factors required for bone marrow stromal fibroblast colony
formation in vitro. Br J Haematol. 97:561–570. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rodriguez AM, Elabd C, Amri EZ, Ailhaud G
and Dani C: The human adipose tissue is a source of multipotent
stem cells. Biochimie. 87:125–128. 2005.PubMed/NCBI
|
|
21
|
Wada MR, Inagawa-Ogashiwa M, Shimizu S,
Yasumoto S and Hashimoto N: Generation of different fates from
multipotent muscle stem cells. Development. 129:2987–2995.
2002.PubMed/NCBI
|
|
22
|
Ballini A, De Frenza G, Cantore S, et al:
In vitro stem cell cultures from human dental pulp and periodontal
ligament: new prospects in dentistry. Int J Immunopathol Pharmacol.
20:9–16. 2007.PubMed/NCBI
|
|
23
|
Marynka-Kalmani K, Treves S, Yafee M, et
al: The lamina propria of adult human oral mucosa harbors a novel
stem cell population. Stem Cells. 28:984–995. 2010.
|
|
24
|
Nagatomo K, Komaki M, Sekiya I, et al:
Stem cell properties of human periodontal ligament cells. J
Periodontal Res. 41:303–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshimura H, Muneta T, Nimura A, et al:
Comparison of rat mesenchymal stem cells derived from bone marrow,
synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res.
327:449–462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hidaka S, Okamoto Y and Liu SY: Natural
products effective on the in vitro formation of calcium phosphate
precipitates. J Trad Med. 26:201–209. 2009.
|