Introduction
Rapamycin, also termed sirolimus, is a carboxycyclic
lactone-lactam macrolide produced by Streptomyces
hygroscopicus. It was first identified as an antifungal agent,
was subsequently investigated as an immunosuppressant and is
clinically used during organ transplantation (1). Rapamycin revealed the mammalian
target of rapamycin (mTOR) signaling pathway, which is important
for normal cell and cancer cell growth (2). mTOR is found in two different
complexes within the cell, mTORC1 and mTORC2; however, only mTORC1
is sensitive to inhibition by rapamycin. Activated mTORC1 regulates
protein synthesis through directly phosphorylating 4E-binding
protein 1 and p70S6 kinase 1 (p70S6K1), which are translation
initiation factors that are important for cap-dependent mRNA
translation, thus, activated mTORC1 increases the levels of
numerous proteins that are required for cell cycle progression,
proliferation, angiogenesis and survival. Several oncogenes and
tumor suppressor genes, which activate mTORC1 primarily through the
phosphatidylinositol 3-kinase (PI3K)/Akt pathway, are frequently
dysregulated in the majority of types of human cancer,
including hepatocellular carcinoma (HCC) (3,4). A
large number of preclinical and clinical trials have demonstrated
that inhibition of the mTOR signaling pathway using rapamycin or
rapamycin analogs may be a promising therapeutic strategy for HCC
(5–8). Despite the potential use of rapamycin
as a chemotherapeutic agent, the immunosuppressive effect
simultaneously induced by rapamycin is problematic. Thus, the
identification of other classes of anticancer agents, which act
synergistically with rapamycin are urgently required in order to
minimize the immunosuppressive effect.
Berberine, a small molecule derived from Coptidis
rhizoma, also termed Huang lian, as well as various other plants,
has strong anticancer properties with no significant side effects
(9,10). Berberine has been shown to
simultaneously induce autophagic cell death and mitochondrial
apoptosis in HCC cells (11). In a
previous study, we demonstrated that berberine induces cell death
in HCC cells through downregulating cluster of differentiation (CD)
147 (12), a transmembrane
glycoprotein that is highly expressed in HCC cells and is strongly
associated with HCC progression and prognosis (13). Berberine has been reported to
inhibit the proliferation and development of HCC cells through
inhibiting the mTOR-signaling pathway (11), thus in the present study, it was
hypothesized that the combination of rapamycin and berberine may
increase the efficacy of chemotherapy for HCC through synergistic
suppression of the mTOR signaling pathway. The rapamycin-induced
immunosuppressive effect is dose-dependent (1); therefore, the present study
investigated the synergistic anticancer effect of different
concentrations of rapamycin (0, 10, 50, 100 and 200 nM) combined
with berberine (62.5 μM) on HCC cells in vitro. The possible
underlying molecular mechanisms were also investigated.
Materials and methods
Materials
SMMC7721 and HepG2 human hepatoma cell lines were
provided by the Institute of Biochemistry and Cell Biology, Chinese
Academy of Sciences (Shanghai, China). RPMI-1640 medium and fetal
bovine serum (FBS) were obtained from Gibco-BRL (Grand Island, NY,
USA). Berberine and rapamycin were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Berberine was dissolved in RPMI-1640 medium
at a final concentration of 1 M, which was used as a stock
solution. Rapamycin was dissolved in dimethyl sulfoxide (DMSO) at a
final concentration of 2 mmol/l. 3-[4, 5-Dimethylthiazol-2-yl]-2,
5-diphenyltetrazolium bromide (MTT) was purchased from
Sigma-Aldrich. The autophagy inhibitor, 3-methyladenine (3-MA), was
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
The caspase-3 inhibitor, z-DEVD-fmk (10 mM stock solution) was
purchased from BD Biosciences (Franklin Lakes, NJ, USA). The mouse
anti-human CD147 and mouse anti-human β-actin monoclonal antibodies
(mAbs) were purchased from BioVision, Inc. (Palo Alto, CA, USA) and
the horseradish peroxidase (HRP)-labeled goat anti-mouse
immunoglobulin G (IgG) was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The mouse anti-human
phosphorylated (p)-mTOR, p70S6K1 and p-p70S6K1 (Thr389) mAbs were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
pcDNA3.1 and pcDNA3.1-CD147 were obtained from the Cell Biology
Department of The Fourth Military Medical University (Xi’an,
China).
Cell culture
Human SMMC7721 and HepG2 cells were cultured in
RPMI-1640 supplemented with 10% FBS and antibiotics (100 U/ml
penicillin and 100 μg/ml streptomycin) at 37°C in a humidified
atmosphere containing 5% CO2 and 95% air. To investigate
the synergistic effect of berberine- and rapamycin-induced cell
death, the SMMC7721 and HepG2 cells were cultured either with or
without berberine and rapamycin at 37°C for 24 h.
Cell growth and proliferation assay
Cell viability was determined using the MTT
quantitative colorimetric assay. Our previous study showed that
berberine inhibited HCC cell growth in a dose- and time-dependent
manner at a concentration of 62.5 μM (12); thus in the present study, berberine
was used at a concentration of 62.5 μM in combination with
rapamycin. In brief, SMMC7721 and HepG2 cells were plated at a
density of 5×103 cells/well on 96-well culture plates in
RPMI-1640 medium supplemented with 10% FBS. Following overnight
incubation, the cells were treated with various concentrations of
rapamycin (0, 10, 50, 100 or 200 nM) with or without 62.5 μM
berberine for 24 h. The effect of the combined treatment of
berberine with rapamycin was assessed at different time points (0,
12, 24, 48 and 72 h). A total of 10 μl of 5 mg/ml MTT was then
added to each well for an additional 4 h and the resulting formazan
crystals were dissolved in 100 μl DMSO. The absorbance was read at
570 nm using an automatic microplate reader (Immuno Mini NJ-2300;
Intermed Inc., Tokyo, Japan).
Analysis of cell death using transmission
electron microscopy (TEM)
TEM analysis was performed as described previously
(14). In brief, following culture
with 200 nM rapamycin in the absence or presence of 62.5 μM
berberine for 24 h, SMMC7721 and HepG2 cells were fixed using 3%
glutaraldehyde in 0.2 M phosphate-buffered saline (PBS; pH 7.3) for
4 h at 4°C. The cells were then post-fixed with 1% osmium tetroxide
and 0.5% tannic acid for 1 h at 4°C, and washed three times using
0.1 M PBS (pH 7.3). Cells were dehydrated and embedded in EPON 812
(Electron Microscopy Sciences, Fort Washington, PA, USA). Sections
were then counterstained with uranyl acetate and lead citrate, and
analyzed using a JEM-2000EX transmission electron microscope (Jeol
Ltd., Tokyo, Japan).
Trypan blue exclusion assay
Cell death was analyzed using a Trypan blue
exclusion assay as described previously (15). In brief, SMMC7721 cells were
cultured for 24 h with 62.5 μM berberine and 50 nM rapamycin in the
absence or presence of the autophagy inhibitor 3-MA (10 mM) and/or
the caspase-3 inhibitor z-DEVD-fmk (100 μM). Adherent and
non-adherent cells were harvested, washed three times with PBS and
resuspended in 100 μl PBS. Subsequent to mixing with 100 μl of 0.8%
Trypan blue, cells were counted using a hemocytometer (Yancheng
Hengtai Glass Instrument, Yancheng, China). The number of dead
cells with disrupted membranes (blue cells) per 200 cells was
counted in three replicates. Cell death was expressed as the mean
percentage of blue cells/total cells.
Western blot analysis
Western blot analysis was performed as described
previously (16). In brief, cells
were lysed on ice for 30 min in radioimmunoprecipitation assay
buffer (Beyotime Biotechnology Inc., Nantong, China) containing 100
μM phenylmethylsulfonyl fluoride. Following centrifugation at
12,000 × g for 15 min, the supernatant was harvested as the total
cellular protein extract and was stored at −70°C. The protein
concentration was determined using a bicinchoninic acid kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal quantities of total
protein were separated using 10% SDS-PAGE, then transferred to
polyvinylidene fluoride membranes (Millipore Corporation,
Billerica, MA, USA). Membranes were blocked with 5% non-fat dry
milk in PBS containing 0.05% Tween-20 and incubated with antibodies
against p70S6K1, p-p70S6K1, CD147, p-mTOR and β-actin. Membranes
were washed three times in PBS, followed by incubation with the
appropriate HRP-linked IgG.
Overexpression of CD147 in SMMC7721
cells
We previously reported that CD147 has an important
role in berberine-induced cell death (12). In order to investigate whether
CD147 also participates in the regulation of the mTOR pathway,
SMMC7721 cells were transfected with pcDNA3.1-CD147 or pcDNA3.1
(Invitrogen Life Technologies, Carlsbad, CA, USA) for 24 h and then
cultured with rapamycin and berberine for 24 h. mTOR expression was
assessed using western blot analysis.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Statistical
significance was determined using Student’s t-test and analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
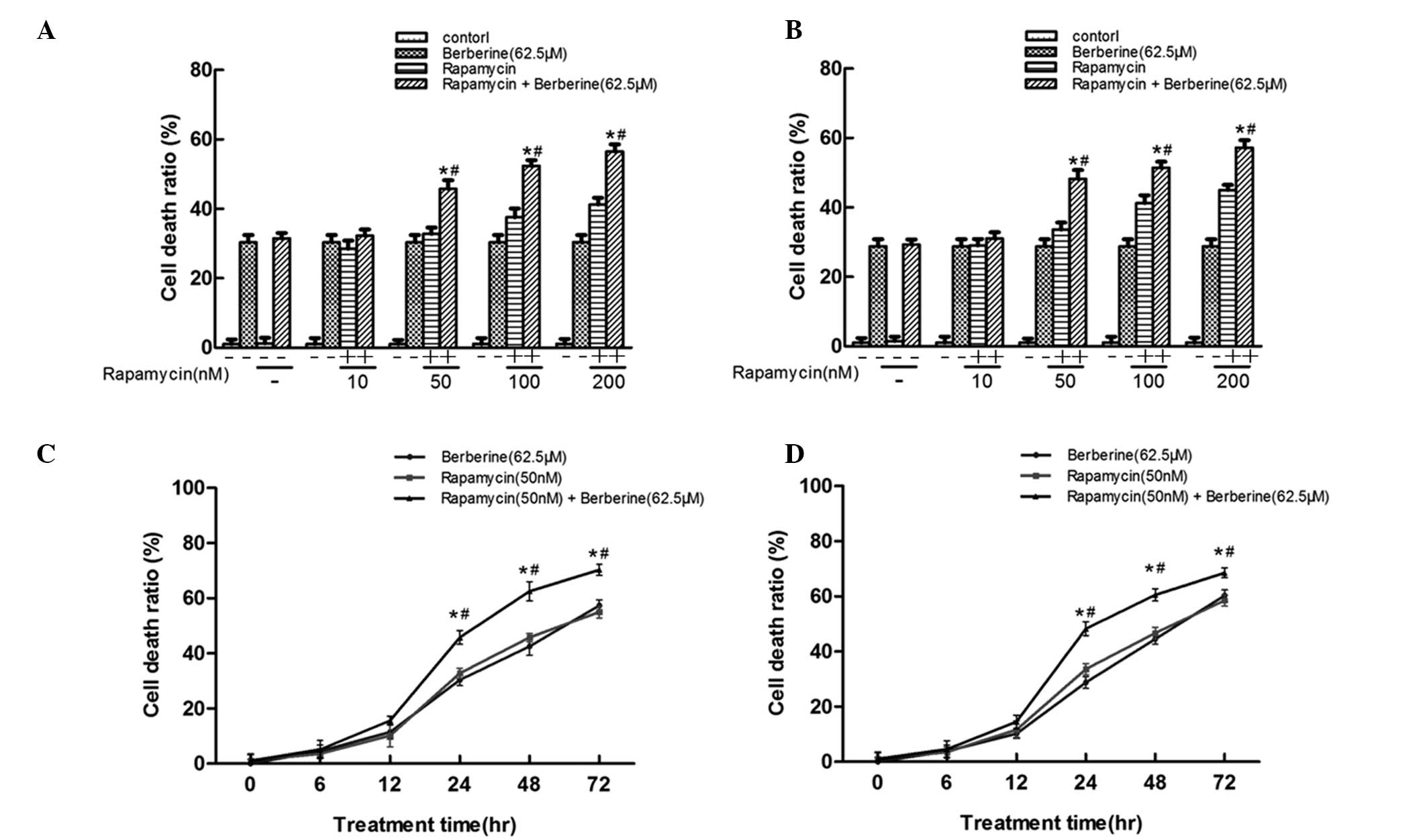
Berberine enhances rapamycin-mediated
cell death in vitro
To determine cell viability following combined
treatment with berberine and different concentrations of rapamycin
in human SMMC7721 and HepG2 cells, an MTT assay was performed.
Initially, the separate effects of rapamycin and berberine were
investigated on the viability of SMMC7721 and HepG2 cells. In our
previous study (12), 62.5 μM
berberine was combined with rapamycin. In the present study, the
HCC cells were treated with various doses of rapamycin for 24 h and
cell viability was subsequently measured. As shown in Fig. 1A and B, the cell death ratio was
significantly increased by rapamycin in a dose-dependent manner.
Furthermore, berberine was found to significantly enhance cell
cytotoxicity when combined with 50 nM rapamycin. Similar anticancer
effects were observed in the cells treated with 62.5 μM berberine
and 50 nM rapamycin and those treated with 100–200 nM rapamycin,
indicating that berberine synergistically enhanced
rapamycin-mediated cell death in vitro. Thus, 62.5 μM
berberine and 50 nM rapamycin was used in order to analyze the
combined effect of berberine and rapamycin in the HCC cells. The
present study also investigated whether the combined effect of
rapamycin and berberine on cell viability was time-dependent.
Fig. 1C and D show that combined
treatment with berberine and rapamycin induced cell death in a
time-dependent manner in SMMC7721 and HepG2 cells.
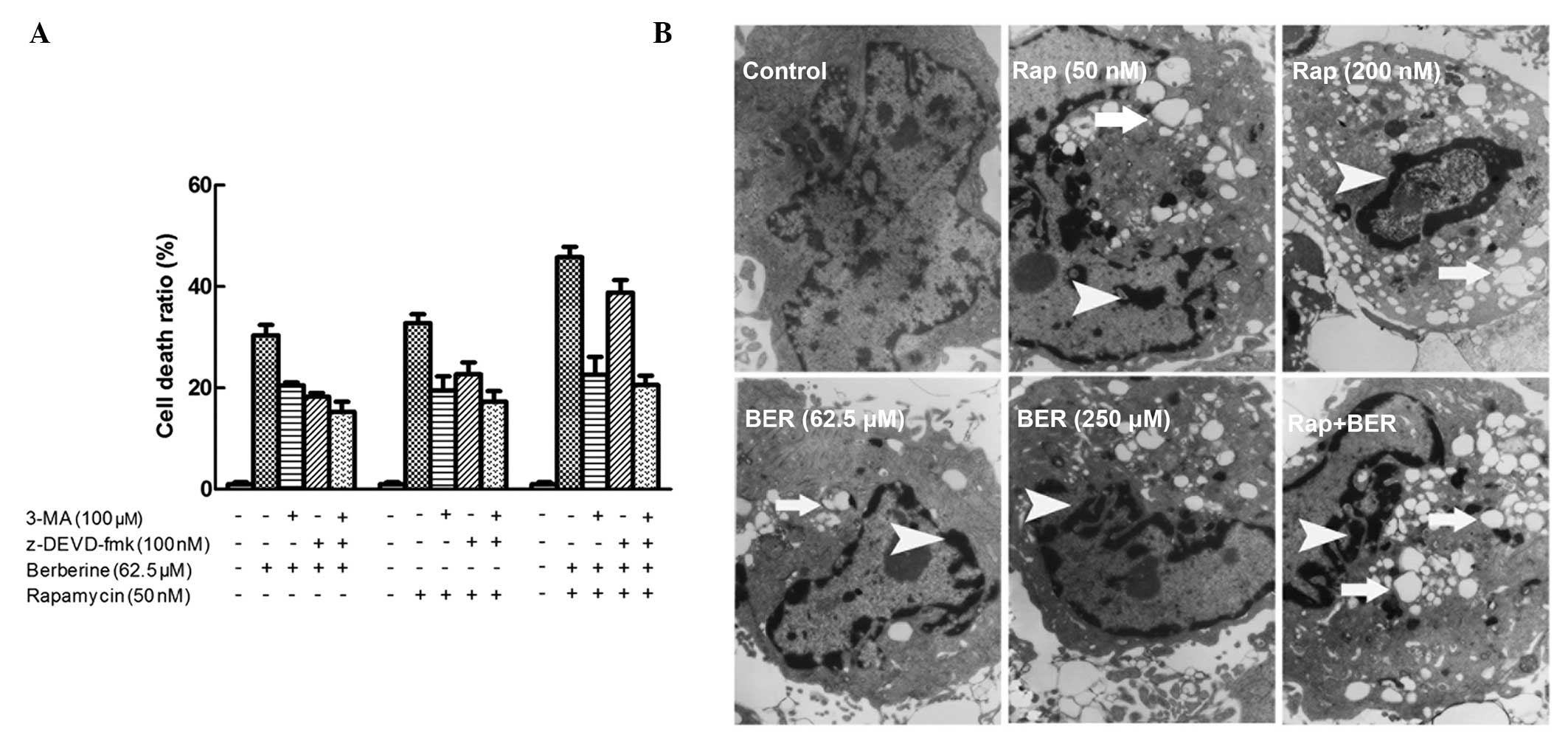
Autophagy is induced by combined
berberine and rapamycin treatment in HCC cells
To determine the specific type of cell death induced
by berberine and rapamycin, the effect of the combined treatment
was assessed in the SMMC7721 cells using a Trypan blue exclusion
assay. 3-MA, a common autophagy inhibitor, was used to inhibit
autophagy and prevent autophagic cell death. 3-MA does not inhibit
cell death induced by other mechanisms. z-DEVD-fmk, an apoptosis
inhibitor, inhibits apoptosis, but no other types of cell death. In
the present study, berberine-induced and rapamycin-induced cell
death were observed to involve autophagy and apoptosis (Fig. 2A). Furthermore, the combined use of
3-MA and z-DEVD-fmk did not completely inhibit cell death,
indicating that cell death may also be induced by other mechanisms,
including necrosis. However, upon combined treatment with berberine
and rapamycin, a significantly stronger inhibition of 3-MA- rather
than z-DEVD-fmk-induced cell death was observed, suggesting that
berberine may enhance rapamycin-induced cell death primarily
through autophagy.
To further analyze the synergistic effect of
berberine and rapamycin on autophagy, the level of autophagy was
assessed in SMMC7721 cells using TEM. Autophagic vacuoles were
observed in the rapamycin-treated SMMC7721 cells (Fig. 2B). However, a significantly higher
level of autophagy was found in the SMMC7721 cells co-treated with
berberine and rapamycin compared with those treated with rapamycin
alone. As shown in Fig. 2B,
treatment with 50 nM rapamycin induced significant cell autophagy
when combined with 62.5 μM berberine compared with treatment with
200 nM rapamycin, indicating that the combined use of berberine and
rapamycin had a synergistic effect on cell autophagy.
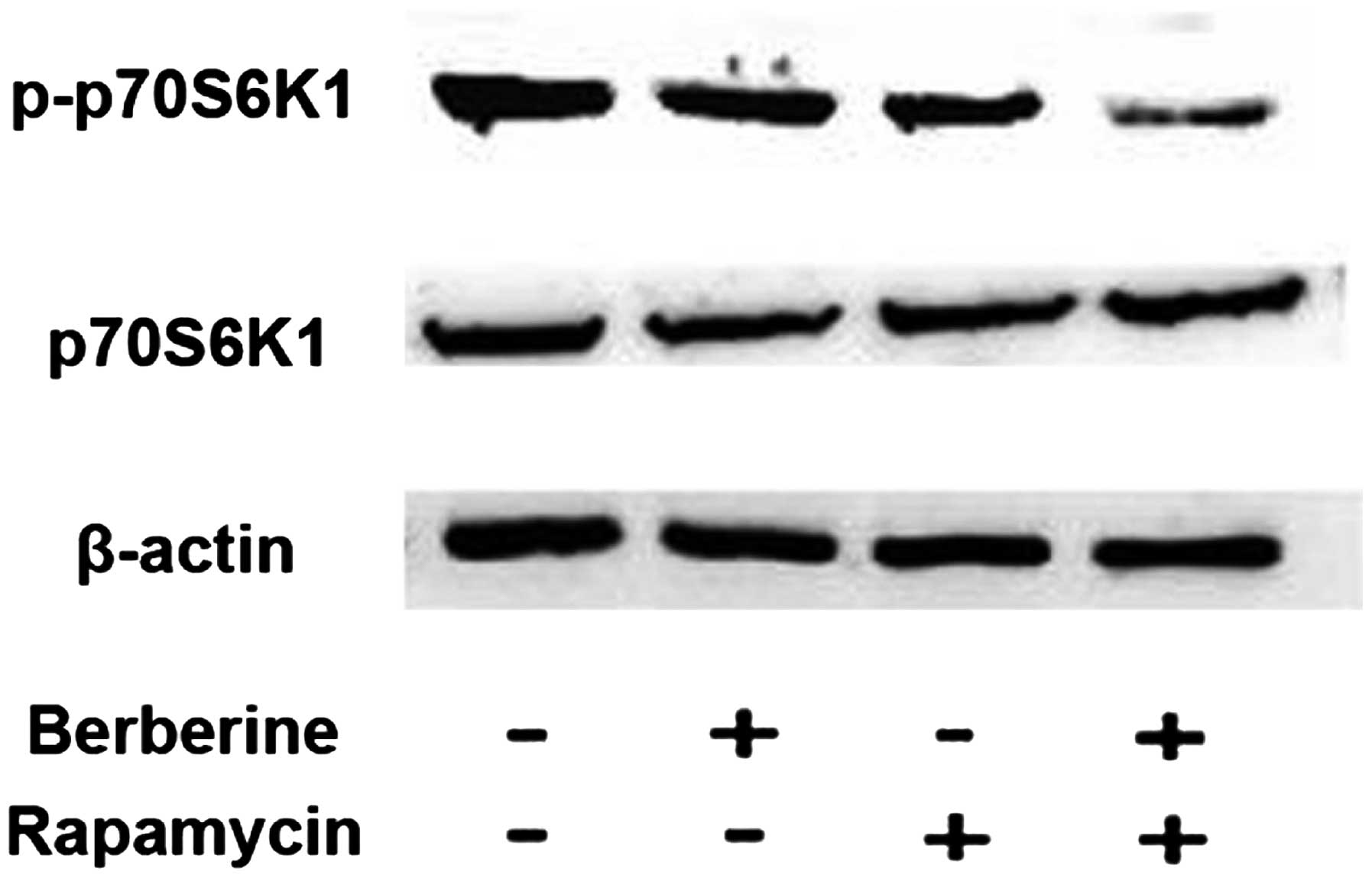
Combined berberine and rapamycin-induced
cell death is associated with inhibition of mTOR-p70S6K1
activity
p70S6K1 is a downstream target of mTOR. To determine
whether the phosphorylation of p70S6K1 is involved in berberine and
rapamycin-induced cell death in HCC cells, SMMC7721 cells were
treated with rapamycin in the absence or presence of berberine for
12 h and the phosphorylation of p70S6K1 at Ser421/Thr424 was
detected using western blot analysis. As shown in Fig. 3, rapamycin and berberine alone were
not found to significantly inhibit the phosphorylation of p70S6K1.
Moreover, a significantly higher reduction in p70S6K1
phosphorylation was observed following berberine addition. These
results suggest that the inhibition of p70S6K1 activation was
associated with berberine and rapamycin-induced cell death.
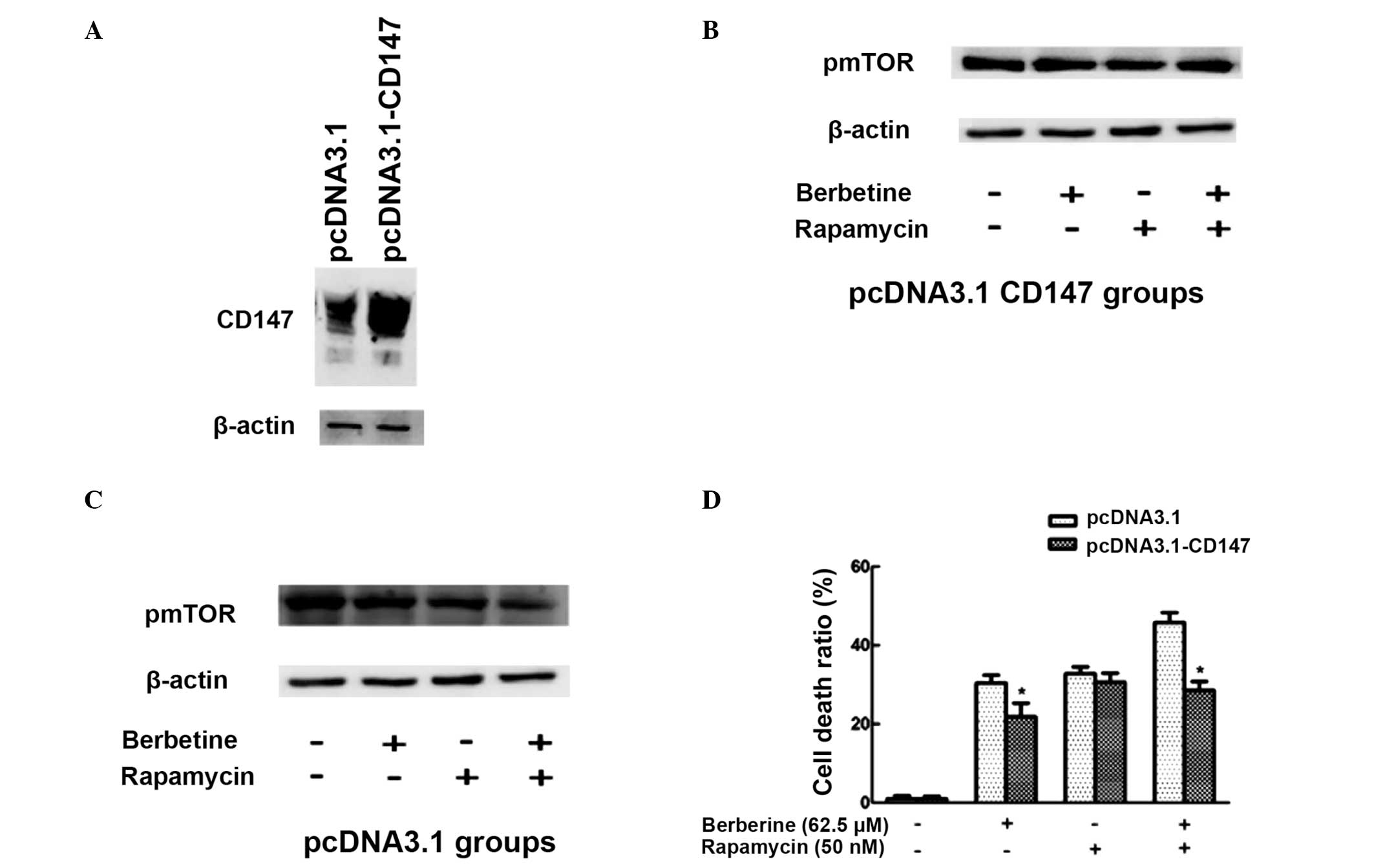
Inhibitory effect of berberine and
rapamycin on p-mTOR is associated with CD147
Our previous studies have suggested that berberine
induces cell death in HCC cells through the downregulation of
CD147. In order to investigate whether CD147 was involved in the
cell death induced by combined berberine and rapamycin treatment,
CD147 was overexpressed in SMMC7721 cells through transfection with
pcDNA3.1-CD147. p-mTOR expression was then detected using western
blot analysis and cell death was assessed using the Trypan blue
exclusion assay. As indicated in Fig.
4A, following transfection with pcDNA3.1-CD147, CD147 was found
to be significantly overexpressed in SMMC7721 cells. Furthermore, a
more significant decrease in p-mTOR expression was observed
following combined treatment with berberine and rapamycin compared
with treatment with berberine or rapamycin alone (Fig. 4C). However, no significant
reduction in p-mTOR expression was observed in SMMC7721 cells
transfected with pcDNA3.1-CD147 (Fig.
4B). SMMC7721 cells transfected with pcDNA3.1-CD147 exhibited
significantly lower levels of cell death, as compared with those
transfected with pcDNA3.1, upon berberine treatment alone or
co-treatment of berberine with rapamycin (Fig. 4D). These results suggest that
overexpression of CD147 inhibits berberine and rapamycin-induced
cell death.
Discussion
While the inhibition of mTOR represents an
attractive anticancer target and clinical trials of rapamycin and
its analogs have shown the importance of mTOR inhibition as a novel
treatment strategy for malignancies, rapamycin-induced
immunosuppression is a problem preventing its clinical application.
Rapamycin-induced immunosuppression is dose-dependent and analyses
of transplant patients who were administered rapamycin have
revealed that through careful therapeutic drug monitoring, it is
possible to maximize the benefits and minimize the hazards of
chronic immunosuppression using a rapamycin-based regimen (1). Thus, rapamycin-induced
immunosuppression may be overcome through combining lower
concentrations of rapamycin with other anticancer agents. Berberine
has been demonstrated to have strong anticancer properties in HCC
cells, which involve inhibition of the mTOR-signaling pathway
through suppressing the activity of Akt (11). Therefore, in the present study, the
anticancer effect of berberine combined with various concentrations
of rapamycin was investigated in HCC cells in vitro. Potent
synergistic cytotoxic effects were observed upon co-treatment with
berberine and rapamycin in SMMC7721 and HepG2 cells. Furthermore,
berberine was found to maintain the cytotoxic effects of rapamycin
on the HCC cells with a lower concentration of rapamycin,
suggesting that berberine may minimize rapamycin-induced
immunosuppression by allowing a lower dose of rapamycin to be used.
Similar synergistic anticancer results have been reported in
SH-SY5Y human neuroblastoma cells, where a low dose combination of
berberine and As2O3, an anticancer agent that
induces various side effects at high concentrations, was found to
markedly decrease cell viability compared with treatment with a low
dose of berberine or As2O3 alone (17). Berberine is a small molecule, which
is easily isolated from a large number of traditional medicinal
plants and has strong anticancer properties without significant
side effects (9,10). Thus, the findings of the present
study suggest that the combined use of berberine with low doses of
rapamycin may be a novel and promising chemotherapeutic strategy
for HCC treatment, with minimal rapamycin-induced immunosuppressive
effects. However, further investigations are required using in
vivo HCC models.
The mode of cell death induced by combined berberine
and rapamycin treatment in HCC cells was also investigated. The
PI3K/Akt/mTOR pathway has been characterized as a key negative
regulator of apoptosis and autophagy in a number of types of cancer
cell and has been proposed to control cellular processes that
contribute to the initiation and maintenance of cancer. Previous
studies have demonstrated that berberine simultaneously induces
autophagic cell death and apoptosis in HCC cells (11,12).
In the present study, apoptosis and autophagy were simultaneously
observed in HCC cells treated with berberine combined with
rapamycin. In addition, autophagic cell death was observed to be
more prominent than apoptosis in the cell death induced by these
two agents.
Notably, the incubation of HCC cells with 3-MA
combined with z-DEVD-fmk was not found to completely prevent cell
death induced by combined berberine and rapamycin treatment. This
finding suggests that besides autophagic cell death and apoptosis,
other types of cell death, including necrosis may be induced in the
HCC cells upon co-treatment with berberine and rapamycin. This was
consistent with the findings of our previous study (12) and is in agreement with a previous
report showing that berberine induces cell lysis/necrosis in B16
murine melanoma cells (18).
To determine the possible signaling molecules
responsible for the synergistic cell killing effect induced by
berberine and rapamycin, the level of p-p70S6K1 (the downstream
target of mTOR) was investigated. p-p70S6K1 expression was found to
be significantly inhibited by treatment with berberine and
rapamycin alone. Moreover, combined treatment with berberine and
rapamycin induced a significant reduction in the level of p-p70S6K1
in the HCC cells compared with that in those treated with rapamycin
or berberine alone. This suggests that p70S6K1, as well as mTOR,
may be the target molecules involved in the synergistic cell
killing effect of combined berberine and rapamycin treatment, and
that the efficacy of this combination may have been induced through
further suppression of mTOR signaling. This was further supported
by the findings of the present study, that in SMMC7721 cells
transfected with pcDNA3.1, berberine and rapamycin alone were
capable of inhibiting p-mTOR expression, while the combination of
berberine and rapamycin induced a lower level of p-mTOR than
berberine or rapamycin alone. Similar synergistic mechanisms have
been reported in HCC cells co-treated with the rapamycin analog
RAD001 and the Akt inhibitor MK-2206 (19), as well as in HCC cells co-treated
with rapamycin and bortezomib, a proteasome inhibitor with
antitumor activity, including the capacity to downregulate p-Akt
(20). However, in the present
study, whether there were other mechanisms involved in the
synergistic anticancer effect of combined berberine and rapamycin
treatment, other than the synergistic suppression of mTOR
signaling, requires further investigation.
In our previous study, berberine was found to induce
cell death in SMMC7721 cells through downregulating CD147 (12). Furthermore, CD147 has been reported
to inhibit starvation-induced autophagic cell death in SMMC7721
cells through increasing the activity of the PI3K/Akt pathway
(16). To the best of our
knowledge, at present, no studies have directly focused on the
interrelation between CD147 and mTOR signaling. In order to
investigate whether CD147 was implicated in the cell death mediated
by mTOR signaling in the HCC cells co-treated with berberine and
rapamycin, CD147 was overexpressed in SMMC7721 cells and its effect
on cell death was observed. Overexpression of CD147 was found to
significantly inhibit the downregulation of p-mTOR expression and
to decrease cell death in cells co-treated with berberine and
rapamycin. Based on these findings and our previous studies
(12,16), it was hypothesized that CD147 may
have a regulatory effect on the activity of the PI3K/Akt/mTOR
signaling pathway, and that the cell death induced by combined
berberine and rapamycin treatment may, at least in part, be due to
berberine-induced CD147 downregulation. However, the effect of
rapamycin on CD147 expression in HCC cells requires further
investigation.
In conclusion, the results of the present study
indicate that berberine sensitizes rapamycin-mediated HCC cell
death, at least in part, through synergistically inhibiting mTOR
signaling through the downregulation of CD147. These findings
suggest the possibility of developing a novel regimen capable of
improving HCC therapy and minimizing the immunosuppression
associated with rapamycin through decreasing its dose.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81101744), the
Natural Science Foundation of Xi’an Medical University (no. 11FZ09
and 11FZ14) and the Scientific Research Program, funded by Shaanxi
Provincial Health Department (nos. 2014D21 and 2014D24).
References
|
1
|
Saunders RN, Metcalfe MS and Nicholson ML:
Rapamycin in transplantation: a review of the evidence. Kidney Int.
59:3–16. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gibbons JJ, Abraham RT and Yu K: Mammalian
target of rapamycin: discovery of rapamycin reveals a signaling
pathway important for normal and cancer cell growth. Semin Oncol.
36(Suppl 3): S3–S17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou L, Huang Y, Li J and Wang Z: The mTOR
pathway is associated with the poor prognosis of human
hepatocellular carcinoma. Med Oncol. 27:255–261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Owonikoko TK and Khuri FR: Targeting the
PI3K/AKT/mTOR pathway: biomarkers of success and tribulation. Am
Soc Clin Oncol Educ Book. 2013:395–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Finn RS, Poon RT, Yau T, et al: Phase I
study investigating everolimus combined with sorafenib in patients
with advanced hepatocellular carcinoma. J Hepatol. 59:1271–1277.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fasolo A and Sessa C: Targeting mTOR
pathways in human malignancies. Curr Pharm Des. 18:2766–2777. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Q, Lui VW and Yeo W: Targeting the
PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol.
7:1149–1167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M: mTOR inhibitor for the treatment
of hepatocellular carcinoma. Dig Dis. 29:310–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang J, Feng Y, Tsao S, Wang N, et al:
Berberine and Coptidis rhizoma as novel antineoplastic agents: a
review of traditional use and biomedical investigations. J
Ethnopharmacol. 126:5–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Feng Y, Zhu M, et al: Berberine
induces autophagic cell death and mitochondrial apoptosis in liver
cancer cells: the cellular mechanism. J Cell Biochem.
111:1426–1436. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hou Q, Tang X, Liu H, et al: Berberine
induces cell death in human hepatoma cells in vitro by
downregulating CD147. Cancer Sci. 102:1287–1292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang X, Guo N, Xu L, et al: CD147/EMMPRIN:
an effective therapeutic target for hepatocellular carcinoma. J
Drug Target. Aug 29–2012.(Epub ahead of print).
|
|
14
|
Espert L, Denizot M, Grimaldi M, et al:
Autophagy is involved in T cell death after binding of HIV-1
envelope proteins to CXCR4. J Clin Invest. 116:2161–2172. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Valentim L, Laurence KM, Townsend PA, et
al: Urocortin inhibits Beclin1-mediated autophagic cell death in
cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol
Cell Cardiol. 40:846–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gou X, Ru Q, Zhang H, et al: HAb18G/CD147
inhibits starvation-induced autophagy in human hepatoma cell
SMMC7721 with an involvement of Beclin 1 down-regulation. Cancer
Sci. 100:837–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim DW, Ahan SH and Kim TY: Enhancement of
arsenic trioxide (As(2)O(3))-mediated apoptosis using berberine in
human neuroblastoma SH-SY5Y cells. J Korean Neurosurg Soc.
42:392–399. 2007. View Article : Google Scholar
|
|
18
|
Letasiová S, Jantová S, Cipák L and
Múcková M: Berberine-antiproliferative activity in vitro and
induction of apoptosis/necrosis of the U937 and B16 cells. Cancer
Lett. 239:254–262. 2006.PubMed/NCBI
|
|
19
|
Grabinski N, Ewald F, Hofmann BT, et al:
Combined targeting of AKT and mTOR synergistically inhibits
proliferation of hepatocellular carcinoma cells. Mol Cancer.
11:852012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu C, Miao X, Huang L, et al: Genome-wide
association study identifies five loci associated with
susceptibility to pancreatic cancer in Chinese populations. Nat
Genet. 44:62–66. 2011. View
Article : Google Scholar : PubMed/NCBI
|