Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune
disease affecting approximately 1% of the population world-wide, in
a female:male ratio of 2.5/1. Over 60 million people suffer from
this disorder, which is characterized by arthrosynovitis and
erosive destruction of peripheral joints. These clinical
manifestations are chronic, occur symmetrically and synovitis is
present in multiple joints (1–4). To
date, the cause of RA has not been fully elucidated. Although a
number of drugs are used for the clinical treatment of RA, an
effective drug with few side effects and of low cost is still
lacking. Micheliolide (MCL) is a new effective compound for the
treatment of RA; it is a guaianolide sesquiterpene lactone
(5) that can be isolated from
magnoliaceae (6) and
semi-synthesized from parthenolide with 90% yield (7). Parthenolide is a traditional drug,
which originated in Europe and has demonstrated potent anticancer
and anti-inflammatory activities (8). Compared with parthenolide, MCL
exhibits greater plasma stability with a more sustained release and
superior in vivo efficacy (9) has a low toxicity, low cost and strong
anti-inflammatory effects. Methotrexate (MTX), is an almost
unsurpassed drug for the clinical treatment of RA and it has been
supported for its safety and convenience, since it is amenable to
administration by the oral route (10–14).
The collagen-induced arthritis (CIA) mouse model is the most
commonly studied autoimmune model of RA. It is induced by
immunization with an emulsion of complete Freund’s anduvant and
type II collagen (CII) (15). In
the present study, the effects of MCL and MTX on type II
collagen-induced arthritis (CIA) in mice were investigated as well
as evaluating whether MCL may be used as a new drug for the
treatment of RA.
Materials and methods
Animals
Experiments were performed according to the
Declaration of Helsinki guidelines and were approved by the
institutional biomedical research ethics committee in Laboratory
Animal Center of Institute of Hematology and Blood Diseases
Hospital, Chinese Academy of Medical Sciences (Tianjin, China). A
total of 40 specific-pathogen free male DBA/1 mice, aged 6–7 weeks,
were purchased from Beijing HFK Bioscience Co., Ltd (Beijing,
China).
CIA and treatment
In total, forty DBA/1 mice were randomly divided
into four groups and numbered. The groups were termed normal (Nor),
model (CIA), MTX and MCL groups. On the first day, DBA/1 mice (with
the exception of the normal group mice) were immunized by
intradermal injections at the base of the tail with an emulsion
containing 100 μg of immunization grade bovine type II collagen
(CII; Chondrex Inc., Richmond, WA, USA) in complete Freund’s
adjuvant containing heat-killed mycobacterium butyricum (Chondrex
Inc., Richmond, WA, USA). On day 21, a booster containing 100 μg
bovine CII in incomplete Freund’s adjuvant (Chondrex Inc.) was
administered (15). Drugs were
administered intraperitoneally (IP) every two days from day 22. The
normal group did not receive treatment; the model group was
administered with equal volumes of solvent, DMSO (Tianjin Fengchuan
Chemical Reagent Science and Technology Co., Ltd., Tianjin, China);
the MTX group was treated with 6.6 mg/kg MTX (Shanxi Powerdone
Pharmaceutics Co., Ltd, Beijing, China) solution (dissolved in
saline) (16); the MCL group was
treated with 30 mg/kg MCL (provided by Prof. Chen Yue, College of
Pharmacy, Nankai University, Tianjin, China) solution (dissolved in
DMSO). All treatments were administrated 28 times to all mice in
the corresponding group.
Evaluation of the disease
To evaluate the disease, the mice were measured on
alternate days for 60 days following the booster immunization. The
clinical severity of paw inflammation was scored periodically by
the arthritis scores. The arthritis scores were evaluated as
follows: 0, Normal; 1, swelling and redness of the paw or one
digit; 2, two joints involved; 3, more than two joints involved and
4, severe arthritis of the entire paw and digits. The final score
for each mouse was the sum of the four paws (17).
Histopathology
The knees and paws of the mice were severed, fixed
in phosphate-buffered saline containing 4% formaldehyde,
decalcified in 10% ethylenediaminetetraacetic acid and embedded in
paraffin. The tissues were sliced and stained with hematoxylin and
eosin (H and E). Histopathological changes were observed under a
light microscope (Eclipse TE300, Nikon Corporation, Tokyo, Japan)’
and scored by two independent observers using the following scoring
systems: Inflammation was scored on a scale of 0–2 as follows: 0,
Normal, 1, local inflammatory infiltration and 2, marked
infiltration with lymphoid aggregates and edema. Pannus formation
and articular cartilage damage were scored on a scale of 0–2 as
follows: 0, Normal, 1, synovial proliferation adjacent to cartilage
but no articular cartilage damage and 2, synovial proliferation and
articular cartilage damage (18).
Measurement of serum cytokines
The cytokines in the mouse serum were measured using
mouse cytokine array panel A (R&D Systems, Minneapolis, MN,
USA). Briefly, blood samples were allowed to clot for 1 h at room
temperature prior to incubation for 4 h at 4°C and subsequently
centrifuged at 1,500 × g for 10 min by (Sorvall Legend Micro 17R,
Thermo Fisher Scientific, Waltham, MA, USA). Serum was removed and
assayed by mouse cytokine array panel A, according to the
manufacturer’s instructions. Membranes were blocked for 1 h,
samples were subsequently added to an antibody detection cocktail
(R&D Systems), mixed and incubated at room temperature for 1 h
prior to overnight incubation at 4°C. Samples were subsequently
washed, streptavidin-horseradish peroxidase conjugate working
solution was added and incubated for 30 min at room temperature on
a rocking platform shaker. Following washing, samples were
incubated for 1 min using a Chemi Reagent Mix (R&D Systems),
samples were then exposed to X-ray film for 1–10 min and the
scanned results were analyzed using Adobe Photoshop CS4 (Adobe
Systems, Inc., San Jose, CA, USA).
Statistical analysis
All data were summarized and are presented as the
mean ± standard deviation (SD) and analyzed using the GraphPad
Prism Version 5 software program (GraphPad Prism, San Diego, CA,
USA). Group comparisons were performed using the unpaired Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
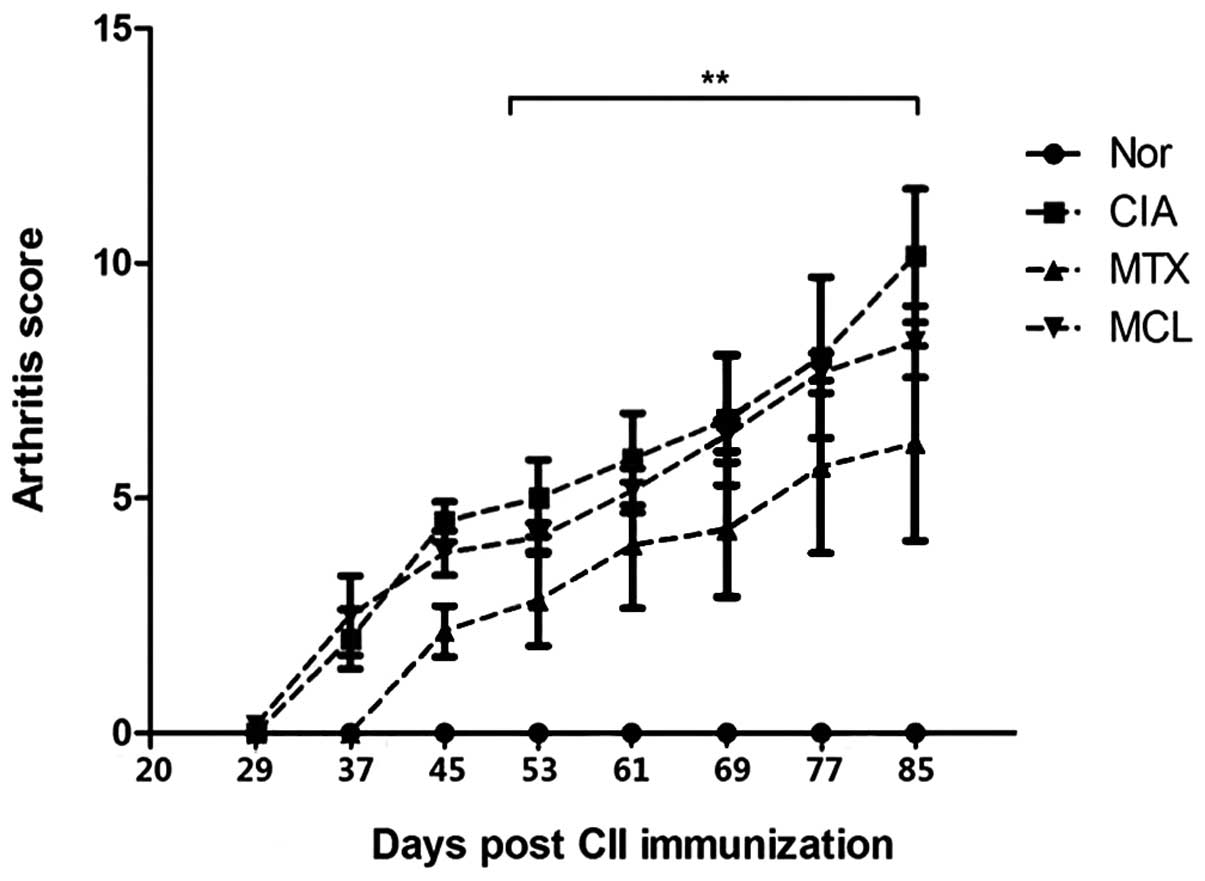
Evaluation of the disease
Compared with the model group, MCL and MTX were able
to significantly reduce swelling of the paws and suppress the
degeneration of articular cartilage, as demonstrated by the lower
arthritis scores compared with the model group. The arthritis
scores of the MCL group were slightly higher than those of the MTX
group, suggesting that the therapeutic effect of MCL on CIA was
less significant than for MTX (Fig.
1).
Effects of MCL on histopathological
changes
H and E staining indicated that the joints in the
normal group exhibited no pathological changes (Fig. 2A: i-iii). However, staining
revealed multiple pathological changes in the model group,
including significant inflammatory cell infiltration (Fig. 2A: iv), hyperplasia of synovial
cells, formation of granulation tissue (Fig. 2A: v) and necrotic tissue present in
the articular cavity (Fig. 2A:
vi). The pathological changes in the MTX and MCL group were
weaker than for the model group. In the MTX group, synovial cells
of fibrous tissue exhibited infiltration and hyperplasia (Fig. 2A: vii), inflammatory cells
infiltrated the tissues surrounding the joints (Fig. 2A: viii) and inflammatory cells
emerged from articular cavities (Fig.
2A: ix). The pathological changes in the MCL group were similar
to those of the MTX group (Fig. 2A:
x-xii). The inflammation score, the score of pannus formation
and articular cartilage destruction further indicated this
(Fig. 2B, C). The present results
revealed that MCL as well as MTX could remit the pathological
changes of CIA.
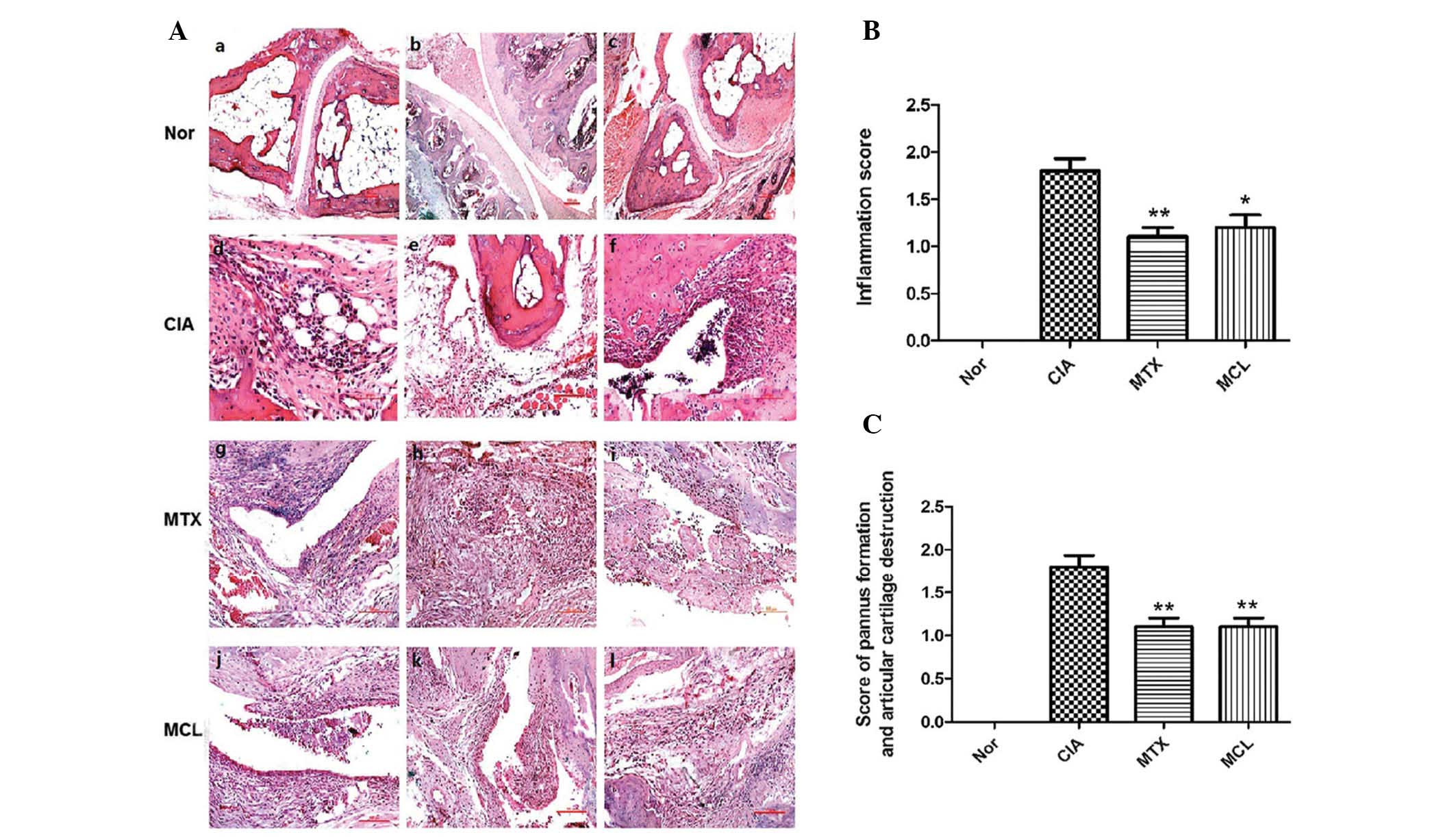 | Figure 2Joint tissues with hematoxylin and
eosin staining of the four groups. (A: i-iii) Normal joint tissue
present from normal control group; (A: iv-vi), (A: vii-ix) and (A:
x-xii) are the corresponding specimens from CIA control group, MTX
control group and MCL group, respectively. (A: iv-xii) Indicate
infiltration by inflammatory cells, synovitis cell infiltration and
hyperplasia of fibrous tissue. (B) Inflammation score of CIA
control group, MTX control group and MCL group. Data is presented
as the mean ± SEM of clinical scores. *P<0.05,
**P<0.01 compared with CIA group. (C) The score of
pannus formation and articular cartilage destruction of CIA control
group, MTX control group and MCL group. Data is presented as the
mean ± SEM of clinical scores. **P<0.01 compared with
CIA group. This data is representative of three separate
experiments, which yielded similar results. CIA, collagen induced
arthritis; MTX, methotrexate; MCL, micheliolide; SEM, standard
error of the mean. |
Effects of MCL on serum cytokines
While forty cytokines were assessed in this
experiment, only six of them were detectable in serum and are
presented in the results (Fig.
3A). These are denoted 1-6. Cytokines 1-4 were all present in
the four experimental groups, these were complement component 5a
(C5/C5a), tissue inhibitors of metalloproteinases-1 (TIMP-1),
macrophage colony-stimulating factor (M-CSF) and soluble
intercellular adhesion molecule-1 (sICAM-1), respectively. Compared
with the normal group, the expression levels of M-CSF, TIMP-1 and
C5/C5a in the CIA group were decreased. Following treatment with
MCL or MTX, all mice recovered to differing degrees; this was
increasingly evident following MTX treatment. The cytokine
B-Lymphocyte chemoattractant (BLC) was only present in the MCL
group (Fig. 3B). The results of
the cytokine analysis and histopathological detection indicated
that MCL and MTX were effective in the CIA model. Notably MCL may
have a more potent effect than MTX with regard to the effects on
autoimmunity.
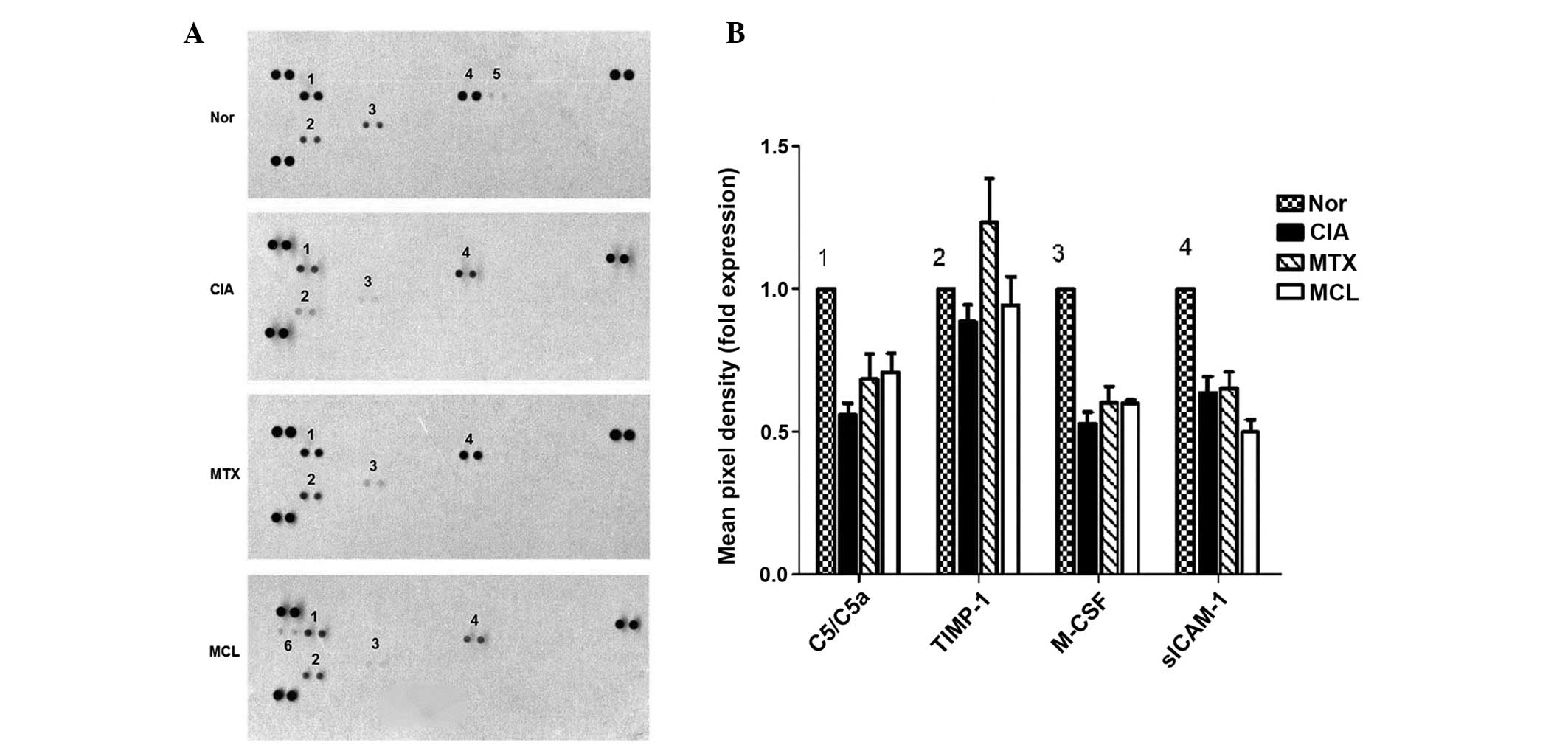 | Figure 3Mouse cytokine test for the four
groups of mice. (A) Six cytokines are presented in this experiment
and they are denoted 1-6. Cytokines 1-4 are present in all four
groups; these are C5/C5a, TIMP-1, M-CSF and (sICAM-1); cytokine 5
refers to interferon-γ, only shown in the normal control group;
cytokine 6 is B-Lymphocyte chemoattractant, only present in the MCL
group. (B) The result of M-CSF is the same as that of C5/C5a: The
normal control group is the highest and the CIA control group is
the lowest, while the MCL group is between the CIA control group
and the MTX control group. **P<0.01 compared with the
CIA group; TIMP-1, the MCL group and the MTX group are higher than
the CIA control group as well as marginally higher than the normal
control group and are very similar to each other.
**P<0.01 compared with the CIA group; sICAM-1; the
MCL group is the lowest group. This data is representative of three
separate experiments, which yielded similar results. CIA, Collagen
induced arthritis; MTX, methotrexate; MCL, micheliolide; SEM,
standard error of the mean; C5/C5A, complement component 5a;
TIMP-1, tissue inhibitors of metalloproteinases-1; M-CSF,
macrophage colony-stimulating factor; sICAM-1, soluble
intercellular adhesion molecule-1. |
Discussion
RA is one of the most common systemic autoimmune
diseases (17). It can result in
the progressive destruction of diseased cartilage and joints,
severe disability, high social cost and even shortened lifespan
(19–21). Previous studies have shown that the
CIA model can lead to peripheral arthritis in multiple joints,
local joint swelling or even joint deformity. The clinical
manifestations, laboratory parameters, immunological and
pathological changes are similar to those exhibited in human RA
(1,22), therefore, it may be utilized as an
animal model of RA. In the present study, DBA/1 mice were used to
establish the CIA model and the results obtained suggested that the
CIA model in this experiment was successful and stable. The
arthritis scores of the MCL group were slightly higher than those
of the MTX group, however, the two scores were lower than that of
the model group. This suggests that, similarly to MTX, MCL
possesses the ability to treat RA. MTX is most commonly used as a
disease-modifying antirheumatic drug (DMARD) and has
antiproliferative and anti-inflammatory effects (10). In the current study, the
histopathological changes of the CIA mice that were treated with
MTX were observed to be significantly improved. As the results of
the MCL group almost unanimously correspond with the MTX group,
this suggests that MCL may also exhibit antiproliferative and
anti-inflammatory potential.
The serum cytokines measured in the present study
were C5/C5a, TIMP-1, M-CSF, sICAM-1, interferon-γ (IFN-γ) and BLC.
C5/C5a is essential in the innate immune response and evidence now
suggests that it may also be involved in adaptive immunity. C5a can
bind to the surface of immune cells, such as macrophages,
neutrophils and T-cells. It is a well-established receptor that
initiates G-protein-coupled signaling via mitogen-activated protein
kinase pathways, thereby inducing the synthesis of cytokines such
as tumor necrosis factor-α and interleukin-6 (23,24).
TIMPs are well recognized for their role in extracellular matrix
remodeling by controlling the activity of matrix metalloproteinases
(MMPs); of these, TIMP-1 with its major target proMMP-9 participate
in the regulation of multiple cell functions, including
proliferation and migration (25,26).
M-CSF is produced by osteoblasts, synovial and periodontal
fibroblasts. Combined with the receptor activation of nuclear
factor-κB ligand, it can regulate osteoclastic differentiation and
participate in the chronic inflammatory processes that often
aggravate bone loss, such as RA (27). The results showed that, following
treatment with MCL and MTX, C5/C5a, TIMP-1 and M-CSF recovered to
different degrees, particularly the level of TIMP-1 in the MTX
group. This suggests that MCL and MTX may modulate the progression
of RA via regulation of immunity, cell proliferation, migration and
the chronic inflammatory process. sICAM-1 has been shown to have
anti-inflammatory effects (28).
In the present study, the level of sICAM-1 in the MCL group is
lower than that of the MTX and CIA groups, however the sICAM-1
level in the MTX group is similar to that of the CIA group. This
indicates that MTX and MCL have extremely limited involvement in
the regulation of sICAM-1, particularly MCL. BLC was only present
in the MCL group; this phenomenon indicated that MCL may also be
involved in autoimmune regulation. IFN-γ was only present in the
normal DBA/1 mice, which may indicate no association with RA.
Although MTX is a commonly used DMARD for the
clinical treatment of RA, there are several disadvantages,
including the fact that a significant number of patients require
additional treatments in order to control the disease process,
either concurrently, or following treatment with MTX. The present
study demonstrated that MCL has extremely similar therapeutic
effects to MTX, suggesting that MCL may be a potential alternative
drug for the treatment of RA. In addition to the existing results,
further investigation is required to gain an improved understanding
of MCL in the treatment of RA. We propose that MCL may participate
in the regulation of the autoimmune system in RA, and therefore
suggest that future studies may investigate the underlying
mechanisms of its effect on the immune function in RA in greater
depth, as well as conducting in vivo studies. Furthermore, studies
on the toxicity of MCL are required to demonstrate the clinical
applications of this therapy.
Acknowledgements
This study was supported by grants from the Natural
Science Key Program Foundation of Tianjin Technology Commission of
China (grant no. 14JCZDJC34900), the National Natural Science
General Program Foundation of China (grant no. 81170510) and the
Major Program Foundation of China (grant no. 81090410).
References
|
1
|
Zhang Y, Xu W, Li H, et al: Therapeutic
effects of total alkaloids of Tripterygium wilfordii Hook f. on
collagen-induced arthritis in rats. J Ethnopharmacol. 145:699–705.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vingsbo-Lundberg C, Nordquist N, Olofsson
P, et al: Genetic control of arthritis onset, severity and
chronicity in a model for rheumatoid arthritis in rats. Nat Genet.
20:401–404. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee Dm and Weinblatt MB: Rheumatoid
arthritis. Lancet. 15:903–911. 2001.
|
|
5
|
Schall A and Reiser O: Synthesis of
biologically active guaianolides with a trans-annulated lactone
moiety. Eur J Org Chem. 2008:2353–2364. 2008. View Article : Google Scholar
|
|
6
|
Ma WW, Shi QQ, Ding YH, et al: Synthesis
of micheliolide derivatives and their activities against AML
progenitor cells. Molecules. 18:5980–5992. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhai JD, Li D, Long J, et al: Biomimetic
semisynthesis of arglabin from parthenolide. J Org Chem.
77:7103–7107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghantous A, Sinjab A, Herceg Z and
Darwiche N: Parthenolide: from plant shoots to cancer roots. Drug
Discov Today. 18:894–905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Q, Lu Y, Ding Y, et al: Guaianolide
sesquiterpene lactones, a source to discover agents that
selectively inhibit acute myelogenous leukemia stem and progenitor
cells. J Med Chem. 55:8757–8769. 2012.
|
|
10
|
Sokka T and Pincus T: Contemporary disease
modifying antirheumatic drugs (DMARD) in patients with recent onset
rheumatoid arthritis in a US private practice:methotrexate as the
anchor drug in 90% and new DMARD in 30% of patients. J Rheumatol.
29:2521–2524. 2002.PubMed/NCBI
|
|
11
|
Swierkot J and Szechiński J: Methotrexate
in rheumatoid arthritis. Pharmacol Rep. 58:473–492. 2006.
|
|
12
|
Cronstein BN: Low-dose methotrexate: a
mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev.
57:163–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pincus T, Yazici Y, Sokka T, et al:
Methotrexate as the “anchor drug” for the treatment of early
rheumatoid arthritis. Clin Exp Rheumatol. 21:S179–S185. 2003.
|
|
14
|
Van der, Heijden JW, Dijkmans BA, et al:
Drug Insight: resistance to methotrexate and other
disease-modifying antirheumatic drugs--from bench to bedside. Nat
Clin Pract Rheumatol. 3:26–34. 2007.PubMed/NCBI
|
|
15
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar
|
|
16
|
Suszko A and Obminska-Mrukowicz B:
Influence of polysaccharide fractions isolated from Caitha
palustris L. on the cellular immune response in collagen-induced
arthritis (CIA) in mice. A comparison with methotrexate. J
Ethnopharmacol. 145:109–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuzzocrea S, Ayroldi E, Di Paola R, et al:
Role of glucocorticoid-induced TNF receptor family gene (GITR) in
collagen-induced arthritis. FASEB J. 19:1253–1265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xinqiang S, Fei L, Nan L, Yuan L, et al:
Therapeutic efficacy of experimental rheumatoid arthritis with
low-dose methotrexate by increasing partially CD4+CD25+Treg cells
and inducing Th1 to Th2 shift in both cells and cytokines. Biomed
Pharmacother. 64:463–471. 2010. View Article : Google Scholar
|
|
19
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wildbaum G, Nahir MA and Karin N:
Beneficial autoimmunity to proinflammatory mediators restrains the
consequences of self-destructive immunity. Immunity. 19:679–688.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sinha AA, Lopez MT and McDevitt HO:
Autoimmune diseases: the failure of self tolerance. Science.
248:1380–1388. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Xiao C, Zhao L, et al: The effect
of triptolide on CD4+ and CD8+ cells in Peyer’s patch of SD rats
with collagen induced arthritis. Int Immunopharmacol. 6:198–203.
2006.
|
|
23
|
Ma N, Xing C, Xiao H, et al: C5a regulates
IL-12 (+) DC migration to induce pathogenic Th1 and Th17 cells in
sepsis. PLoS One. 8:e697792013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laudes IJ, Chu JC, Huber-Lang M, et al:
Expression and function of C5a receptor in mouse microvascular
endothelial cells. J Immunol. 169:5962–5970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ries C: Cytokine functions of TIMP-1. Cell
Mol Life Sci. 71:659–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Djafarzadeh R, Mojaat A, Vicente AB, et
al: Exogenously added GPI-anchored tissue inhibitor of matrix
metalloproteinase-1 (TIMP-1) displays enhanced and novel biological
activities. Biol Chem. 385:655–663. 2004. View Article : Google Scholar
|
|
27
|
Souza PP and Lerner UH: The role of
cytokines in inflammatory bone loss. Immunol Invest. 42:555–622.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fei X, Hongxiang Z, Qi C and Daozhen C:
Maternal plasma levels of endothelial dysfunction mediators
including AM, CGRP, sICAM-1 and tHcy in pre-eclampsia. Adv Clin Exp
Med. 21:573–579. 2012.PubMed/NCBI
|