Introduction
Leukocyte differentiation antigens are a series of
glycoproteins and glycolipids attached to or inserted into the
membrane of leukocytes, which are the major cells of the immune
system and malignant cells that derive from them (1). These antigens are able to transmit
membrane signals, regulate cell activation and differentiation as
well as mediate numerous interactions between the immune system and
antigens, other physiological systems or other components of the
immune system (1–3). Numerous leukocyte differentiation
antigens were reported to be involved in the pathogenesis and
development of hematopoietic malignancies; in addition, certain
antigens may be used as markers for diagnosis, classification, risk
stratification and eventually as treatment targets in these
malignant diseases (4,5).
In previous unpublished experiments conducted in
2008, we aimed to identify novel potential leukocyte
differentiation antigens using an integrated bioinformatics
analysis at a genome-wide level to select proteins matching the
following criteria: 1, No reported functional association with the
immune system; 2, predominant expression in cells of the immune
system; 3, high expression in certain specific subsets of immune
cells; and 4, type I and type II membrane proteins preferred. V-set
and transmembrane domain-containing 1 (VSTM1) was identified
by the data mining as a candidate gene. VSTM1 was found to
be located at human chromosome 19q13.4 and to encode two primary
splicing forms, VSTM1-v1 and VSTM1-v2. VSTM1-v1 contains 236 amino
acids and is a type I membrane molecule, which has an
immunoglobulin (Ig)V-like domain in its extracellular region and
two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in its
cytoplasmic tail. It has been suggested that VSTM1-v1 may be a
novel ITIM-bearing inhibitory immune receptor involved in the
regulation of phagocytes (6).
VSTM1-v2 is composed of 205 amino acids and was reported to be a
classical secretory glycoprotein, which does not contain a
transmembrane domain. It was also reported that recombinant
VSTM1-v2 promoted the differentiation and activation of human Th17
cells (7).
Genetic instabilities are widely accepted to be one
of the major causes of cancer pathogenesis in humans. Chromosome
19q13 has been reported to be involved in numerous types of cancer,
including hematopoietic malignancies, due to its instable genomic
region. These instabilities include chromosome translocations,
single nucleotide polymorphisms, amplifications, mutations and
deletions, which occur frequently in certain types of myeloid and
lymphoid leukemia and lymphoma (8–12).
Furthermore, epigenetic modifications such as DNA methylation were
reported to be involed in the dysregulation of genes located on
19q13.3–4 (13–15).
DNA methylation is an epigenetic code that controls
the expression of lineage- and development-specific genes. It has
been reported that the aberrant methylation of genes regulating the
differentiation of hematopoietic lineages induced leukemic
transformations (16–19). The aim of the present study was to
examine the expression of VSTM1 in normal human peripheral blood
leukocytes and hematopoietic tumor cell lines.
Materials and methods
Isolation of peripheral blood
granulocytes, monocytes and lymphocytes
Peripheral blood mononuclear cells (PBMCs) were
isolated from granulocytes and red blood cells in the buffy coats
of healthy donors [obtained at the Beijing Red Cross Blood Center
(Beijing, China) between December 2012 and April 2013] using
Ficoll/Hypaque density-gradient centrifugation, as described
previously (20), and cultured in
RPMI 1640 medium supplemented with 10% fetal calf serum (FCS;
Hyclone Laboratories, Inc, Logan, UT, USA) in petri dishes to
enable monocyte adhesion. Non-adherent cells, predominantly
peripheral blood lymphocytes (PBLs), and adherent monocytes were
collected separately (20).
Granulocytes were isolated through lysing red blood cells with a
hypotonic buffer as previously described (21). Protocols performed in the present
study were approved by the Ethics Committee of Peking University
Health Science Center (Bejing, China). Written informed consent was
obtained from all healthy donors.
Cell lines
KG-1, HL-60, HuT-78, Jurkat, K562, MOLT-4, Raji,
Thp-1, U937 and RPMI 8866 cells, purchased from the American Type
Culture Collection (Manassas, VA, USA), were cultured in RPMI 1640
medium supplemented with 10% FCS at 37°C in a humidified atmosphere
with 5% CO2. Jurkat cells were treated with 2 μmol/l
5-aza-2′-deoxycytidine (Sigma-Aldrich, St. Louis, MO, USA) for 3
days, and a proportion of these cells were then further treated
with 100 nmol/l trichostatin A (Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 16 h as previously described (22,23).
Genomic DNA samples were extracted from Jurkat cells using a ZR
Genomic DNA II Kit™ (Zymo Research. Irvine, CA, USA) according to
the manufacturer’s instructions. Jurkat cell transfection was
performed using electroporation; in brief, 25 μg plasmid was mixed
with 2×107 cells in 350 μl serum-free medium and
electroporated at a single pulse (135 V; 20 ms) in a 2-mm gap
cuvette using an ECM 830 Square Wave Electroporation System (BTX
Harvard Apparatus, Holliston, MA, USA).
Semiquantitative reverse transcription
polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells using TRIzol
reagent (Life Technologies Inc., Carlsbad, CA, USA). Reverse
transcription was performed according to standard protocols using
the RevertAid™ II First Strand complementary (c)DNA synthesis kit
(Fermentas, Waltham, MA, USA). Human Multiple Tissue and Immune
System MTC™ panels containing cDNA from a pool of donors were
purchased from Clontech Laboratories, Inc. (Mountain View, CA,
USA). Semiquantitative PCR was performed as previously described
(7). GAPDH was then amplified and
used as an internal control. qPCR was performed as previously
described (23) using the primers
5′-ACTTGCAGCTGGTGGTCACA-3′ and 5′-CCGGAAGTTTGGAATGGCT-3′. Samples
with unspecific amplification according to the dissolving curve and
electrophoresis were regarded as zero. All samples were normalized
to GAPDH using the comparative Ct method (ΔΔCt).
Cytosine-phosphate-guanine (CpG) island
screening, DNA bisulfite treatment and methylation analysis
CpG island searcher (http://cpgislands.usc.edu/) was used to screen for CpG
islands in the promoter region of VSTM1. Based on the search
results, primers for the detection of methylated or unmethylated
alleles of the VSTM1 promoter and for bisulfite genomic
sequencing (BGS) were designed. Bisulfite modification of DNA,
methylation-specific PCR (MSP) and BGS were then performed as
previously described (22,23). The bisulfite-treated DNA was
amplified using the methylation-specific primer set m3,
5′-TTGTATATTTTGGGGACGAATC-3′ and m4, 5′-AAAAAAAATTCTACGATCATAACG-3′
or the unmethylation-specific primer set u3, 5′-TTTGTATAT
TTTGGGGATGAATT-3′ and u4, 5′-AAAAAAAAA TTCTACAATCATAACA-3′ (Beijing
AuGCT DNA-SYN Biotechnology Co Ltd., Beijing, China). MSP was
performed for 40 cycles using Ampli-Taq Gold (Perkin Elmer Applied
Systems, Foster City, CA, USA) and hot start. MSP primers were
tested in order to confirm that they did not amplify unbisulfited
DNA; in addition, the specificity of MSP was confirmed by direct
sequencing of PCR products. For BGS, bisulfite-treated DNA was
amplified using the following primers: b1,
5′-AGAGTGGGGTAGAGAATTAGAGTGTTA-3′ and b2,
5′-ACCTAAACTACCACTCCCACATAAAT-3′. The PCR products were cloned into
the pGEM-T easy vector (Promega Corp., Maddison, WI, USA) and
sequenced using ten randomly selected colonies.
Flow cytometric analysis
Cells were blocked using fluorescence-activated cell
sorting (FACS) buffer [phosphate-buffered saline (PBS) containing
2% FCS] on ice for 30 min. A total of 1×106 cells were
then incubated on ice for 1 h with rabbit anti-VSTM1 polyclonal
antibody (prepared by authors as previously described; 2 μg/sample
in 100 μl FACS buffer) (24).
Cells were then washed in PBS and stained with fluorescein
isothiocyanate-conjugated anti-rabbit immunoglobulin (Ig; dilution,
1:100 in FACS buffer; Biolegend, Inc., San Diego, CA, USA) in the
dark at 4°C for 30 min. Cells were susequently washed in PBS and
analyzed using a BD FACSCalibur flow cytometer (Becton-Dickinson,
Franklin Lakes, NJ, USA).
Protein extraction and western blot
analysis
Cells were harvested and lysed using 20 mmol/l
Tris-HCl (pH 7.5), 150 mmol/l NaCl, 1 mmol/l EDTA, 1% Triton X-100,
and 1% protease inhibitor cocktail (Pierce, Rockford, IL, USA).
Proteins were applied to 10% SDS-PAGE and electrotransferred onto
polyvinylidene difluoride membranes (Hybond® Inc.,
Escondido, CA, USA). VSTM1 was detected using rabbit anti-VSTM1
polyclonal antibodies (pAb; 2 μg/ml) and secondary horseradish
peroxidase-conjugated goat anti-rabbit IgG antibodies (dilution,
1:5,000; Cell Signaling Technology, Inc., Danvers, MA, USA).
Signals were visualized using enhanced chemiluminescence western
blot detection reagents (GE Healthcare, Little Chalfont, UK) on an
ImageQuant LAS 500 imaging device (GE Healthcare). β-actin was
detected using mouse anti-β-actin monoclonal antibody (mAb;
dilution, 1:3,000; Clone AC-74; Sigma-Aldrich Shanghai Trading Co.,
Ltd., Shanghai, China) and used as an internal control to determine
lysate input.
Capacity of rabbit anti-VSTM1 polyclonal
antibody (pAb) to cross-link VSTM1-v1
In order to analyze whether the rabbit anti-VSTM1
pAb influenced the functional cross-linking of VSTM1, a reporter
construct (pcDB-VSTM1-v1-CD3ζ) was generated that expressed a
chimeric protein consisting of the extracellular domain of VSTM1-v1
as well as the transmembrane and intracellular domains of CD3ζ
through overlapping PCRs as previously described (25). Dual-luciferase reporter assays were
then performed. A total of 2×107 Jurkat cells were
cotransfected with 20 μg pcDB-VSTM1-v1-CD3ζ or empty vector, 8 μg
p-nuclear factor kappa-light-chain-enhancer of activated B cells
(NFκB)-Luc plasmid (a construct encoding the firefly luciferase
reporter gene under the influence of NFκB binding) and 0.4 μg
p-Renilla luciferase (RL)-thymadine kinase (TK) plasmid (a
construct encoding the RL gene; Promega Corporation, Madison, WI,
USA) as the internal control. 12 h post-transfection, cells were
stimulated with anti-CD28 (2 μg/ml; clone 15E8) in combination with
different concentrations (1, 3 or 5 μg/ml) of rabbit anti-VSTM1 pAb
or normal rabbit IgG (5 μg/ml) (BD Biosciences, San Jose, CA, USA).
Plate-bound anti-CD3 (1 μg/ml; clone OKT3) and tumor necrosis
factor-α (20 ng/ml; eBioscience, San Diego, CA, USA) were used as
positive controls. Following 20 h of stimulation, cells were
collected and lysed using Passive Lysis Buffer (Promega Corp.).
Firefly and RL activities were measured with the dual-luciferase
reporter (DLR) assay system (Promega Corp.) according to the
manufacturer’s instructions using a Veritas™ Microplated
Luminometer (Promega Corp.).
Cell proliferation assay
Jurkat cells transfected with
pCDNA3.1/Myc-His(-)B-VSTM1-v1 (pcDB-VSTM1-v1), or
pCDNA3.1/Myc-His(-)B (pcDB) vector plasmids were seeded in 96-well
plates at a density of 20,000 per well in the presence or absence
of 5 μg/ml anti-VSTM1 pAb or normal rabbit IgG and then incubated
at 37°C in a humidified atmosphere with 5% CO2. Cell
proliferation was analyzed using the Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Inc., Rockville, MA, USA). At 0,
24, 48 and 72 h post-transfection, 10 μl CCK-8 solution was added
into each well and incubated for 2 h. Absorbance was measured with
a Thermo Scientific Multiskan GO microplate spectrophotometer
(Thermo Fisher Scientific Inc., Waltham, MA, USA) at 450 nm in
order to assess the number of viable cells (background absorbance
was measured at 630 nm). Results were obtained from at least three
independent experiments.
Statistical analysis
Values are presented as the mean ± standard error of
the mean/standard deviation. Statistical analysis was performed
using the Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
VSTM1 is expressed in peripheral blood
leukocytes
A previous study reported that VSTM1 expression was
higher in cells of the immune system (7). In the present study qPCR was
performed in order to quantify the expression levels of VSTM1. As
shown in Fig. 1A, VSTM1 was
primarily expressed in immune tissues and cells, including bone
marrow and peripheral blood leukocytes. In order to further
investigate the expression profile of VSTM1 in leukocytes, RT-qPCR
was performed on PBMCs and granulocytes of ten healthy donors. The
results showed that VSTM1 was detected in each of the samples
(Fig. 1B), and according to the
molecular size of the spliced variants, it was found that VSTM1-v1
was the predominantly expressed form. PBLs were then separated from
monocytes of the PBMCs in samples from a further three individuals
(numbered 1–3) and qPCR was performed in order to quantify the
expression levels of VSTM1 in each cell population. As shown in
Fig. 1C, VSTM1 was highly
expressed in granulocytes of each sample; however, the expression
levels of VSTM1 in monocytes and PBL were variable. These results
were not consistent with a previous study by Steevels et al
(6), in which the monoclonal
antibody 1A5 was used to detect VSTM1 expression. This previous
study reported that VSTM1 was expressed exclusively by myeloid
cells and was absent in lymphoid cells. In order to confirm the
existence of VSTM1 in PBLs in the present study, flow cytometric
and western blot analyses were performed at the protein level with
samples from a further nine donors (numbered 4–12). These results
revealed a considerable individual difference in expression levels
of VSTM1 among donors. Flow cytometric analysis demonstrated that
the proportion of surface VSTM1-v1-positive cells in PBL varied
from 4.05 to 50.05% (Fig. 1D). The
representative data from three donors (4, 6 and 11) are shown in
Fig. 1E and F. In samples 4 and
11, surface VSTM1-v1 expression was detected with the positive
percentages of 50.05% and 21.72% respectively; however, in sample
6, VSTM1-v1 was detected in markedly fewer cells (6.78%) (Fig. 1E). Consistent results were obtained
from representative western blots, which demonstrated a ~60-kDa
band of VSTM1 in PBL of donors 4 and 11, which was, however, not
observed in sample 6. This band was identical to that of the band
observed in VSTM1 overexpressing Jurkat cells; however, the pattern
of bands (~55, ~40 and ~37 kDa) in granulocytes was markedly
different from that in PBLs. In addition, these results were not
comparable to those of a previous study in VSTM1-overexpressing
HEK293T cells, which reported bands at ~55 and ~45 kDa (21). This therefore indicated that VSTM1
was diversely modified in cells of different origin.
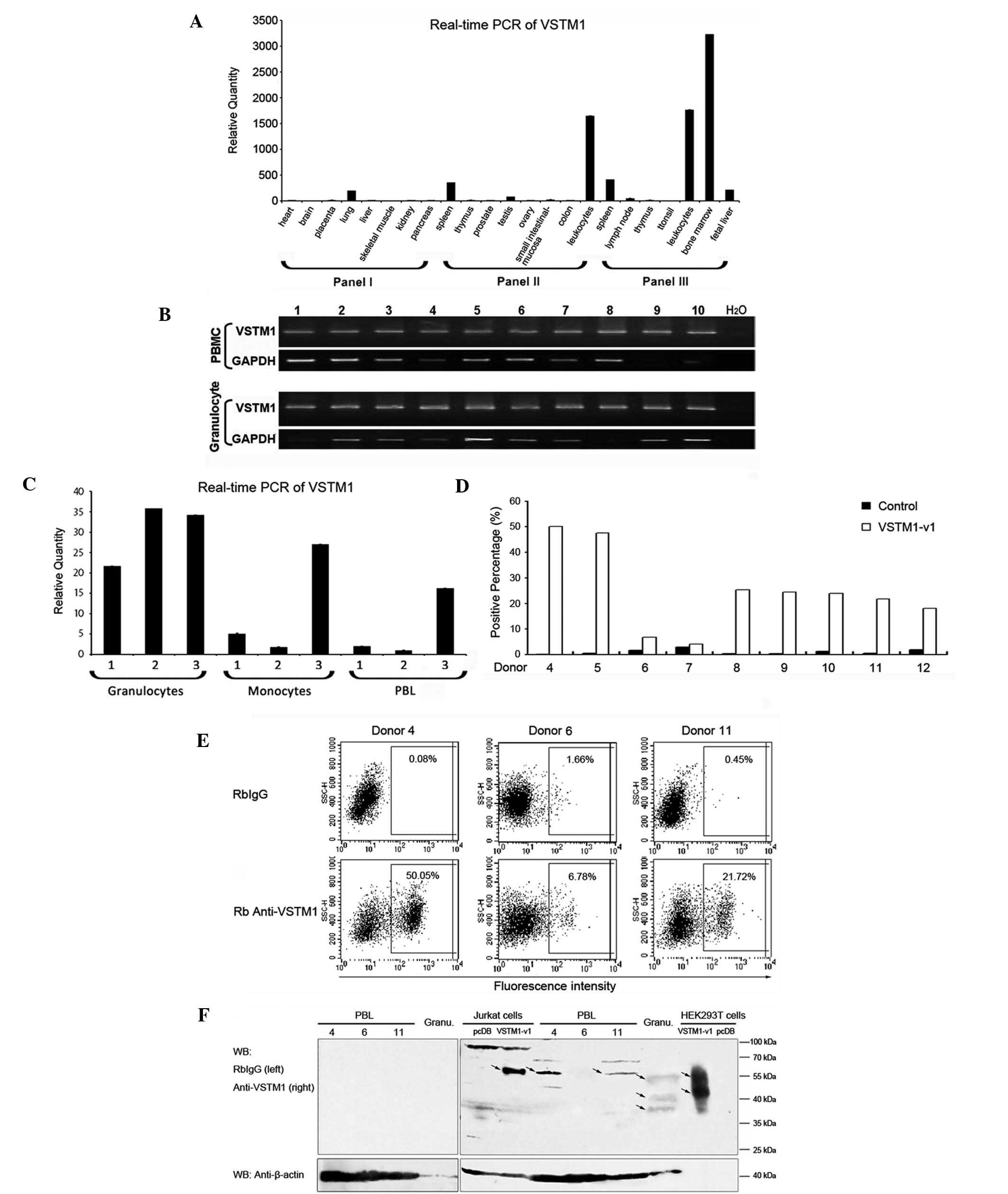 | Figure 1Expression profile of VSTM1. (A) qPCR
analysis of VSTM1 expression in numerous tissue type using human
MTC™ panels (I and II; nos. 636742 and 636743, respectively) and
human immune system MTC™ panel (III; no. 636748). Expression levels
of VSTM1 were measured relative to levels in the kidneys. (B)
RT-qPCR of VSTM1 in peripheral blood mononuclear cells and
granulocytes from ten healthy donors. GAPDH was used as the
internal control. (C) VSTM1 expression in granulocytes, monocytes
and PBLs from three individuals (1–3) was
analyzed using qPCR. GAPDH was used as the internal control and the
expression levels of VSTM1 were measured relative to those of PBLs
from donor two. (D) Flow cytometric analysis of surface VSTM1-v1
expression in PBLs from nine donors (4–12).
Quadrants were set relative to RbIgG stainings and positive
percentages of VSTM1-v1-expressing cells were counted. (E) Flow
cytometric analysis of PBLs of three donors (4, 6 and 11). Images
are representative results of the nine independent experiments
shown in (D). (F) Western blot analysis of VSTM1-v1 expression in
PBLs from donors 4,6 and 11. VSTM1-v1-transfected Jurkat and
HEK293T cells and granulocytes were examined as positive controls.
Loading volumes of lysates were: PBL, 150 μg; granulocyte, 150 μg;
Jurkat cells, 100 μg; and HEK293T, 1 μg. RbIgG was used as the
control antibody and β-actin was used as the internal control.
Arrows indicate specific bands of VSTM1. Values are presented as
the mean ± standard deviation. VSTM1, V-set and transmembrane
domain-containing 1; qPCR, quantitative polymerase chain reaction;
MTC, mutiple tissue complementary DNA; RT-qPCR, reverse
transcription-qPCR; VSTM1-v1, VSTM1 spliced variant 1; PBL,
peripheral blood lymphocyte; HEK293T, human embryonic kidney 293T;
RbIgG, normal rabbit immunoglobulin G. |
VSTM1 is silenced following promoter
methylation in numerous hematopoietic tumor cell lines
VSTM1 was found to be broadly expressed in normal
human PBLs; therefore, RT-qPCR was performed in order to further
investigate whether VSTM1 was also expressed in hematopoietic tumor
cell lines. The results showed that VSTM1 was silenced in the
majority of the examined cell lines (Fig. 2A). The aberrant promoter CpG
methylation was previously reported to be associated with gene
silencing (16); therefore, in the
present study, the VSTM1 promoter was screened for CpG
islands using the CpG Island Searcher. The results indicated that
the VSTM1 promoter contained a typical CpG island (Fig. 2B). VSTM1 promoter
methylation was then analyzed using MSP and it was demonstrated
that the promoters of VSTM1-silenced cell lines were
methylated to varying degrees (Fig.
2C). This therefore indicated that there was an association
between the loss of VSTM1 and CpG hypermethylation in the promoter
region.
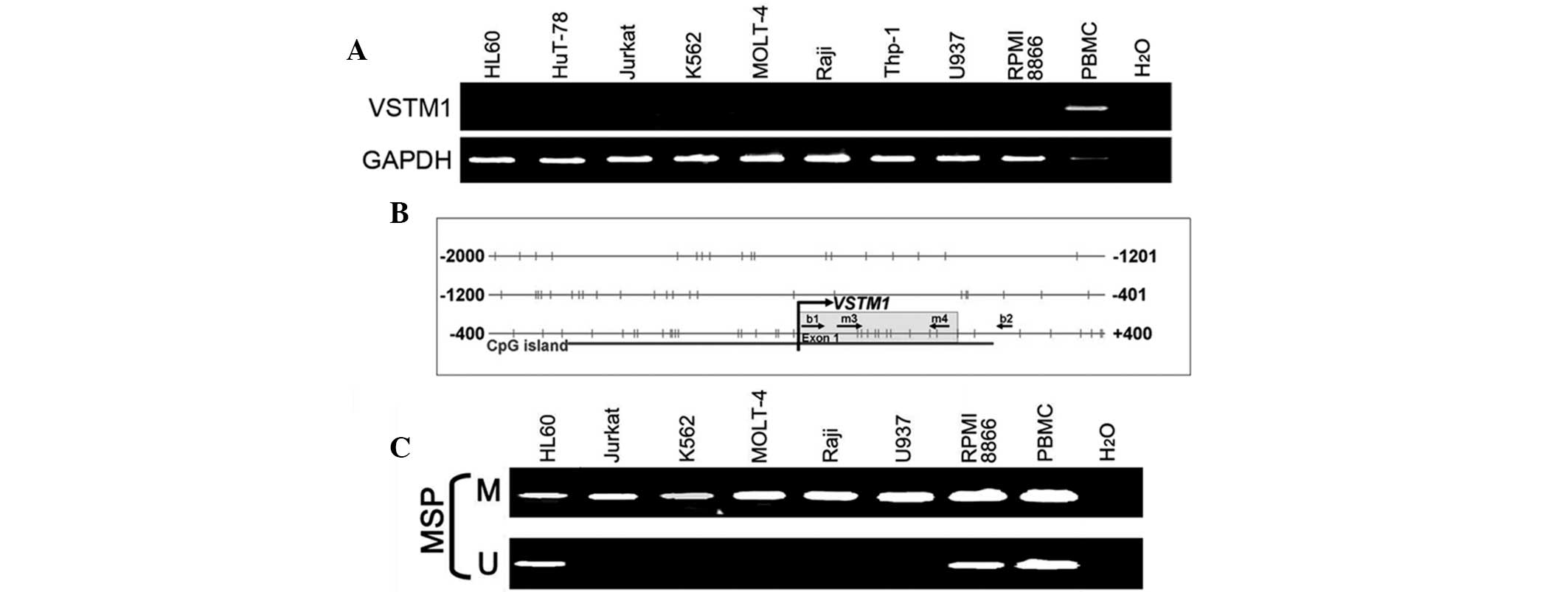 | Figure 2VSTM1 is silenced in numerous
hematopoietic tumor cell lines with promoter methylation. (A)
RT-PCR of VSTM1 in hematopoietic tumor cell lines. PBMCs were used
as a positive control. (B) A CpG island covers the promoter and
exon 1 of VSTM1. Horizontal bars, CpG sites; primers for
methylation analysis, MSP primers (m3/u3 and m4/u4) and bisulfite
genomic sequencing primers (b1 and b2) are indicated; curved arrow,
transcription start site. (C) MSP analysis of VSTM1 showed
complete methylation of the sites where the MSP primers were
located in Jurkat, K562, MOLT4, Raji and U937 cells and partial
methylation in HL60 and RPMI 8866 cells, as well as in PBMCs. M,
methylated; U, unmethylated; VSTM1, V-set and transmembrane
domain-containing 1; RT-PCR, reverse transcription polymerase chain
reaction; PBMC, peripheral blood mononuclear cell; MSP,
methylation-specific PCR; CpG, cytosine-phosphate-guanine. |
Expression of VSTM1 was restored
following pharmacological demethylation
Promoter hypermethylation was performed in Jurkat
cells in order to silence VSTM1; cells were then treated
with the methyltransferase inhibitor 5-aza-2′-deoxycytidine alone
or combination with trichostatin A, a histone deacetylase
inhibitor. This treatment was shown to restore mRNA and protein
expression levels of VSTM1 (Fig. 3A
and B); in addition, MSP analysis demonstrated that this
treatment resulted in promoter demethylation (Fig. 3C) and BGS revealed specific
demethylation of individual CpG sites (Fig. 3D). These results indicated that
VSTM1 silencing in Jurkat cells was due to epigenetic
regulation.
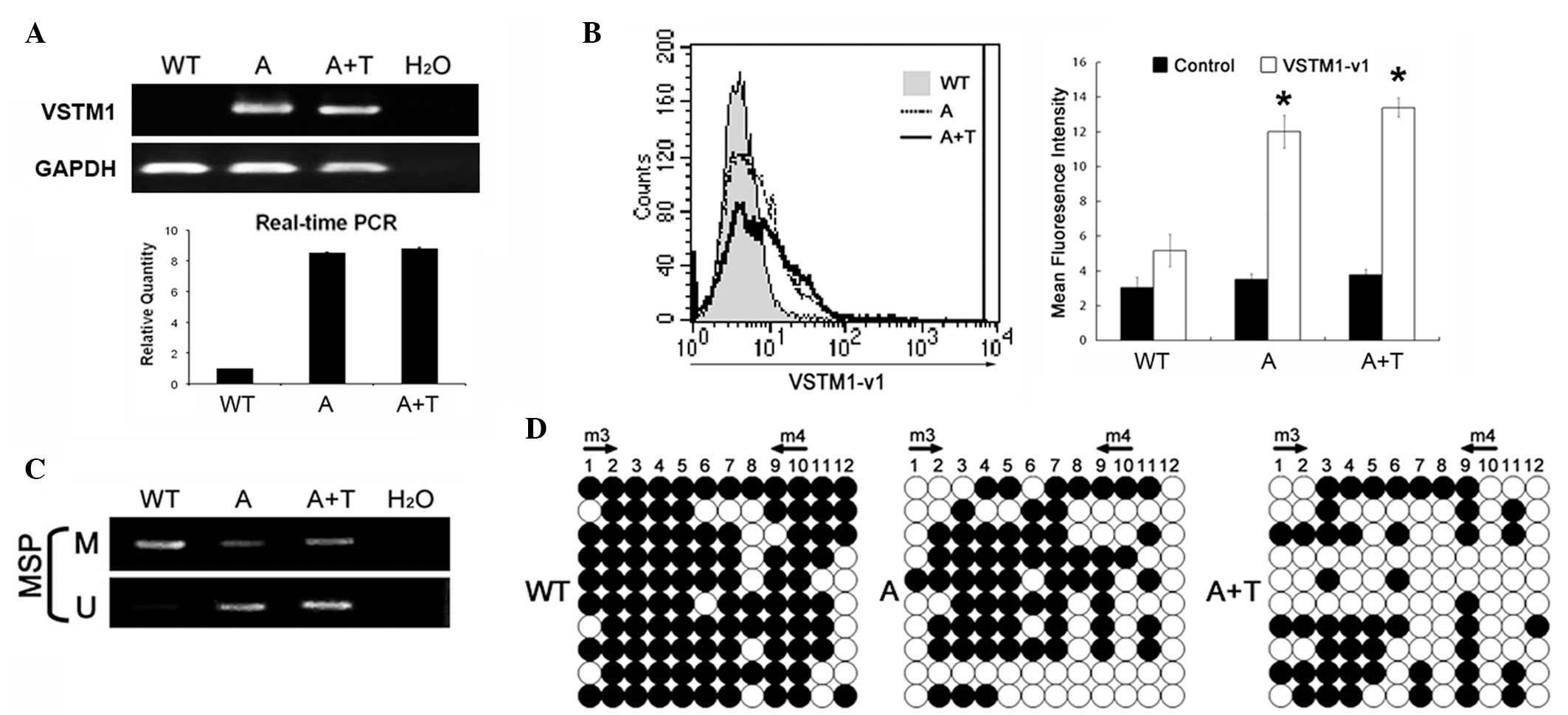 | Figure 3Pharmacological demethylation
restores VSTM1 expression in Jurkat cells. (A) Semiquantitative
RT-PCR and qPCR analyses using WT Jurkat cells and Jurkat cells
treated with the methyltransferase inhibitor A alone or in
combination with the histone deacetylase inhibitor T. GAPDH was
used as an internal control. (B) Flow cytometric analysis confirmed
the restoration of surface VSTM1-v1 expression in Jurkat cells
following pharmacological demethylation. Representative results and
the mean fluorescence intensity of VSTM1 expression for three
independent experiments. Normal rabbit immunoglobulin G was used
for control staining. Values are presented as the mean ± standard
error of the mean. *P<0.05 vs. WT cells. (C) MSP
verified the pharmacological demethylation of the VSTM1
promoter. (D) Detailed bisulfite genomic sequencing analysis of the
VSTM1 promoter. Circles, CpG sites analyzed; row of circles,
an individual promoter allele that was cloned, randomly selected
and sequenced; filled circle, methylated CpG site; open circle,
unmethylated CpG site. A, 5-aza-2′-deoxycytidine; T, trichostatin
A; WT, wild-type; VSTM1, V-set and transmembrane domain-containing
1; RT-PCR, reverse transcription polymerase chain reaction; qPCR,
quantitative PCR; VSTM1-v1, VSTM1 spliced variant 1; MSP,
methylation-specific PCR; CpG, cytosine-phosphate-guanine. |
Overexpression of VSTM1-v1 inhibits
Jurkat cell growth
Results of the present study showed that
VSTM1 was expressed in normal human leukocytes and silenced
in hematopoietic tumor cells; therefore, subsequent experiments
were performed in order to determine effects of exogenous VSTM1-v1
expression on the growth of Jurkat cells following VSTM1
promoter hypermethylation and gene silencing. As shown in Fig. 1E, VSTM1-v1 was successfully
expressed in Jurkat cells following transfection. CCK-8 assays
revealed that VSTM1-v1 inhibited the growth of Jurkat cells
compared with that of vector-transfected control cells (Fig. 4A). VSTM1-v1 is a type I
transmembrane protein, which contains two cytoplasmic ITIMs.
Therefore, it was hypothesized that ligand interaction with the
extracellular region may induce the transmission of an inhibitory
signal into the cell; however, ligands for VSTM1-v1 have not yet
been identified. Therefore, in the present study, the VSTM1
antibody was used as an agonist to bind to the extracellular region
of VSTM1. The influence of rabbit anti-VSTM1 pAb on the functional
cross-linking of VSTM1 was determined using NFκB-firefly luciferase
reporter assays in Jurkat cells with the construct expressing the
VSTM1-v1-CD3ζ chimera (25).
Incubation with the anti-VSTM1 pAb instead of normal rabbit IgG and
in the presence of anti-CD28 resulted in NFκB activation and
consequently increased firefly luciferase activity. In addition,
firefly luciferase activity was enhanced following increased doses
of the anti-VSTM1 pAb (Fig. 4B),
which indicated that this antibody was capable of cross-linking
VSTM1-v1. VSTM1-v1- or vector-transfected Jurkat cells were then
treated with the anti-VSTM1 pAb or normal rabbit IgG (Fig. 4C). A CCK8 assay showed that
incubation with the anti-VSTM1 pAb further augmented the inhibitory
effect of VSTM1-v1 on Jurkat cell growth.
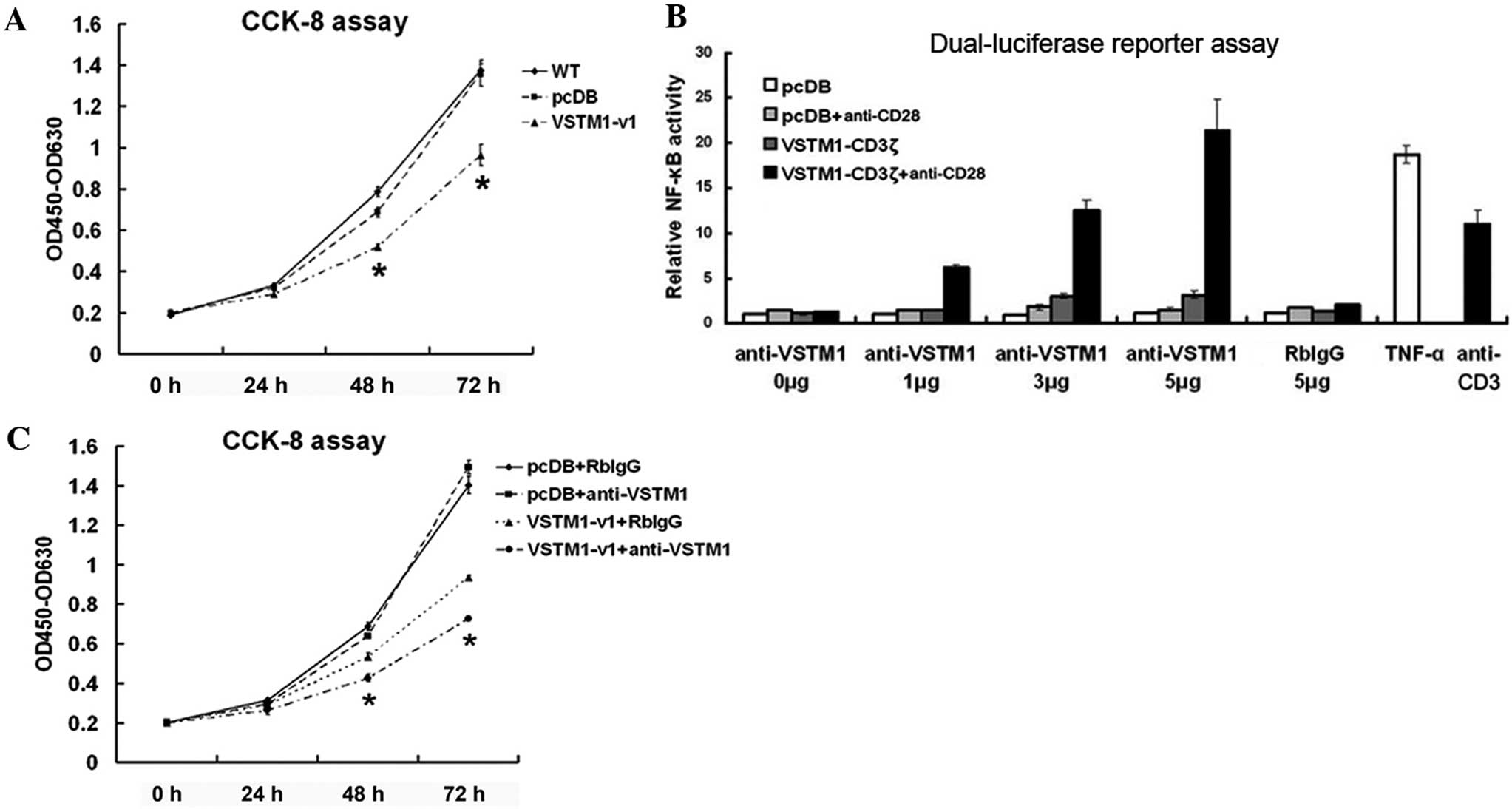 | Figure 4Overexpression of VSTM1-v1 inhibits
Jurkat cell growth. (A) Representative results of ≥five independent
CCK-8 assays in WT, pcDB- and VSTM1-v1-transfected cells. Values
are presented as the difference between absorbance at 450 and 630
nm (OD450-OD630). (B) NFκB-firefly luciferase reporter assays in
Jurkat cells demonstrated the capacity of rabbit anti-VSTM1 pAb to
cross-link VSTM1-v1. Representative results from three individual
experiments are shown. Relative NFκB activity was the mean of the
ratio of firefly to renilla luciferase activities ± SD. TNF-α and
plate-bound anti-CD3 were used as positive controls for activating
NFκB. (C) CCK-8 assays of VSTM1-v1- and pcDB-transfected Jurkat
cells following interaction with anti-VSTM1 pAb. RbIgG control was
used as the internal control. Anti-VSTM1 pAb was shown to further
inhibit the growth of VSTM1-v1-transfected cells. Representative
result of three independent experiments. Values are presented as
the mean ± SD. *P<0.05 vs. controls at each
time-point. VSTM1, V-set and transmembrane domain-containing 1;
VSTM1-v1, VSTM1 spliced variant 1; CCK-8, cell counting kit 8; WT,
wild-type; NFκB, nuclear factor kappa-light-chain-enhancer of
activated B cells; pAb, polyclonal antibody; TNF-α, tumor necrosis
factor-α; pcDB, pcDB-VSTM1-v1-CD3ζ reporter construct; RbIgG,
normal rabbit immunoglobulin G; SD, standard deviation. |
Discussion
VSTM1 is a potential leukocyte
differentiation antigen gene that was previously identified using
immunogenomics. VSTM1 encodes numerous splicing isoforms, of
which VSTM1-v1 is the most predominant and widely expressed form
(7). VSTM1-v1 is a type I
transmembrane molecule that contains an extracellular IgV-like
domain and two ITIMs in its cytoplasmic region. VSTM1 was
previously reported to be expressed exclusively in myeloid cells
and absent in lymphoid cells as indicated by detection using the
monoclonal antibody 1A5 (6).
However, in the present study, VSTM1 mRNA expression was
observed not only in granulocytes and monocytes but also in PBLs
from several healthy donors using RT-qPCR; in addition, there were
considerable differences in VSTM1 expression levels among
individuals. Flow cytometric analysis using rabbit anti-VSTM1 pAb
confirmed the expression patterns at the protein level. However,
the results of the western blot analysis may provide a possible
explanation for the contradictory results from the present and
previous study about the expression of VSTM1 in PBLs. The present
study determined that the molecular size of VSTM1 detected in PBLs
was consistent with that of the overexpressed VSTM1-v1 in Jurkat
cells, which was different from that of endogenous VSTM1 in
granulocytes and overexpressed VSTM1-v1 in HEK293T cells (21). This indicated that VSTM1 may be
diversely modified in cells of different origins, with different
molecular sizes and conformations; therefore, the application of
monoclonal antibodies for the detection of this molecule is likely
to be limited as a monoclonal antibody only recognizes a single
epitope of a molecule (26).
VSTM1 is broadly expressed in normal human PBLs;
however, it was found to be silenced in multiple leukemia cell
lines. VSTM1 is located on chromosome 19q13.4, a genomic
region widely reported to be prone to genetic and epigenetic
modifications in numerous hematopoietic malignancies (8–12).
In the present study, a bioinformatic search tool was used, which
indicated that a typical CpG island was present in the promoter of
VSTM1. RT-PCR and MSP analyses revealed that there was a
link between the expression of silenced VSTM1 in
hematopoietic tumor cell lines and CpG hypermethylation in the
promoter region. This therefore confirmed that epigenetic
modifications were an important mechanism in the regulation of
VSTM1, which may be of use as a promising novel diagnostic
and prognostic genetic marker for hematopoietic malignancies
(27). However, the involvement of
genetic alterations in this process remains to be elucidated.
Previous studies have identified numerous inhibitory
receptors containing ITIMs, which prevent excessive immune
reactions and autoimmunity, thereby maintaining the balance of the
immune system (28–30). These studies may therefore indicate
the function of the two ITIMs in the cytoplasmic tail of VSTM1-v1.
Following interaction with the extracellular portion of the
receptor by ligands, cytoplasmic ITIMs bind to the sarcoma homology
2 domain of phosphatases (SHP), resulting in the inactivation of
different kinases and downregulation of cell activation (28–30).
ITIMs in VSTM1-v1 were also reported to recruit SHP-1 and SHP-2,
which inhibited the fragment, crystallizable epsilon receptor
I-mediated signaling involved in reactive oxygen species production
and the microbicidal activity of phagocytes (6,31).
Further studies are required to determine whether the inhibitory
effect of VSTM1-v1 on cell growth may also be achieved via SHP
recruitment through its cytoplasmic ITIMs.
In conclusion, the results of the present study
demonstrated that VSTM1 was expressed in myeloid cells as well as
lymphocytes, which were of different molecular size and exhibited
considerable variation in their expression levels among donors.
VSTM1 was found to be silenced in numerous hematopoietic
malignancy cell lines, with CpG hypermethylation within the
promoter; however, pharmacological demethylation in Jurkat cells
was shown to be able to restore VSTM1 expression. In addition,
overexpression of VSTM1-v1 in Jurkat cells inhibited cell growth,
which was further restricted following the interaction with the
extracellular portion of VSTM1-v1 using anti-VSTM1 antibodies.
These results therefore elucidated the expression regulation and
functional roles of VSTM1 in the inhibition of hematopoietic
tumor cells, which may contribute towards the development of novel
diagnostic and treatment strategies for hematopoietic
malignancies.
Acknowledgements
The present study was supported by grants from the
Specialized Research Fund for the Doctoral Program of Higher
Education of China (no. 20110001110016) and the National Natural
Science Foundation of China (no. 31400736).
References
|
1
|
Zola H: Human leukocyte differentiation
antigens as therapeutic targets: the CD molecules and CD
antibodies. Expert Opin Biol Ther. 1:375–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zola H and Swart B: The human leucocyte
differentiation antigens (HLDA) workshops: the evolving role of
antibodies in research, diagnosis and therapy. Cell Res.
15:691–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bisig B, Gaulard P and de Leval L: New
biomarkers in T-cell lymphomas. Best Pract Res Clin Haematol.
25:13–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang PY, Best OG, Almazi JG, et al: Cell
surface phenotype profiles distinguish stable and progressive
chronic lymphocytic leukaemia. Leuk Lymphoma. 55:2085–2092. 2014.
View Article : Google Scholar
|
|
5
|
Walter RB, Appelbaum FR, Estey EH and
Bernstein ID: Acute myeloid leukemia stem cells and CD33-targeted
immunotherapy. Blood. 119:6198–6208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steevels TA, Lebbink RJ, Westerlaken GH,
Coffer PJ and Meyaard L: Signal inhibitory receptor on leukocytes-1
is a novel functional inhibitory immune receptor expressed on human
phagocytes. J Immunol. 184:4741–4748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo X, Zhang Y, Wang P, et al: VSTM1-v2, a
novel soluble glycoprotein, promotes the differentiation and
activation of Th17 cells. Cell Immunol. 278:136–142. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brambillasca F, Mosna G, Colombo M, et al:
Identification of a novel molecular partner of the E2A gene in
childhood leukemia. Leukemia. 13:369–375. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Di Bernardo MC, Crowther-Swanepoel D,
Broderick P, et al: A genome-wide association study identifies six
susceptibility loci for chronic lymphocytic leukemia. Nat Genet.
40:1204–1210. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaudhary K, Deb S, Moniaux N, Ponnusamy
MP and Batra SK: Human RNA polymerase II-associated factor complex:
dysregulation in cancer. Oncogene. 26:7499–7507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fuchs O, Provaznikova D, Kocova M, et al:
CEBPA polymorphisms and mutations in patients with acute myeloid
leukemia, myelodysplastic syndrome, multiple myeloma and
non-Hodgkin’s lymphoma. Blood Cells Mol Dis. 40:401–405. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abdool A, Donahue AC, Wohlgemuth JG and
Yeh CH: Detection, analysis and clinical validation of chromosomal
aberrations by multiplex ligation-dependent probe amplification in
chronic leukemia. PLoS One. 5:e154072010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kunitz A, Wolter M, van den Boom J, et al:
DNA hypermethylation and aberrant expression of the EMP3 gene at
19q13.3 in human gliomas. Brain Pathol. 17:363–370. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Bhat I, Zeng M, et al: Human
kallikrein 10, a predictive marker for breast cancer. Biol Chem.
387:715–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roman-Gomez J, Jimenez-Velasco A, Agirre
X, et al: The normal epithelial cell-specific 1 (NES1) gene, a
candidate tumor suppressor gene on chromosome 19q13.3–4, is
downregulated by hypermethylation in acute lymphoblastic leukemia.
Leukemia. 18:362–365. 2004. View Article : Google Scholar
|
|
16
|
Rüter B, Wijermans PW and Lübbert M: DNA
methylation as a therapeutic target in hematologic disorders:
recent results in older patients with myelodysplasia and acute
myeloid leukemia. Int J Hematol. 80:128–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Claus R and Lübbert M: Epigenetic targets
in hematopoietic malignancies. Oncogene. 22:6489–6496. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Xiao XQ, Jiang YF, et al: DNA
demethylation in PD-1 gene promoter induced by 5-azacytidine
activates PD-1 expression on Molt-4 cells. Cell Immunol.
271:450–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gattazzo C, Teramo A, Miorin M, et al:
Lack of expression of inhibitory KIR3DL1 receptor in patients with
natural killer cell-type lymphoproliferative disease of granular
lymphocytes. Haematologica. 95:1722–1729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li T, Zhong J, Chen Y, et al: Expression
of chemokine-like factor 1 is upregulated during T lymphocyte
activation. Life Sci. 79:519–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Wang W, Chen Y and Han W:
Preparation and characterization of monoclonal antibodies against
VSTM1. Monoclon Antib Immunodiagn Immunother. 32:283–289. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shao L, Cui Y, Li H, et al: CMTM5 exhibits
tumor suppressor activities and is frequently silenced by
methylation in carcinoma cell lines. Clin Cancer Res. 13:5756–5762.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Li J, Cui Y, et al: CMTM3, located
at the critical tumor suppressor locus 16q22.1, is silenced by CpG
methylation in carcinomas and inhibits tumor cell growth through
inducing apoptosis. Cancer Res. 69:5194–5201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li T, Guo XH, Wang PZ, Song QS, Ma DL and
Han WL: Preparation, purification, and characterization of the
polyclonal antibody against human VSTM1. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 28:1291–1294. 2012.(In Chinese). PubMed/NCBI
|
|
25
|
Lebbink RJ, de Ruiter T, Adelmeijer J, et
al: Collagens are functional, high affinity ligands for the
inhibitory immune receptor LAIR-1. J Exp Med. 203:1419–1425. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kammerer R1, Hahn S, Singer BB, Luo JS and
von Kleist S: Biliary glycoprotein (CD66a), a cell adhesion
molecule of the immunoglobulin superfamily, on human lymphocytes:
structure, expression and involvement in T cell activation. Eur J
Immunol. 28:3664–3674. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ushijima T: Detection and interpretation
of altered methylation patterns in cancer cells. Nat Rev Cancer.
5:223–231. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ravetch JV and Lanier LL: Immune
inhibitory receptors. Science. 290:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bléry M, Olcese L and Vivier E: Early
signaling via inhibitory and activating NK receptors. Hum Immunol.
61:51–64. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daëron M, Jaeger S, Du Pasquier L and
Vivier E: Immunoreceptor tyrosine-based inhibition motifs: a quest
in the past and future. Immunol Rev. 224:11–43. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Steevels TA, van Avondt K, Westerlaken GH,
et al: Signal inhibitory receptor on leukocytes-1 (SIRL-1)
negatively regulates the oxidative burst in human phagocytes. Eur J
Immunol. 43:1297–1308. 2013. View Article : Google Scholar : PubMed/NCBI
|


















