Introduction
Ruditapes philippinarum, a member of the
Veneridae family, is predominantly found along the coasts of the
Philippines, Japan and China. It is an intertidal bivalve, which
preferentially inhabits the inner bay. It has a high commercial
value and is also an important aquaculture species in intertidal
zones. RPOI-1 (Ruditapes philippinarum oligopeptide), is a
water-soluble tetrapeptide that can be extracted from Ruditapes
philippinarum by means of enzymolysis.
A number of studies have demonstrated that certain
products from marine sources, including Sepia ink,
Dolabella auricularia, Mactromeris polynyma and
Halichondria okadai, have significant anticancer activity
(1–4). Sepia ink oligopeptide (SIO) is
an antitumor tripeptide that was first isolated from Sepia
esculenta using enzymolysis. SIO was shown to significantly
inhibit the proliferation of DU-145, PC-3 and LNCaP human prostate
cancer (PCa) cells in a time- and dose-dependent manner (5). SIO has been shown to induce apoptosis
in prostate cancer cell lines via activation of caspase-3 and
elevation of the Bax/Bcl-2 ratio (5).
PCa is the most common type of malignancy worldwide
and second only to lung cancer as the leading cause of
cancer-related mortality (6).
During the early stages of PCa, growth of prostatic epithelial
cells is androgen-dependent. Therefore, hormone therapy has been an
initial option with which to treat patients with PCa. However,
tumor cells ultimately become androgen-independent, necessitating
the development of different forms of treatment (7). Cytotoxic chemotherapy aims to kill
cancer cells, but is not a targeted treatment, and therefore also
affects healthy cells. Dox (doxorubicin) has potent and
broad-spectrum therapeutic activity against PCa. Despite its
effectiveness, the use of Dox is limited, as concentrations
required to kill cancerous cells cannot be employed without causing
systemic toxicity (8).
Numerous interventions have been tried in order to
remove cancerous tissues, including surgery and chemotherapy,
without universal success (9). In
addition, many cancer drugs exhibit a relatively short clinical
life span and become ineffective, due to the development of
resistance. Furthermore, a number of potent drugs frequently cause
serious side effects. Therefore, there is an urgent requirement to
identify and develop novel anticancer agents that are safe and
effective (10). The present study
evaluated the anticancer activity and mechanisms of action of
RPOI-1 against prostate cancer in a Du-145 PCa cell line. The
objectives of the current study were to investigate the
antiproliferative effects of RPOI-1 against DU-145 cells and to
clarify whether apoptosis and cell-cycle arrest were involved in
any observed anticancer activity.
Materials and methods
Materials
Ruditapes philippinarum was collected from
the Zhoushan coastal area. DU-145 human prostate cancer cells were
obtained from the Cell Bank of the Chinese Academy of Science
(Shanghai, China). The ultrafiltrate was applied to a column
saturated in diethyaminoethyl (DEAE)-sepharose fast flow (FF;
Amersham Pharmacia Biotech, Shanghai, China). The Ruditapes
philippinarum peptide fraction was further purified using
reverse-phase high performance liquid chromatography (HPLC) on a
Primesphere C18 column (Phenomenex Co., Ltd., Shanghai, China). The
degree of inhibition of proliferation of DU-145 cells was detected
by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay (Hangzhou Haotian Biotechnology Co., Ltd., Hangzhou,
China). F-12 medium, fetal bovine serum (FBS) and an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit were
purchased from BD Biosciences (Franklin Lakes, NJ, USA). Other
reagents were of analytical grade and produced in China.
Enzymatic hydrolysis
Ruditapes philippinarum tissues were
homogenized and digested with protease at 45°C using trypsin [300
U/g, pH 7.0; solid-liquid ratio (w/v), 1:3] for 6 h. Following
enzymatic hydrolysis, the mixture was immediately heated to 95°C
for 15 min in order to inactivate the enzyme. The hydrolysates were
clarified by centrifugation at 8580 × g for 20 min to remove the
unhydrolyzed residue.
Isolation and purification of the
anticancer peptide
Ultrafiltration
The Ruditapes philippinarum hydrolysate (RH)
filtrate was filtered through a 3 kDa molecular weight cut-off
membrane (Amicon Ultra-15; Millipore, Billerica, MA, USA). It was
then fractionated into two ranges of molecular weight (RH1>3 kDa
and RH2≤3 kDa). The respective permeates were lyophilized and their
antitumor activity was measured using an MTT assay.
DEAE-sepharose FF ion exchange
chromatography
The lyophilized fraction exhibiting the highest
antitumor activity was dissolved in 0.05 mmol/l Tris/HCl buffer (pH
7.4) and applied to a DEAE-sepharose FF column (115×50 mm), which
had previously been equilibrated with the same buffer. The column
was eluted with a linear gradient of NaCl concentrations in
Tris/HCl buffer from 0 to 1.0 mol/l. Fractions of 4 ml were
collected at a flow rate of 2 ml/min. Chromatography was performed
using an ÄKTA-explorer system (GE Healthcare, Little Chalfont, UK)
and the elution peaks were monitored at 280 nm.
Reverse-phase (RP)-HPLC
The fraction exhibiting the highest anticancer
activity was further purified using reverse-phase HPLC on a
Primesphere C18 column (10×250 mm) with a linear gradient of
acetonitrile (0–50% for 20 min) containing 0.1% trifluoroacetic
acid at a flow rate of 1 ml/min. The absorbance of eluent was
monitored at 280 nm.
DU-145 cell culture
DU-145 cells were cultured in F-12 medium,
supplemented with 10% FBS (v/v), 2 mM glutamine, 100 IU/ml
penicillin and 100 IU/ml streptomycin. Cultures were maintained in
a humidified incubator at 37°C, with 5% CO2 and 95%
air.
Antiproliferative activity using an MTT
assay
The cytotoxicity of RPOI-1 against the DU-145 cells
was assessed using a colorimetric MTT assay. DU-145 cells were
seeded (1×105 cells/ml) together with various
concentrations of the RPOI-1 sample (10, 15, 20, 25 and 30 mg/ml)
and incubated for up to 24, 48 or 72 h prior to MTT treatment. A
stock solution of 50 μl of 2 mg/ml MTT in phosphate-buffered saline
(PBS) was added to each well to achieve a total reaction volume of
250 μl. Following 5 h incubation, the formazan crystals in each
well were dissolved in dimethyl sulfoxide. The quantity of purple
formazan was assessed by measuring the absorbance at 490 nm using
an enzyme-linked immunosorbent assay reader (Thermo Multiskan
Spectrum; Thermo Electron Corporation, Dreieich, Germany).
Morphological analyses
DU-145 cells in the exponential growth phase were
plated at 1×104 cells/well in 6-well plates. Following
24 h growth, cells were treated with RPOI-1 at 10, 20 or 30 mg/ml
for 24 h. At the end of the treatment, the effect of RPOI-1 on the
morphology of the DU-145 cells was assessed by the inverted and
phase-contrast microscope (Olympus Corporation, Tokyo, Japan) at
×200 magnification.
Annexin V-FITC/propidium iodide (PI)
double-staining analysis
Annexin V-FITC/PI double-staining analysis was used
to evaluate the induction of apoptosis in cancer cells by RPOI-1.
Exposed phosphatidylserine on the outside of apoptotic cells was
determined using the Annexin V-FITC Apoptosis kit. To detect the
early and late stages of apoptosis, as well as necrosis induced by
RPOI-1, the DU-145 cells were plated at a density of
1×105 cells/well in 6-well plates and incubated for 24 h
at 37°C in 5% CO2. Cells were harvested by
centrifugation at 4,000 × g for 7 ; they were then washed with
ice-cold PBS twice and stained with 10 μl PI and 5 μl Annexin
V-FITC at room temperature for 15 min in darkness. Annexin V-FITC
and PI emissions were detected in the FL1 and FL2 channels of a
FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA).
The distribution percentages of normal cells, necrotic cells, and
cells in the early and late stages of apoptosis were calculated by
Cell-Quest software (BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle analysis
Flow cytometric analyses were conducted in order to
determine the proportion of apoptotic sub-G1 hypodiploid cells.
DU-145 cells were placed in 6-well plates at a concentration of
1×105 cells/ml for 16 h and treated with 10 or 30 mg/ml
RPOI-1. Following incubation for 24 h, the cells were harvested, as
previously described, at the indicated times and fixed for 30 min
in 1 ml of 70% ethanol at 4°C. The cells were then washed twice
with PBS and incubated for 30 min in darkness in 1 ml PBS
containing 100 μg PI and 100 μg RNase A (Sigma, St. Louis, MO,
USA), at 37°C. Flow cytometric analysis was conducted using a FACS
Calibur flow cytometer (Becton Dickinson). Effects on the cell
cycle were determined by measuring changes in the percentage of
cell distribution at each phase of the cell cycle and were assessed
by histograms generated by the Cell Quest and Mod-Fit computer
programs (Verity Software House, Topsham, ME, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Significance was determined by an unpaired Student’s t-test using
SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results and Discussion
The lyophilized trypsin digestion was dissolved in
Tris/HCl buffer (0.05 mmol/l, pH 7.14) and loaded onto a
DEAE-Sepharose FF column with a linear gradient of NaCl (0.01
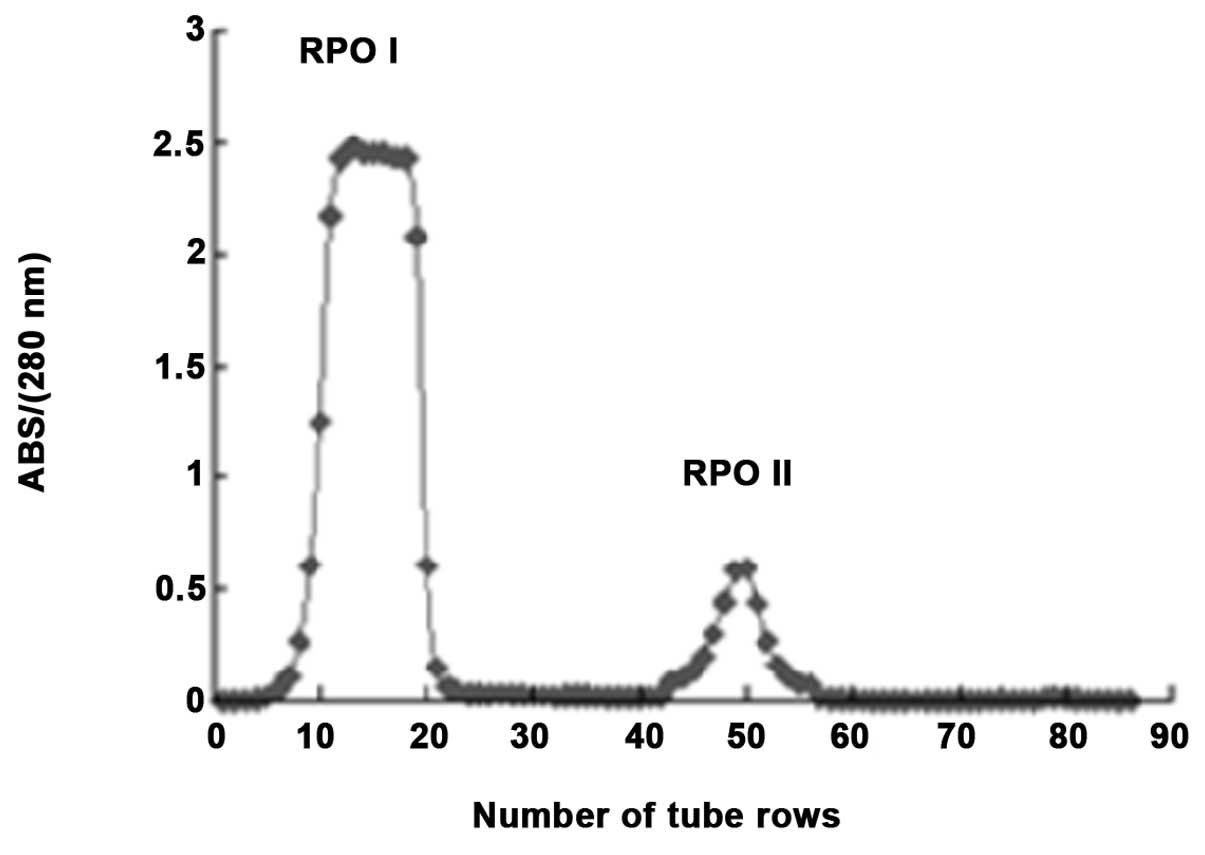
mol/l). As shown in Fig. 1, the
elution peaks were monitored at 280 nm, and two major fractions
(RPOI and RPOII) were identified. Due to the higher anticancer
activity of RPOI, this molecule was further separated using
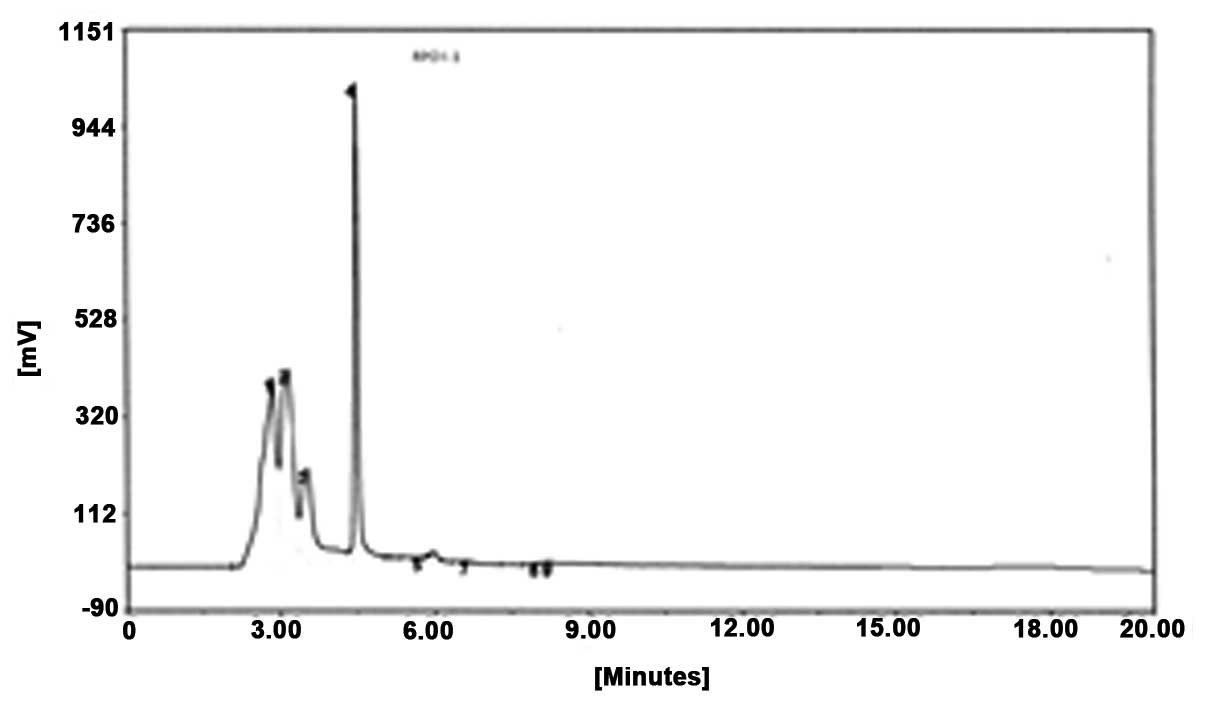
reverse-phase HPLC. As shown in Fig.
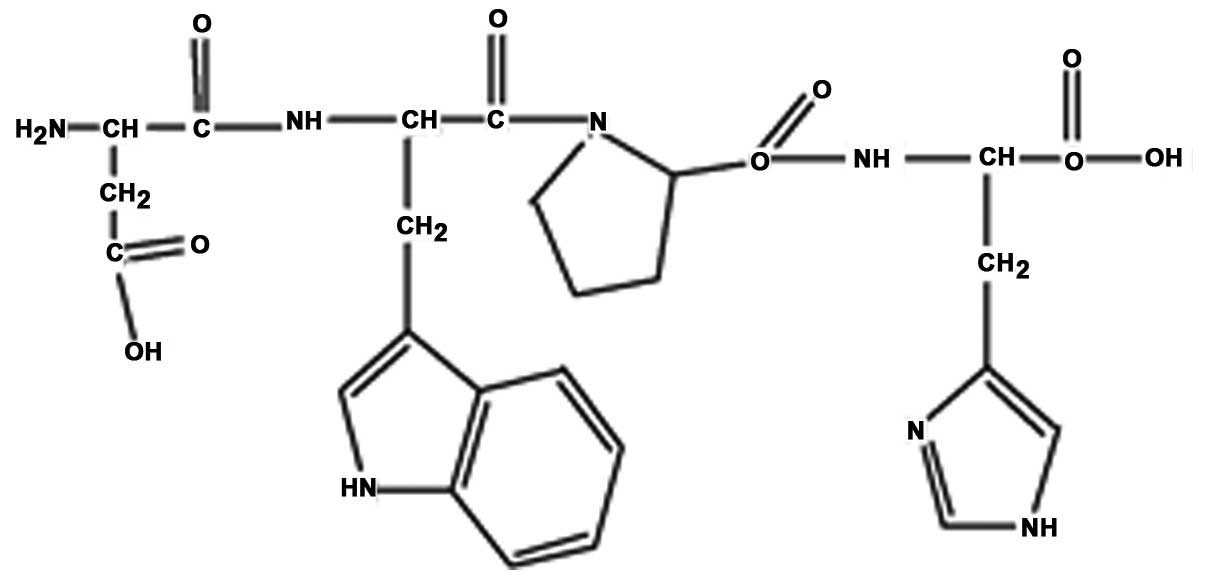
2, peak 4 was collected and termed RPOI-1. Its sequence was
identified as N-Asp-Trp-Pro-His, with a molecular mass of 607.6
kDa. The amino-acid backbone of RPOI-1 is shown in Fig. 3.
Evaluation of RPOI-1 for potential anticancer
activity on DU-145 cells was conducted by investigating its
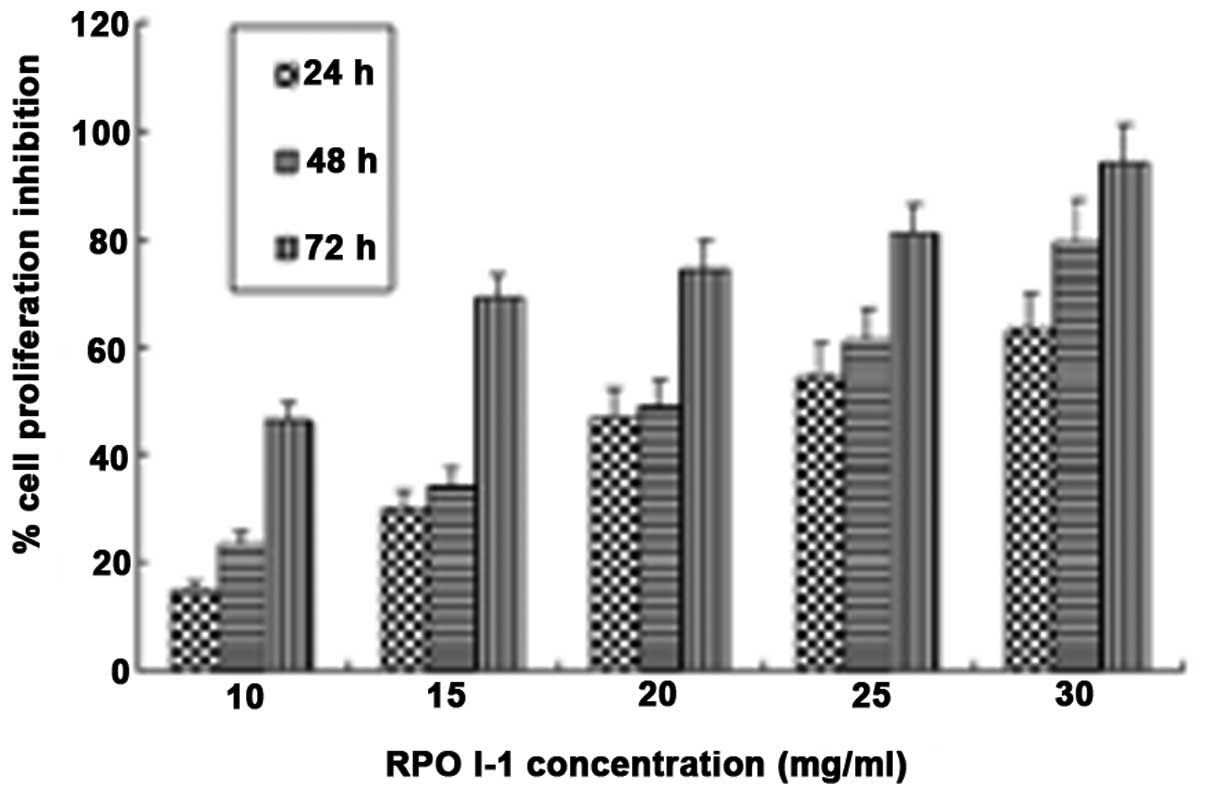
inhibitory effects on cell growth using an MTT assay. As shown in
Fig. 4, RPOI-1 inhibited DU-145
cell proliferation in a dose- and time-dependent manner.
Furthermore, RPOI-1 exhibited >90% cell proliferation inhibition
at a concentration of 30 mg/ml following 72 h treatment.
Morphological observation of apoptotic cells by
fluorescence microscopy using acridine orange (AO) staining is a
well-established biological assay for detecting apoptotic
cells.
Apoptosis, also termed programmed cell death, is
characterized by typical cellular morphology and biochemical
features, including cell shrinkage, cytoplasm vacuolization,
chromatin condensation, DNA fragmentation and ultimately cellular
breakdown into apoptotic bodies (11).
To clarify whether the cell death of RPOI-1 treated
cells occurred via induction of apoptosis, the presence of
apoptotic features in the treated cells was confirmed by double
AO/ethidium bromide staining.
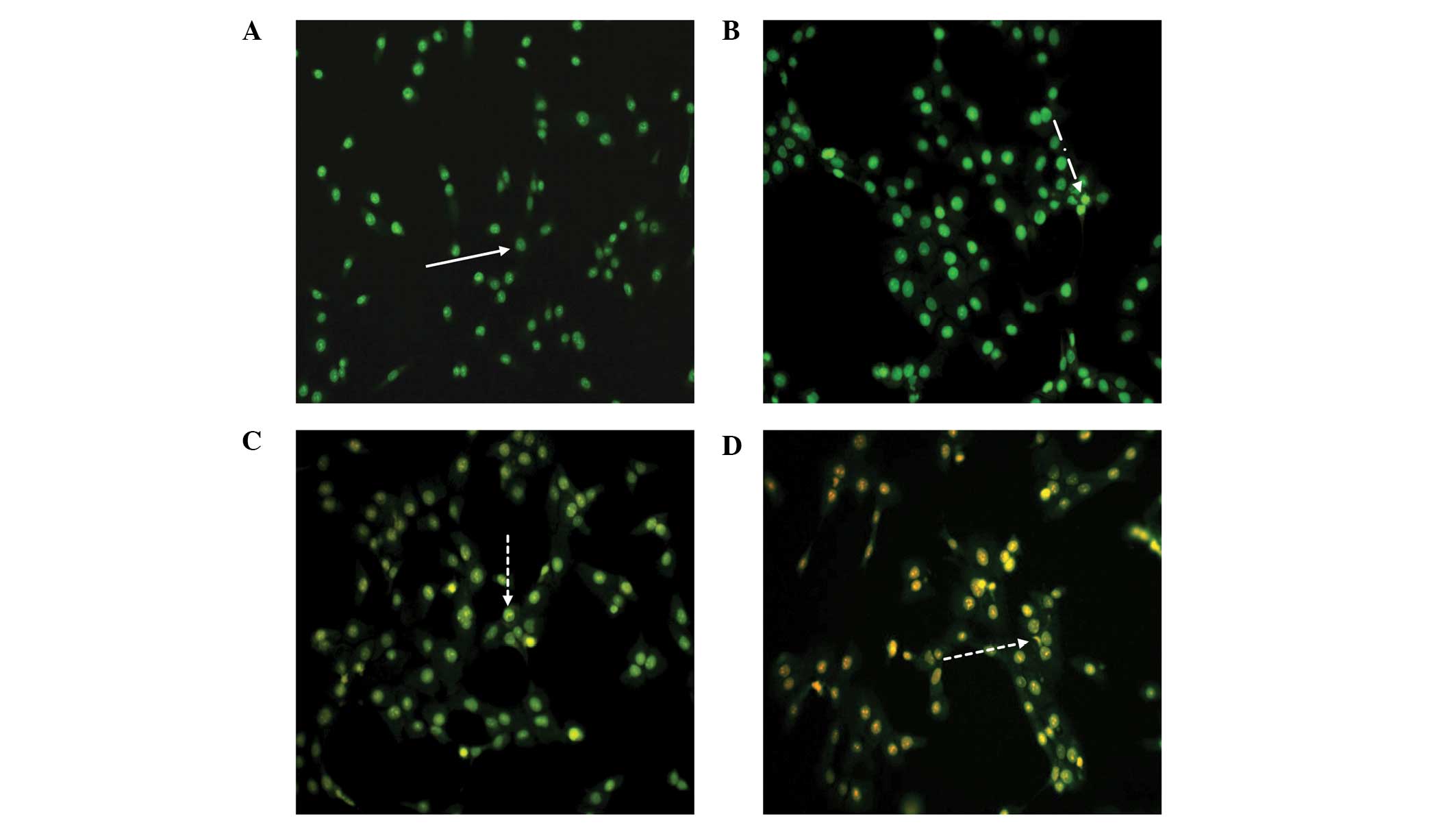
As a control, DU-145 cells were cultured in complete
media and stained with AO/EB (Fig.
5A). Viable cells appear homogeneous, with bright green nuclei
and an intact structure, as shown in Fig. 5A. As displayed in Fig. 5B, early apoptotic cells contained
green nuclei, but perinuclear chromatin condensation was also
visible as bright green patches or fragments. Fig. 5C and D show cells in later stages
of apoptosis, which contain orange-red nuclei with condensed or
fragmented chromatin, and necrotic cells, which contained a
homogeneous orange-red nuclei with an intact structure.
These changes indicated that RPOI-1 may induce
DU-145 cell apoptosis. More apoptotic cells in later stages of
apoptosis were observed with higher concentrations of RPOI-1.
In order to quantify the proportion of cells that
had undergone apoptosis, DU-145 cells were treated with RPOI-1 for
24 h, followed by simultaneous staining with Annexin V-FITC and PI
and analyzed by BD FACSCalibur flow cytometry. Annexin V-FITC/PI
double-staining analysis demonstrated that the treatment with
RPOI-1 decreased the number of healthy DU-145 cells in a
dose-dependent manner. As shown in Fig. 6, the number of apoptotic cells in
the early and late stages of apoptosis increased in a
dose-dependent manner from 3.01 to 13.40% and from 2.69 to 24.20%
at the highest dose, respectively.
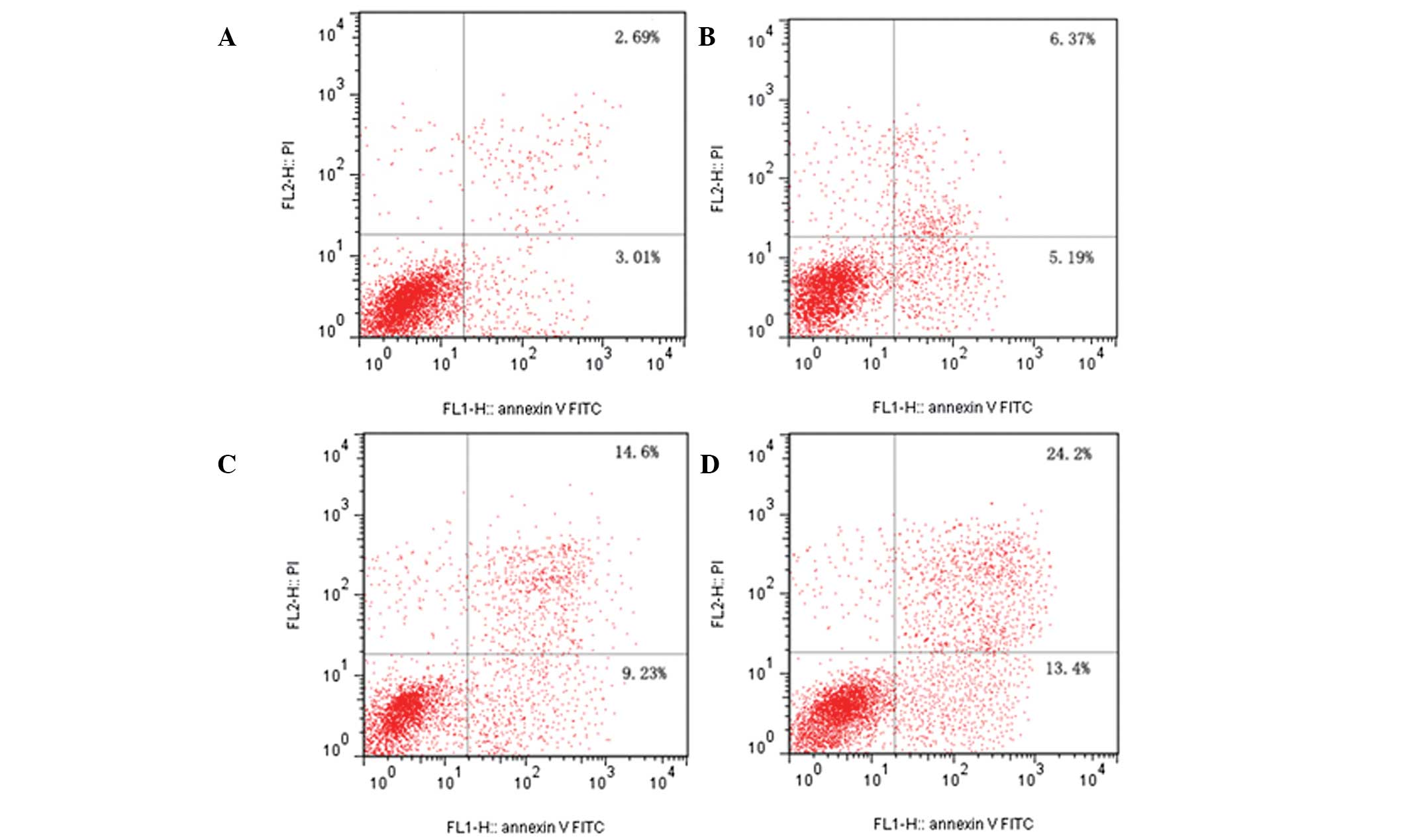 | Figure 6Flow cytometric analysis of DU-145
cells by double-labeling with Annexin V-FITC and PI. Lower left
quadrant, live cells; upper left quadrant, early apoptotic cells;
lower right quadrant, necrotic cells; and upper right quadrant,
late apoptotic cells. (A) control cells; (B) RPOI-1 (5 mg/ml); (C)
RPOI-1 (10 mg/ml); and (D) RPOI-1 (15 mg/ml). Percentages of early
apoptotic cells were 3.01±0.75%, 5.19±1.29%, 9.23±1.02%, and
13.40±2.64%, respectively. One representative
fluorescence-activated cell sorting assay of three independent
experiments is shown for each group. FITC, fluorescein
isothiocyanate; PI, propidium iodide; RPOI-1, Ruditapes
philippinarum oligopeptide. |
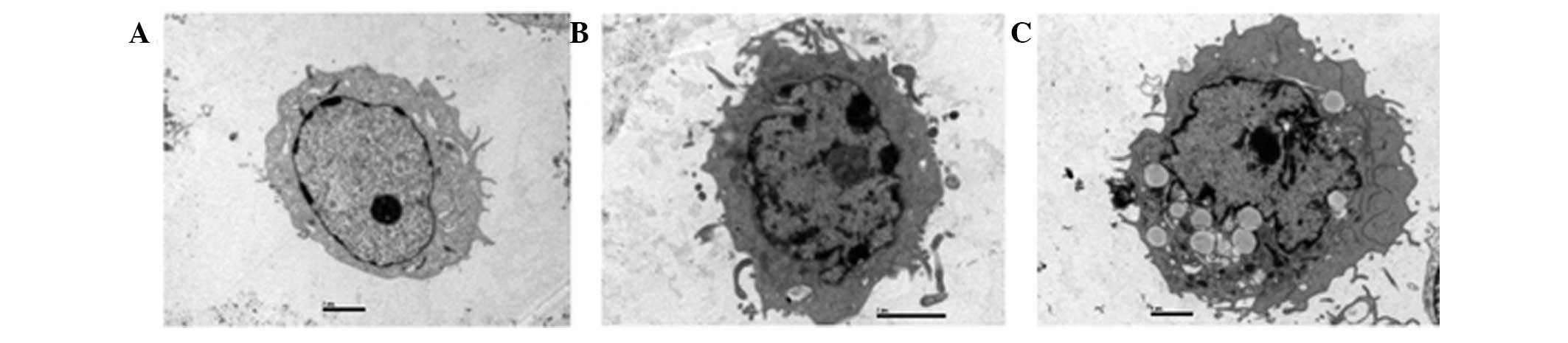
Transmission electron microscopy is the preferred
method for morphological observation by which to clearly
differentiate nuclei and organelles. It was used to confirm
apoptosis in DU-145 cells in this study. As shown in Fig. 7A, there were no typical
morphological changes of apoptosis observed in control cells.
However, when cells were exposed to RPOI-1 for 48 h, characteristic
apoptotic morphological changes were observed, as shown in Fig. 7B and C. These results demonstrate
that RPOI-1 induced apoptosis in DU-145 cells. Following 48 h of
incubation with 10 mg/ml RPOI-1, the nuclei were shrunken,
irregularly shaped and chromatin was highly condensed at the
nuclear periphery. Furthermore, apoptotic bodies were observed in
the cytoplasm following treatment with 30 mg/ml RPOI-1 for 48 h. In
addition, overall cell size was reduced and the nuclei were
shrunken. These findings were highly suggestive of apoptosis in
those cells exposed to RPOI-1. However, characteristics of necrotic
change, such as swelling of cytoplasm or intracytoplasmic
organelles, were not observed.
Cancer is a disease state characterized by
disordered proliferation and the inhibition of apoptosis. The
uncontrolled cellular growth and proliferation that distinguishes
cancer cells from normal cells are key processes in carcinogenesis
(12). The inhibition of
proliferation and induction of apoptosis are efficient methods by
which to treat tumors (13). For
example, a number of anticancer agents induce cell death by
interfering with processes in the cell cycle (14) and others lead to cell death by
increasing apoptosis (15).
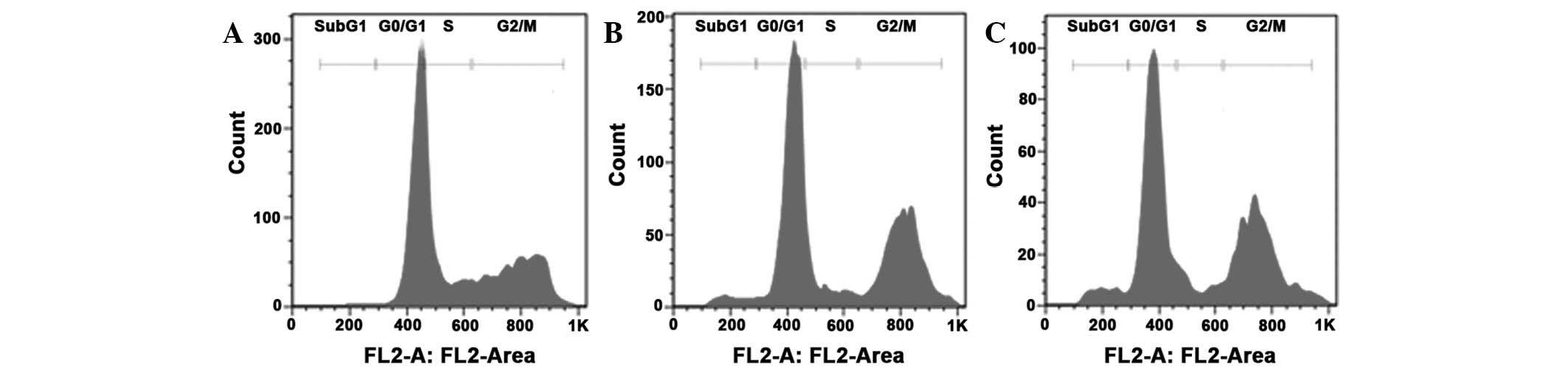
In order to quantify the induction of apoptosis by
RPOI-1 treatment, flow cytometric analysis was conducted. As shown
in Fig. 8, the percentage of cells
in the sub-G1 phase was 0.66% in the control group. However, as
shown in Fig. 7, RPOI-1 treatment
in the DU-145 cells at concentrations of 10 or 30 mg/ml induced a
dose-dependent increase in the proportion of cells in different
phases of the cell cycle, as reflected in the change in the
percentage of cells in the sub-G1 phase (3.67 and 5.74%,
respectively). Table I shows that
there was a significant increase from 0.66±0.61 to 5.74±0.59% in
the sub-G1 phase and from 31.10±1.67 to 48.67±2.40% in the G2/M
phase in DU-145 cells treated with 30 mg/ml RPOI-1. These results
demonstrate that RPOI-1 induced G2 phase arrest and apoptosis in
DU-145 cells.
 | Table IPercentage of DU-145 cells in sub-G1,
G0/G1, S and G2/M phases. |
Table I
Percentage of DU-145 cells in sub-G1,
G0/G1, S and G2/M phases.
| DU-145 cells (%) |
|---|
|
|
|---|
| RPOI-1 (mg/ml) | Sub-G1 | G0/G1 | S | G2/M |
|---|
| 0 | 0.66±0.61 | 48.82±1.34 | 19.33±3.15 | 31.10±1.67 |
| 10 | 3.67±0.27a | 48.80±3.21 | 10.40±1.04a | 40.37±3.33a |
| 30 | 5.74±0.59a | 45.63±2.12 | 6.83±1.18a | 48.67±2.40a |
In conclusion, RPOI-1 had a toxic effect on DU-145
cells and typical apoptotic morphological changes were observed
with AO/EB staining, FCM analysis and TEM. The present study
demonstrated that RPOI-1 inhibited DU-145 cell proliferation
through induction of the apoptotic pathway and that Ruditapes
philippinarum may possess anticancer properties. To the best of
our knowledge, these findings demonstrate for the first time the
anticancer potential of Ruditapes philippinarum. This
evidence for biological activity in the Ruditapes
philippinarum extract indicates that its consumption may be
beneficial to health. In addition, the findings of this study may
increase awareness regarding the potential anticancer properties of
RPOI-1 and aid future developments in anticancer therapeutics on an
industrial scale.
Acknowledgements
This study was supported by the General Projects of
National Natural Science Foundation (grant no. 81273429), the Major
Project of Zhejiang Provincial Science and Technology Office (grant
no. 2010C13009) and the Natural Science Foundation of Zhejiang
Province (grant nos. Y2100805, Y3100129, LY12C2008 and
LY12C2005).
References
|
1
|
Goel S, Mita AC, Mita M, et al: A phase I
study of eribulin mesylate (E7389), a mechanistically novel
inhibitor of microtubule dynamics, in patients with advanced solid
malignancies. Clin Cancer Res. 15:4207–4212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hadaschik BA, Adomat H, Fazli L, et al:
Intravesical chemotherapy of high-grade bladder cancer with
HTI-286, a synthetic analogue of the marine sponge product
hemiasterlin. Clin Cancer Res. 14:1510–1518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Den Brok MW, Nuijen B, Meijer DM, Millán
E, Manada C and Beijnen JH: Pharmaceutical development of a
parenteral lyophilised formulation of the investigational
anticancer agent ES-285. HCl PDA J Pharm Sci Technol. 59:246–257.
2005.
|
|
4
|
Garg V, Zhang W, Gidwani P, Kim M and Kolb
EA: Preclinical analysis of tasidotin HCl in Ewing’s sarcoma,
rhabdomyosarcoma, synovial sarcoma, and osteosarcoma. Clin Cancer
Res. 13:5446–5454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang F, Yang Z, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sánchez C, Mendoza P, Contreras HR, et al:
Expression of multidrug resistance proteins in prostate cancer is
related with cell sensitivity to chemotherapeutic drugs. Prostate.
69:1448–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nehmé A, Varadarajan P, Sellakumar G, et
al: Modulation of docetaxel-induced apoptosis and cell cycle arrest
by all- trans retinoic acid in prostate cancer cells. Br J Cancer.
84:1571–1576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tyagi AK, Singh RP, Agarwal C, Chan DC and
Agarwal R: Silibinin strongly synergizes human prostate carcinoma
DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest,
and apoptosis. Clin Cancer Res. 8:3512–3519. 2002.PubMed/NCBI
|
|
9
|
Dong H, Bai LP, Wong VK, et al: The in
vitro structure-related anti-cancer activity of ginsenosides and
their derivatives. Molecules. 16:10619–10630. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sondhi SM, Singh J, Rani R, Gupta PP,
Agrawal SK and Saxena AK: Synthesis, anti-inflammatory and
anticancer activity evaluation of some novel acridine derivatives.
Eur J Med Chem. 45:555–563. 2010. View Article : Google Scholar
|
|
11
|
Heo SJ, Kim KN, Yoon WJ, et al: Chromene
induces apoptosis via caspase-3 activation in human leukemia HL-60
cells. Food Chem Toxicol. 49:1998–2004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bartek J, Lukas C and Lukas J: Checking on
DNA damage in S phase. Nat Rev Mol Cell Biol. 5:792–804. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinloch RA, Treherne JM, Furness LM and
Hajimohamadreza I: The pharmacology of apoptosis. Trends Pharmacol
Sci. 20:35–42. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dirsch VM, Antlsperger DS, Hentze H and
Vollmar AM: Ajoene, an experimental anti-leukemic drug: mechanism
of cell death. Leukemia. 16:74–83. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|