Introduction
Chronic inflammation is a major driving force in the
initiation and progression of various types of tumor in numerous
tissues (1). Ulcerative colitis
(UC) is one of the major forms of chronic inflammatory bowel
diseases in humans. Patients with UC have an increased risk of
developing colitis-associated colon cancer (CAC) and the level of
risk is associated with the extent and duration of the disease and
the severity of microscopic inflammation (2). There are different opinions regarding
the mechanisms underlying UC (3–5).
Immune cells, including T helper (Th)1, Th17 and follicular T
helper (Tfh) cells, infiltrate the nidus producing cytokines, which
is considered to be important in the progression of
inflammation.
Tfh cells, which are located in germinal centers
(GC), have emerged as a separate subset of cluster of
differentiation 4 (CD4)+ T helper cells that express
high levels of inducible costimulator (ICOS), C-X-C chemokine
receptor type 5 (CXCR5), CD40 ligand (CD40L), programmed cell
death-1 (PD-1) and the important transcription factor B-cell
lymphoma 6 (Bcl-6) (6,7). Interleukin (IL)-21 is a pleiotropic
cytokine synthesized by Tfh cells and is also expressed by other
CD4+ T cells, including Th1, Th17 and activated natural
killer T cells (8–10). IL-21 modulates innate and adaptive
immune responses (11).
Furthermore, IL-21 is crucial in the proliferation of T cells, B
cells and natural killer cells, and affects regulatory T cells
(12). IL-21 performs functions
through a heterodimeric receptor, IL-21 receptor (IL-21R), which is
highly expressed by CD4+ T cells (13,14).
Studies have indicated that IL-21 controls the functional activity
of epithelial cells and fibroblasts in the gut, thus it is an
important mediator in the crosstalk between immune and nonimmune
cells (15,16). The present study examined whether
Tfh cells and IL-21 are necessary for the development of
chemically-induced colitides and examined their interconnections in
UC.
Materials and methods
Patients
Colonic tissue sections were obtained from patients
comprising 20 cases of UC and 15 healthy controls, the latter
suffering from non-UC inflammatory diseases, including colonic
ileus and vascular malformation. The diagnosis of UC was determined
according to established guidelines based on endoscopic and
histopathological examination. Each sample was divided into two
sections. One section was used for histopathological analysis, in
which the sample was deparaffinized, dehydrated through xylene and
ethanol and incubated with a rabbit anti-human polyclonal IL-21
antibody (R&D Systems, Minneapolis, MN, USA) for 2 h at room
temperature. It was then counterstained with hematoxylin (Sangon
Biotech, Shanghai, China). The other section was frozen in liquid
nitrogen and stored at −80°C until Tfh cell and IL-21 RNA analysis.
All the samples were collected at the Department of General
Surgery, The First Affiliated Hospital of Soochow University
(Suzhou, China), between February 2012 and March 2013. The human
studies were approved by the ethics committee of the First
Affiliated Hospital of Soochow University and each patient provided
written informed consent.
Animals
Female wild-type (WT) and IL-21 knockout (IL-21KO)
mice, 6–8-weeks of age, with the same genetic background
(C57BL/6J), were purchased from the Chinese Academy of Sciences
(Shanghai, China). The mice were bred and maintained under specific
pathogen-free conditions in the animal facility at the Experimental
Department of The First Affiliated Hospital of Soochow University.
The IL-21KO mice were viable and did not exhibit any phenotype. All
animal experiments were approved by the local (the Regulation of
Animal Experiments, 1988.10.31) and national (Measures of Jiangsu
Province on Administration of Affairs Concerning Experimental
Animals, 200.10.01) institutional guidelines.
Model of UC
On the basis of the preliminary experiments
(unpublished data), the WT and IL-21KO mice were administered 3%
dextran sulphate sodium (DSS) in drinking water for 14 days. The
control experiment animals received water only. The result of the
preliminary study demonstrated that 100% of the WT mice developed
colitis at day 14.
Histological examination
Colonic tissues of the mice treated with DSS were
removed and fixed in formalin solution overnight at 4°C, embedded
in paraffin, processed, sectioned at a thickness of 5 μm and
stained with hematoxylin & eosin (H&E). Colitis was graded
at day 4 using a scale, which was originally developed for grading
inflammation in UC patients (17).
The samples were graded as follows: Grade 1, normal mucosa; grade
2, mild inflammation (gland enlargement, many intraepithelial
granulocytes, and influx of cells and/or eosinophils into the
stroma); grade 3, intermediate inflammation (gradual reduction in
goblet cells, tubular parallelism, mucin secretion and aggregation
of inflammatory cells in the stroma); grade 4, severe inflammation
(obvious atrophy of glands and mucosa, abundant proliferation of
inflammatory cells and follicle formation in deeper cell layers,
crypt abscesses and pus on the surface); and grade 5, fulminated
inflammation (ulceration with pus, gland and mucosal atrophy, crypt
abscesses, and inflammation of the stroma and deep follicles). The
average score of the WT mice group and IL-21KO mice group in the
examination was then calculated and compared.
RNA preparation and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissues using an
miRNeasy minikit, according to the manufacturer’s instructions
(Invitrogen Life Technologies, Carlsbad, CA, USA). A sample of
total RNA (1 μg) was used to produce first-strand cDNA with a
random primer (Random Hexamers Primer) in a final reaction volume
of 40 μl. The RNA templates were treated with DNase to avoid
genomic DNA contamination. The cDNA/sample (1 μl) was then
amplified by qPCR in an Applied Biosystems 7300 detection system
(Applied Biosystems, Foster City, CA, USA). Combined murine primers
were purchased from Shanghai Generay Biotech Co., Ltd. (Shanghai,
China). The following primers were used: IL-21, forward
5′-CGCCTCCTGATTAGACTTCG-3′ and reverse 5′-TGGGTGTCCTTTTCTCATACG-3′;
ICOS, forward 5′-TGACCCACCTCCTTTTCAAG-3′ and reverse
5′-TTAGGGTCATGCACACTGGA-3′; CXCR5, forward
5′-CTTCGCCAAAGTCAGCCAAG-3′ and reverse 5′-TGGTAGAGGAATCGGGAGGT-3′;
PD-1, forward 5′-AAGCTTATGTGGGTCCGGC-3′ and reverse
5′-AAGCTTATGTGGGTCCGGC-3′; Bcl-6, forward
5′-CAGATTTGTACAGGTGGCCCA-3′ and reverse 5′-AGAGTCTGAAGGTGCCGGAA-3′
and GAPDH, forward 5′-AGAACATCATCCCTGCATCC-3′ and reverse
5′-AGCCGTATTCATTGTCATACC-3′. qPCR reactions were performed
according to the manufacturer’s instructions of the iQ SYBR Green
Supermix (Takara Bio, Inc., Shiga, Japan). Data was normalized with
the levels of GAPDH in the samples. Human IL-21RNA was evaluated
using a TaqMan assay (Applied Biosystems).
Protein extraction and ELISA
analysis
IL-21 in the colonic tissues of mice was measured
using ELISA, according to the manufacturer’s instructions (Sangon
Biotech, Shanghai, China). Optical densities were measured at 450
nm using a Multiskan MK3 (Thermo Fisher Scientific, Waltham, MA,
USA; purchased from Vedeng, Nanjing, China).
Lamina propria mononuclear cell (LPMC)
isolation
LPMCs were isolated, as follows: Colonic tissues
from human and mice were cut longitudinally and washed with Hank’s
balanced salt solution (HBSS; Sangon Biotech) to remove feces and
debris. The colon sections were then finely minced and incubated in
HBSS containing 10% fetal bovine serum (FBS; Hangzhou Evergreen
Biological Engineering Materials Co., Ltd., Hangzhou, China), 0.145
mg/ml dithiothreitol, 5 mM EDTA, 1% penicillin/streptomycin (P/S)
and 1 M Hepes (all from the laboratory of The First Affiliated
Hospital of Soochow University) at 37°C for 15 min for two cycles.
The EDTA was then removed by washing three times in HBSS and the
tissue was digested in RPMI-1640 (Hangzhou Evergreen Biological
Engineering Materials Co., Ltd.) containing 0.4 mg/ml collagenase D
(Sangon Biotech) and 0.01 mg/ml DNase I (Sangon Biotech) for 60 min
at 37°C on an agitating platform. Following collagenase digestion,
the medium containing the mononuclear cells was collected and
centrifuged at 500 × g for 10 min and the resulting cells were
resuspended in RPMI-1640 supplemented with 10% FBS and 1% P/S. The
LPMCs were incubated for 50 min with PE-conjugated CD4
(eBioscience, San Diego, CA, USA), FITC-conjugated CXCR5 (R&D
Systems) and PerCP/Cy5.5-conjugated ICOS (BioLegend, San Diego, CA,
USA) at 4°C and washed twice with staining buffer (1X PBS, 2 mmol
EDTA and 0.5% BSA). The cells were then resuspended in 400 ml PBS
and assayed using flow cytometry (Beckman Coulter, Brea, CA, USA).
The percentages of CXCR5+ ICOS+ cells in
CD4+ lymphocytes were determined. The cells were then
analyzed using the supporting flow cytometry software (Cytomics FC
500, Beckman Coulter, Brea, CA, USA).
Lamina propria T lymphocyte culture and
IL-21 analysis
CD3+ lamina propria T lymphocytes were
purified from the LPMC using magnetically labeled CD3 antibodies
(R&D Systems), resuspended in RPMI-1640 supplemented with 10%
FBS (Hangzhou Evergreen Biological Engineering Materials Co., Ltd.)
and cultured with anti-CD3 beads (Miltenyi Biotec, Bergisch
Gladbach, Germany). They were then divided into three equal
portions and added to 10 μg/ml anti-IL-21 (eBioscience), 10 μg/ml
control IgG (eBioscience) and 10 μg/ml IL-21 (eBioscience),
respectively. Following culture for 72 h, these were used for flow
cytometric analysis and RNA extraction.
Statistical analysis
The data were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Differences among the
groups were compared using Student’s t-test, analysis of variance
and Wilcoxon tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Overexpression of Tfh cells and IL-21 in
human and mouse UC
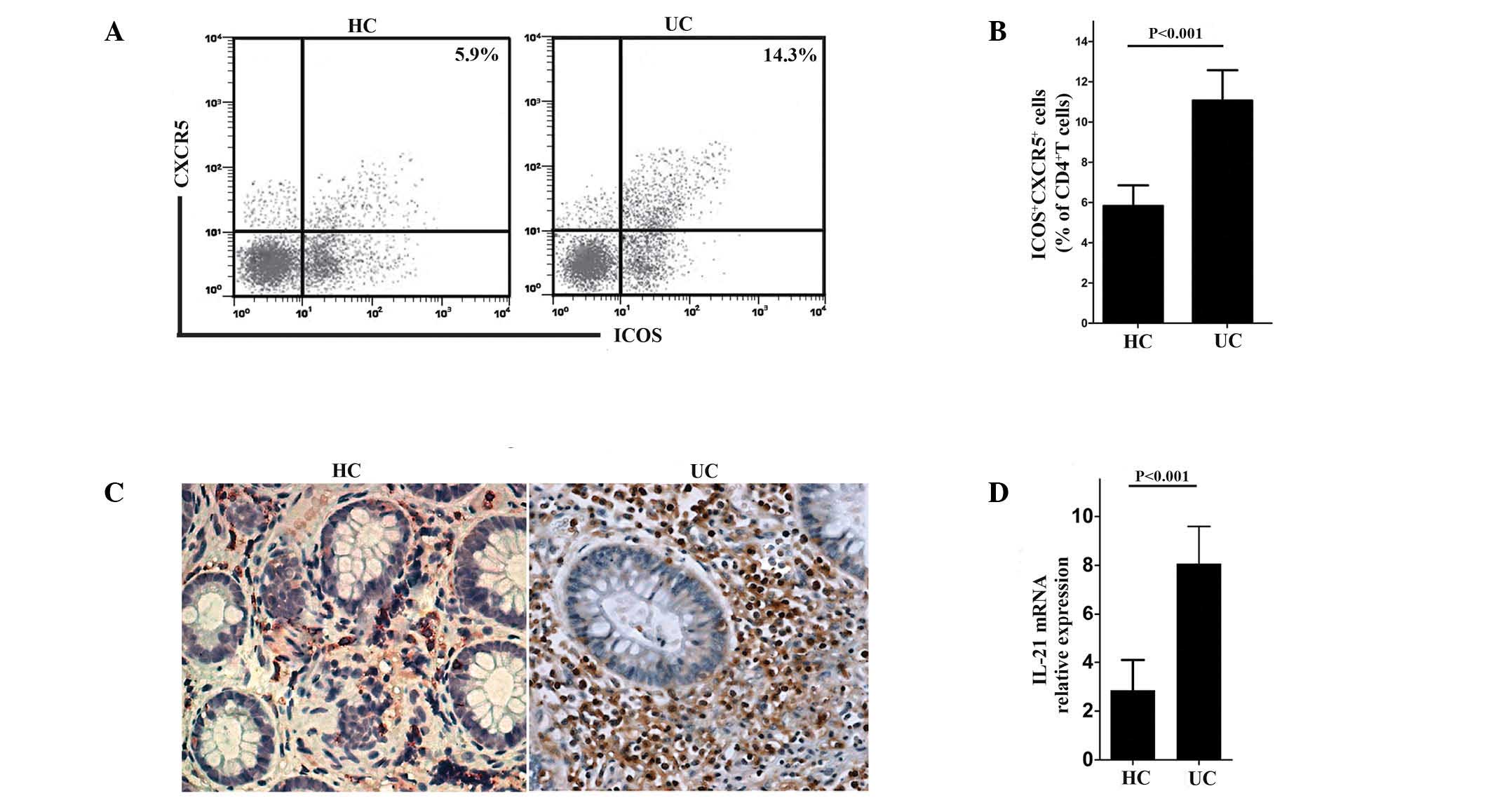
The percentage of ICOS+ CXCR5+
cells among the CD4+ T lymphocytes were examined and
were considered to represent Tfh cells (17). The results of the present study
revealed a significant upregulation of Tfh cells among the
CD4+ T lymphocytes in the UC patients compared with
those in the controls (Fig. 1A and
B). Immunohistochemistry was then used to assess the expression
of IL-21 in the mucosa of patients with UC and in the controls. The
results confirmed the increased expression of IL-21 in UC (Fig. 1C). In addition, more pronounced RNA
expression of IL-21 was detected in patients with UC compared with
the controls (Fig. 1D).
IL-21-deficient mice are resistant to DSS
colitis and produce fewer Tfh cells
WT and IL-21KO mice were treated with DSS and the
degree of inflammation was assessed by histological examination at
day 14. The WT mice demonstrated marked gland and mucosal atrophy,
evident crypt abscesses with pus on the surface, a considerable
influx of acute inflammatory cells and follicle formation in deeper
cell layers. By contrast, IL-21-deficient mice demonstrated mild
infiltration of inflammatory cells in the mucosa, with a reduction
in goblet cells, minimal loss of crypts and lymphoid aggregates
(Fig. 2A). Furthermore, the
average inflammatory grade score of the IL-21KO mice was lower than
the WT mice (Fig. 2C). In order to
examine whether the level of IL-21 was associated with Tfh cell
differentiation and proliferation, the percentage of Tfh cells was
compared between the WT and IL-21KO mice. It was found that the
percentage of Tfh cells in the WT mice was significantly higher
than in the IL-21KO mice regardless of DSS treatment and Tfh cells
were increased in the WT mice, however, not in the IL-21KO mice,
following DSS treatment (Fig.
2B).
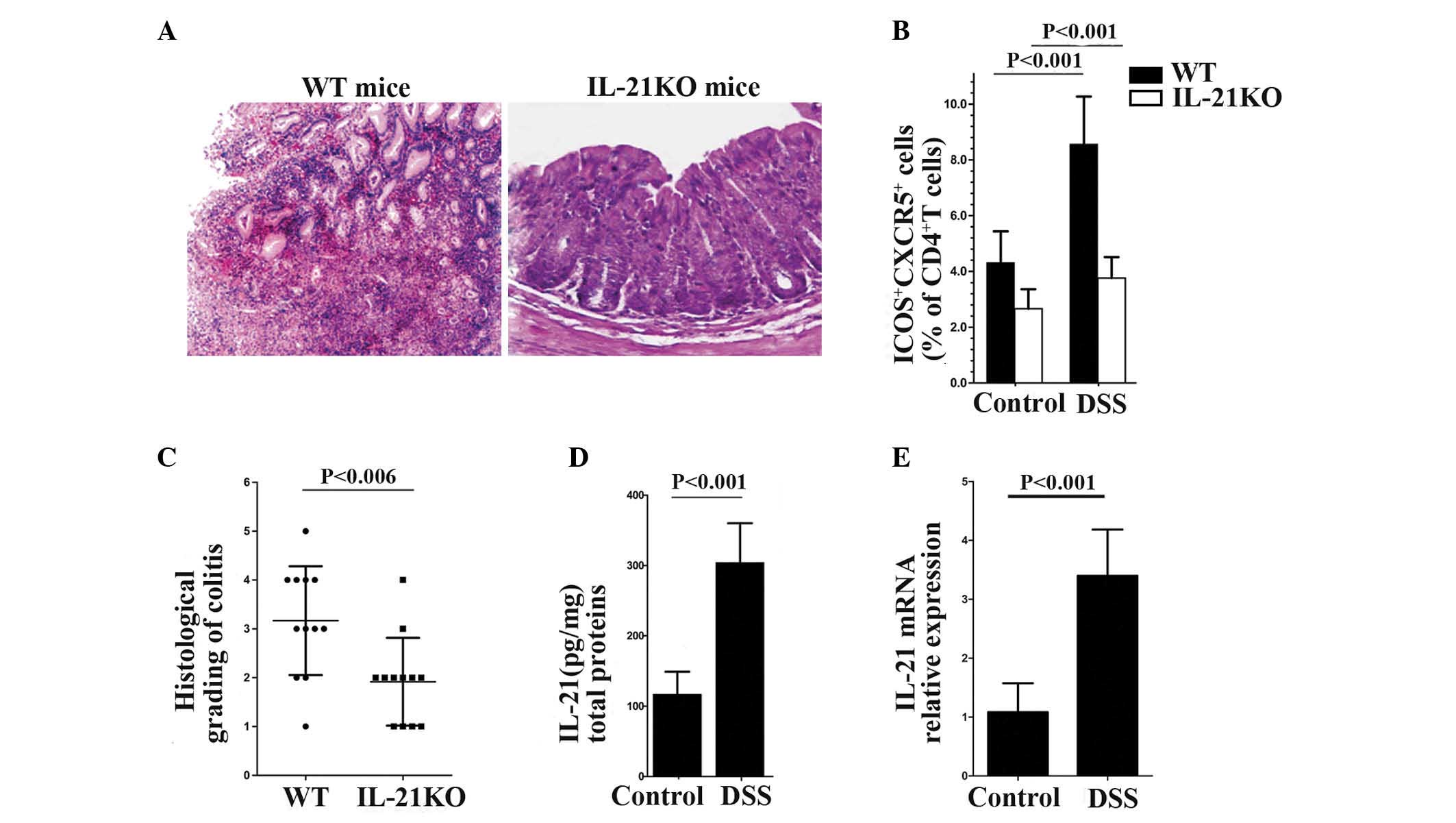 | Figure 2Induction of DSS-colitis in WT mice
is associated with enhanced Tfh cell proliferation and IL-21
synthesis. IL-21-deficient mice were resistant to DSS colitis and
produced fewer Tfh cells. (A) Representative hematoxylin &
eosin-stained sections of the colon obtained from WT and IL-21KO
mice. Induction of colitis in IL-21-deficient mice demonstrated
mild mucosal infiltration of inflammatory cells and a minimal
reduction in goblet cells. Magnification, ×20. (B) Percentage of
ICOS+ CXCR5+ cells in the CD4+ T
lymphocytes of mice colonic tissues. (C) Inflammatory score of 12
WT mice and 12 IL-21KO mice following DSS treatment. (D) IL-21
protein was analyzed by ELISA in the colonic extracts of WT mice
treated with or without DSS. Data are expressed as
picogram/milligram total protein. In each experiment, six mice per
group were used. (E) The mRNA expression of IL-21 in the colonic
extracts of WT mice with or without DSS treatment. IL-21KO,
interleukin-21 knockout; WT, wild-type; DSS, dextran sulphate
sodium; IL-21, interleukin-21; Tfh, follicular T helper; ICOS,
inducible costimulator; CXCR5, C-X-C chemokine receptor type 5;
CD4, cluster of differentiation 4. |
Subsequently, the expression of Tfh cells and IL-21
in mice with DSS-induced colitis was analyzed. WT mice were
provided with DSS-supplemented water (3%) and then sacrificed at
day 14. ELISA and qPCR analysis of IL-21 in the colonic extracts
obtained from the DSS-treated and control mice revealed that the
mRNA and protein levels of IL-21 were upregulated in colitis
(Fig. 2D and E). Flow cytometric
analysis of the mononuclear cells isolated from the DSS-treated and
control mice also demonstrated that Tfh cells were increased in
DSS-induced colitis (Fig. 2B).
IL-21-deficient mice are unable to
upregulate Tfh-associated molecules following DSS treatment
To examine whether IL-21 controls Tfh cell response
in UC, RNA was extracted from colonic specimens of WT and IL-21KO
mice and the content of various mediators of Tfh cells was
analyzed. Initially, the expression of PD-1, ICOS, CXCR5 and CD40L
was analyzed. A significant increase in ICOS, CXCR5 and CD40L RNA
was observed in the colonic tissues of mice that received DSS,
independently of the presence of IL-21 (Fig. 3A, C and D), whereas PD-1
transcripts did not differ between the WT and IL-21KO mice
(Fig. 3B). By contrast, following
DSS treatment, the WT mice, but not the IL-21-deficient mice,
demonstrated a significant increase in ICOS and CD40L (Fig. 3C and D), indicating that IL-21 was
necessary for the induction of ICOS and CD40L during colitis. The
expression of Bcl-6, a key transcription factor of Tfh cells, was
then examined. Following DSS administration, the quantity of bcl-6
transcripts increased significantly in the WT, but not in the
IL-21-deficient mice (Fig.
3E).
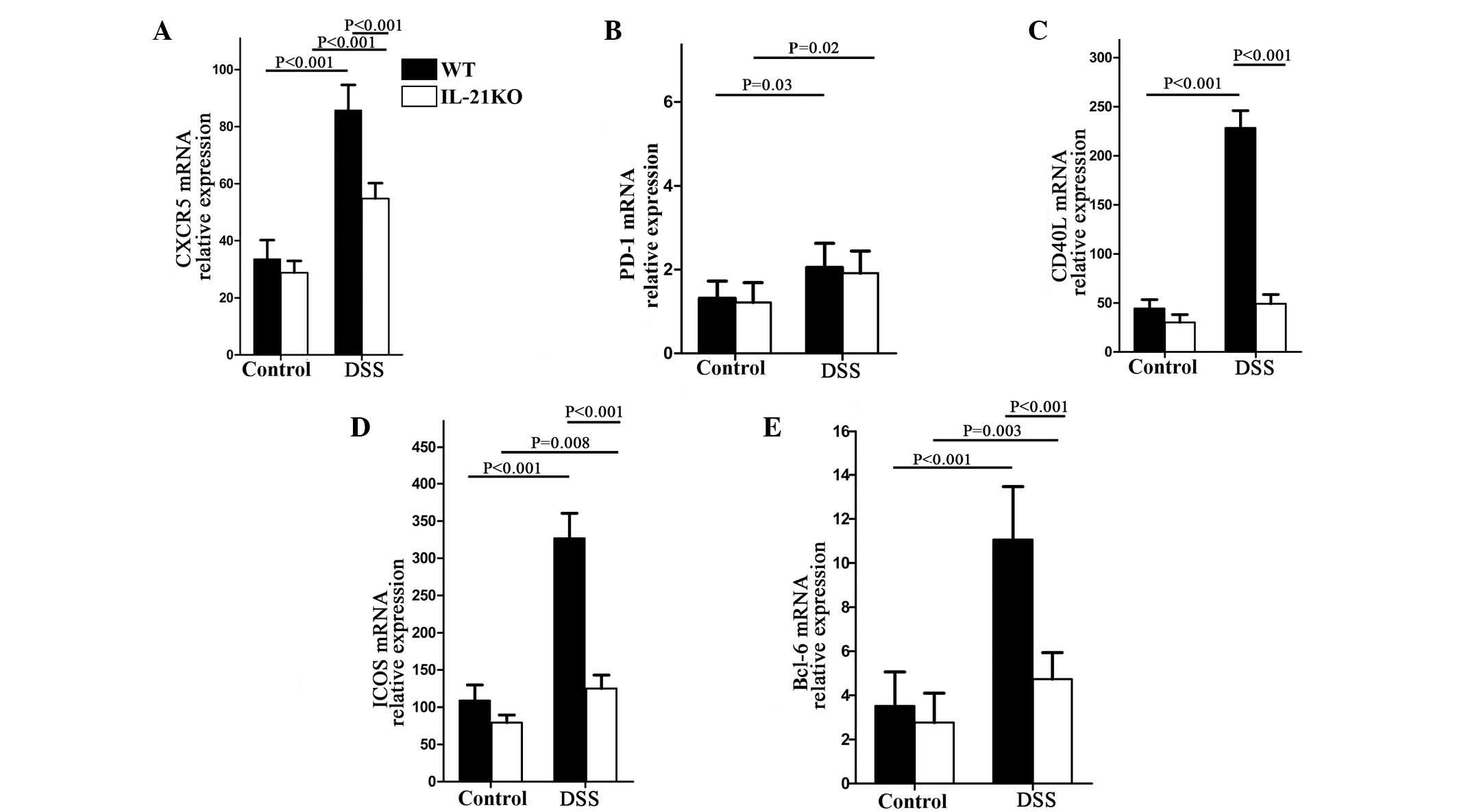 | Figure 3WT and IL-21KO mice were treated with
or without oral DSS and sacrificed for the collection of colonic
samples at day 14. The expression of the Tfh-associated markers,
CXCR5, ICOS, CD40L, PD-1 and Bcl-6, were analyzed by quantitative
polymerase chain reaction. In each experiment, six mice per group
were used; (A) CXCR5, (B) PD-1, (C) CD40L, (D) ICOS and (E) Bcl-6.
WT, wild-type; IL-21KO, interleukin-21 knockout; DSS, dextran
sulphate sodium; Tfh, follicular T helper; CXCR5, C-X-C chemokine
receptor type 5; ICOS, inducible costimulator; CD40L, CD40 ligand;
PD-1, programmed cell death-1; Bcl-6, B-cell lymphoma 6. |
IL-21 is necessary for Tfh cell
proliferation and secretion in vivo
The above results indicated that IL-21 was necessary
for enhancing the proliferation of Tfh cells and the expression of
Tfh-associated molecules. In order to confirm this, the
CD4+ T cells of WT mice were divided into three groups,
and cultured and stimulated with anti-IL-21, control IgG or IL-21.
Subsequently, the percentage of Tfh cells and its associated
molecules were analyzed after 10 days. Flow cytometric analysis
confirmed that the percentage of Tfh cells in the anti-IL-21 group
was less than in the control IgG group and was highest in the IL-21
group (Fig. 4A). The possibility
that IL-21 enhanced the secretion of Tfh cells was then examined.
RNA was extracted from each group and analyzed by qPCR. The results
demonstrated that the expression of ICOS and CD40L was lowest in
the anti-IL-21 group and highest in the IL-21 group (Fig. 4B and C). The same results were
observed for the key transcription factor, Bcl-6 (Fig. 4D).
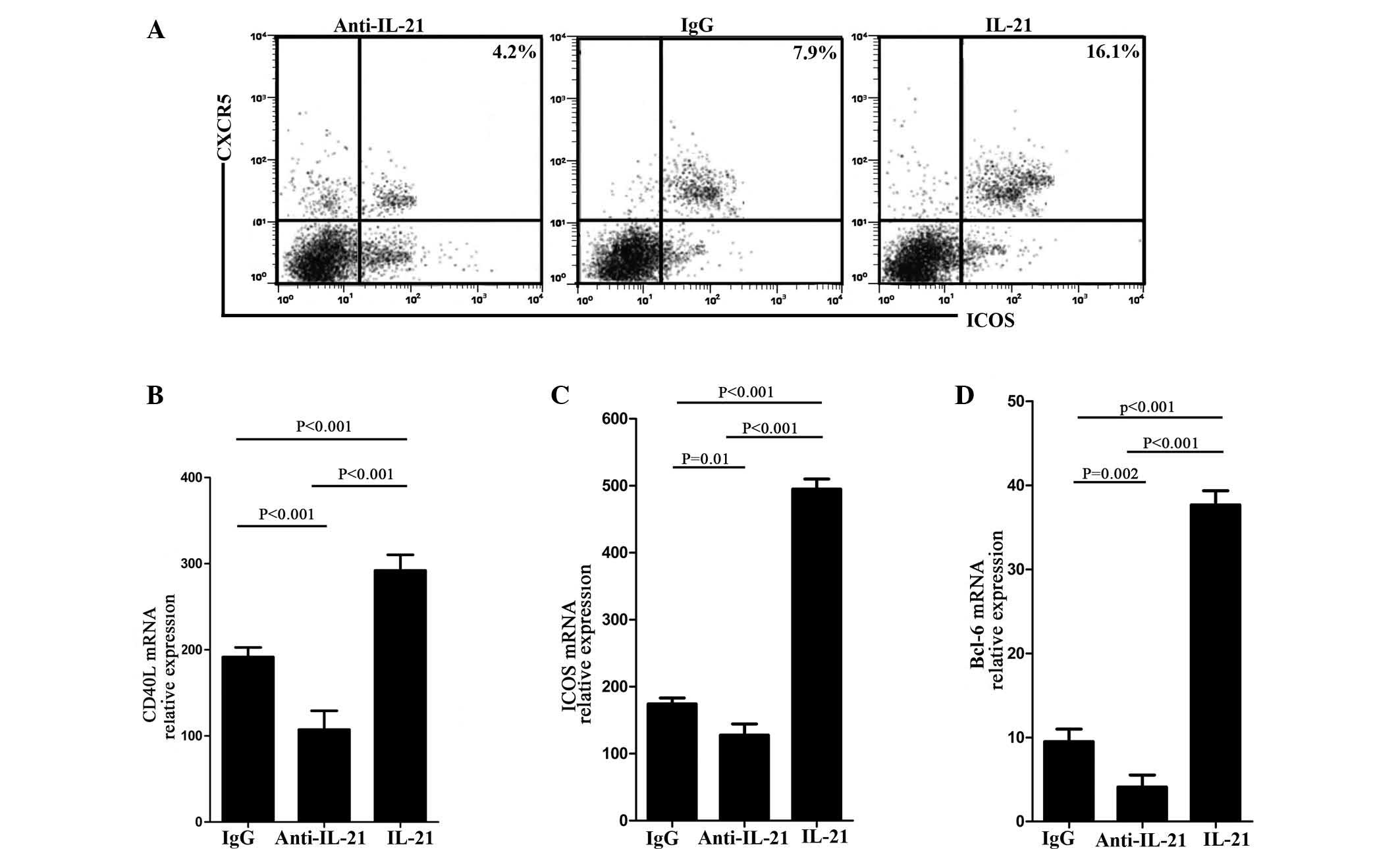 | Figure 4T cells from WT mice fail to promote
the proliferation and secretion of Tfh cells. (A) Representative
dot-plots showing ICOS+ CXCR5+ cells in a
CD4+ T cell culture system undergoing different
treatments. CD4+ T cells were obtained from the colonic
tissues of WT mice without DSS treatment. (B–D) The expression of
Tfh-associated markers, ICOS, CD40L and the transcription factor
Bcl-6 was analyzed by quantitative polymerase chain reaction. In
each experiment, six mice per group were used. WT, wild-type; Tfh,
follicular T helper; DSS, dextran sulphate sodium; ICOS, inducible
costimulator; CXCR5, C-X-C chemokine receptor type 5; CD4, cluster
of differentiation 4; CD40L, CD40 ligand; Bcl-6, B-cell lymphoma
6. |
Inhibition of endogenous IL-21
ameliorates DSS-induced colitis and reduces Tfh cells in WT
mice
The aforementioned findings suggested that elevated
levels of IL-21 in WT mice affected the chronic phase of
DSS-induced colitis and enhanced the percentage of Tfh cells in the
CD4+ T lymphocytes. To assess this hypothesis, certain
DSS-treated WT mice randomly received either neutralizing IL-21
(anti-IL-21; 150 μg/mouse) or control IgG (150 μg/mouse), once per
week intraperitoneally (eBioscience) until day 14. Flow cytometric
analysis of the CD4+ T cells isolated from the
anti-IL-21-treated and control mice indicated that Tfh cells were
decreased in the anti-IL-21-treated mice (Fig. 5A). Histological examination at day
14 demonstrated that inhibition of endogenous IL-21 significantly
reduced the inflammatory score compared with the control mice
(Fig. 5B).
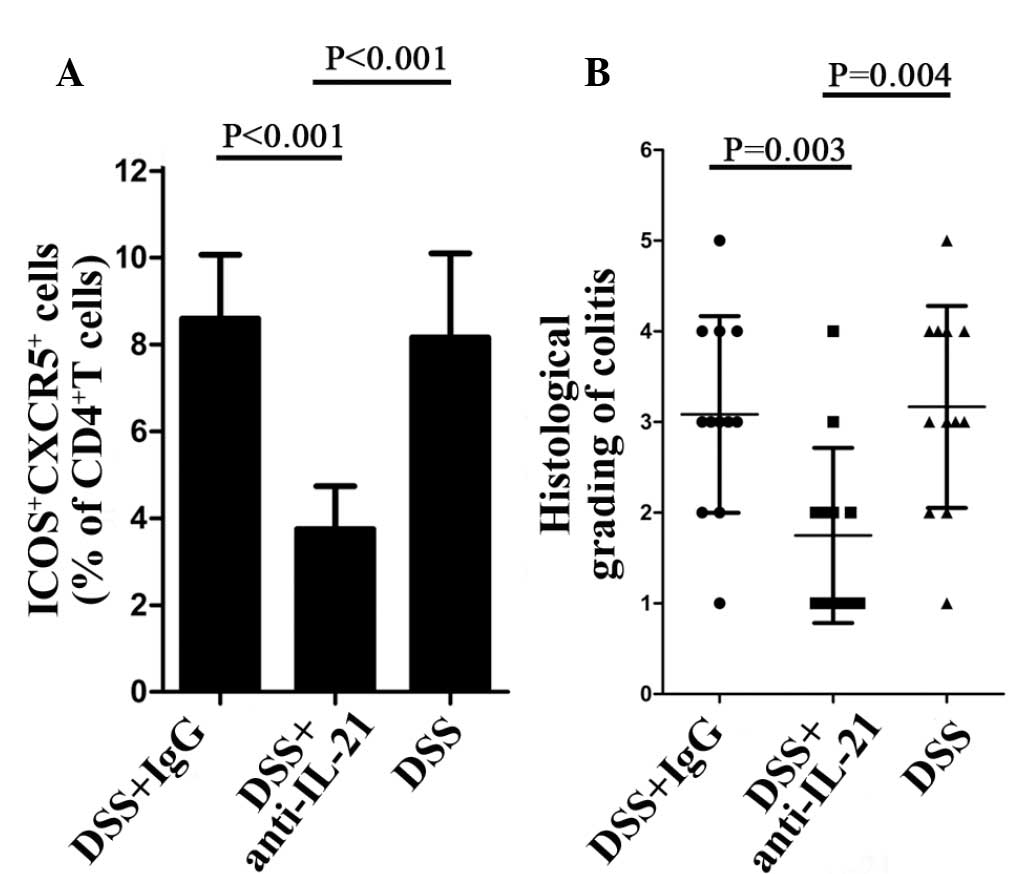 | Figure 5WT mice treated with IL-21
neutralizing antibody (anti-IL-21) are less susceptible to UC and
unable to upregulate Tfh cells. (A) Percentage of ICOS+
CXCR5+ cells in CD4+ T lymphocytes.
CD4+ T lymphocytes were isolated from WT mice following
DSS + IgG, DSS + anti-IL or DSS treatment, respectively. (B)
Inflammatory score of WT mice following DSS + IgG, DSS + anti-IL or
DSS treatment, respectively. WT, wild-type; Tfh, follicular T
helper; IL, interleukin; IgG, immunoglobulin G; DSS, dextran
sulphate sodium; CD4, cluster of differentiation 4; ICOS, inducible
costimulator; CXCR5, C-X-C chemokine receptor type 5. |
Discussion
Tfh cells have attracted increasing attention in
recent years and have been demonstrated to be involved in several
immune diseases (18–20). As a separate subset of
CD4+ T helper cells, the relevant transcription factor
for these cells is Bcl-6, which distinguishes them from Th1, Th2
and Th17 cells (21,22). These Tfh cells express high levels
of CXCR5, which allows their chemotaxis and retention in the
lymphoid follicle and promotes the formation of the GC. ICOS, CD40L
and PD-1 are markers of Tfh cells (6,7),
whereas IL-21 is the major cytokine secreted by Tfh cells and has a
dual function in inflammation (23). IL-21 is able to trigger the
inflammatory pathway and promote tissue damage in numerous organs.
The pathogenic effect of IL-21 has been described in psoriasis
(24), rheumatoid arthritis
(25), Type I diabetes (26) and systemic lupus erythematosus
(27). The present study examined
whether Tfh cells and IL-21 are involved in the process of
DSS-induced colitis and examined the association between them.
Initially, the present study demonstrated
upregulation of IL-21 in the colonic tissues of patients with UC,
suggesting that IL-21 may be important in UC. This investigation
was extended through examination of UC in animals and the same
conclusion was reached. The present study provided evidence that
IL-21-deficient mice were largely protected against the development
of colonic inflammation. These results were confirmed by further
studies demonstrating that WT mice (treated with DSS), which were
administered with neutralizing IL-21 antibody, developed milder
inflammation compared with treatment with a control antibody.
Although, the importance of IL-21 in various
antibacterial responses and immune-inflammatory diseases has been
understood for several years, it is now recognized that the
majority of IL-21 is secreted by a separate Th cell subset, termed
Tfh cells (6,28). Previous studies have demonstrated
that the important function of Tfh cells is to assist B cells in
producing high-affinity antibodies through cognate Tfh-B cell
interaction in the GC of B cell follicles and to adjust antibody
type conversion, therefore they are involved in autoimmune diseases
(20). Few studies have indicated
the involvement of Tfh cells in inflammatory disease, however,
Tfh-associated cytokines, including CXCR5, IL-21 and ICOS play a
decisive role in the production of chemokines (22,29).
Therefore, the present study aimed to examine the interconnections
between IL-21 and Tfh cells.
Analysis of the mechanisms underlying the
IL-21-mediated progression of UC revealed that the lack of IL-21
was paralleled by a marked reduction in the number of Tfh cells,
suggesting that IL-21 was not only an effector molecule, but also a
key regulator of Tfh cells. These results were confirmed by further
evidence.
Initially, whether the level of Tfh cells in WT mice
is higher than in IL-21KO mice following treatment with DSS was
examined. The results demonstrated that the expression of CXCR5,
ICOS, CD40L and Bcl-6 were increased in WT, but not in IL-21KO,
mice.
Secondly, the lack of IL-21 during culture of
CD4+ T cells caused a significant inhibition of Tfh cell
proliferation and downregulated the expression of cytokines. The
opposite effect was observed when stimulated with IL-21.
Finally, in WT mice administered with a neutralizing
IL-21 antibody, the production of Tfh cells was markedly
impaired.
However, the mechanism underlying the IL-21
regulation of Tfh cell polarization has yet to be fully elucidated.
A plausible explanation is that IL-21 induces the activation of
signal transducer and activator of transcription 3 (STAT3) via
IL-21R, promotes expression of the STAT3-induced anti-apoptotic
protein B-cell lymphoma-extra large (Bcl-XL), directly restrains
Tfh cell apoptosis and enhances its transcriptional activity
(30). These conclusions are
consistent with previous studies demonstrating that IL-21R is
broadly expressed by T cells. The expression of STAT3 and Bcl-XL
are increased in UC and CAC (31–33),
suggesting that IL-21 activates STAT3 in the tumor initiating cells
of WT and IL-21KO mice, but not in epithelial cells. Another
possibility is that active STAT3 facilitates the induction of Bcl-6
and promotes the differentiation of Tfh cells from the original
lymphocyte.
ICOS is the only homolog of CD28 found in humans and
mice. In the absence of ICOS, reduced Tfh cell numbers are observed
in mice and humans (34–36). These results suggest that ICOS is
significantly important for the development of Tfh cells. Further
studies are required to clarify whether ICOS is necessary for Bcl-6
upregulation and subsequent Bcl-6 controls the Tfh cell
differentiation program (37).
ICOS signals can also promote the production of IL-21 (37,38).
Several studies have suggested that the transcription factor c-Maf,
which is downstream of ICOS, regulates the production of IL-21 in
developing Tfh cells (39–42). ICOS-deficiency may lead to a defect
in c-Maf upregulation, IL-21 production and consequently a defect
in the upregulation of IL-21R on Tfh cells. These studies
demonstrate that ICOS has a significant effect on the survival of
Tfh cells and regulates the IL-21 production of Tfh cells (18,37,43).
In conclusion, the present study assessed the Tfh
cell counts and levels of IL-21 to examine whether Tfh cells have
an effect during UC. The results confirmed that Tfh cells and IL-21
were upregulated during colitis and indicated that Tfh cells are
important in sustaining pathogenicity in UC. The present study also
demonstrated that IL-21 promotes the proliferation and secretion of
Tfh cells. In addition, more Tfh cells enables the production of
more IL-21. Associated cytokines, including ICOS, are able to
stimulate Tfh cells, which enhance the production of IL-21 and
amplify a positive feedback loop, which assists in stabilizing the
Tfh cell line and magnifying inflammation (35–38,43).
These results suggested that IL-21 could be a new and potential
target for therapeutic agents in UC patients. This was further
substantiated by the demonstration that administration of
anti-IL-21 following DSS-induced colitis partly attenuated the
ongoing inflammation. This may be successful in inhibiting the
development of UC when Tfh cell differentiation is restrained in a
specific way, however, further investigation is required to
demonstrate this.
Acknowledgements
The authors would like to thank all the subjects for
their participation in this study. This study was supported by the
Project of Medical Science and Technology Development Foundation of
Jiangsu Province (grant no. H201209), the Project of Nature Science
Foundation of China (grant no. 81201905), the Nature Science
Research Grants of the University of Jiangsu Province (grant no.
12KJB320009), the Nature Science Research Grants of the University
of Jiangsu Province (grant no. 13KJB320019) and the Science and
Technology Research Project in the Science and Technology Bureau of
Suzhou City (grant no. SYS201220).
References
|
1
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta RB, Harpaz N, Itzkowitz S, et al:
Histologic inflammation is a risk factor for progression to
colorectal neoplasia in ulcerative colitis: a cohort study.
Gastroenterology. 133:1099–1105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ananthakrishnan AN, Khalili H, Konijeti
GG, et al: A prospective study of long-term intake of dietary fiber
and risk of Crohn’s disease and ulcerative colitis.
Gastroenterology. 145:970–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sayyed HG, Jaumdally RJ, Idriss NK, El
Sers DA and Blann A: The effect of melatonin on plasma markers of
inflammation and on expression of nuclear factor-kappa beta in
acetic acid-induced colitis in the rat. Dig Dis Sci. 58:3156–3164.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada K, Tanaka H, Maeda K, et al: Vitamin
D receptor expression is associated with colon cancer in ulcerative
colitis. Oncol Rep. 22:1021–1025. 2009.PubMed/NCBI
|
|
6
|
Fazilleau N, Mark L, McHeyzer-Williams LJ
and McHeyzer-Williams MG: Follicular helper T cells: lineage and
location. Immunity. 30:324–335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crotty S: Follicular helper CD4 T cells
(TFH). Annu Rev Immunol. 29:621–663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monteleone G, Pallone F and Macdonald TT:
Interleukin-21 (IL-21)-mediated pathways in T cell-mediated
disease. Cytokine Growth Factor Rev. 20:185–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
King C, Tangye SG and Mackay CR: T
follicular helper (TFH) cells in normal and dysregulated immune
responses. Annu Rev Immunol. 26:741–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Chen J, Liu Y, et al: Prevalence of
Th17 and Treg cells in gastric cancer patients and its correlation
with clinical parameters. Oncol Rep. 30:1215–1222. 2013.PubMed/NCBI
|
|
11
|
Habib T, Nelson A and Kaushansky K: IL-21:
a novel IL-2-family lymphokine that modulates B, T, and natural
killer cell responses. J Allergy Clin Immunol. 112:1033–1045. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leonard WJ and Spolski R: Interleukin-21:
a modulator of lymphoid proliferation, apoptosis and
differentiation. Nat Rev Immunol. 5:688–698. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins M, Whitters MJ and Young DA: IL-21
and IL-21 receptor: a new cytokine pathway modulates innate and
adaptive immunity. Immunol Res. 28:131–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Parrish-Novak J, Foster DC, Holly RD and
Clegg CH: Interleukin-21 and the IL-21 receptor: novel effectors of
NK and T cell responses. J Leukoc Biol. 72:856–863. 2002.PubMed/NCBI
|
|
15
|
Fina D, Caruso R, Pallone F and Monteleone
G: Interleukin-21 (IL-21) controls inflammatory pathways in the
gut. Endocr Metab Immune Disord Drug Targets. 7:288–291. 2007.
View Article : Google Scholar
|
|
16
|
Caruso R, Fina D, Peluso I, et al: A
functional role for interleukin-21 in promoting the synthesis of
the T-cell chemoattractant, MIP-3alpha, by gut epithelial cells.
Gastroenterology. 132:166–175. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Florén CH, Benoni C and Willén R:
Histologic and colonoscopic assessment of disease extension in
ulcerative colitis. Scand J Gastroenterol. 22:459–462. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linterman MA and Vinuesa CG: Signals that
influence T follicular helper cell differentiation and function.
Semin Immunopathol. 32:183–196. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita R, Schmitt N, Bentebibel SE, et al:
Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular
cells and contain specific subsets that differentially support
antibody secretion. Immunity. 34:108–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Liu Z, Dang E, et al: Follicular
helper T cells (Tfh) and IL-21 involvement in the pathogenesis of
bullous pemphigoid. PLoS One. 8:e681452013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spolski R and Leonard WJ: IL-21 and T
follicular helper cells. Int Immunol. 22:7–12. 2010. View Article : Google Scholar
|
|
22
|
Yu D, Batten M, Mackay CR and King C:
Lineage specification and heterogeneity of T follicular helper
cells. Curr Opin Immunol. 21:619–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stolfi C, Pallone F, Macdonald TT and
Monteleone G: Interleukin-21 in cancer immunotherapy: Friend or
foe? Oncoimmunology. 1:351–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Botti E, Caruso R, Sarra M, et al: IL-21,
a new player in pathogenesis of psoriasis. Journal of Investigative
Dermatology (39th Annual Meeting of the European Society for
Dermatological Research abstracts). 129:S872009.
|
|
25
|
Rasmussen TK, Andersen T, Hvid M, et al:
Increased interleukin 21 (IL-21) and IL-23 are associated with
increased disease activity and with radiographic status in patients
with early rheumatoid arthritis. J Rheumatol. 37:2014–2020. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spolski R, Kashyap M, Robinson C, Yu ZX
and Leonard WJ: IL-21 signaling is critical for the development of
type I diabetes in the NOD mouse. Proc Natl Acad Sci USA.
105:14028–14033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McPhee CG, Bubier JA, Sproule TJ, et al:
IL-21 is a double-edged sword in the systemic lupus
erythematosus-like disease of BXSB. Yaa mice J Immunol.
191:4581–4588. 2013. View Article : Google Scholar
|
|
28
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008. View Article : Google Scholar
|
|
29
|
Annamalai T and Selvaraj RK: Chemokine
receptor CCR7 and CXCR5 mRNA in chickens following inflammation or
vaccination. Poult Sci. 90:1695–1700. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caprioli F, Sarra M, Caruso R, et al:
Autocrine regulation of IL-21 production in human T lymphocytes. J
Immunol. 180:1800–1807. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stolfi C, Rizzo A, Franzè E, et al:
Involvement of interleukin-21 in the regulation of
colitis-associated colon cancer. J Exp Med. 208:2279–2290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hemdan NY: Anti-cancer versus
cancer-promoting effects of the interleukin-17-producing T helper
cells. Immunol Lett. 149:123–133. 2013. View Article : Google Scholar
|
|
33
|
Ysebrant de Lendonck L, Eddahri F,
Delmarcelle Y, et al: STAT3 signaling induces the differentiation
of human ICOS(+) CD4 T cells helping B lymphocytes. PLoS One.
8:e710292013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi YS, Kageyama R, Eto D, et al: ICOS
receptor instructs T follicular helper cell versus effector cell
differentiation via induction of the transcriptional repressor
Bcl6. Immunity. 34:932–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akiba H, Takeda K, Kojima Y, et al: The
role of ICOS in the CXCR5+ follicular B helper T cell
maintenance in vivo. J Immunol. 175:2340–2348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bossaller L, Burger J, Draeger R, et al:
ICOS deficiency is associated with a severe reduction of CXCR5+CD4
germinal center Th cells. J Immunol. 177:4927–4932. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauquet AT, Jin H, Paterson AM, et al: The
costimulatory molecule ICOS regulates the expression of c-Maf and
IL-21 in the development of follicular T helper cells and TH-17
cells. Nat Immunol. 10:167–175. 2009. View
Article : Google Scholar :
|
|
38
|
Galicia G, Kasran A, Uyttenhove C, De
Swert K, Van Snick J and Ceuppens JL: ICOS deficiency results in
exacerbated IL-17 mediated experimental autoimmune
encephalomyelitis. J Clin Immunol. 29:426–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bauquet AT, Jin HL, Paterson AM, et al:
The costimulatory molecule ICOS regulates the expression of c-Maf
and IL-21 in the development of follicular T helper cells and TH-17
cells. Nat Immunol. 10:167–175. 2009. View
Article : Google Scholar :
|
|
40
|
Spolski R and Leonard WJ: IL-21 and T
follicular helper cells. Int Immunol. 22:7–12. 2010. View Article : Google Scholar
|
|
41
|
Awasthi A and Kuchroo VK: The yin and yang
of follicular helper T cells. Science. 325:953–955. 2009.PubMed/NCBI
|
|
42
|
Choi YS, Kageyama R, Eto D, et al: ICOS
receptor instructs T follicular helper cell versus effector cell
differentiation via induction of the transcriptional repressor
BcI6. Immunity. 34:932–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kroenke MA, Eto D, Locci M, et al: Bcl6
and Maf cooperate to instruct human follicular helper CD4 T cell
differentiation. J Immunol. 188:3734–3744. 2012. View Article : Google Scholar : PubMed/NCBI
|