Introduction
A study from the American Federation for Aging
Research states that as normal cells become senescent, whether due
to ongoing cell division, direct DNA damage, activated oncogenes or
other causes, they incur hundreds of biological alterations that
affect several of their activities (1). A number of these changes are similar,
if not identical, to the types of changes that are observed in
aging humans. It is hypothesized that the numerous losses in
function that occur in cells as they approach senescence leads to
an increased vulnerability to age-related diseases or pathologies.
Therefore, the study of cellular senescence continues to provide
important insights into the aging process at the level of the cell
and the pathways within the cell.
An imbalance in antioxidants and pro-oxidants
results in oxidative stress, thus, the destruction of toxic free
radicals, produced as a result of normal metabolism, is essential.
Cataracts are the leading cause of blindness worldwide and opacity
of the lens is a direct result of oxidative stress (2). Visual dysfunction and blindness
induced by age-related cataracts (ARCs) increases as the world’s
population ages. Environmental components and genetic
predisposition contribute to the development of ARC. In addition,
increased age and being female are associated with an increased
risk for ARC (3).
One of the main antioxidant protective mechanisms is
the activation of nuclear factor erythroid 2-related factor 2
(Nrf2). Nrf2 is a transcriptional activator, which binds to the
antioxidant response element. This leads to the transcription of
~200 protective genes, including numerous antioxidant-associated
enzymatic genes (4,5). Previous studies have investigated the
antioxidant components sulforaphane, curcumin and
acetyl-L-carnitine for the prevention of cataracts (6–9).
However, the molecular mechanism underlying the failure of the
antioxidant system in human lenses remains to be elucidated.
Several studies have reported that oxidative stress stimulates the
modification of DNA, including DNA methylation (10–13).
The present study aimed to investigate the
underlying mechanism of the Nrf2-mediated suppression of the
antioxidant system in lenses with age-associated cataracts by
comparing them with normal clear lenses of different age.
Materials and methods
HLECs and bisulphite conversion
Two different types of sets of lenses were used in
the present study: Sets of healthy clear lenses from donors aged
between 15–80 years were obtained from the eye bank of Shandong Eye
Institute (Shandong, China) and the other sets of lenses were
obtained from patients aged between 45–90 years who had undergone
cataract surgery. These patients participated in the present study
at the Department of Ophthalmology, Linyi People’s Hospital (Linyi,
China). Patients with other systemic disease, including diabetic
cataracts, were excluded in order to avoid misleading parameters.
In total, ~120 samples with at least one sample from each subject
were used. The Institutional Review Board of Linyi People’s
Hospital approved the study and written informed consent was
obtained from each participant.
Detection of Nrf2/Keap1 protein
levels
HLECs were harvested and lysed with 200 μl
immunoprecipitation assay buffer (Pierce IP Lysis Buffer; Thermo
Fisher Scientific Inc., Rockford, IL, USA). The lysates were
centrifuged at 13,500 × g for 15 min at 4°C and the protein content
of the supernatant was determined using the Bradford method
(14). The soluble proteins (10–20
μg) were loaded and separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and blotted onto a
polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc.).
Subsequently, the membranes were blocked with 5% non-fat dry milk
powder solution for 1 h at room temperature prior to overnight
incubation with Nrf2 and Keap1 rabbit polyclonal antibodies (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C. Following
rinsing the membranes, the membranes were incubated with a
secondary antibody for 1 h at room temperature and the bands were
made more visible using enhanced chemiluminescence. The intensity
of each band was normalized to that of β-actin and quantified using
Image J analysis software (National Institutes of Health, Bethesda,
MD, USA).
Evaluation of Nrf2/Keap1 gene
expression
Total RNA was extracted from the HLECs using a
Quick-RNA MicroPrep solution (Zymo Research, Orange, CA, USA)
according to the manufacturer’s instructions. The purified total
RNA was reverse transcribed using iScript Reverse Transcription
Supermix for quantitative PCR (qPCR; Bio-Rad, Hercules, CA, USA)
according to the manufacturer’s instructions. The reverse
transcribed RNA was analyzed by qPCR using the SsoFast EvaGreen
supermix (Bio-Rad). Roche’s ProbeFinder designed the optimal qPCR
assay for the Nrf2 and Keap1 genes. The primer sequences used were
as follows: Nrf2, forward 5′-ACACGGTCCACAGCTCATC-3′ and
reverse 5′-TGCCTCCAAAGTATGTCAATCA-3′ with a product size of 96 bp;
Keap1, forward 5′-GGGTCCCCTACAGCCAAG-3′ and reverse
5′-TGGGGTTCCAGAAGATAAGC-3′ with a product size of 66 bp and
β-actin, forward 5′-CCAACCGCGAGAAGATGA-3′ and reverse
5′-CCAGAGGCGTACAGGGATAG-3′ with a product size of 97 bp. Each
reaction was performed in triplicate and a standard curve was
prepared using a serial dilution of a reference sample. The
relative copy numbers were obtained from the standard curve and
were normalized to the values obtained for β-actin as the internal
control.
Sample preparation bisulfite
conversion
The genomic DNA of HLECs was subjected to bisulfite
conversion using an EZ DNA Methylation-Direct™ kit (Zymo Research).
HLECs from the area of capsulotomy were isolated from the center of
the anterior surface of lenses and of age-related cataractous
lenses under a dissection microscope (Carl Zeiss AG, Oberkochen,
Germany). The bisulfite converted DNA was then used for bisulfite
genomic DNA sequencing.
Genomic DNA bisulfite sequencing
The bisulfite-modified DNA was amplified by
bisulfite sequencing PCR using Platinum PCR SuperMix High Fidelity
(Invitrogen Life Technologies, Carlsbad, CA, USA) with primers
specific to the human Keap1 promoter region 1 with 330 bp (forward
5′-TTAGTTATTTAGGAGGTTGAGGTT-3′ and reverse 5′-AACCCCCCTTCTCACTA-3′)
and region 2 with 448 bp (forward 5′-AGTGAGAAGGGGGGTTTGG-3′ and
reverse 5′-CCAAAAATAAAATAAACACC-3′). The primers were designed
using the Methyl Primer Express software from Applied Biosystems
(Foster City, CA, USA). The PCR products were purified by gel
extraction using the Zymoclean™ Gel DNA recovery kit (Zymo
Research) and then cloned into the pCR4-TOPO vectors using a TOPO
TA Cloning kit (Invitrogen Life Technologies). The recombinant
plasmids were transformed into One Shot TOP10 chemically competent
Escherichia coli (Invitrogen Life Technologies) using the
regular chemical transformation method, according to the
manufacturer’s instructions. Plasmid DNA was prepared from ~12
independent clones of each amplicon using a PureLink Quick Plasmid
Miniprep kit (Invitrogen Life Technologies) and sequenced to
determine the status of cytodine-phosphate-guanine methylation. The
sequenced data of each clone was then compared with the in
silico reference human Keap1 promoter bisulfite converted DNA
sequence, derived using the Methyl Primer Express software (Applied
Biosytems).
Results
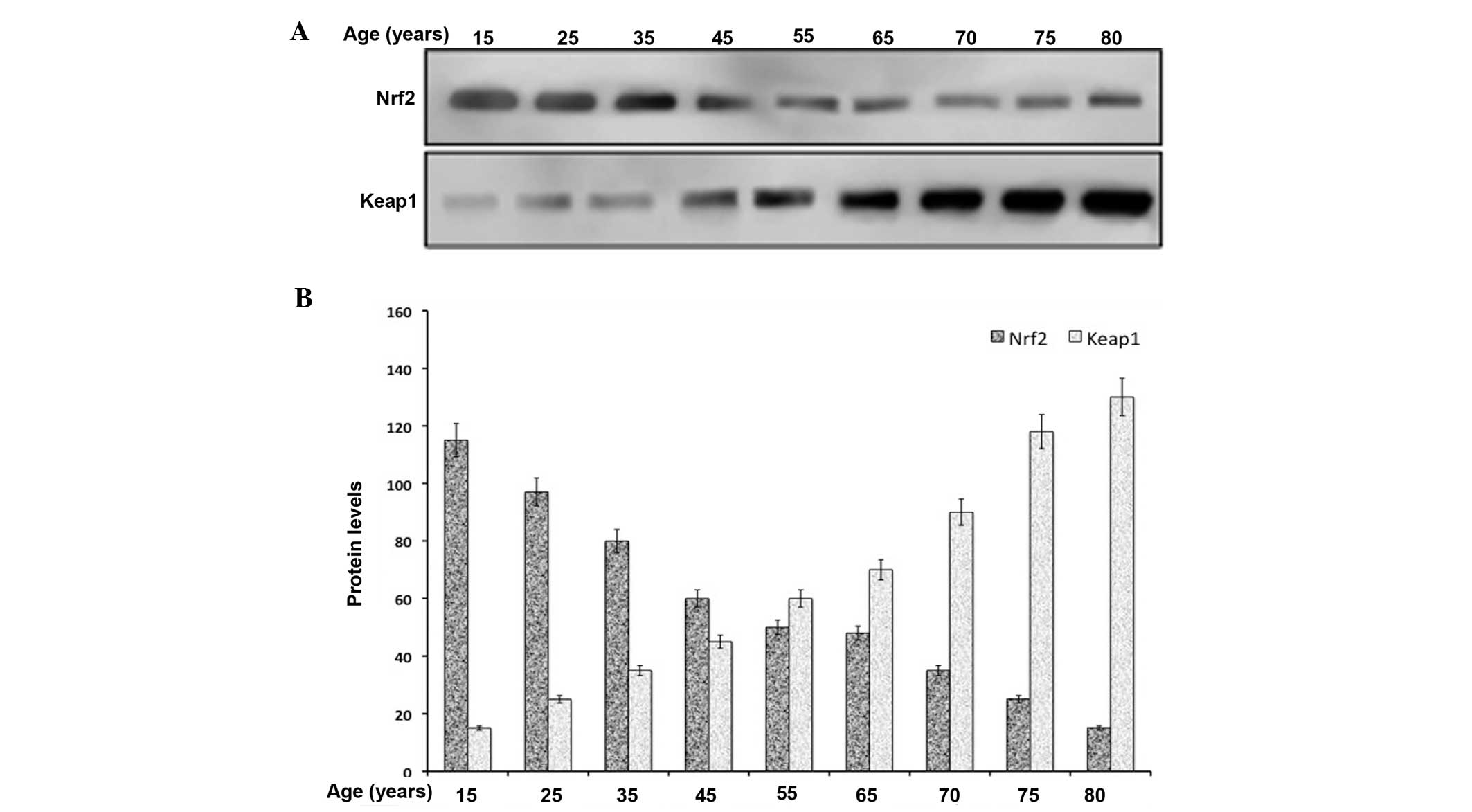
Detection of protein levels
Initially, the protein levels of Nrf2 and Keap1 from
human clear lenses of different ages (15, 25, 35, 45, 55, 65, 70,
75 and 80 years) were examined. An age-dependent decrease in the
level of Nrf2 was detected by protein immunoblot analysis (Fig. 1). A significant decrease in the
protein level of Nrf2 was detected in lenses from the group aged
between 65 and 80 years compared with that observed in the group
aged between 15 and 45 years. However, a contrast in results was
observed in the Keap1 immunoblots. A significant increase in the
protein level of Keap1 was detected in lenses of group aged between
65 and 80 years compared with the group aged between 15 and 45
years (Fig. 1). An increase in the
protein level of Keap1 may explain the decrease in the level of
Nrf2, as Keap1 is a regulator protein, which binds to Nrf2 and
targets it for proteasomal degradation. Therefore, the mechanism
underlying this marked increase in the protein levels of Keap1 with
increasing age was next investigated.
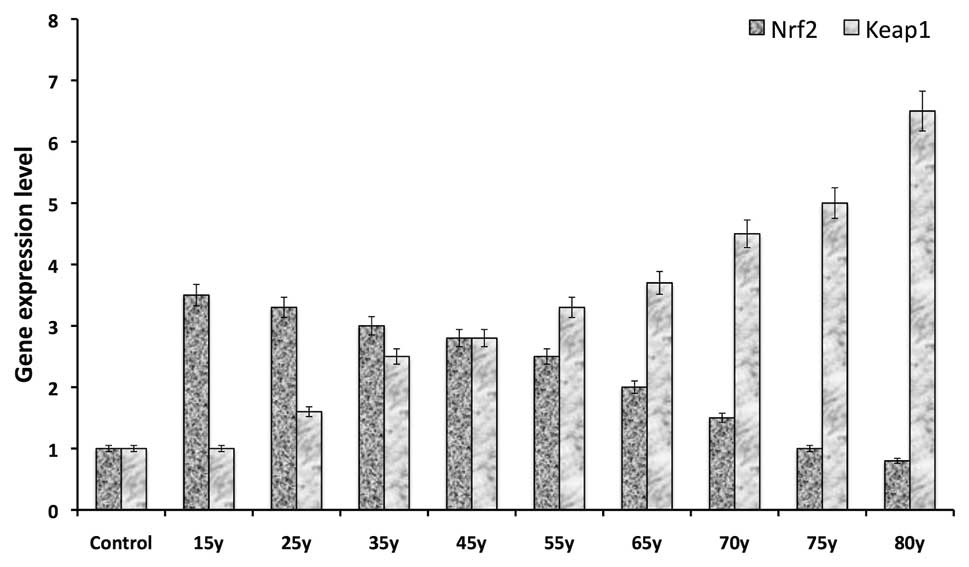
Gene expression study
Following the results of the protein blotting, the
mRNA and gene expression levels of Nrf2 and Keap1
were examined in human clear lenses from different age groups
(15–80 years). qPCR was performed to analyze or quantify the level
of mRNA transcription, the results of which reflected the protein
immunoblotting results. A gradual decrease was observed in the
level of Nrf2 gene expression as age increased (Fig. 2). In addition, a significant
decrease in the expression of Nrf2 was observed in lenses
from the group aged between 65 and 80 years compared with those
<40 years old. However, a gradual increase was observed in the
gene expression of Keap1 in lenses from the group aged between 15
and 65 years, while a significant increase was observed after 65
years (Fig. 2). This result
suggested that the marked increase in the gene expression of Keap1
with increasing age may have been regulated by epigenetic
modification, including DNA methylation or demethylation.
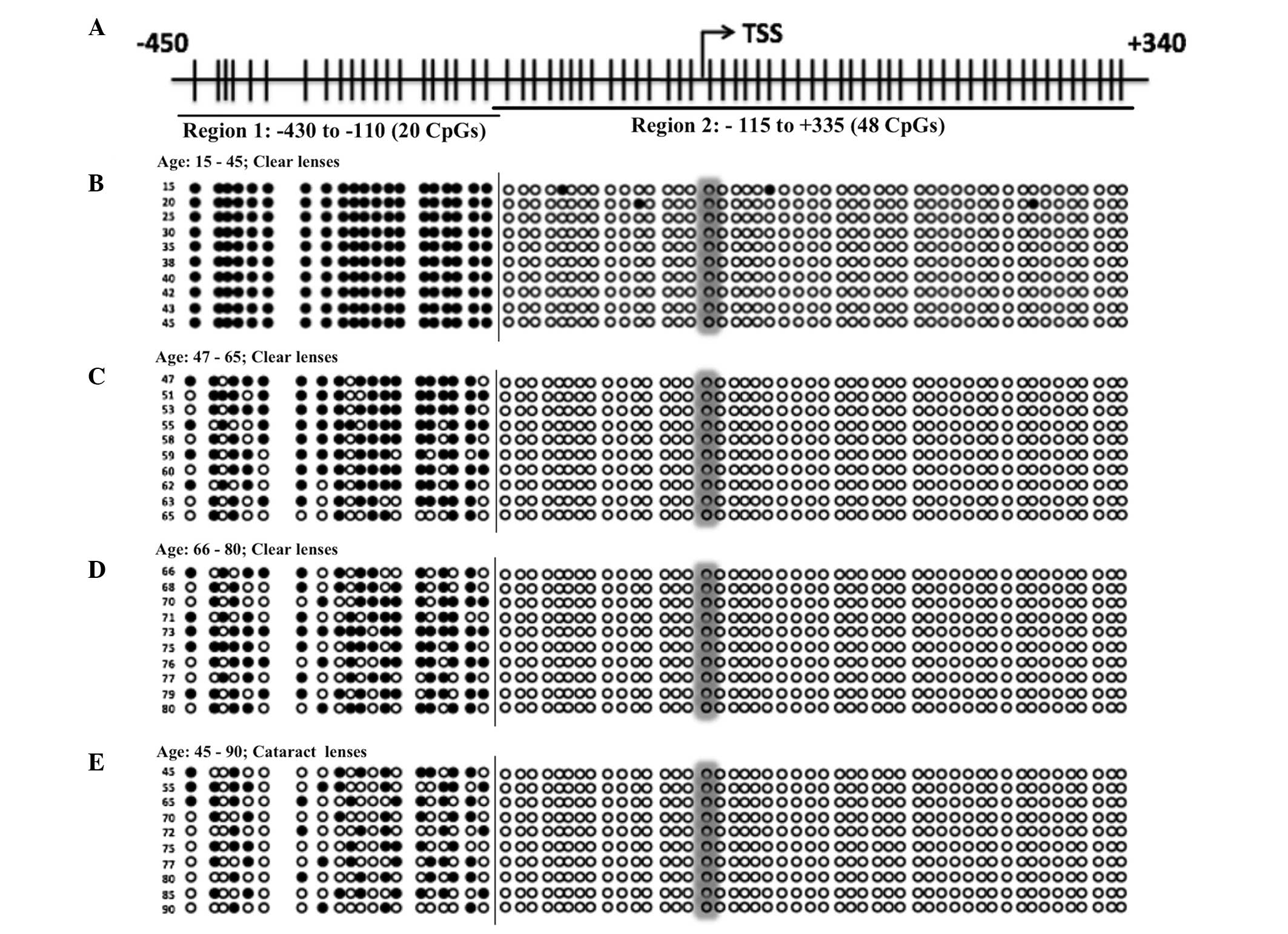
Gene specific DNA methylation study
The present study aimed to investigate whether DNA
methylation was involved in Keap1 gene expression.
Consequently, the gene specific DNA methylation in the promoter
region of the Keap1 gene was examined by implementing the genomic
DNA Bisulfite sequencing method. The keap1 promoter region was
analyzed using two sets of primers as region 1 (−430 to −110) and
region 2 (−115 to +335; Fig. 3A).
The clear lenses from different age groups (15–80 years) and the
age-related cataract lenses from patients aged between 45 and 90
years were selected for the investigation. Complete methylation was
identified in the clear lenses from the group aged between 15 and
45 years (Fig. 3B). However,
gradual demethylation was observed in the group aged between 45 and
65 years (Fig. 3C) and significant
demethylation was found in lenses from the group aged between 65
and 80 years (Fig. 3D). Notably,
similar significant demethylation was observed in the age-related
cataract lenses from patients aged between 45 and 90 years
(Fig. 3E). These results
demonstrated that gradual demethylation occurred throughout aging,
which was due to the exposure of oxidative stress in the cells.
Depending on the level of exposure, demethylation of the Keap1
promoter DNA or increase in the gene expression of Keap1 occurred
and suppressed the protein levels of Nrf2. This led to the failure
of the antioxidant system in the lenses and ultimately to cataract
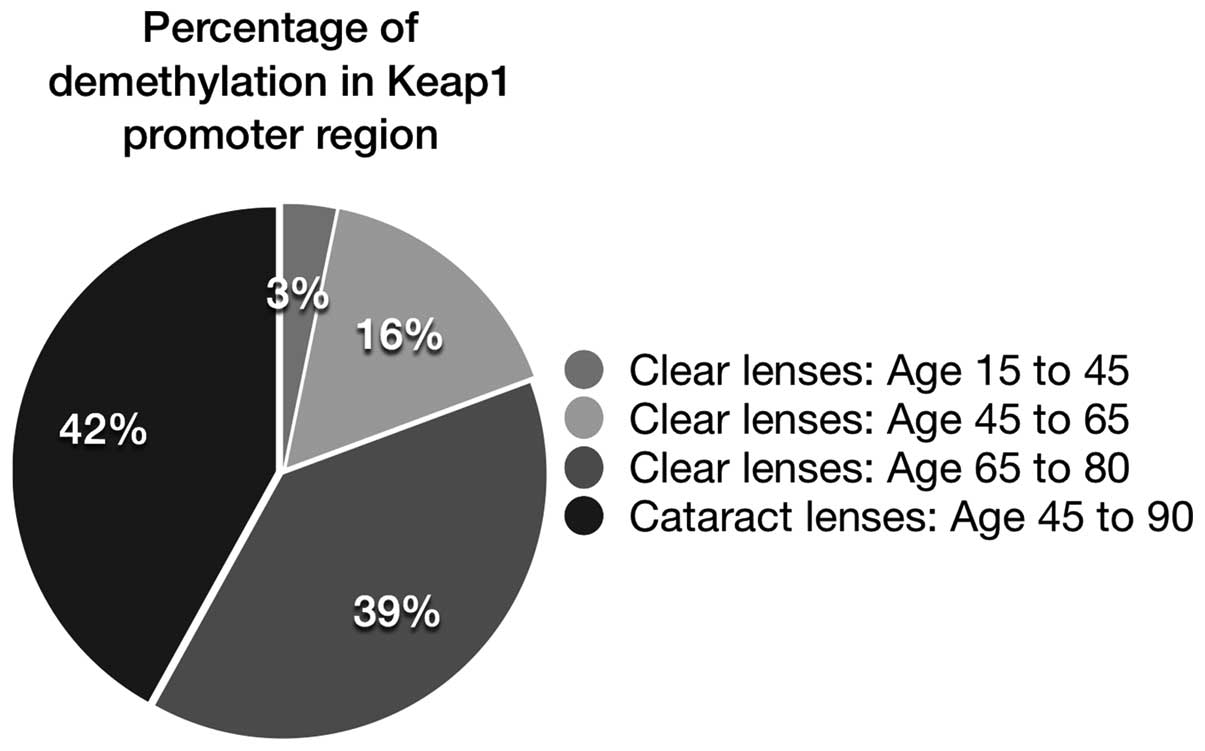
formation. The methylation study revealed the percentage of
demethylation in different age groups, which was estimated as 3%
(age 15–45 years), 16% (age 45–65 years), 39% (age 65–80 years) and
42% in cataract lenses from patients aged between 45 and 90 years
(Fig. 4). This result clearly
indicated that demethylation of the Keap1 gene led to lens
oxidation or cataract formation, which was observed in aging
lenses. The percentage of demethylation in lenses from the group
aged between 65 and 80 years (39%) was almost equal to the
percentage observed in cataract lenses (42%). Therefore, due to
Keap1 demethylation, the aged lenses were susceptible to cataract
formation.
Discussion
The combination of aging with environmental and
genetic stresses is considered to be the main factor contributing
to oxidation, modification and aggregation of lenticular proteins.
The human lens grows throughout life by generating new fiber cells
on old lens fiber cells. The lens epithelial cells in the
germinative zone, which differentiate into the cortical fiber
cells, are more prone to reactive oxygen species (2). This suggests that these lens fiber
cells have less Nrf2-dependent antioxidant protection and the
changes result in oxidation and crystallin aggregation in the lens
cortical and posterior regions (15). In the present study, the level of
antioxidant system proteins was investigated and the results
demonstrated that the protein level of Nrf2 decreased as the age of
the human lens increased. However, the protein level of Keap1
increased upon aging (Fig. 1),
which is consistent with previous studies demonstrating that Keap1
is a negative regulatory protein of Nrf2, increased Keap1
stimulates the degradation of Nrf2 due to proteasomal degradation
and a reduction in Nrf2 suppresses Nrf2-dependent antioxidant
protection (16).
The gene expression of Nrf2 and Keap1
was then determined in human clear lens epithelial cells from
different age groups (15–80 years). A similar pattern of expression
to the protein blotting was observed. The results revealed an
age-dependent increase in Keap1 gene expression but an
age-dependent decrease in Nrf2 gene expression (Fig. 2). These results are consistent with
those of a previous study demonstrating that increased activation
of Nrf2 increases the expression of cytoprotective genes
that detoxify electrophiles to a greater extent than those that
detoxify reactive oxygen species in livers from Keap1-knockdown
mice (17). This previous study
supports the observation in aging human lens in the present study,
with gene expression indicating the impact of epigenetic
modification in the DNA promoter region of these genes. It may be
either gene specific methylation or demethylation that occurs
during aging. This transcriptional regulation of Keap1 and
the proteasomal degradation of Nrf2 leads to the failure of
Nrf2-dependent oxidative stress protection, which may result
in the oxidation of HLECs and formation of cataracts.
The present study also investigated the gene
specific DNA promoter methylation/demethylation for Keap1 gene
expression. The present study examined the normal aging of clear
lenses in from different age group ranging between 15 and 80 years
and the age-related cataract lenses from patients aged between 45
and 90 years. The clear lens from the group aged between 15 and 45
years demonstrated complete methylation (3%), the group aged
between 45 and 65 years demonstrated mild demethylation (16%) and
the group aged between 65 and 80 years demonstrated significant
demethylation (39%) of the Keap1 promoter DNA (Fig. 4). The normal aging lenses from the
group aged between 65 and 80 years revealed almost equal
demethylation status with the cataract lenses from patients aged
between 45 and 90 years old (Fig.
4). This is consistent with previous studies investigating the
occurrence of DNA hypomethylation in cancer and age-dependent
increases in demethylation in normal tissues in vertebrates,
including humans (18–20). The present study revealed the
age-dependent epigenetic modification of the Nrf2-mediated
antioxidant system in the human lens. These results are also
consistent with previous data suggesting that the development of
age-related inflammatory diseases, including rheumatoid arthritis
and polymyalgia rheumatica, in which TNF is an important mediator,
may be affected by changes in DNA methylation (21).
The present study demonstrated that the
Nrf2/Keap1-mediated antioxidant system has an important role in the
lens antioxidant system. ARCs may be caused by an imbalance in the
Nrf2 and Keap1 regulatory mechanism. Imbalance in the gene
expression of Keap1 directly affects Nrf2-mediated protection in
the lens. The present study revealed that the age-dependent gene
specific DNA promoter demethylation of Keap1 gene expression
was the key mechanism underlying the formation of human ARCs.
However, a previous study demonstrated that hypermethylation of the
Keap1 promoter region suppressed the mRNA expression of
Keap1 and increased the expression of nuclear Nrf2 and the
downstream antioxidant response element gene in colorectal cancer
cells and tissues (22). In
conclusion, the present study provided a greater understanding of
the novel mechanism underlying the development of human ARCs.
Further detailed studies in this area may provide support for the
development of treatments.
References
|
1
|
American Federation for Aging Research
(AFAR). Cellular Senescence. http://www.afar.org/docs/migrated/110930_INFOAGING_GUIDE_CELLULAR_SCENESCENCE_Web.pdf.
Accessed October 9, 2014
|
|
2
|
Vinson JA: Oxidative stress in cataracts.
Pathophysiology. 13:151–162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Q, Zhou N, Zhang N, Zhu B, Hu S, Zhou
Z and Qi Y: Genetic variations and polymorphisms in the ezrin gene
are associated with age-related cataract. Mol Vis. 19:1572–1579.
2013.PubMed/NCBI
|
|
4
|
Enomoto A, Itoh K, Nagayoshi E, Haruta J,
Kimura T, O’Connor T, Harada T and Yamamoto M: High sensitivity of
Nrf2 knockout mice to acetaminophen hepatotoxicity associated with
decreased expression of ARE-regulated drug metabolizing enzymes and
antioxidant genes. Toxicol Sci. 59:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu H, Smith AJ, Lott MC, Bao Y, Bowater
RP, Reddan JR and Wormstone IM: Sulforaphane can protect lens cells
against oxidative stress: implications for cataract prevention.
Invest Ophthalmol Vis Sci. 54:5236–5248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grama CN, Suryanarayana P, Patil MA, Raghu
G, Balakrishna N, Kumar MN and Reddy GB: Efficacy of biodegradable
curcumin nanoparticles in delaying cataract in diabetic rat model.
PLoS One. 8:e782172013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elanchezhian R, Sakthivel M, Geraldine P
and Thomas PA: The effect of acetyl-Lcarnitine on lenticular
calpain activity in prevention of selenite-induced
cataractogenesis. Exp Eye Res. 88:938–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elanchezhian R, Sakthivel M, Geraldine P
and Thomas PA: Regulatory effect of acetyl-l-carnitine on
expression of lenticular antioxidant and apoptotic genes in
selenite-induced cataract. Chem Biol Interact. 184:346–351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muscarella LA, Parrella P, D’Alessandro
VD, la Torre A, Barbano R, Fontana A, Tancredi A, et al: Frequent
epigenetics inactivation of Keap1 gene in non-small cell lung
cancer. Epigenetics. 6:710–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu S, Khor TO, Cheung KL, Li W, Wu TY,
Huang Y, Foster BA, et al: Nrf2 expression is regulated by
epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One.
5:e85792011. View Article : Google Scholar
|
|
12
|
Zhang P, Singh A, Yegnasubramanian S,
Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG and Biswal S: Loss of
Kelch-like ECH-associated protein 1 function in prostate cancer
cells causes chemoresistance and radioresistance and promotes tumor
growth. Mol Cancer Ther. 9:336–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang R, An J, Ji F, Jiao H, Sun H and Zhou
D: Hypermethylation of the Keap1 gene in human lung cancer cell
lines and lung cancer tissues. Biochem Biophys Res Commun.
373:151–154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elanchezhian R, Palsamy P, Madson CJ,
Mulhern ML, Lynch DW, Troia AM, Usukura J and Shinohara T: Low
glucose under hypoxic conditions induces unfolded protein response
and produces reactive oxygen species in lens epithelial cells. Cell
Death Dis. 3:e3012012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McMahon M, Itoh K, Yamamoto M and Hayes
JD: Keap1-dependent proteasomal degradation of transcription factor
Nrf2 contributes to the negative regulation of antioxidant response
element-driven gene expression. J Biol Chem. 13:21592–21600. 2003.
View Article : Google Scholar
|
|
17
|
Reisman SA, Yeager RL, Yamamoto M and
Klaassen CD: Increased Nrf2 activation in livers from
Keap1-knockdown mice increases expression of cytoprotective genes
that detoxify electrophiles more than those that detoxify reactive
oxygen species. Toxicol Sci. 108:35–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ehrlich M: DNA methylation in cancer: too
much, but also too little. Oncogene. 21:5400–5413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Frigola J, Solé X, Paz MF, Moreno V,
Esteller M, Capellà G and Peinado MA: Differential DNA
hypermethylation and hypomethylation signatures in colorectal
cancer. Hum Mol Genet. 4:319–326. 2005.
|
|
20
|
Suzuki K, Suzuki I, Leodolter A, Alonso S,
Horiuchi S, Yamashita K and Perucho M: Global DNA demethylation in
gastrointestinal cancer is age dependent and precedes genomic
damage. Cancer Cell. 9:199–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gowers IR, Walters K, Kiss-Toth E, Read
RC, Duff GW and Wilson AG: Age-related loss of CpG methylation in
the tumour necrosis factor promoter. Cytokine. 56:792–797. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanada N, Takahata T, Zhou Q, Ye X, Sun R,
Itoh J, Ishiguro A, et al: Methylation of the KEAP1 gene promoter
region in human colorectal cancer. BMC Cancer. 13:662012.
View Article : Google Scholar
|