Introduction
The ability to maintain a standing position is
fundamental in gait and the initiation of rapid voluntary
movements, the two of which are essential for undertaking everyday
activities. This capability is commonly investigated through the
traces of the center of pressure (COP). Several factors influence
postural control, including the time of day (1–3),
general and local fatigue (4), and
age (5–7). It has been proposed that diminished
central cognitive processing is primarily responsible for the
impairment in balance performance with advancing age (8).
Emotions and mood can have a direct impact on the
physical and mental health of an individual, such as sadness,
apprehension, addiction and stress (9), and the physiological responses to
stress are important determinants of overall health and
susceptibility to disease (10).
The consequences of stress can be either harmful or beneficial,
depending on the intensity, duration, and frequency of the stress
(11). It has been observed that
high levels of stress are associated with worsening of executive
functions, abstract reasoning, processing speed, and visual-spatial
memory (10,12–14).
Furthermore, it has been observed that mood states are capable of
influencing balance control (15)
and anticipatory postural adjustments (16).
The purpose of the present study was to assess
whether heightened stress and negative mood experiences adversely
affect the postural control in young females. The task undertaken
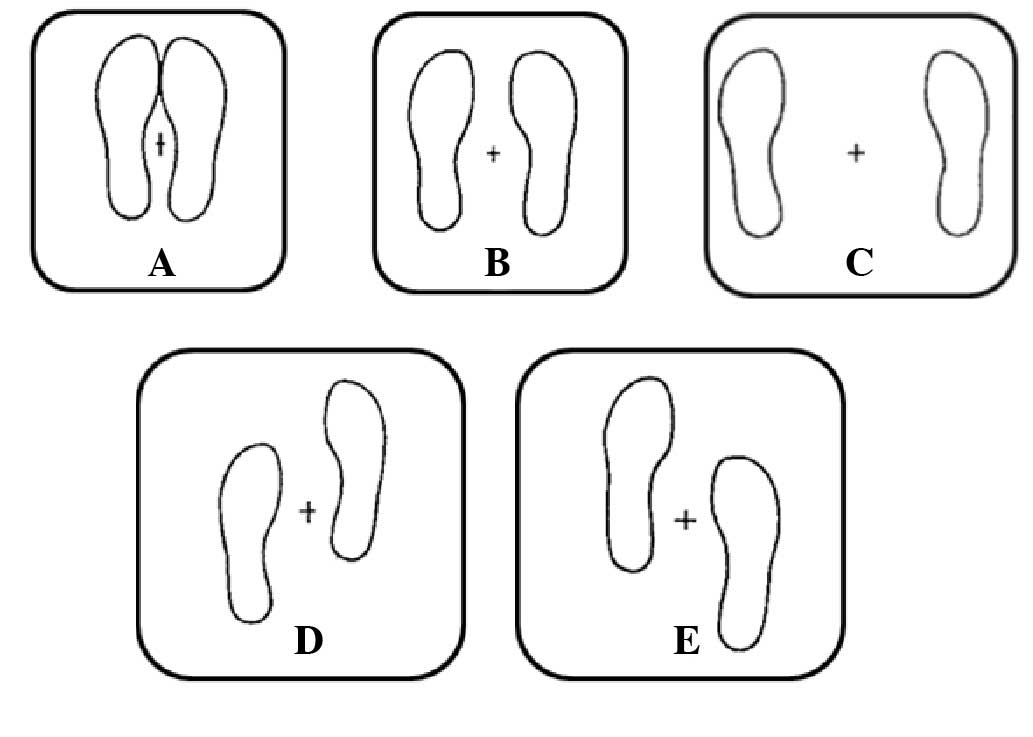
by participants consisted of quiet standing in 5 positions selected
from those proposed by Kirby et al (17): 3 of these positions had the feet on
the same anteroposterior level but separated by different
distances, whilst in the other 2 positions one of the feet was
placed 10 cm in front of the other (in position D, the dominant
foot and in position E, the non-dominant foot).
The perceived stress of the participants was
assessed using the perceived stress scale (PSS), devised by Cohen
et al (18), whilst overall
mood disturbance was measured with the profile of mood states
(POMS). As a second measure of stress, the cortisol awakening
response (CAR) of participants was studied. The CAR is a rapid
increase of free cortisol levels which occurs within 30 min of
awakening and subsequently returns to baseline levels ~1 h later
(19). It has previously been
reported that chronic stress has an important role in the CAR (as
reviewed in 20,21); and so in the present study saliva
samples were collected from participants in order to measure their
CAR.
Materials and methods
Participants
A total of 14 healthy young females, students at the
University of Catania (Catania, Italy), participated in this study.
Only female participants were recruited for this study due to the
gender-related differences in the brain structures that control the
activity of the hypothalamic-pituitary-adrenal (HPA) axis, as well
as differences in the levels of corticosteroid-binding globulins,
which influence the basal and stress-induced activation of the HPA
axis (as reviewed in 22).
Participants were selected on the basis of similar anthropometric
characteristics. The participants had a mean age of 23.79 years (±
3.49), a mean height of 163.86 cm (± 4.88), a mean weight of 57.36
kg (± 3.77), a mean body mass index of 21.35 (± 0.89) and all were
right-handed. The dominant hand was determined by the Edinburgh
Handedness Inventory (23). All
the subjects signed informed consent documentation in accordance
with the Ethical Committee of the University of Catania prior to
their participation in the study.
Stress measurement
Participants’ perceptions of stress were evaluated
using the 10-item version of the PSS (18), which consists of 10 questions
graded on a five-point Likert scale. Scores range from 0–40, with a
higher score indicating greater subjective distress. The PSS is
designed to allow individuals to rate how frequently they felt
overwhelmed by stressful events during the previous 30 days, using
a scale which ranges from 0 (never) to 4 (very often). One such
question from the test is as follows: ‘In the past week, how often
have you felt difficulties were piling up so high that you could
not overcome them?’. The mean score for normative females in the
general population is 13.7±26.6 (24).
Overall mood disturbance was evaluated using an
abbreviated 30-item version of the POMS test developed by McNair
et al (25). Participants
rated each item on a 5-point Likert scale with anchors ranging from
‘Not at all’ to ‘Extremely’. Items were combined to form 6 separate
subscales: Tension-anxiety (T), depression-dejection (D),
anger-hostility (A), vigor-activity (V), fatigue-inertia (F), and
confusion-bewilderment (C). For each of the 6 subscales, the raw
scores were subjected to a T-score transformation using the
following formula: T = 50 + 10(n − m)/s where n= raw score, m =
mean and s = standard deviation. This transformation converted the
raw scores to scores on a standard scale with a mean value of 50 ±
10 (26). The 6 subscale T-scores
were then be combined to form an overall measure of affect that is
known as total mood disturbance (TMD = T+D+A−V+F+C).
Salivary cortisol assay
Saliva samples were collected on two consecutive
workdays, immediately upon awakening (sample 1), and 15 min (sample
2), 30 min (sample 3), 45 min (sample 4) and 60 min (sample 5)
thereafter, resulting in five samples per day and a total of ten
samples for each individual. Participants were requested to wake at
the same time between 06.00 and 08:00 h on the two days and asked
to remain in bed until all 5 saliva samples were obtained. Each
sample was obtained by instructing participants to chew on a dental
roll (Richmond Dental, Charlotte, NC, USA) for ~1 min and then
samples were stored in sterile containers which were provided.
Samples were frozen at −70°C until assay. All saliva profiles were
sampled during the winter between January and February 2013. A
radioimmunoassay cortisol test (AKS18EW; Radim SpA, Rome, Italy)
was used to measure salivary free cortisol levels according to
previously described methods (27).
Balance performance
COP excursion data were collected using an AMTI
force platform (model OR-6-7-1000; AMTI, Newton, MA, USA). As shown
in Fig. 1, COP measurements were
conducted in 5 balance positions adapted from a study by Kirby
et al (17): (A) Feet
together; (B) feet 15 cm apart; (C) feet 30 cm apart; (D) right
foot forward 10 cm; and (E) left foot forward 10 cm. Each of these
5 positions was held for 52 sec with eyes open and then 52 sec with
eyes closed.
To avoid influences of time of day on postural
stability (1), the experiments
were performed between 10:00 and 13:00 h. The force plate of the
platform had a metal surface and all tests were conducted with
subjects barefoot. The AMTI force platform simultaneously measures
three force components along the x (medio-lateral); y
(antero-posterior); z (vertical) axes and three moment components
about the x-, y- and z-axes. Signals from the force platform were
amplified through an AMTI MiniAmp MSA-6 Strain Gauge Amplifier
system (AMTI) prior to being digitized into an IBM-compatible
Pentium computer (Asus S550C; ASUSTeK Computer Inc., Taipei,
Taiwan). The signals were digitized using a Cambridge Electronic
Design 1401 acquisition unit (CED, Cambridge, England) at a
sampling rate of 100 Hz. Routines were developed with MATLAB
software (The MathWorks Inc., Natick, MA, USA) to calculate the
area corresponding to 95% of the area described by the COP
trajectory (A95), since previous studies have demonstrated that
this is a more sensitive measure of postural stability (28,29).
Data analysis
All data are reported as the mean±standard
deviation. For each of the 5 time points, cortisol levels were
averaged over the 2 days to obtain a single mean cortisol
level.
The cortisol increase was defined as the difference
between the individual cortisol peak value (e.g., sample 2, 3, 4 or
5) and the cortisol level immediately after awakening (sample 1).
The area under the response curve (AUCr) was computed including
individual baseline cortisol levels, as described in a previous
study (30).
Data collected from the participants were compared
by means of a non-parametric Wilcoxon signed-rank test. The
correlation between variables was analyzed using a linear
regression. Statistical analysis was performed according to the
guidelines for reporting statistics in journals published by the
American Physiological Society (31).
Results
PSS and TMD vary greatly between
participants
Table I summarizes
the results obtained for the mood measurements of all 14 subjects
in the group. The level of stress experienced by participants
varied greatly, the PSS ranged from 12 to 27 (mean: 18.21±4.59),
TMD ranged from 143 to 297 (mean: 187.93±39.14) and the salivary
cortisol, expressed as area under the response curve (AUCr), ranged
from 20.63 to 26.74 nmol/l (mean: 23.52 nmol/l±1.45).
 | Table IScores obtained in the 14
subjects. |
Table I
Scores obtained in the 14
subjects.
| Subject | PSS | POMS - TMD | Mean AUCr
(nmol/l) |
|---|
| 1 | 12 | 143 | 20.63 |
| 2 | 14 | 157 | 24.93 |
| 3 | 24 | 239 | 22.46 |
| 4 | 21 | 195 | 24.28 |
| 5 | 16 | 169 | 26.74 |
| 6 | 18 | 175 | 23.15 |
| 7 | 19 | 184 | 24.27 |
| 8 | 27 | 297 | 23.13 |
| 9 | 22 | 198 | 23.24 |
| 10 | 17 | 176 | 22.57 |
| 11 | 14 | 168 | 23.37 |
| 12 | 13 | 166 | 24.72 |
| 13 | 23 | 164 | 22.23 |
| 14 | 15 | 200 | 23.57 |
| Mean | 18.21 | 187.93 | 23.52 |
| SD | 4.59 | 39.14 | 1.45 |
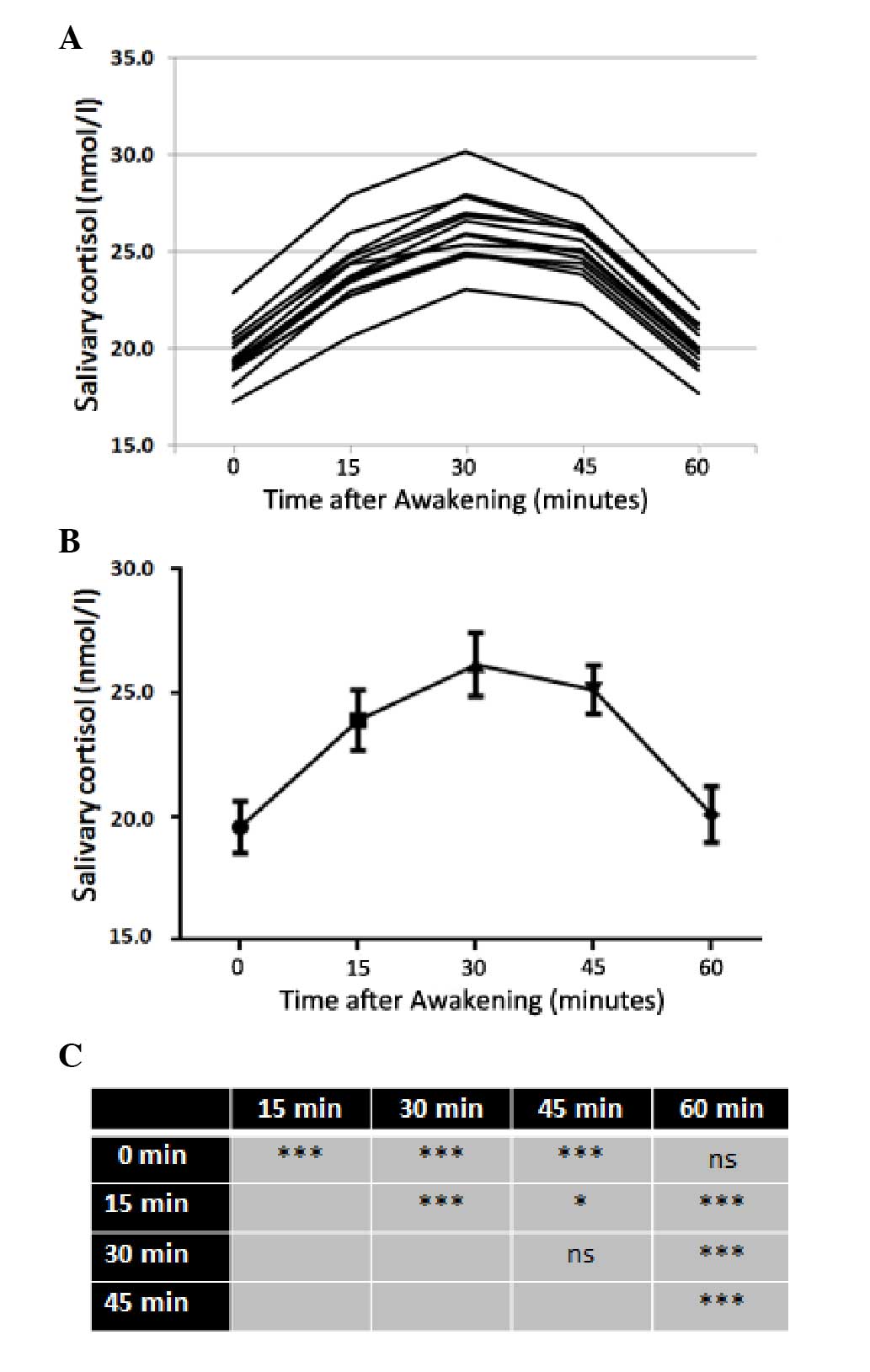
Salivary cortisol increases rapidly
within 30 min of awakening
Fig. 2 shows the
cortisol awakening profiles of the single subjects of the sample,
as well as the mean values of the cortisol awakening profiles and
the results of Tukey’s multiple comparisons test between the mean
values. In all studied subjects, there was a significant increase
in the levels of salivary cortisol immediately after awakening
(time 0), with levels reaching their maximum value after 30 min.
However, 60 min after awakening, salivary cortisol concentration
was not significantly different from that at time 0.
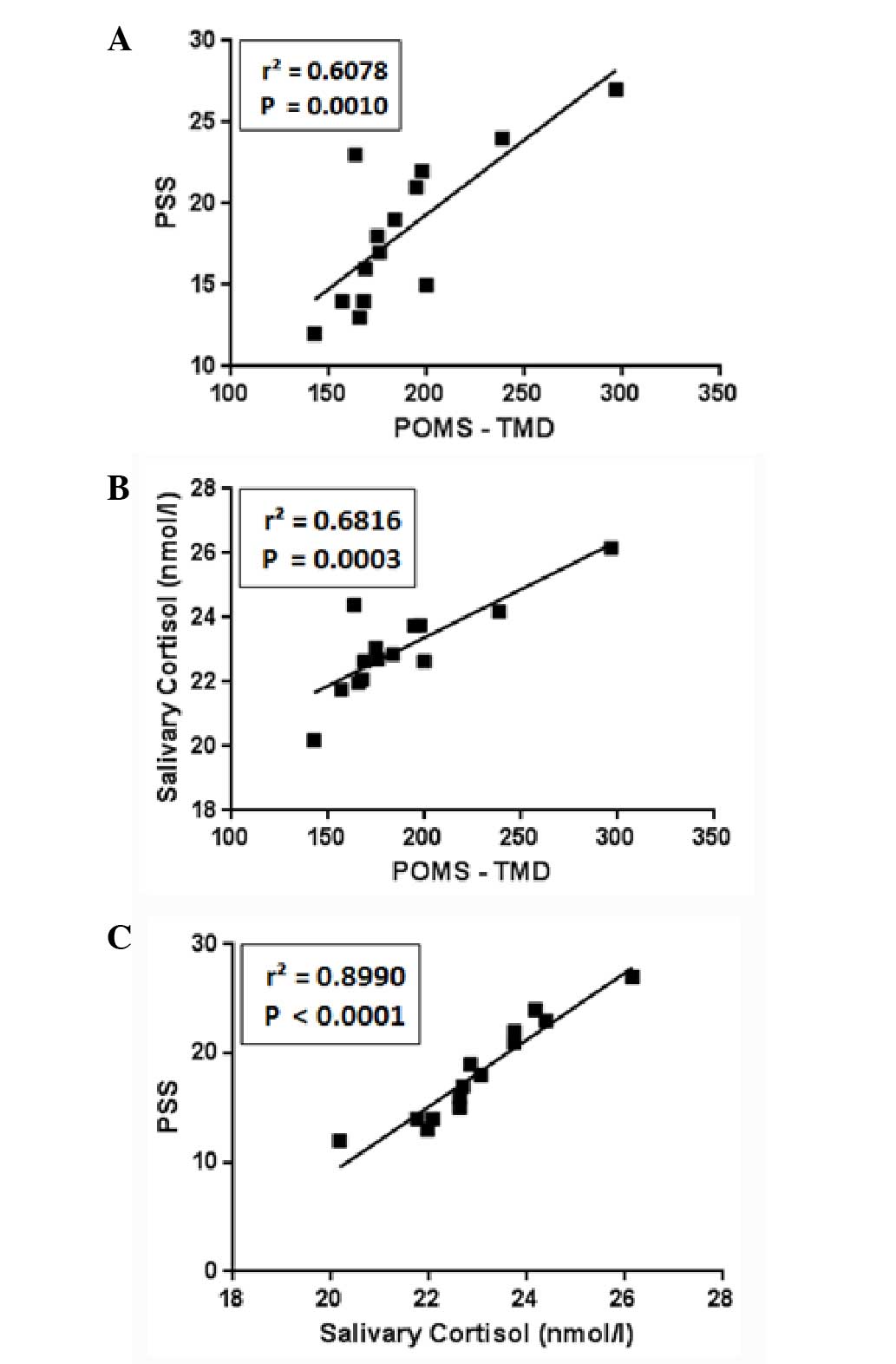
There is a strong positive correlation
between TMD, PSS and salivary cortisol
Fig. 3 illustrates
the correlation between the POMS, expressed as TMD, the PSS and the
salivary cortisol levels for each of the studied subjects. A strong
significant positive correlation between these three measures was
identified.
A95 varies greatly between
participants
Regarding the postural control, Table II summarizes the results obtained
when measuring (in cm2) the A95 in positions A–E
(Fig. 1). A significant variation
was observed between individuals, with the differences between 39
and 66% in the eyes open experiments and between 43 and 76% with
eyes closed. Furthermore, when comparing the data from a single
position measured with open eyes with those calculated with closed
eyes, significant differences (P>0.05) were observed only in
positions D and E, with the worst values recorded in with eyes
closed (Table II).
 | Table IIArea (95% confidence ellipse
expressed in cm2) of the center of pressure measured in
the 14 subjects when stood in each of the five balance positions
(A–E) presented in Fig. 1. |
Table II
Area (95% confidence ellipse
expressed in cm2) of the center of pressure measured in
the 14 subjects when stood in each of the five balance positions
(A–E) presented in Fig. 1.
| A | B | C | D | E | Mean value |
|---|
|
|
|
|
|
|
|
|---|
| Subjects | OE | CE | OE | CE | OE | CE | OE | CE | OE | CE | OE | CE |
|---|
| 1 | 1.29 | 2.27 | 0.41 | 0.29 | 0.45 | 0.80 | 0.67 | 0.97 | 1.37 | 1.71 | 0.84 | 1.21 |
| 2 | 1.13 | 1.38 | 1.59 | 3.58 | 2.88 | 0.80 | 2.24 | 2.38 | 3.84 | 4.05 | 2.34 | 2.44 |
| 3 | 5.69 | 8.68 | 3.28 | 3.23 | 2.06 | 3.83 | 4.31 | 6.86 | 3.67 | 5.77 | 3.80 | 5.67 |
| 4 | 5.06 | 4.60 | 3.84 | 1.71 | 1.52 | 1.69 | 1.95 | 2.01 | 3.53 | 3.57 | 3.18 | 2.72 |
| 5 | 2.82 | 1.99 | 2.35 | 2.69 | 1.86 | 2.36 | 1.60 | 3.49 | 2.30 | 3.23 | 2.19 | 2.75 |
| 6 | 3.11 | 4.96 | 1.39 | 2.37 | 1.05 | 0.77 | 3.46 | 3.61 | 1.41 | 1.80 | 2.08 | 2.70 |
| 7 | 3.35 | 6.41 | 2.01 | 2.12 | 0.98 | 1.35 | 3.81 | 5.50 | 3.17 | 4.17 | 2.66 | 3.91 |
| 8 | 0.10 | 5.87 | 1.35 | 1.53 | 2.28 | 2.36 | 6.37 | 13.35 | 4.61 | 6.98 | 2.94 | 6.02 |
| 9 | 2.27 | 1.97 | 2.27 | 1.36 | 0.91 | 1.12 | 2.35 | 6.06 | 2.23 | 4.94 | 2.01 | 3.09 |
| 10 | 2.93 | 2.85 | 2.18 | 2.27 | 1.20 | 0.98 | 2.88 | 3.51 | 1.66 | 1.95 | 2.17 | 2.31 |
| 11 | 2.15 | 2.20 | 3.05 | 2.92 | 1.75 | 1.85 | 2.05 | 2.18 | 3.75 | 3.80 | 2.55 | 2.59 |
| 12 | 1.44 | 1.97 | 1.05 | 1.15 | 0.80 | 0.80 | 1.02 | 1.15 | 1.58 | 1.82 | 1.18 | 1.38 |
| 13 | 3.55 | 3.45 | 3.56 | 3.80 | 2.55 | 3.75 | 4.05 | 5.23 | 3.88 | 4.85 | 3.52 | 4.22 |
| 14 | 1.55 | 1.60 | 1.78 | 2.22 | 2.45 | 2.35 | 2.54 | 2.66 | 3.54 | 4.56 | 2.37 | 2.68 |
| Mean | 2.60 | 3.59 | 2.15 | 2.23 | 1.62 | 1.77 | 2.81 | 4.21 | 2.90 | 3.80 | 2.42 | 3.12 |
| SD | 1.53 | 2.20 | 1.00 | 0.98 | 0.75 | 1.05 | 1.50 | 3.19 | 1.10 | 1.60 | 0.81 | 1.40 |
| W-test | NS | NS | NS | P<0.05 | P<0.01 | P<0.05 |
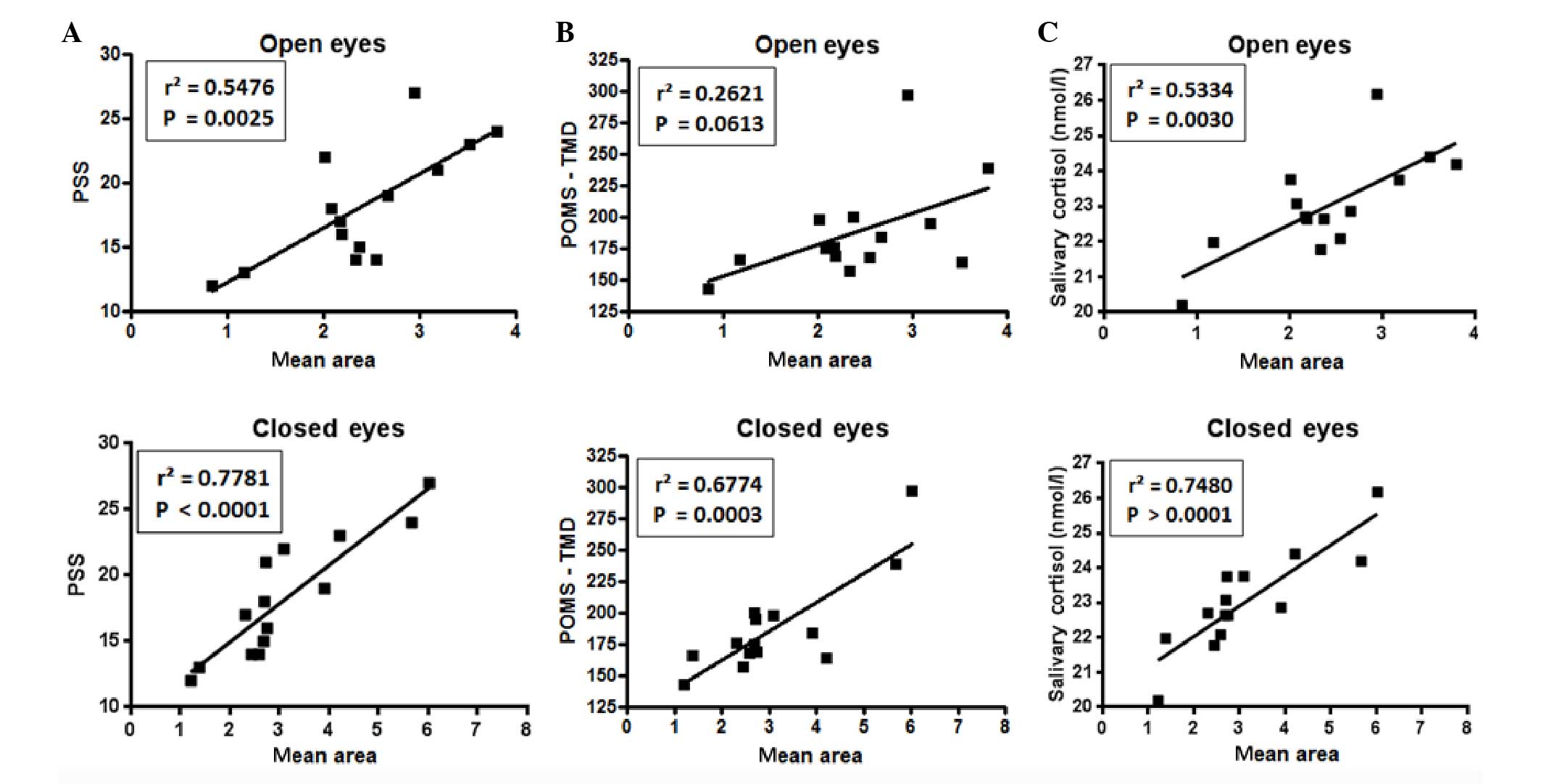
When comparing, for each subject, the scores of PSS,
TMD and salivary cortisol with the A95 mean value measured in the
position E (Fig. 4), a significant
correlation was observed between the A95 and the salivary cortisol
levels and the PSS with open and closed eyes. For POMS no
significant correlation was observed when eyes were open, whereas a
strong positive correlation was detected when eyes were closed.
Discussion
Previous evaluations of kinematic and kinetic
variables have revealed their cognitive influences on gait and
postural stability (32–36) and it has been observed that high
levels of stress are associated with poorer executive function,
abstract reasoning, processing speed, and visual-spatial memory
(12–14). Furthermore, mood state is capable
of influencing balance control (15) and anticipatory postural adjustments
(16).
The main aim of the present study was to investigate
the impact of stress on postural stability with or without visual
input, whilst the secondary objective was to evaluate the effect of
laterality.
The level of stress was measured with PSS whilst
overall mood disturbance was assessed with POMS as TMD. Stress was
also evaluated by measuring the rapid increase of free cortisol
levels after awakening. Chronic stress exhibits an important role
in the CAR but studies examining how chronic stress modulates the
CAR have reported conflicting results (reviewed in 20,21).
Certain studies observed a reduced CAR in association with chronic
stress (37–41), whereas other studies described an
increased CAR in individuals chronically exposed to stressful
events (42–45).
Several elements have been suggested to explain
these contrasting results. Fries et al (21) postulated that the duration of
chronic stress may exert positive or negative influences on the
CAR. This theory is supported by the results of a meta-analysis
conducted by Miller et al (20), which concluded that ‘timing is an
especially critical element, as hormonal activity is elevated at
stressor onset but reduces as time passes’. In the present study, a
strong positive correlation was observed between CAR and
psychological rating scales evaluating stress (PSS) or, more in
general, mood states (POMS). Therefore, by accepting the
conclusions of Miller et al (20), it is reasonable to assume that the
subjects in the present study were exposed to relatively recent
stressful conditions.
Postural stability was inferred from the A95
measured in five postural conditions, which were maintained for at
least 52 sec with open and closed eyes. A95 was selected as
previous studies showed that this measure is the primary measure
for postural stability (28,29).
Data regarding the COP indicated an increased significant body sway
in two specific conditions, i.e. when eyes were closed in position
D and E, where the dominant foot was 10 cm behind the other.
Therefore, when the two feet are not at the same level, maintenance
of posture is easier when the dominant foot is ahead, regardless of
visual input. This effect of laterality is in agreement with the
observations of previous studies (46,47).
In the present study, a strong correlation for each
subject was observed between the PSS and TMD scores, as well as the
levels of salivary cortisol and the mean value of A95 measured in
the 5 positions when eyes were closed, whereas between the mean of
A95 and PSS a significant correlation was only observed only with
open eyes. It is worth noting that in previous studies aimed at
evaluating the association between mood and postural control
(15,16) only the POMS was used. Although PSS
and POMS co-vary linearly, on the basis of the results of the
present study the former may be more appropriate than the latter to
demonstrate heightened stress.
In conclusion, the present study demonstrated not
only that the level of stress is capable of influencing postural
stability, but also that this influence is particularly evident
when no visual information is available during postural
control.
Abbreviations:
|
COP
|
center of pressure
|
|
POMS
|
profile of mood states
|
|
PSS
|
perceived stress scale
|
|
A95
|
95% confidence ellipse area of COP
|
|
SD
|
standard deviation
|
|
TMD
|
total mood disturbance
|
References
|
1
|
Deschamps T, Magnard J and Cornu C:
Postural control as a function of time-of-day: influence of a prior
strenuous running exercise or demanding sustained-attention task. J
Neuroeng Rehabil. 10:262013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gribble PA, Tucker WS and White PA:
Time-of-day influences on static and dynamic postural control. J
Athl Train. 42:35–41. 2007.PubMed/NCBI
|
|
3
|
Jorgensen MG, Rathleff MS, Laessoe U,
Caserotti P, Nielsen OBF and Aagaard P: Time-of-day influences
postural balance in older adults. Gait Posture. 35:653–667. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paillard T: Effects of general and local
fatigue on postural control: a review. Neurosci Biobehav Rev.
36:162–176. 2012. View Article : Google Scholar
|
|
5
|
Era P and Heikkinen E: Postural sway
during standing and unexpected disturbance of balance in random
samples of men of different ages. J Gerontol. 40:287–295. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernie GR, Gryfe CI, Holliday PJ and
Llewellyn A: The relationship of postural sway in standing to the
incidence of falls in geriatric subjects. Age Ageing. 11:11–16.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Laudani L, Casabona A, Perciavalle V and
Macaluso A: Control of head stability during gait initiation in
young and older women. J Electromyogr Kinesiol. 16:603–610. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marsh AP and Geel SE: The effect of age on
the attentional demands of postural control. Gait Posture.
12:105–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joormann J: Cognitive inhibition and
emotion regulation in depression. Curr Dir Psychol Sci. 19:161–166.
2010. View Article : Google Scholar
|
|
10
|
McEwen BS and Stellar E: Stress and the
individual. Mechanisms leading to disease. Arch Intern Med.
153:2093–2101. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demirovic D and Rattan SI: Establishing
cellular stress response profiles as biomarkers of homeodynamics,
health and hormesis. Exp Gerontol. 48:94–98. 2013. View Article : Google Scholar
|
|
12
|
Arnsten AF: The biology of being frazzled.
Science. 280:1711–1712. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liston C, McEwen BS and Casey BJ:
Psychosocial stress reversibly disrupts prefrontal processing and
attentional control. Proc Natl Acad Sci USA. 106:912–917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oaten M and Cheng K: Academic examination
stress impairs self-control. J Soc Clin Psychol. 24:254–279. 2005.
View Article : Google Scholar
|
|
15
|
Bolmont B, Gangloff P, Vouriot A and
Perrin PP: Mood states and anxiety influence abilities to maintain
balance control in healthy human subjects. Neurosci Lett.
329:96–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitaoka K, Ito R, Araki H, Sei H and
Morita Y: Effect of mood state on anticipatory postural
adjustments. Neurosci Lett. 370:65–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kirby RL, Price NA and MacLeod DA: The
influence of foot position on standing balance. J Biomech.
20:423–427. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen S, Kamarck T and Mermelstein R: A
global measure of perceived stress. J Health Soc Behav. 24:385–396.
1983. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pruessner J, Wolf O, Hellhammer D,
Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F and Kirschbaum
C: Free cortisol levels after awakening: a reliable biological
marker for the assessment of adrenocortical activity. Life Sci.
61:2539–2549. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miller GE, Chen E and Zhou ES: If it goes
up, must it come down? Chronic stress and the
hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull.
133:25–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fries E, Dettenborn L and Kirschbaum C:
The cortisol awakening response (CAR): facts and future directions.
Int J Psychophysiol. 72:67–73. 2009. View Article : Google Scholar
|
|
22
|
Kudielka BM and Kirschbaum C: Sex
differences in HPA axis responses to stress: a review. Biol
Psychol. 69:113–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oldfield RC: The assessment and analysis
of handedness: the Edinburgh inventory. Neuropsychologia. 9:97–113.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Demitrack MA and Crofford LJ: Evidence for
and pathophysiological implications of hypothalamic pituitary
adrenal axis dysregulation in fibromyalgia and chronic fatigue
syndrome. Ann NY Acad Sci. 840:684–697. 1998. View Article : Google Scholar
|
|
25
|
McNair DM, Lorr M and Dropplema LF: Manual
for the profile of mood states. Education and Industrial Testing
Service; San Diego, CA: 1971
|
|
26
|
Terry PC and Lane AM: Normative values for
the profile of mood states for use with athletic samples. J Appl
Sport Psychol. 12:93–109. 2000. View Article : Google Scholar
|
|
27
|
Di Corrado D, Agostini T, Bonifazi M and
Perciavalle V: Changes in mood states and salivary cortisol levels
following two months of training in elite female water polo
players. Mol Med Rep. 9:2441–2446. 2014.PubMed/NCBI
|
|
28
|
Boyle J, Danjou P, Alexander R, Calder N,
Gargano C, Agrawal N, Fu I, McCrea JB and Murphy MG: Tolerability,
pharmacokinetics and night-time effects on postural sway and
critical flicker fusion of gaboxadol and zolpidem in elderly
subjects. Br J Clin Pharm. 67:180–190. 2009. View Article : Google Scholar
|
|
29
|
Norris V, Baisley KJ, Calder N, van
Troostenburg AR and Warrington SJ: Assessment of the
AccuSwayPLUS system in measuring the effect of lorazepam
on body sway in healthy volunteers. Int J Pharm Med. 19:233–238.
2005. View Article : Google Scholar
|
|
30
|
Kudielka BM and Kirschbaum C: Awakening
cortisol responses are influenced by health status and awakening
time but not by menstrual cycle phase. Psychoneuroendocrinology.
28:35–47. 2003. View Article : Google Scholar
|
|
31
|
Curran-Everett D and Benos DJ: Guidelines
for reporting statistics in journals published by the American
Physiological Society. J Appl Physiol. 97:457–459. 2004. View Article : Google Scholar
|
|
32
|
Ebersbach G, Dimitrijevic MR and Poewe W:
Influence of concurrent tasks on gait: A dual-task approach.
Percept Mot Skills. 81:107–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang HG and Lipsitz LA: Stiffness control
of balance during quiet standing and dual task in older adults: the
MOBILIZE Boston Study. J Neurophysiol. 104:3510–3517. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lajoie Y, Teasdale N, Bard C and Fleury M:
Attentional demands for static and dynamic equilibrium. Exp Brain
Res. 97:139–144. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Riley MA, Baker AA and Schmit JM: Inverse
relation between postural variability and difficulty of a
concurrent short-term memory task. Brain Res Bull. 62:191–195.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vuillerme N and Vincent H: How performing
a mental arithmetic task modify the regulation of centre of foot
pressure displacements during bipedal quiet standing. Exp Brain
Res. 169:130–134. 2006. View Article : Google Scholar
|
|
37
|
Barker ET, Greenberg JS, Seltzer MM and
Almeida DM: Daily stress and cortisol patterns in parents of adult
children with a serious mental illness. Health Psychol. 31:130–134.
2012. View
Article : Google Scholar :
|
|
38
|
Buchanan TW, Kern S, Allen JS, Tranel D
and Kirschbaum C: Circadian regulation of cortisol after
hippocampal damage in humans. Biol Psychiatry. 56:651–656. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meinlschmidt G and Heim C: Decreased
cortisol awakening response after early loss experience.
Psychoneuroendocrinology. 30:568–576. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Quevedo K, Johnson A, Loman M, Lafavor T
and Gunnar M: The confluence of adverse early experience and
puberty on the cortisol awakening response. Int J Behav Dev.
36:19–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duan H, Yuan Y, Zhang L, Qin S, Zhang K,
Buchanan TW and Wu J: Chronic stress exposure decreases the
cortisol awakening response in healthy young men. Stress.
16:630–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gustafsson PE, Janlert U, Virtanen P and
Hammarström A: The association between long-term accumulation of
temporary employment, the cortisol awakening response and circadian
cortisol levels. Psychoneuroendocrinology. 37:789–800. 2012.
View Article : Google Scholar
|
|
43
|
Schlotz W, Hellhammer J, Schulz P and
Stone AA: Perceived work overload and chronic worrying predict
weekend-weekday differences in the cortisol awakening response.
Psychosom Med. 66:207–214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Steptoe A, Brydon L and Kunz-Ebrecht S:
Changes in financial strain over three years, ambulatory blood
pressure, and cortisol responses to awakening. Psychosom Med.
67:281–287. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wust S, Federenko I, Hellhammer DH and
Kirschbaum C: Genetic factors, perceived chronic stress, and the
free cortisol response to awakening. Psychoneuroendocrinology.
25:707–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Conson M, Mazzarella E and Trojano L:
Self-touch affects motor imagery: a study on posture interference
effect. Exp Brain Res. 215:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mezaour M, Yiou E and Le Bozec S: Does
symmetrical upper limb task involve symmetrical postural
adjustments? Gait Posture. 30:239–244. 2009. View Article : Google Scholar : PubMed/NCBI
|