Introduction
Lung cancer is the most common tumor worldwide.
Surgical resection to remove the tumor together with surrounding
lung tissue remains the best treatment for this disease. However,
only 10–15% of patients are suitable for surgical resection
(1). Cisplatin is the major
component of the chemotherapeutic combination that is used in
transcatheter arterial chemoembolization (cisplatinum, interferon,
doxorubicin and 5-fluorouracil). However, the drug resistance of
lung cancer to chemotherapy remains a major challenge (2). There are several intrinsic or
acquired mechanisms that have been elucidated. Lung cancer cells
frequently possess intrinsic drug resistance mediated by enhanced
cellular drug efflux of several cytotoxic agents. This phenomenon
is associated with an increased expression of ATP-binding cassette
(ABC) proteins, a drug transporter family, including multidrug
resistance gene 1 (MDR1) and multidrug resistance-associated
protein (MRP1) (3).
Soluble resistance-related calcium-binding protein
(Sorcin) is a 22 kDa calcium-binding protein that was initially
found to be overexpressed in vincristine-resistant cells. The
binding of Ca2+ triggers the translocation of Sorcin
from the cytoplasm to the cell membrane, where it interacts with
specific proteins involved in the signaling cascades of several
physiological processes (4).
Further studies revealed that Sorcin was not only overexpressed in
vincristine-resistant cells, but also in several tumor cell types
with different drug-resistant profiles (5). Previous studies indicated that
overexpression of Sorcin is associated with resistance in human
ovarian, breast cancer, lung cancer, leukemia and gastric carcinoma
(6). Downregulation of Sorcin
expression in K562/A02 cells, which are resistant to doxorubicin,
restored drug sensitivity (7–9).
These findings suggest that Sorcin may be a potential therapeutic
target for reversing MDR in cancer. However, the role of Sorcin in
lung cancer MDR remains to be elucidated.
The current study aimed to investigate the effect of
Sorcin on multidrug resistance reversal of human lung cancer
A549/DDP cells and its mechanism.
Materials and methods
Cell lines and cell culture
The human lung cancer cell line A549 and cisplatin
resistant cell line A549/DDP were purchased from the Cell Bank of
the Chinese Academy of Sciences (Shanghai, China) and maintained at
37°C in a humidified atmosphere with 5% CO2. The culture
medium was Eagle’s minimal essential medium supplemented with 10%
fetal bovine serum (FBS; Gibco-BRL, Carlsbad, CA, USA). The present
study was approved by the Ethics Committee of Weifang Medical
University (Weifang, China).
Silencing of Sorcin by siRNA
The Sorcin siRNA and blank vector were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and the
transfection of siRNA was performed according to the manufacturer’s
instructions. Briefly, 4×105 A549/DDP cells were seeded
into 6-well plates for 24 h in the culture medium. Then, Sorcin
siRNA and the blank vector were transfected with Lipofectamine
2000. Following 24 h of incubation, normal growth medium containing
15% FBS was added and the cells were incubated for another 18 h.
The medium was aspirated and replaced with fresh normal growth
medium (10% FBS) and was added after 72 h with 400 μg/ml G418 to
generate Sorcin silencing and negative control sublines. Sorcin
expression was measured by western blotting and quantitative
polymerase chain reaction (qPCR).
MTS assay to determine the drug
sensitivity of the cells
The cells (0.1 ml) were seeded into each well of the
96-well microplate at a density of 1×105/ml and
incubated overnight to allow the adherence of cells. Then,
different concentrations of cisplatin (Sigma-Aldrich, St. Louis,
MO, USA) were added to each well and the cells were cultured for
another 72 h. At the end of the incubation, CellTiter 96 Aqueous
One Solution Reagent (Promega Corporation, Madison, WI, USA) was
employed according to the manufacturer’s instructions. After 4 h,
the cell viability was determined by measuring the absorbance at
490 nm using a microplate reader (US Biotek Laboratories, Seattle,
WA, USA).
Analysis of intracellular accumulation of
Rhod-123, membrane expression of P-gp, the cell cycle and cell
apoptosis using flow cytometry
Approximately 3×105 cells were seeded
into each well of the 6-well microplate and incubated with 10 μM
cisplatin for 24 h. Cells were then harvested and the apoptosis
rate was determined using an Annexin V-FITC Apoptosis kit purchased
from BD Biosciences (Franklin Lakes, NJ, USA). Briefly, 20 μl of
Annexin V-FITC and propidium iodide (PI) were added to the
suspension and incubated for 15 min in the dark at room
temperature. Subsequently, the cells were washed twice with
phosphate-buffered saline (PBS), resuspended with PBS and analyzed
by flow cytometry at an excitation wavelength of 488 nm.
Approximately 1×106 cells were harvested
and fixed in cold alcohol at 4°C overnight. The cells were washed
twice with PBS, stained with 10 μM PI with DnaseA for 30 min and
analyzed using flow cytometry (FACSCalibur; BD Biosciences) at an
excitation wavelength of 488 nm.
Approximately 1×106 cells were harvested
and resuspended in 0.1 ml culture medium. The cells were stained
with 10 μM Rhod-123 (Sigma, St. Louis, MO, USA) for 1 h and the
intracellular concentration was determined by flow cytometry
(FACSCalibur; BD Biosciences) at an excitation wavelength of 488
nm.
The expression of P-gp on the cell surface was
determined using a direct immunofluorescence staining kit purchased
from BD Biosciences. Briefly, 20 μl of reagent containing
anti-P-gp-PE was added to the suspension and incubated for 30 min
in the dark at room temperature. Cells were then washed twice with
PBS, resuspended with 0.5 ml of 1% paraformaldehyde and analyzed by
flow cytometry at an excitation wavelength of 488 nm.
Determination of glutathione (GSH)
expression levels
The intracellular GSH was measured using a Total
Glutathione Assay kit (Beyotime Institute of Biotechnology, Haimen,
Jiangsu, China). Analysis was performed according to the
manufacturer’s instructions. Briefly, cells were incubated with
cisplatin for 4 h. Cell lysates were harvested and reacted with
assay solution for 5 min at 25°C. The absorbance at 412 nm was
measured on a Spectra Max M5 microplate reader (Molecular Devices,
Sunnyvale, CA, USA).
qPCR analysis
Approximately 3×106 cells were harvested
for qPCR analysis. Total mRNA was extracted from the cells using a
Dynabeads mRNA Direct kit (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. Total mRNA
was then reverse transcribed for 1 h at 42°C in incubation buffer
containing 250 μM of each deoxynucleotide triphosphate, 5 μM oligo
(dT)20, 25 units of RNase inhibitor and 20 units of avian
myeloblastosis virus reverse transcriptase (Roche Diagnostics,
Mannheim, Germany). The transcription level of MDR1, MRP1, ABCA2,
ABCA5 and Bcl-2 was detected by semiquantitative PCR using the
iCycler iQ detection system (Bio-Rad, Hercules, CA, USA). The PCR
conditions were as follows: decontamination at 65°C for 2 min,
denaturation at 95°C for 2 min, followed by 40 cycles at 95°C for
20 sec and at 65°C for 40 sec. β-actin was used as the internal
control. The full details are shown in Table I.
 | Table IOligonucleotide sequences for
quantitative polymerase chain reaction. |
Table I
Oligonucleotide sequences for
quantitative polymerase chain reaction.
| Gene | Sequence (5′-3′) |
|---|
| MDR1 | Forward:
AAAAAGATCAACTCGTACCACTC
Reverse: GCACAAAATACACCAACAA |
| MRP1 | Forward:
ACTTCCACATCTGCTTCGTCAGTG
Reverse: ATTCAGCCACAGGAGGTAGAGAGC |
| LRP | Forward:
GCCTGACTTCTTCACAGACGTC
Reverse: TCA AAGTGCCAGTTGTAGGCC |
| GST-π | Forward:
CTGGAAGGAGGAGGTGGTG
Reverse: GACGCAGGATGGTATTGGAC |
| ABCA2 | Forward:
CCCGGAAGATTGGCCGTATCCTGG
Reverse: TTGAAGGACAGCTGGGCCCGC |
| ABCA5 | Forward:
GATGATTCACTGAAGTGTATGGGTTA
Reverse: ATCTTAACTGCCCAGACACCATGAT |
| BCL-2 | Forward:
ACGGGGTGAACTGGGGGAGGA
Reverse: TGTTTGGGGCAGGCATGTTGACTT |
| β-actin | Forward:
TGAGCGCGGCTACAGCTT
Reverse: TCCTTAATGTCACGCACGATTT |
Western blotting
Cells were eliminated by trypsinization and the
whole proteins were obtained using radioimmunoprecipitation assay
lysis buffer (Millipore, Billerica, MA, USA) extraction and
centrifugation at 12,000 × g for 10 min. Total protein
concentrations of the supernatants were measured using the
bicinchoninic acid kit (Sigma-Aldrich, St. Louis, MO, USA).
Proteins (20 μg) were separated on 12% sodium dodecyl sulfate
polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked for 2
h at room temperature in Tris-NaCl-Tween (TNT) buffer (10 mM
Tris-HCl and 150 mM NaCl, pH 7.4, 0.1% Tween-20) with 5% non-fat
dried milk, followed by incubation overnight at 4°C with rabbit
anti-MDR1, MRP1, LRP, GST-π, ABCA2, ABCA5, Bcl-2, p-ERK, p-Akt and
β-actin antibody (Santa Cruz Biotechnology, Inc.). All primary
antibodies were diluted according to the manufacturer’s
instructions. The membranes were washed and incubated for 1 h with
peroxidase-labelled anti-rabbit IgG (Santa Cruz Biotechnology,
Inc., diluted at 1:2,000). Finally, the membranes were washed three
times in TNT buffer and exposed to the Immobilon™ western
chemiluminescent horseradish peroxidase substrate (Millipore) for 1
min, and then exposed to autoradiography film for 2–3 min in the
dark. β-actin was used as the internal control.
NF-κB, STAT3, STAT5 and NFAT
transcriptional activities assay
The activity of NF-κB was determined by the reporter
gene system (Promega Corporation) according to the manufacturer’s
instructions with moderate modification. Briefly, 1×104
cells were seeded into each well of a 96-well plate and incubated
overnight to allow the adherence of cells. Then, the activity of
transcription factors was measured using a TransAM transcription
factor activity ELISA kit (Active Motif, Carlsbad, CA, USA)
according to the manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analysis was performed using one-way analysis of
variance and P<0.05 was considered to indicate a statistically
significant difference.
Results
Sorcin siRNA transfection results in
downregulation of Sorcin expression
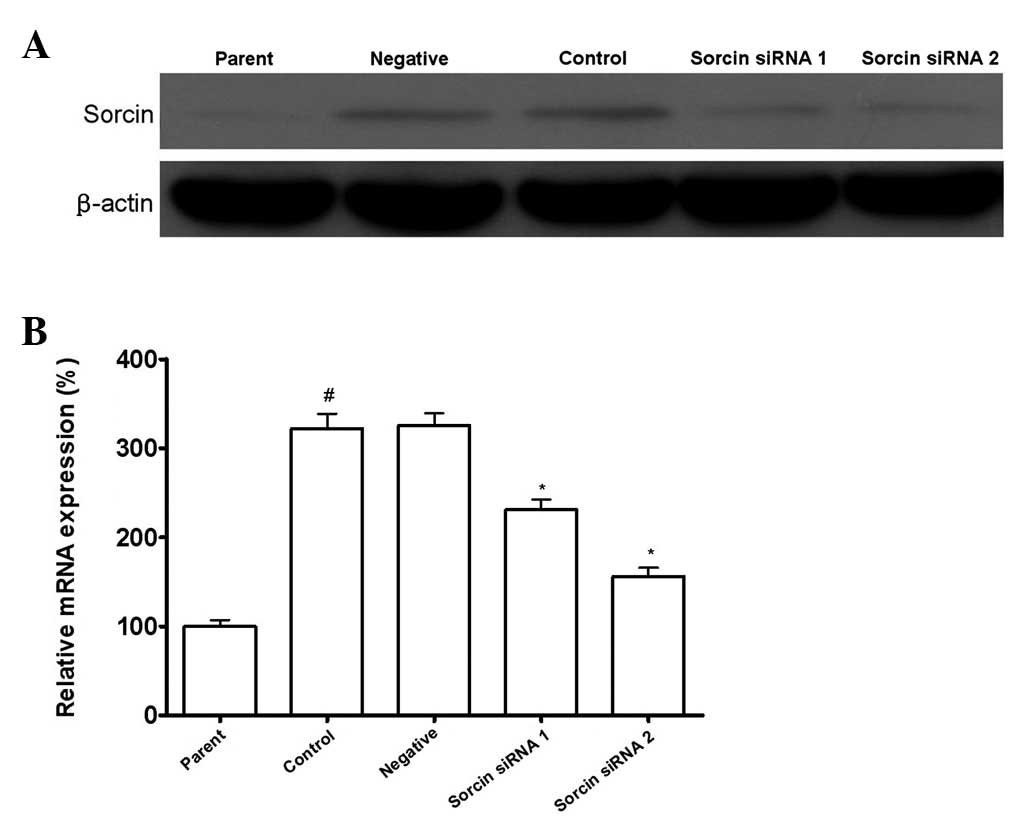
As shown in Fig. 1,
the Sorcin level in A549/DDP cells was elevated significantly
compared with the parental A549 cells. After A549/DDP cells were
transfected with Sorcin siRNA, the Sorcin level in A549/DDP
Sorcin silenced cells was decreased significantly compared with the
normal A549/DDP cells. With different silencing levels of Sorcin,
A549/DDP cells were divided into the Sorcin siRNA group 1 and
Sorcin siRNA group 2. A549/DDP cells transfected with control siRNA
were the negative group, A549/DDP cells without treatment were the
control group and A549 cells were the parent group.
Sorcin silencing increases cisplatin
sensitivity
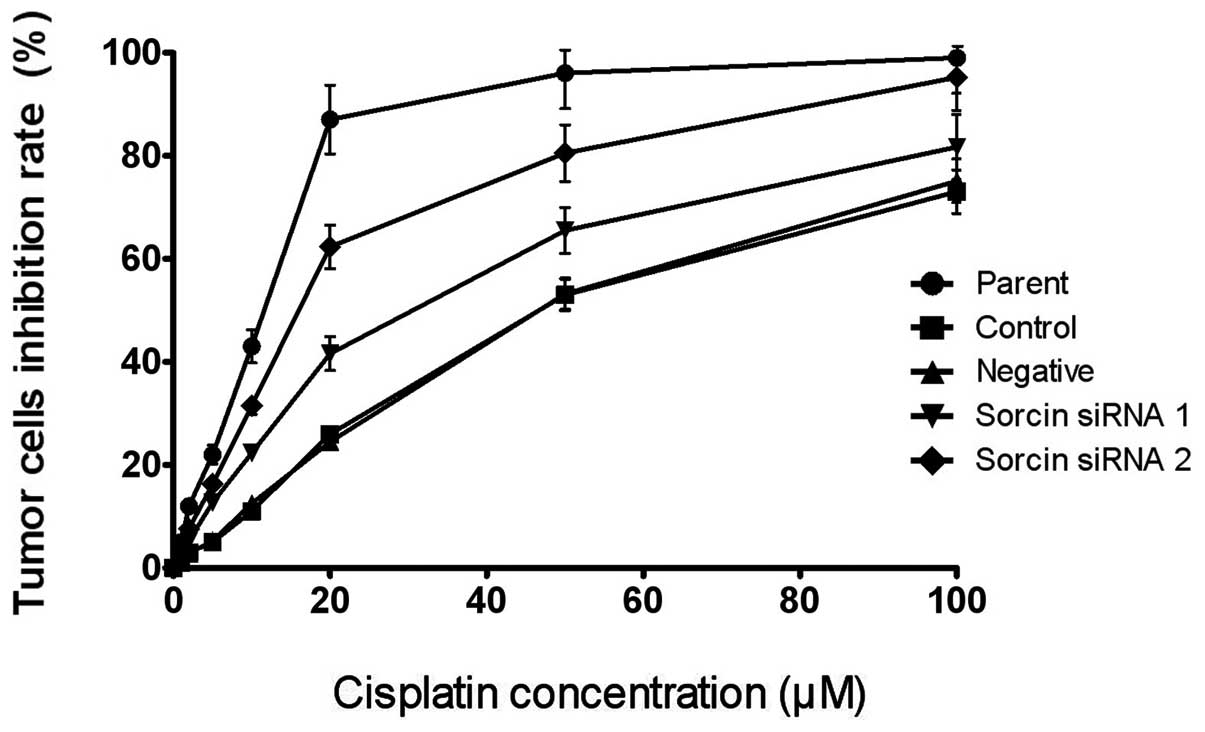
The present study determined the sensitivity of the
cells to cisplatin following Sorcin silencing using an MTS assay to
examine whether Sorcin contributed to the cisplatin resistance of
A549/DDP. The drug sensitivity of each group of cells was
represented by the IC50. As shown in Fig. 2, the IC50 values of the
Sorcin silenced cells were lower than that of the parental A549/DDP
cells. The IC50 of the Sorcin siRNA group 1 and the
Sorcin siRNA group 2 were 36.4 and 17.6 μM, respectively, however,
the IC50 of the control group and the negative group
were 46.8 and 45.6 μM, respectively, suggesting that the reversal
fold of Sorcin silencing were 1.29 and 2.66 fold, respectively
[Reversal fold = IC50 (resistance cells) /
IC50 (reversal cells)]. These results indicated that
Sorcin has a positive function in the cisplatin resistance of the
cells.
Sorcin silencing increases cell
apoptosis, cell cycle arrest in the G2/M phase and intracellular
accumulation of Rhod-123, and decreases the expression of P-gp
It was possible that the restoration of cisplatin
sensitivity by Sorcin silencing was associated with the regulation
of accumulation of intracellular drug, since increasing drug efflux
is a major drug resistance mechanism. Rhod-123 is a fluorescent
pigment that shares the same membrane transporter protein with
cisplatin and is able to reflect the intracellular drug
accumulation potency. The results from the flow cytometry
demonstrated that the Sorcin silenced cells have a greater
intracellular fluorescent activity, which indicated that more
Rhod-123 was retained in the cell. In addition, flow cytometric
analysis demonstrated that Sorcin silencing was able to
downregulate the expression of P-gp, increase cell apoptosis and
arrest the cell cycle in G2/M phase, indicating that Sorcin
silencing was able to restore cisplatin sensitivity through
regulating the drug pump and the cell cycle in A549/DDP cells
(Fig. 3).
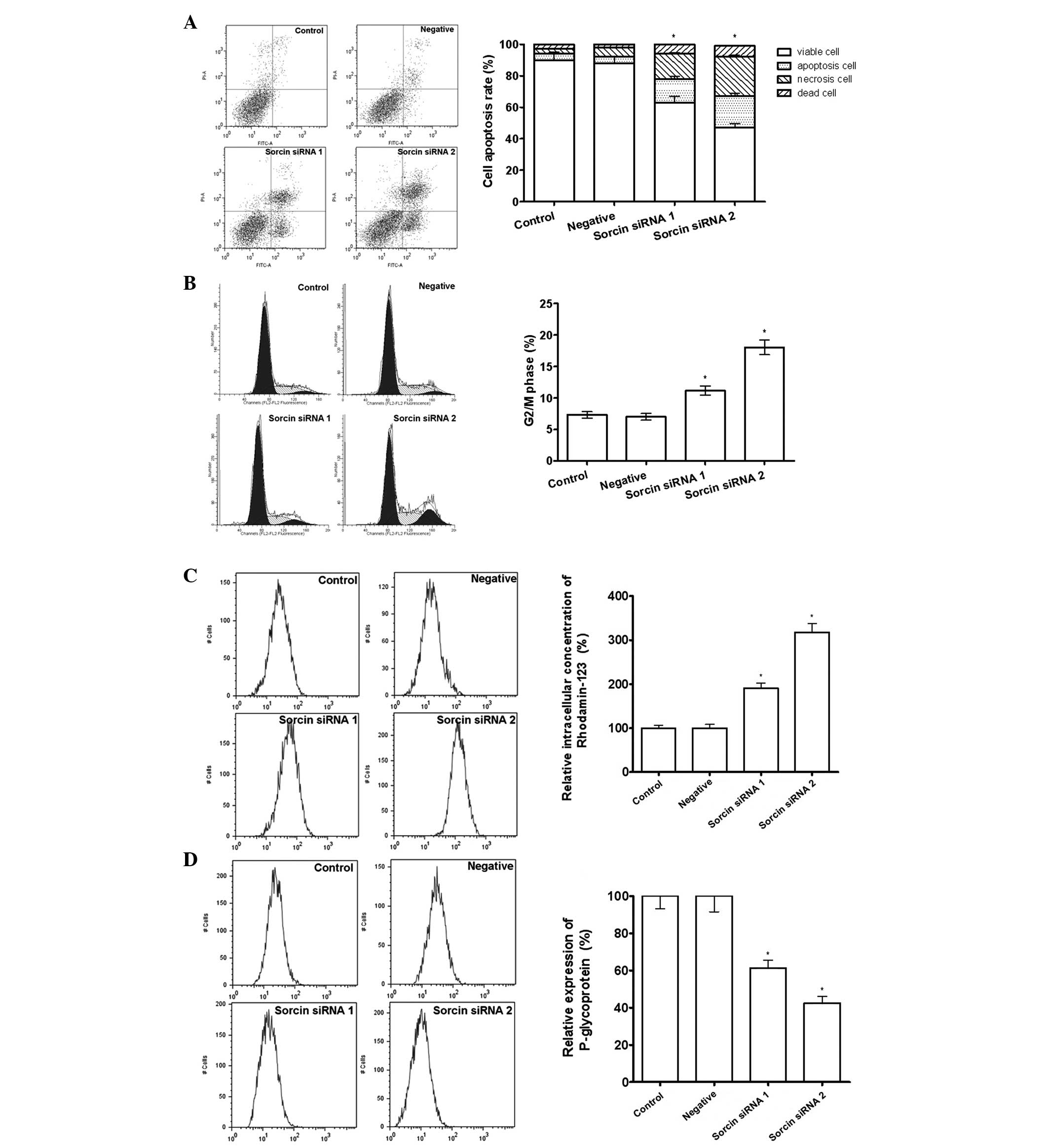 | Figure 3Effect of Sorcin silencing on drug
efflux pumps measured by flow cytometry. (A) Percentage of
apoptotic cells assessed by Annexin V and propidium iodide
staining. *P<0.05, compared with the control group,
n=3. (B) Percentage of cells arrested in the G2/M phase of the cell
cycle. *P<0.05, compared with the control group,
P<0.05, n=3. (C) Intracellular accumulation of drug assessed by
rhodamine-123 staining. *P<0.05, compared with the
control group, n=3. (D) Drug excretion assessed by P-glycoprotein
staining. *P<0.05, compared with the control group,
n=3. All the data are expressed as the mean ± standard deviation.
Sorcin, soluble resistance-related calcium-binding protein; siRNA,
small interfering RNA. |
Sorcin reverses MDR by downregulating the
expression of MDR1, MRP1, LRP, GST-π, ABCA2, ABCA5 and Bcl-2
MDR1, LRP and MRP1 are major membrane transporter
proteins that lead to MDR due to their efflux activities. The
present study was interested in whether transporters from other sub
families of ABC, including ABCA2 and ABCA5 were also involved in
the drug resistance. GST-π is a major antioxidant molecule leading
to MDR in tumor cells. Bcl-2 has been implicated in several types
of cancer and is also considered to be involved in resistance to
conventional cancer treatment. Western blot analysis demonstrated
that A549/DDP cells expressed a high level of MDR1, MRP1, LRP,
GST-π, ABCA2, ABCA5 and Bcl-2. It was then investigated whether
Sorcin was able to modulate the expression of these proteins. The
qPCR and western blotting results demonstrated that, when compared
with the high expression level observed in the A549/DDP cells, the
expression of MDR1, MRP1, LRP, GST-π, ABCA2, ABCA5 and Bcl-2
decreased in the Sorcin silenced cells (Fig. 4).
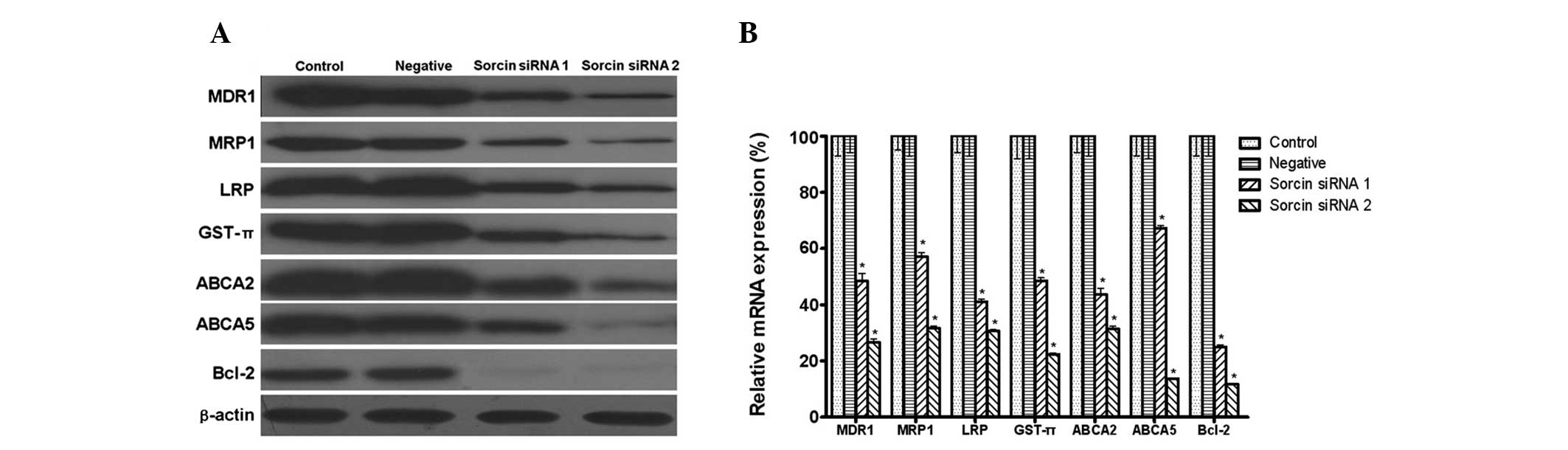 | Figure 4Expression level of multidrug
resistance genes measured by western blot analysis and quantitative
polymerase chain reaction following Sorcin silencing. (A) Protein
expression of MDR1, MRP1, LRP, GST-π, ABCA2, ABCA5 and Bcl-2 with
β-actin as a loading control (n=3 per lane). (B) mRNA expression of
MDR1, MRP1, LRP, GST-π, ABCA2, ABCA5 and Bcl-2 with β-actin as a
loading control. Data are expressed as the mean ± standard
deviation. *P<0.05, compared with the control group,
n=5. MDR1, multidrug resistance gene 1; MRP1, multidrug
resistance-associated protein 1; LRP, lung resistance protein;
GST-π, glutathione S-transferase π; ABC, ATP-binding cassette
transporter; Bcl-2, B-cell lymphoma 2; Sorcin, soluble
resistance-related calcium-binding protein; siRNA, small
interfering RNA. |
Intracellular level of GSH decreases as
the cells gain sensitivity to cisplatin
Highly cisplatin resistant cancer cells are
frequently associated with a marked increase of GSH synthesis. The
reduced intracellular GSH was able to rescue the cells from
cytotoxicity. The results demonstrated that the intracellular
concentration of GSH was decreased in Sorcin-silenced A549/DDP
cells compared with the control and negative groups. The
intracellular concentration of GSH of the Sorcin siRNA group 1 and
Sorcin siRNA group 2 were 52.7 and 31.6% compared with the control
group, respectively (Fig. 5).
Sorcin modulates the phosphorylation
level of AKT and ERK, and the transcriptional activities of NF-κB,
STAT3, STAT5 and NFAT
The present study aimed to investigate whether the
PI3K/AKT and MEK/ERK pathways acted as linkers between Sorcin and
drug resistance. The western blotting assays demonstrated that the
phosphorylation level of AKT and ERK was downregulated in
Sorcin-silenced A549/DDP cells (Fig.
6A).
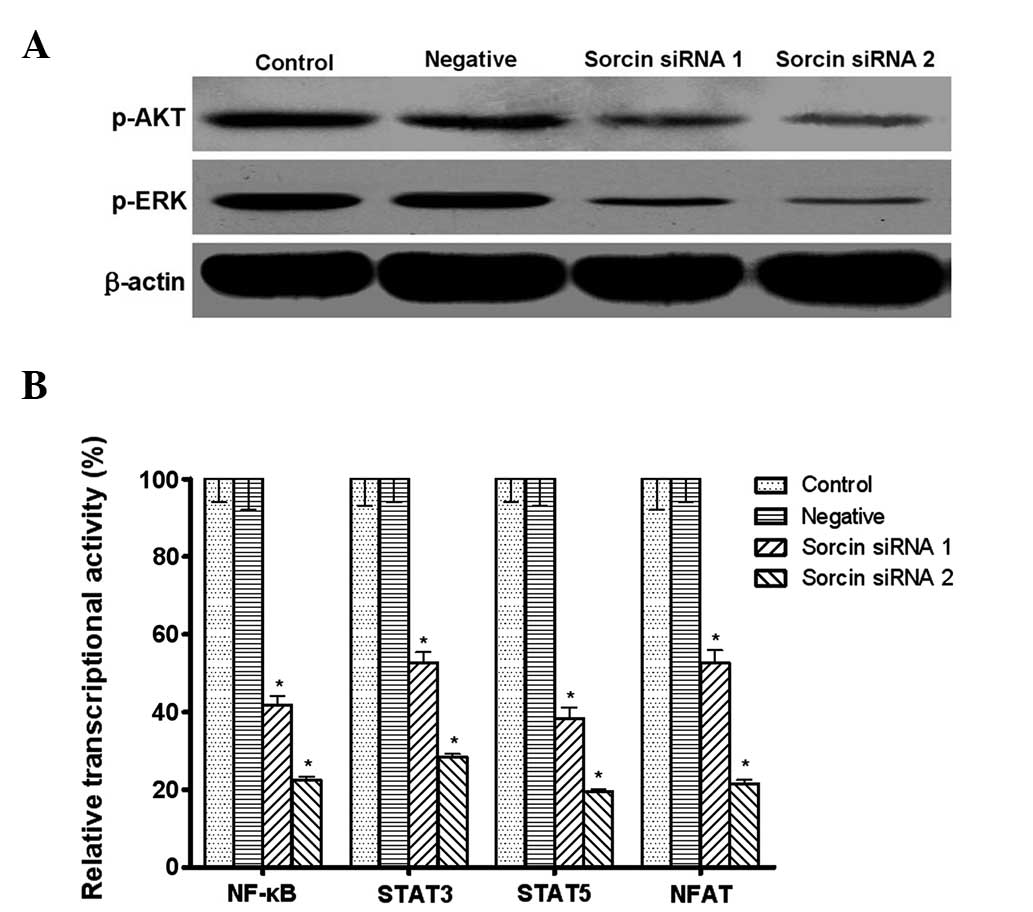 | Figure 6Effect of Sorcin silencing on
signaling molecules measured by western blot analysis and
enzyme-linked immunosorbent assay. (A) Phosphorylation of AKT and
ERK with β-actin as a loading control (n=3 per lane). (B)
Transcriptional activities of NF-κB, STAT3, STAT5 and NFAT. Data
are expressed as the mean ± standard deviation.
*P<0.05, compared with the control group, n=5. ERK,
extracellular signal-regulated kinase; NF-κB, nuclear factor κB;
STAT, signal transducer and activator of transcription; NFAT,
nuclear factor of activated T-cells; Sorcin, soluble
resistance-related calcium-binding protein; siRNA, small
interfering RNA. |
NF-κB is activated by PI3K/AKT and its activation
can lead to the expression of drug resistance-associated genes,
including MDR1 and MRP1. STAT3, STAT5 and NFAT are also shown to
control EMT and the transition is reported to contribute to the
chemoresistance of cancer cells. The present study aimed to
elucidate whether NF-κB, STAT3, STAT5 and NFAT transcriptional
activities were also modified by Sorcin. The reporter gene system
assay demonstrated that the transcriptional activities of NF-κB,
STAT3, STAT5 and NFAT were significantly decreased following Sorcin
silencing (Fig. 6B).
Discussion
A large amount of evidence has connected the
overexpression of Sorcin with MDR in cancer cells. However, the
exact roles of Sorcin in cisplatin-resistant lung cancer remain to
be elucidated. The present study demonstrated that in a human lung
cancer cell line A549/DDP, which was resistant to cisplatin, the
Sorcin protein was overexpressed. Furthermore, it was demonstrated
that several mechanisms including drug efflux were involved in
Sorcin-mediated drug resistance.
Lung cancer is one of the most common types of tumor
worldwide. The treatment of lung cancer is of great important in
China since approximately half of the patients worldwide are from
China (10). Cisplatin is widely
used as part of the combination chemotherapy (cisplatinum,
interferon, doxorubicin and 5-fluorouracil) for unresectable
disease. However, the response rate to this chemotherapy treatment
is poor. Therefore, the investigation of the mechanisms involved in
MDR is valuable for identifying new strategies to overcome the
treatment failure. The present study aimed to elucidate whether
Sorcin silencing was able to restore the drug sensitivity of
A549/DDP. The MTS assay indicated an increased cisplatin
cytotoxicity of A549/DDP cells by sorcin silencing. In other words,
the sensitivity of cancer cells to cisplatin was inversely
correlated with the cellular Sorcin level. This result was
consistent with previous studies demonstrating that Sorcin
contributed to the drug resistance of tumor cells.
One major mechanism involved in drug resistance is
increasing the drug efflux from cancer cells (11,12).
The intracellular concentration of Rhod-123 and expression of P-gp
were determined by flow cytometry. The results demonstrated that
Sorcin silencing increased the intracellular concentration of
Rhod-123 and decreased the expression of P-gp to reverse the drug
resistance of tumor cells. MDR1, LRP and MRP1 are the most common
membrane transporters that efflux drugs out of cells and are
frequently found to be overexpressed in the drug resistant
environment (13–15). The expression of these proteins in
A549/DDP cells prior to and following Sorcin silencing was
investigated. There was a significant downregulation of MDR1, LRP
and MRP1 mRNAs and proteins in Sorcin silenced A549/DDP cells,
providing solid evidence to support the theory that these membrane
transporters are involved in Sorcin-mediated cisplatin resistance
of A549/DDP cells. It has been demonstrated that Sorcin possibly
interacts with MDR1 through two distinct mechanisms (16), one is that the Sorcin and
MDR1 genes are located in the same homogeneously staining
region, and they are most likely to co-amplify under drug selection
pressure; the other is that MDR1 activation requires
Ca2+, Sorcin can bind to Ca2+ with high
affinity and regulate the concentration of Ca2+ and thus
MDR1 activation. The mechanisms of MRP1 regulation remain to be
elucidated, which makes our interpretation of the association
between Sorcin and MRP1 difficult.
MDR1 and MRP1 belong to the sub-family B and C of
the ABC family, respectively. Other sub families of ABC are also
involved in drug resistance since they pump the drugs out of the
cells. The present study focused on ABCA2 and ABCA5, which are from
the sub-family A. It has been reported that the overexpression of
ABCA2 is a response to the resistance to estramustine (17). However, to the best of our
knowledge, there are no studies investigating the role of ABCA2 and
ABCA5 in cisplatin resistance. A considerable body of evidence
highlights the importance of ABC proteins in cancer extending
beyond drug transport to fundamental roles in tumor biology
(18). Huang et al
demonstrated that ABCA5 was upregulated in the stem cell-like side
population of lung cancer PLC/PRF/5 cells. Drug resistance is one
major property of cancer stem cells (19–21).
The present study demonstrated that the expression of ABCA2 and 5
was elevated in A549/DDP cells and mediated by Sorcin. Whether
cisplatin is the substrate of these two ABC proteins is unclear.
There is a possibility that ABCA2 and 5 are important in cisplatin
resistance in A549/DDP cells other than drug efflux, which requires
further investigation.
Bcl-2 is a protein that has an anti-apoptotic effect
in cancer cells. The finding that Bcl-2 was upregulated in A549/DDP
cells indicated that the resistance to cisplatin-induced apoptosis
is also involved in these cells. The regulation of Bcl-2 is
complicated. The present study demonstrated that ERK activity was
modified by Sorcin, which lead to the consequent regulation of
Bcl-2. In addition, it has also been reported that GSH is able to
promote Bcl-2-mediated cisplatin resistance (22).
Other mechanisms are also involved in tumor
resistance to cisplatin. One is GSH accumulation in cancer cells.
The present study demonstrated that the level of GSH and GST-π was
downregulated following Sorcin silencing. The synthesis of GSH is
mediated by several enzymes. γ-glutamylcysteine synthetase (γ-GCS),
the rate-limiting enzyme for GSH biosynthesis, is found to be
elevated in numerous cases of cisplatin resistance (23). Transcriptional and
post-transcriptional regulations have been reported for the
upregulation of γ-GCS (22).
Oxidative stress is able to stabilize the mRNA of
γ-GCS through the MEK/ERK pathway (24). In the present study, the p-ERK
level, which indicated the activity of MEK/ERK, was attenuated by
Sorcin silencing. This may be the possible regulatory mechanism of
γ-GCS in A549/DDP cells. It was revealed that Sorcin silencing led
to the downregulation of AKT phosphorylation indicating that the
PI3K/AKT pathway was modulated by Sorcin. Further investigation
confirmed that the activity of NF-κB, which is downstream to
PI3K/AKT, was also inhibited by Sorcin silencing (25). To the best of our knowledge, there
are no studies investigating the association between Sorcin and
STAT3, STAT5 and NFAT. The present study was interested in these
proteins since they are known to be involved in EMT, which is
correlated with cancer malignancy and drug resistance (26–28).
In conclusion, by downregulating the expression of
Sorcin in A549/DDP cells, the present study revealed that Sorcin
was important in the drug resistance of A549/DDP cells. Targeting
Sorcin remains a promising strategy to reverse drug resistance by
several mechanisms. In addition, the PI3K/Akt and MEK/ERK pathways
are important.
References
|
1
|
Sereno M, Rodríguez-Esteban I,
Gómez-Raposo C, et al: Lung cancer and peritoneal carcinomatosis.
Oncol Lett. 6:705–708. 2013.PubMed/NCBI
|
|
2
|
Chung AS, Wu X, Zhuang G, et al: An
interleukin-17-mediated paracrine network promotes tumor resistance
to anti-angiogenic therapy. Nat Med. 19:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadava D and Kane SE: Silibinin reverses
drug resistance in human small-cell lung carcinoma cells. Cancer
Lett. 339:102–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li X, Wang J, Liu J, et al: Engagement of
soluble resistance-related calcium binding protein (sorcin) with
foot-and-mouth disease virus (FMDV) VP1 inhibits type I interferon
response in cells. Vet Microbiol. 166:35–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Li S, Yang M, et al: Sorcin
silencing inhibits epithelial-to-mesenchymal transition and
suppresses breast cancer metastasis in vivo. Breast Cancer Res
Treat. 143:287–299. 2014. View Article : Google Scholar
|
|
6
|
Li GY, Liu JZ, Zhang B, et al: Tegillarca
granosa extract Haishengsu (HSS) suppresses expression of mdr1,
BCR/ABL and sorcin in drug-resistant K562/ADM tumors in mice. Adv
Med Sci. 58:112–117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maddalena F, Laudiero G, Piscazzi A, et
al: Sorcin induces a drug-resistant phenotype in human colorectal
cancer by modulating Ca(2+) homeostasis. Cancer Res.
71:7659–7669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landriscina M, Laudiero G, Maddalena F, et
al: Mitochondrial chaperone Trap1 and the calcium binding protein
Sorcin interact and protect cells against apoptosis induced by
antiblastic agents. Cancer Res. 70:6577–6586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Cheng X, Li S, et al: Inhibition of
sorcin reverses multidrug resistance of K562/A02 cells and
MCF-7/A02 cells via regulating apoptosis-related proteins. Cancer
Chemother Pharmacol. 72:789–798. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qu Y, Yang Y, Liu B, et al: Comparative
proteomic profiling identified sorcin being associated with
gemcitabine resistance in non-small cell lung cancer. Med Oncol.
27:1303–1308. 2010. View Article : Google Scholar
|
|
11
|
Kawakami M, Nakamura T, Okamura N, et al:
Knock-down of sorcin induces up-regulation of MDR1 in HeLa cells.
Biol Pharm Bull. 30:1065–1073. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akazawa Y, Kawaguchi H, Funahashi M, et
al: Effect of interferons on P-glycoprotein-mediated rhodamine-123
efflux in cultured rat hepatocytes. J Pharm Sci. 91:2110–2115.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Toner AP, McLaughlin F, Giles FJ, et al:
The novel toluidine sulphonamide EL102 shows pre-clinical in vitro
and in vivo activity against prostate cancer and circumvents MDR1
resistance. Br J Cancer. 109:2131–2141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tajitsu Y, Ikeda R, Nishizawa Y, et al:
Molecular basis for the expression of major vault protein induced
by hyperosmotic stress in SW620 human colon cancer cells. Int J Mol
Med. 32:703–708. 2013.PubMed/NCBI
|
|
15
|
Keppler D: Multidrug resistance proteins
(MRPs, ABCCs): importance for pathophysiology and drug therapy.
Handb Exp Pharmacol. 201:299–323. 2011. View Article : Google Scholar
|
|
16
|
Zheng BB, Zhang P, Jia WW, Yu LG and Guo
XL: Sorcin, a potential therapeutic target for reversing multidrug
resistance in cancer. J Physiol Biochem. 68:281–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boonstra R, Timmer-Bosscha H, van
Echten-Arends J, et al: Mitoxantrone resistance in a small cell
lung cancer cell line is associated with ABCA2 upregulation. Br J
Cancer. 90:2411–2417. 2004.PubMed/NCBI
|
|
18
|
Noguchi K, Katayama K and Sugimoto Y:
Human ABC transporter ABCG2/BCRP expression in chemoresistance:
basic and clinical perspectives for molecular cancer therapeutics.
Pharmgenomics Pers Med. 7:53–64. 2014.PubMed/NCBI
|
|
19
|
Huang L, Lu Q, Han Y, Li Z, Zhang Z and Li
X: ABCG2/V-ATPase was associated with the drug resistance and tumor
metastasis of esophageal squamous cancer cells. Diagn Pathol.
7:1802012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cuestas ML, Sosnik A and Mathet VL:
Poloxamines display a multiple inhibitory activity of ATP-binding
cassette (ABC) transporters in cancer cell lines. Mol Pharm.
8:1152–1164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Y, Liu JH, Chai K, Tashiro S, Onodera
S and Ikejima T: Inhibition of c-Met promoted apoptosis, autophagy
and loss of the mitochondrial transmembrane potential in
oridonin-induced A549 lung cancer cells. J Pharm Pharmacol.
65:1622–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kohsaka S, Takahashi K, Wang L, et al:
Inhibition of GSH synthesis potentiates temozolomide-induced
bystander effect in glioblastoma. Cancer Lett. 331:68–75. 2013.
View Article : Google Scholar
|
|
24
|
Tang SC, Wu CH, Lai CH, et al: Glutathione
S-transferase mu2 suppresses cancer cell metastasis in non-small
cell lung cancer. Mol Cancer Res. 11:518–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paolo M, Assunta S, Antonio R, et al:
Selumetinib in advanced non small cell lung cancer (NSCLC)
harbouring KRAS mutation: endless clinical challenge to KRAS-mutant
NSCLC. Rev Recent Clin Trials. 8:93–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dauphin M, Barbe C, Lemaire S, et al:
Vimentin expression predicts the occurrence of metastases in non
small cell lung carcinomas. Lung Cancer. 81:117–122. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Zhang JG, Shi Y, et al: MiR-130b
is a prognostic marker and inhibits cell proliferation and invasion
in pancreatic cancer through targeting STAT3. PLoS One.
8:e738032013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jans R, Mottram L, Johnson DL, et al:
Lysophosphatidic acid promotes cell migration through STIM1- and
Orai1-mediated Ca2+(i) mobilization and NFAT2
activation. J Invest Dermatol. 133:793–802. 2013. View Article : Google Scholar :
|