Introduction
Nephrolithiasis is associated with multiple
metabolic abnormalities and is one of the most prevalent diseases
with uncertain etiology amongst adults (1). Idiopathic hypercalciuria (IH) is the
most common metabolic disorder to occur in adult calcium stone
formers, present in ~30–60% of such patients, and is also common
among pediatric calcium stone formers (2). The development of IH is influenced by
genetic background and environment (2). Previous studies have demonstrated
that hypercalciuria may involve the dysregulation of multiple
calcium transport systems, including increased intestinal
absorption of calcium, primary renal calcium leakage and increased
bone demineralization (3,4). Despite extensive study, the molecular
and genetic causes of stone diseases have remained elusive.
Members of the transforming growth factor β (TGF-β)
family participate in numerous physiological and pathological
processes, have vital roles in biological processes, including cell
differentiation, proliferation and apoptosis, and regulate the
growth, development and regeneration of extracellular materials
(5). The function of the
profibrotic cytokine TGF-β1 in the initiation and progression of
fibrosis in the kidney has been extensively studied and certain
studies have indicated that TGF-β1 may have an important function
in mediating the epithelial to mesenchymal transition (EMT), where
renal tubular epithelial cells lose their epithelial phenotype and
a novel mesenchymal phenotype is obtained following treatment with
TGF-β1 (6,7). Furthermore, studies have revealed
that Ca2+ is a potent, suppressive effector that induces
undifferentiated mesenchymal stem cells (MSCs) to differentiate
into numerous cell types via intracellular Ca2+ release
(8). However, to date, the
specific effects of TGF-β1 and Ca2+ in IH, as well as in
renal stone formation, have remained to be elucidated.
In the present study, the expression of bone
morphogenetic protein 2 (BMP2), osteopontin (OPN) and
1,25-dihydroxyvitamin D3 receptor (VDR) were evaluated
in nephrolithiasis patients with IH, compared with renal stone
patients without IH and healthy age-matched normal controls. These
factors are closely associated with osteoblastic differentiation
and bone formation. The results of the present study indicated that
TGF-β1 and Ca2+ may regulate the expression of
bone-associated factors, including BMP2, OPN and VDR, in renal
tubular epithelial cells in IH to further promote calculus
formation in situ. Taken together, it was hypothesized that
TGF-β1-induced EMT and Ca2+-induced mesenchymal
osteogenetic differentiation were involved in nephrolithiasis in
IH.
Materials and methods
Patients
In the present study, 29 nephrolithiasis patients
with idiopathic hypercalciuria (mean ± standard deviation; age,
32.1±9.5 years; range, 20–57 years) were recruited as group IH and
29 healthy age-matched volunteers (30.5±10.6 years; range, 22–54
years) were recruited as normal controls (NC group). Furthermore,
29 renal stone patients without idiopathic hypercalciuria
(35.6±12.1 years; range, 21–58 years) were recruited as the nIH
group. IH was diagnosed by 24-h urine Ca2+ excretion
rates >140 mg Ca2+/g urine creatinine on outpatients
without dietary restrictions (9).
Patients with systemic Ca2+ disorders, including
hyperthyroidism and diabetic nephropathy, were excluded. All
procedures were approved by the Ethical Committee of Tongji
Hospital of Tongji Medical College, Huazhong University of Science
and Technology (Wuhan, China). Prior to participation in the study,
informed consent was obtained from all subjects.
Measurement of TGF-β1, BMP2 and OPN by
ELISA
Blood samples (5 ml) were drawn from patients in the
IH, NC and nIH groups. Following centrifugation at 20,000xg for 15
min, sera were collected and aliquoted into 96-well plates (100
μl/well). The expression levels of TGF-β1, BMP2 and OPN were
detected using corresponding TGF-β1-, BMP2-and OPN-specific human
ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA). Sera
samples were acidified with 1 mol/l HCl solution to activate the
cytokines to their immunoreactive states (pH 2.0–3.0), prior to
neutralization according to the manufacturer’s instructions and the
addition of 50 μl diluent RD-121 to each well. Subsequently, a 50
μl sample from each group was added in triplicate to each well for
2 h. Following washing three times with phosphate-buffered saline
(PBS; pH 7.2) containing 0.05% Tween 20 (Sigma-Aldrich), substrate
solution was added to each well for 30 min until the reaction was
stopped by the additon of Stop Solution. Optical density was
measured at 450 nm using microplate reader SPR-960 (Sunostik
Medical Technology Co., Ltd, Changchun, China) and cytokine levels
were calculated by standard curves.
Preparation of primary renal epithelial
cells (PRECs) from patients with IH
All patients with IH received invasive surgical
treatment for urolithiasis. Two patients were required to receive a
nephrectomy due to ipsilateral renal severe hydronephrosis and
renal failure, according to diagnosis by computed creatinine and
emission tomography tests. A section of renal tissue was removed
from each subject under sterile conditions. Isolation and culture
of human renal epithelial cells was performed as described by a
previous study (10). Briefly,
cortical tissue was finely minced, washed multiple times and
agitated for 20 min at 37°C in Hanks’ balanced salt solution (HBSS)
containing collagenase type II and calcium (Merck Co., Shanghai,
China). HBSS was added and the solution containing tubular
fragments was passed through a 100-μm sieve (LFJ706; EHSY Co.,
Shanghai, China). The tubular fragments were subsequently
resuspended in 45% percoll in phosphate-buffered saline (PBS), and
centrifuged at 20,000 xg for 10 min. High-density tubular fragments
were removed and cultured in serum-free, hormonally defined
Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (HyClone, GE
Healthcare, Little Chalfont, UK) containing penicillin (50 U/ml)
and streptomycin (50 μg/ml; Invitrogen Life Technologies, Carlsbad,
CA, USA).
HK-2 cells, a permanent and well-characterized human
proximal tubular cell line immortalized by transduction with human
papillomavirus 16 E6/E7 genes, were purchased from the China Center
for Type Culture Collection (Wuhan, China). HK-2 cells were
cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS;
Gibco-BRL, Invitrogen Life Technologies), 50 U/ml penicillin and 50
μg/ml streptomycin. Serum-free medium was used when serum
starvation was required.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction was performed using
TRIzol® reagent (Invitrogen Life Technologies). cDNA
synthesis (2 μg total RNA) was performed using Omniscript Reverse
Transcriptase (Qiagen, Hilden, Germany) for first-strand cDNA
synthesis and oligo-deoxythymine primer generation according to the
manufacturer’s instructions. The mRNA expression levels of BMP2,
OPN, VDR and housekeeping gene β-actin were analyzed using qPCR on
a Rotor Gene 3000 (Corbett Research, Sydney, Australia) using SYBR
Premix Ex TaqII (Takara Bio, Inc., Otsu, Japan).
Amplification reactions were performed under the following
conditions: 95°C denaturation for 10 min, followed by 40 cycles of
95°C for 15 sec, 59°C for 15 sec and 72°C for 30 sec. The primer
sequences used were as follows: BMP2 forward,
5′-CTTCTAGCGTTGCTGCTTCC-3′ and reverse, 5′-AGAGCCTGCGATACAGGTCT-3′;
OPN forward, 5′-GCCAAACGCCGACCAAGGTACA-3′ and reverse,
5′-TTCCTGCACAGTCACCCACTGAA-3′; VDR forward,
5′-GCCCACCATAAGACCTACGA-3′ and reverse, 5′-AGATTGGAGAAGCTGGACGA-3′;
β-actin forward, 5′-CACGATGGAGGGGCCGGACTCATC-3′ and reverse,
5′-TAAAGACCTCTATGCCAACACAGT-3′. The Ct values were calculated using
the comparative Ct (ΔΔCt) method. The fold difference was
calculated using the 2−ΔΔCt method.
Western blot analysis
Extracts of total cellular proteins were prepared by
scraping the cells into Mammalian Protein Extraction reagent
(78503; Pierce, Thermo Fisher Scientific Inc., Rockford, IL, USA).
Protein concentration was detected using the bicinchoninic acid
(BCA) protein assay kits (#23235; Pierce). The protein samples were
separated by 12% SDS-PAGE and electrotransferred onto
polyvinylidene difluoride-nitrocellulose membranes (Immobilon-P;
EMD Millipore, Billerica, MA, USA). Blots were incubated with mouse
monoclonal immunoglobulin (Ig)G2a BMP2 antibody
(SC-137087; 1:200; Santa Cruz Biotechnology Inc., Dallas, TX, USA),
polyclonal rabbit anti-human IgG OPN antibody (BA1678; 1:400;
Boster Systems, Inc., Pleasanton, CA, USA) or polyclonal rabbit
anti-human IgG VDR antibody (BA2877-2; 1:400; Boster Systems,
Inc.). In addition, polyclonal rabbit anti-GAPDH IgG antibody
(2275-PC-100; 1:1,000; R&D Systems, Inc.) was usedto ensure
equal loading. The membranes were incubated with secondary
antibodies (goat anti-human IgG-horseradish peroxidase; 1:2,000;
Pierce). Proteins were detected using enhanced chemiluminescence
(ECL) Prime western blotting detection reagent (GE Healthcare). The
blots were quantified by densitometry using TotalLab Quant software
(TL120 v2009; TotalLab Ltd, Newcastle upon Tyne, UK).
Treatment of TGF-β1 and Ca2+
for PRECs and HK-2
PRECs and HK-2 cells were cultured and passaged to
the third generation prior to treatment with TGF-β1 (PeproTech
Inc., Rocky Hill, NJ, USA) and/or Ca2+ in the form of
calcium chloride (Fortuna Chemical Co., Wuhan, China) at different
concentrations. To inhibit translation, cycloheximide (5 μg/ml,
Sigma-Aldrich, St. Louis, MO, USA) was added 30 min prior to the
addition of TGF-β1 for the indicated time-period. The
concentrations of TGF-β1 were 0.5, 2.0 and 5.0 ng/ml, while the
Ca2+ concentrations were 0.5, 1.5 and 2.5 mM. All cells
were plated in 24-well plates at 4×109 cells/well in
DMEM supplemented with 10% FBS for 24 h prior to experiments.
Statistical analysis
Differences among groups were assessed using one-way
analysis of variance combined with the Bonferroni test using SPSS
13.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test was used to
compare two independent samples. Values are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
Serum TGF-β1, BMP2 and OPN concentrations
are significantly higher in patients with IH than those in
age-matched controls
Serum samples from a total of 58 patients from the
IH and nIH groups, and 29 subjects from the NC group were analyzed
by ELISA to detect serum TGF-β1, BMP2 and OPN levels. As shown in
Fig. 1, TGF-β1, BMP2 and OPN serum
levels were all significantly higher in IH patients than those in
the NC group (P<0.01). The higher expression levels of TGF-β
(Fig. 1A) and OPN (Fig. 1B) in the nIH group compared with
those in the NC group (P<0.01) suggested that these factors were
significantly increased in patients with renal stones. Furthermore,
the results also indicated that the expression levels of all three
cytokines in the IH group were significantly higher than those in
the nIH group (P<0.01), suggesting that Ca2+ may be a
critical factor in regulating cytokine secretion.
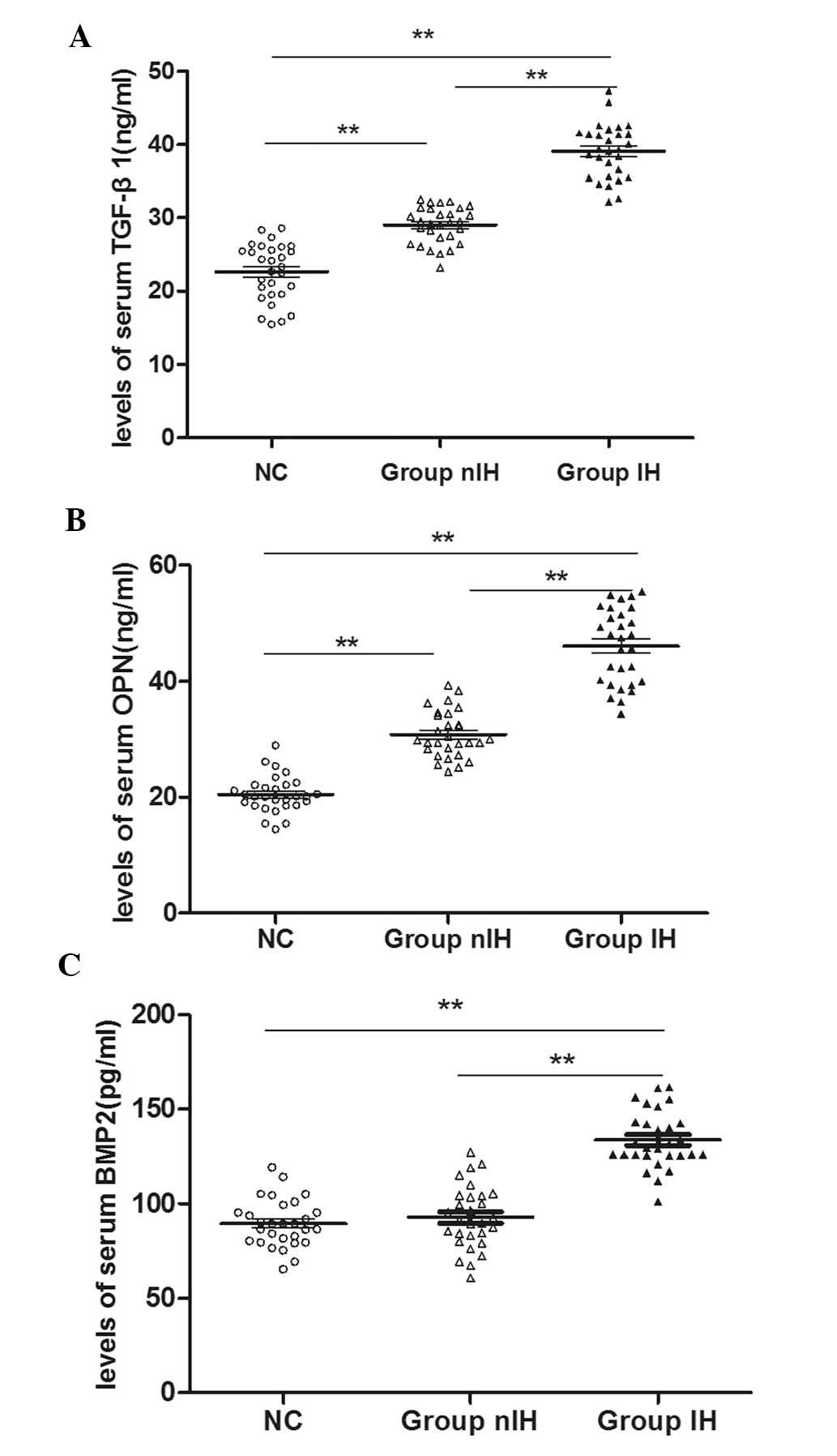 | Figure 1Serum expression levels of TGF-β1,
BMP2 and OPN in the IH, nIH and NC groups. Expression levels were
determined by ELISA. (A) TGF-β1 expression levels in the IH group
were significantly higher than those in the NC group. Expression
levels of TGF-β1 in the nIH group were also significantly higher
than those in the NC group. Serum TGF-β1 expression was also
significantly different between the nIH and NC groups. (B)
Significant differences were also detected in the serum expression
levels of OPN between each group. (C) VDR expression levels in the
IH group were significantly higher than those in the NC group and
levels of VDR in the IH group were significantly higher than those
in the nIH group. There were no significant differences in serum
VDR expression levels between the nIH and NC groups. Values are
expressed as the mean ± standard deviation (n=29).
**P<0.01. TGF-β1, transforming growth factor-β1;
BMP2, bone morphogenetic protein 2; OPN, osteopontin; VDR,
1,25-dihydroxyvitamin D3 receptor; IH, idiopathic
hypercalciuria; group IH, patients with IH; group nIH, renal stone
patients without IH; NC, healthy age-matched controls. |
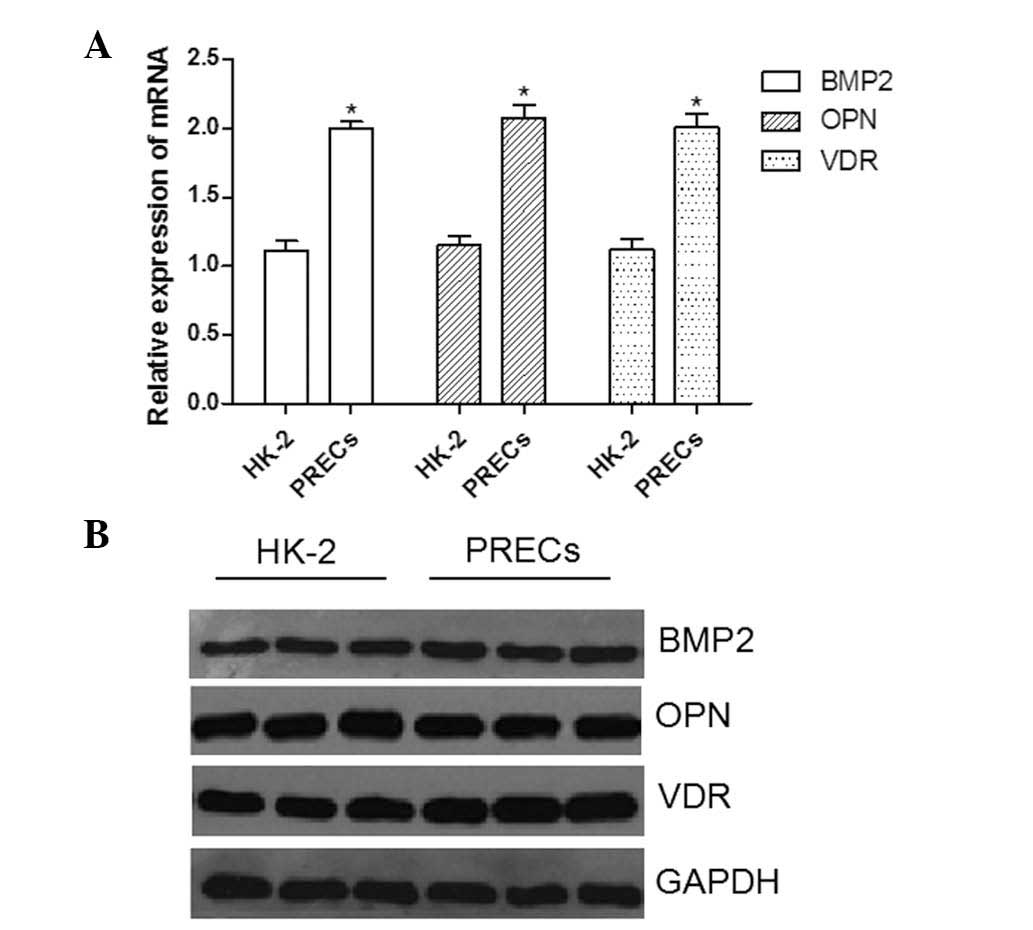
Basal expression levels of BMP2 and VDR
are higher in PRECs than those in HK-2 cells
Basal levels of bone-associated factors BMP2, OPN
and VDR were evaluated in PRECs and HK-2 cells. As shown in
Fig. 2A, the results of RT-qPCR
analysis demonstrated that mRNA expression levels of BMP2, OPN and
VDR were significantly higher in PRECs than those in HK-2 cells
(P<0.05). Western blot analysis (Fig. 2B) confirmed that there were
analogous variations in BMP2 and VDR protein expression between
PRECs and HK-2 cells (P<0.05). By contrast, no significant
difference was detected in OPN protein expression levels between
the two types of cell (P=0.076).
Bone-associated factors are significantly
increased by TGF-β1 or Ca2+ stimulation alone in PRECs
compared with those in HK-2 cells
To determine whether TGF-β1 or Ca2+
influenced the changes in bone-associated factors in PRECs and HK-2
cells in vitro, the mRNA expression levels of BMP2, OPN and
VDR were examined in each group. Cells were cultured with
cycloheximide to inhibit further protein synthesis so that only
mRNAs of direct TGF-β1 targets were activated. Subsequently,
various concentrations of TGF-β1 (0.5, 2.0 and 5.0 ng/ml) and
calcium chloride (0.5, 1.5 and 2.5 mM) were added, and RNA was
assessed following 48 h of incubation. Total mRNA from PRECs
treated with TGF-β1 and Ca2+, respectively, was compared
with mRNA extracted from analogously treated HK-2 cells. As shown
in Fig. 3, following incubation
with TGF-β1, the mRNA expression levels of BMP2, OPN and VDR in
PRECs steadily increased in a dose-dependent manner; however, no
significant differences were detected in HK-2 cells with increasing
TGF-β1 dosage. The levels of BMP2, OPN and VDR in PRECs treated
with TGF-β1 at doses of 2.0–5.0 ng/ml were significantly higher
than those in PRECs prior to treatment (Fig. 3A–C). Furthermore, the BMP2, OPN and
VDR mRNA expression levels induced by treatment with 5.0 ng/ml
TGF-β1 were significantly higher than those induced by 2.0 ng/ml
TGF-β1 (P<0.05). Analogously with these trends, when the dose of
Ca2+ was increased from 0.5 to 2.5 mM, the gene
expression levels of bone-associated factors in PRECs increased. In
particular, a statistically significant increase in expression
levels was observed following treatment with 2.5 mM
Ca2+, compared to those in untreated PRECs (P<0.01;
Fig. 3). The results also
indicated that the fold change in VDR mRNA expression levels in
PRECs induced by Ca2+ treatment at concentrations of 1.5
and 2.5 mM were significantly different (Fig. 3F). These data indicated that the
primary isolated cells from patients with IH exhibited a consistent
and dose-dependent response to TGF-β1 and Ca2+ alone,
via the activation of bone-associated genes.
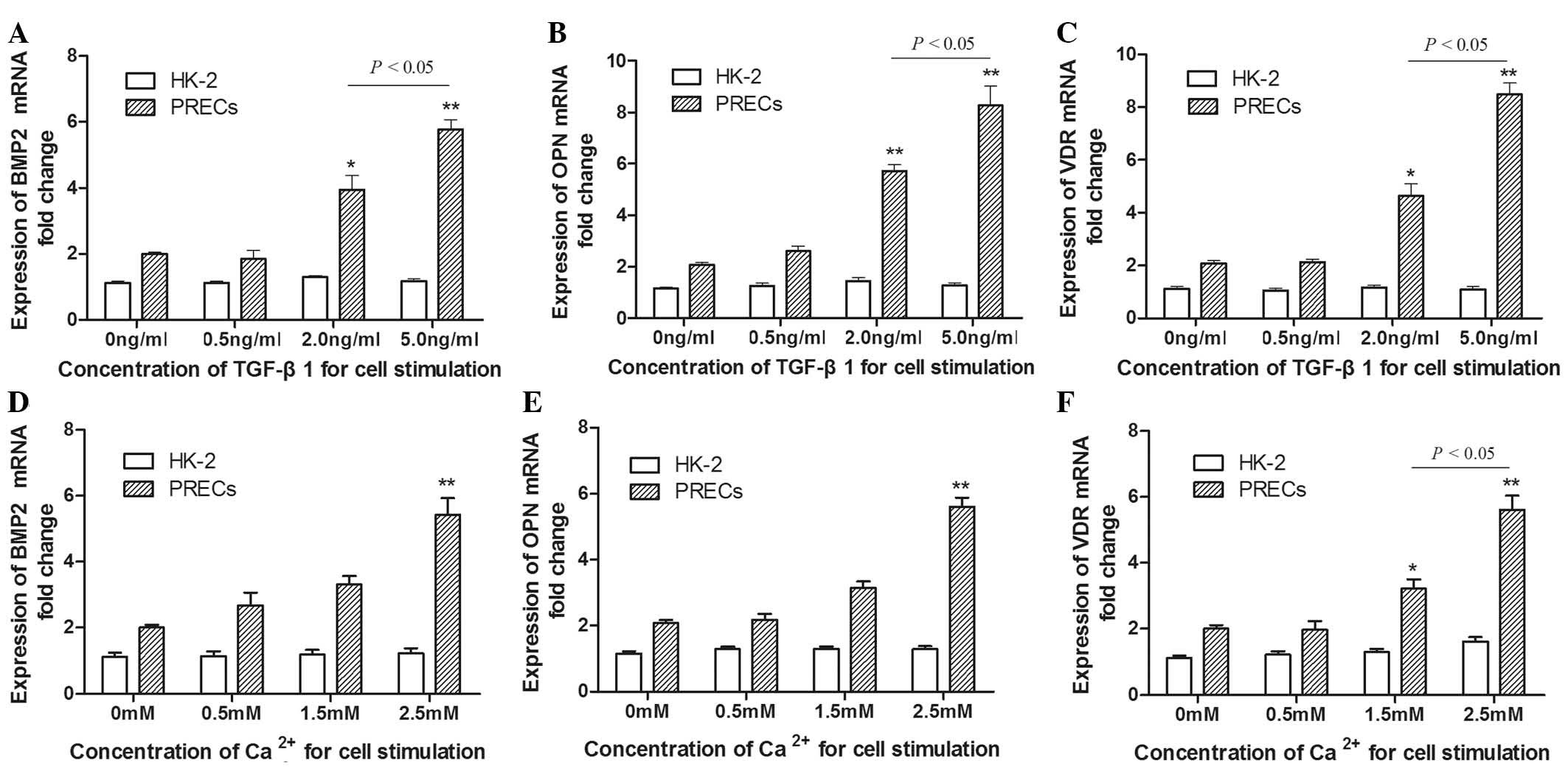 | Figure 3Bone-associated factors were detected
using reverse transcription quantitative polymerase chain reaction
in PRECs and HK-2 cell lines following incubation with TGF-β1 or
Ca2+ alone for 48 h. (A) mRNA expression levels of BMP2
in PRECs increased in a dose-dependent manner following incubation
with TGF-β1 at various concentrations. BMP2 mRNA expression levels
were significantly higher following incubation with TGF-β1 at 2.0
and 5.0 ng/ml than those prior to treatment. No significant
differences were detected in HK-2 cells following identical
treatment with TGF-β1. (B) OPN and (C) VDR mRNA expression levels
in PRECs treated with TGF-β1 also increased in a dose-dependent
manner. OPN and VDR expression levels were statistically different
at concentrations of 2.0 and 5.0 ng/ml compared with those in PRECs
prior to treatment. (D) When the dose of Ca2+ was
increased from 0.5 to 2.5 mM, the gene expression profile of BMP2
increased, and this increase was statistically significant at 2.5
mM. An analagous effect was observed in the expression profiles of
(E) OPN and (F) VDR. Furthermore, the increase in VDR mRNA
expression caused by Ca2+ treatment at 2.5 mM was
significantly greater than that at 1.5 mM. Values are expressed as
the mean ± standard deviation. *P<0.05,
**P<0.01 vs. untreated cells. TGF-β1, transforming
growth factor-β1; BMP2, bone morphogenetic protein 2; OPN,
osteopontin; VDR, 1,25-dihydroxyvitamin D3 receptor;
PRECs, primary renal epithelial cells; mRNA, messenger RNA. |
Combined stimulation with TGF-β1 and
Ca2+ significantly increases cellular bone-associated
factor expression in PRECs and HK-2 cells
The effects of treatment with a combination of
TGF-β1 (5.0 ng/ml) and Ca2+ (2.5 mM) (TGF-β1 +
Ca2+) were evaluated in order to elucidate whether this
induced the upregulation of cellular bone-associated factors in
HK-2 cells and PRECs. A previous study revealed that in renal
epithelial cell culture, TGF-β1 activated a program of mesenchymal
marker gene expression associated with the transition of epithelial
cells to more mesenchymal phenotypes (5). In the present study, it was therefore
hypothesized that Ca2+ may have a significant role in
enhancing TGF-β-induced EMT. As shown in Fig. 4, the mRNA expression levels of
BMP2, OPN and VDR in HK-2 cells and PRECs which were treated with
TGF-β1 + Ca2+ were significantly higher than those of
the control group (P<0.01). This result suggested that
Ca2+ may have a synergistic effect on the mechanism of
TGF-β-induced EMT, and promote cells expressing mesenchymal markers
to differentiate into cells with bone-associated phenotypes. The
results displayed in Fig. 4A
confirmed that there were no significant differences in BMP2 mRNA
expression fold-change between HK-2 cells and PRECs following
TGF-β1 + Ca2+ treatment (P>0.05). It was also
demonstrated that there were no significant differences in the mRNA
expression levels of OPN between HK-2 cells and PRECs following
TGF-β1 + Ca2+ treatment (P>0.05). By contrast,
significant differences between HK-2 cells and PRECs were detected
in the expression of VDR mRNA following TGF-β1 + Ca2+
treatment (P<0.05; Fig.
4C).
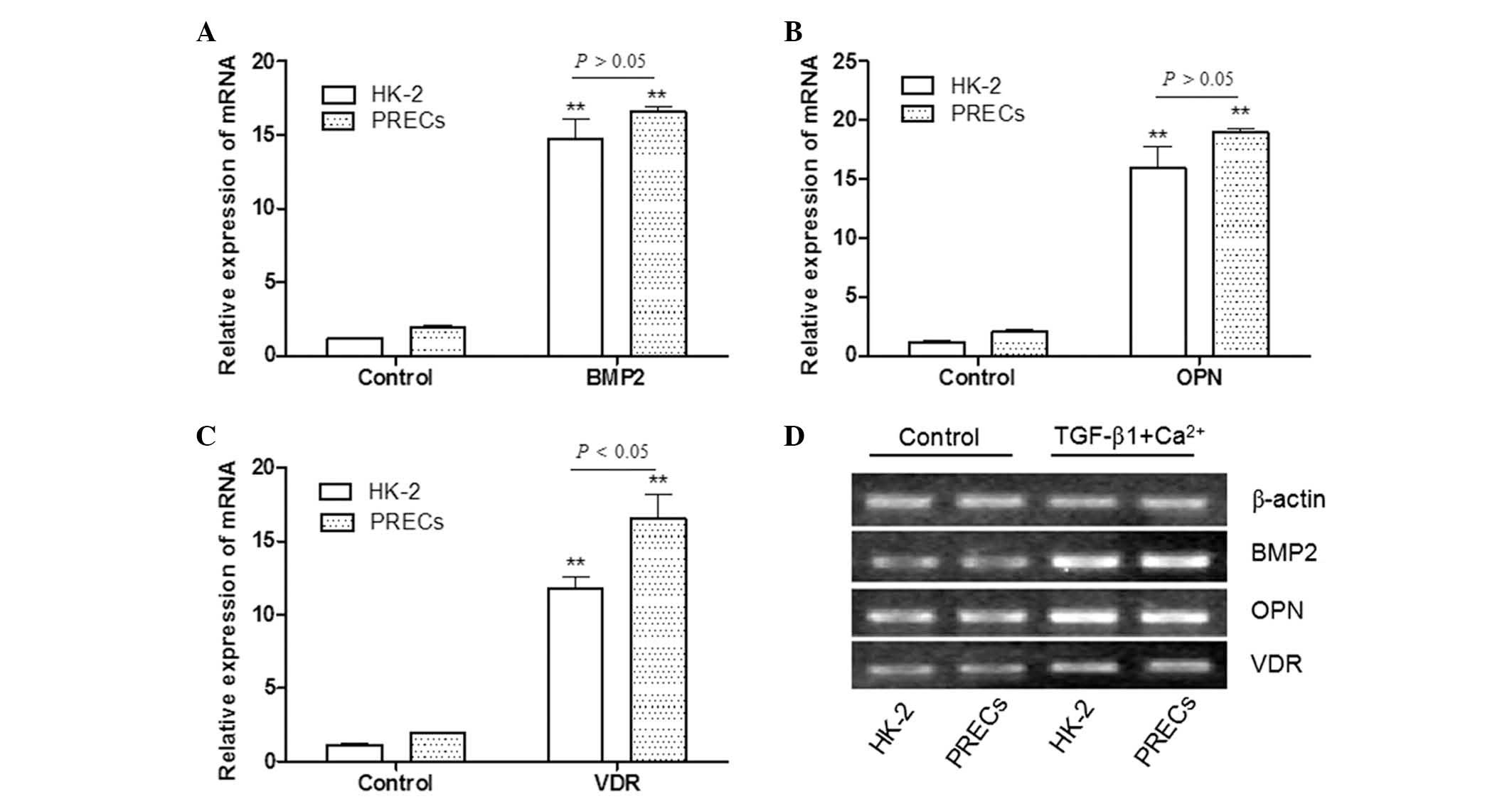 | Figure 4Effects of combined stimulation with
TGF-β1 and Ca2+ on bone-associated factor expression in
HK-2 cells and PRECs. mRNA expression levels of (A) BMP2, (B) OPN
and (C) VDR in HK-2 and PRECs following incubation with TGF-β1 (5.0
ng/ml) and Ca2+ (2.5 mM), in combination, were
significantly higher than those in the control group. VDR mRNA
expression levels following co-incubation with TGF-β1 and
Ca2+ were significantly different between HK-2 cells and
PRECs. Values are expressed as the mean ± standard deviation.
**P<0.01 vs. control group. (D) Representative gel
from three independent experiments. TGF-β1, transforming growth
factor-β1; BMP2, bone morphogenetic protein 2; OPN, osteopontin;
VDR, 1,25-dihydroxyvitamin D3 receptor; PRECs, primary
renal epithelial cells; mRNA, messenger RNA. |
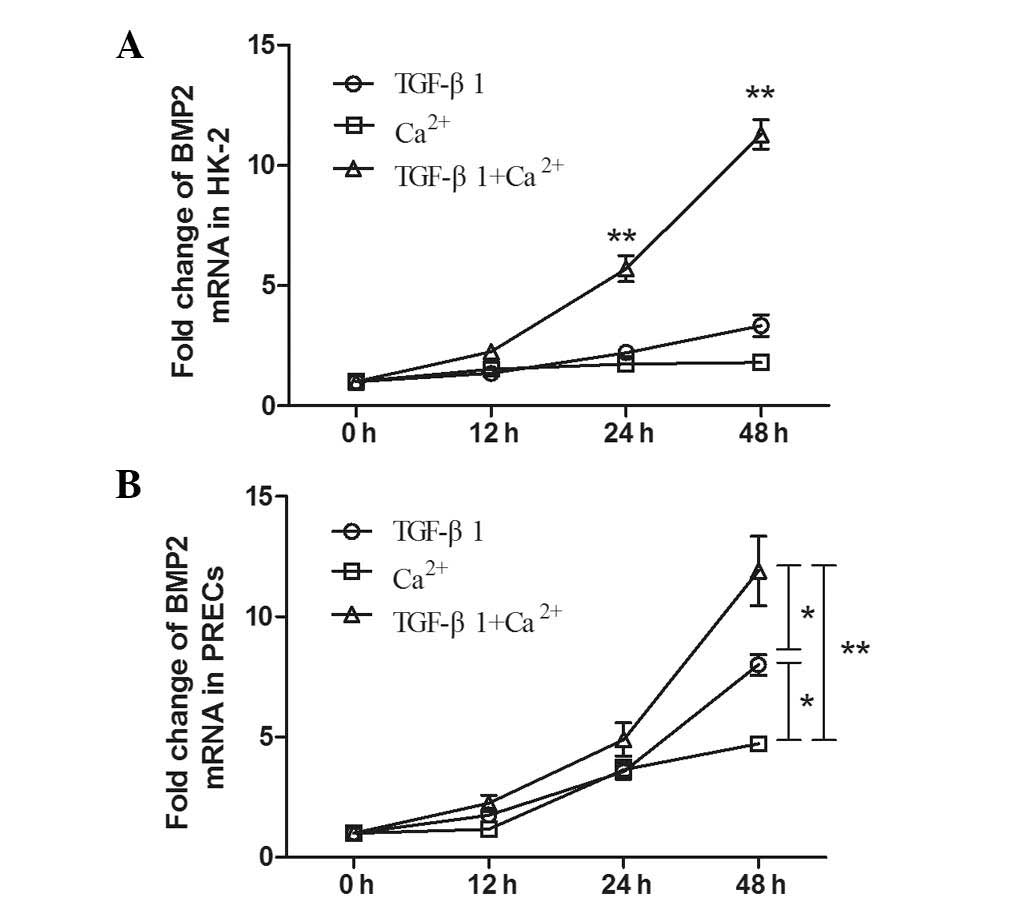
BMP2 mRNA levels increase in a
time-dependent manner in response to TGF-β1 and Ca2+
stimulation in HK-2 cells and PRECs
In HK-2 cells, examination of BMP2 mRNA expression
levels following treatment with TGF-β1 and Ca2+ by
RT-qPCR analysis suggested that levels began to increase at 12 h
following co-culture and continued to increase up until 48 h. At 48
h, BMP2 mRNA expression levels were significantly higher than those
induced by TGF-β1 or Ca2+ stimulation alone (P<0.01).
Of note, an analogous trend was observed in PRECs following
incubation with TGF-β1 + Ca2+ (Fig. 5B). Furthermore, the results
demonstrated that BMP2 mRNA levels stimulated by TGF-β1 +
Ca2+ treatment were significantly increased compared
with those in PRECs treated with TGF-β1 (P<0.05) or
Ca2+ (P<0.01) alone at 48 h. Furthermore, a
significant difference was observed in BMP2 expression levels in
PRECs induced by treatment with TGF-β1 and Ca2+ alone
(P<0.05).
Discussion
The results of the present study indicated that
TGF-β1 and Ca2+ had a synergistic effect on the
differentiation of PRECs and HK-2 cell lines, and regulated the
mRNA and protein expression levels of bone- and
nephrolithiasis-associated factors BMP2, OPN and VDR. In addition,
expression levels of these factors were significantly increased
following stimulation with TGF-β1 and Ca2+ alone in
PRECs, but not in HK-2 cells. Furthermore, serum TGF-β1, BMP2 and
OPN levels were significantly upregulated in patients with renal
stones compared with those in healthy controls. The mechanism
underlying these changes may be associated with TGF-β-induced EMT
and Ca2+-linked ion channel activation for
differentiation of renal epithelial cells. It was therefore
hypothesized that this process may be a vital physiological and
pathological mechanism underlying renal stone formation in IH.
TGF-βs are multifunctional growth factors which
participate in numerous pathophysiological processes, including
cell differentiation, proliferation, embryonic development, wound
healing, extracellular matrix (ECM) formation, development of the
immune and nervous systems, conferment of immunity and
tumorigenesis (11–13). Amongst mammals, the most abundant
form of TGF-β is TGF-β1, which is synthesized by multiple cells,
including all types of kidney cell, and is secreted as a latent
precursor complexed with TGF-β-binding proteins (14). Stimulation of TGF-β receptors in
the cell membrane induces the activation of intracellular signaling
pathways, which modulate numerous developmental, physiological and
pathological processes, including renal fibrosis and podocyte
injury, which influence renal glomerular filtration barrier
function. The TGF-β membrane receptor complex is made up of
proteins from two families which have serine/threonine kinase
activity: Type II (TβRII) (13)
and type I (TβRI) receptors, which include activin-like kinase
receptors. TGF-β binds to TβRII, which recruits TβRI to form a
receptor complex, which is subsequently phosphorylated and
activates multiple intracellular signaling cascades, including
Smad2/3. Smad2/3 heterotrimerizes with co-activator Smad4,
resulting in nuclear translocation of the complex, which
subsequently induces the transcription of TGF-β-responsive genes
via the activation of Smad-binding elements on their promoters
(15). TGF-β1 additionally
recruits non-Smad pathways in order to activate mitogen-activated
protein kinases, including Wnt/β-catenin. These effectors modulate
the expression of specific target genes, which may be involved in
physiological or kidney disease-associated events, including
cellular growth, differentiation, apoptosis, ECM deposition and EMT
(16). Previous studies have also
revealed that TGF-β1 is a pivotal signaling molecule in the Wnt
pathway. Of note, the canonical Wnt/β-catenin signaling pathway was
found to be involved in mediating the EMT and TGF-β1-mediated
fibrosis (17–19). Activation of canonical Wnt
signaling with a Wnt ligand was found to be triggered by
TGF-β1-induced upregulation of mesenchymal marker genes, including
Zeb1, Snail1 and αSMA, in renal epithelial cells, whereas
inhibition of the TGF-β1 pathway alleviated the severity of
progressive renal fibrosis and EMT (20). This effect was also investigated in
asthmatic bronchial epithelial cells, which were induced by
treatment with a combination of interleukin-22 and TGF-β1 (21). A previous study also revealed that
Wnt signaling prevented proteolytic processing of β-catenin by the
proteasome, resulting in β-catenin accumulation in the cytoplasm
and interaction with T-cell factor/lymphoid enhancer-binding factor
proteins to regulate gene and protein expression (22). Furthermore, Hill et al
(23) observed that the BMP and
Wnt signaling pathways tightly regulate each other. Activation of
the Wnt signaling pathway induces the expression of members of the
BMP family, including BMP2, BMP4, and BMP7, as well as increasing
the expression of BMP target genes, for example Msh homeobox 2
(Msx2) and gremlin, in the mesenchyme, a process which also occurs
during osteoblast differentiation and bone formation (24). In brief, downstream genes were
activated by TGF-β1 via the Wnt signaling pathway. At present, the
BMP2 signaling pathway is known to include BMP2, Runt-related
transcription factor 2 (Runx2), Msx and Osterix. BMP2, which
belongs to the TGF-β superfamily, is a critical mediator of
osteogenesis physiology, due to its ability to upregulate Msx2 and
Runx2, which are key regulators of osteoblastic differentiation
(25). A previous study by our
group indicated that the upregulation of 1,25-dihydroxyvitamin
D3 induced an increase in the expression of BMP2, Runx2
and Osterix in PRECs (26). In
addition, it was also found that bone-associated factors, including
BMP2, Runx2, Osterix and OPN, had an important role in renal stone
formation in IH (data not shown). Furthermore, VDR knockdown
reduced the expression levels of BMP2, Runx2, Osterix and OPN, as
well as decreasing the tubular calcium phosphate deposits in the
genetic hypercalciuric rat model of IH (26). It was therefore postulated that
TGF-β1 was a crucial factor in urolithiasis formation in patients
with IH, and that upregulation of TGF-β1 may increase the
expression of markers of osteoblastic cells in PRECs and renal
tissue from patients with IH. The results of the present study
indicated that the mRNA expression levels of BMP2, OPN and VDR in
PRECs increased in a dose-dependent manner following incubation
with TGF-β1. The expression levels of BMP2, OPN and VDR in PRECs,
which were treated with TGF-β at doses of 2.0–5.0 ng/ml, were
significantly higher than those in PRECs prior to treatment. It was
also demonstrated that TGF-β expression levels were significantly
higher in patients with IH than those in the NC and nIH groups.
These results confirmed our prior hypothesis stating the
essentiality of TGF-β-induced differentiation of renal epithelial
cells in urolithiasis formation.
Hypercalciuria is the most common abnormality
identified amongst calcium stone formers (27). However, the role of hypercalciuria
in stone formation remains to be elucidated. High urinary calcium
concentrations lead to increased saturation of urinary calcium
salts and inhibitory activity via complexion with negatively
charged inhibitors, including citrate and chondroitin sulfate
(4). The intracellular
concentration of calcium has been shown to be an important
regulator of gene expression patterns during numerous cellular
physiological development processes (28). An association between extracellular
calcium stimulation and vascular calcification has emerged and
studies have revealed that increasing calcium concentration to
levels observed in hypercalcemic individuals increased the
mineralization of human smooth muscle cell cultures at normal
phosphorus levels (29,30). Furthermore, examination of
calcified vessels in humans and animals revealed the expression of
BMP2, OPN, VDR and osteoblast transcription factors, including
Runx2/Cbfa1, Osterix and Msx2, which suggested that calcium has an
essential role in ectopic calcification (25). The effects of calcium on cells may
be mediated through multiple receptors. Chang et al
(31) investigated the hypothesis
that extracellular calcium may enhance terminal differentiation and
mineralization of osteoblast precursor cells via a calcium
receptor-sensitive mechanism. Similarly, L-type calcium channels
have been implicated in the calcium-mediated regulation of
osteoblastic differentiation (24). In the present study, levels of
BMP2, OPN, VDR in PRECs of the IH group were found to exhibit a
consistent and dose-dependent response to Ca2+
stimulation alone. These results suggested that Ca2+ may
be closely associated with ectopic calcification in calcium stone
formation in patients with IH, and it was concluded that
Ca2+ may promote the cellular differentiation of PRECs
and enhance TGF-β-induced EMT. However, the mechanisms underlying
the cross-talk between TGF-β1 and calcium in PRECs in the nephron
remain to be elucidated.
In conclusion, the present study demonstrated that
TGF-β1 regulated the expression of BMP2, OPN and VDR in PRECs, but
not in HK-2 cells. Furthermore, co-incubation with TGF-β1 and
Ca2+ significantly increased the expression levels of
bone-associated factors in PRECs and HK-2 cells. Further studies
are required in order to elucidate the specific mechanism
underlying the association between Ca2+-stimulated and
TGF-β1-induced EMT and the process of cell differentiation in
hypercalciuria. Furthermore, the present study supplied
experimental evidence that the pathogenesis of calcium stone
development is associated with bone formation.
Acknowledgements
The present study was funded by grants from the
National Natural Science Foundation of China (nos. 30972985 and
81270787).
References
|
1
|
Worcester EM and Coe FL: Nephrolithiasis.
Prim Care. 35:369–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van’t Hoff WG: Aetiological factors in
paediatric urolithiasis. Nephron Clin Pract. 98:c45–c48. 2004.
View Article : Google Scholar
|
|
3
|
Worcester EM, Bergsland KJ, Gillen DL, et
al: Evidence for increased renal tubule and parathyroid gland
sensitivity to serum calcium in human idiopathic hypercalciuria. Am
J Physiol Renal Physiol. 305:F853–F860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon V, Adams-Huet B, Sakhaee K and
Maalouf NM: Hyperinsulinemia and urinary calcium excretion in
calcium stone formers with idiopathic hypercalciuria. J Clin
Endocrinol Metab. 98:2589–2594. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Massagué Joan: TGFβ in cancer. Cell.
134:215–230. 2008. View Article : Google Scholar
|
|
6
|
Böttinger EP and Bitzer M: TGF-beta
signaling in renal disease. J Am Soc Nephrol. 13:2600–2610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeisberg EM, Tarnavski O, Zeisberg M, et
al: Endothelial to mesenchymal transition contributes to cardiac
fibrosis. Nat Med. 13:952–961. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawano S, Shoji S, Ichinose S, et al:
Characterization of Ca(2+) signaling pathways in human mesenchymal
stem cells. Cell Calcium. 32:165–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coe FL, Parks JH and Asplin JR: The
pathogenesis and treatment of kidney stones. N Engl J Med.
327:1141–1152. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van der Hauwaert C, Savary G, Gnemmi V, et
al: Isolation and characterization of a primary proximal tubular
epithelial cell model from human kidney by CD10/CD13 double
labeling. PLoS One. 14:e667502013. View Article : Google Scholar
|
|
11
|
Xu L, Kitani A and Strober W: Molecular
mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal
Immunol. 3:230–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Pang Y and Moses HL: TGF-beta and
immune cells: an important regulatory axis in the tumor
microenvironment and progression. Trends Immunol. 31:220–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng XM, Huang XR, Xiao J, et al: Diverse
roles of TGF-β receptor II in renal fibrosis and inflammation in
vivo and in vitro. J Pathol. 227:175–188. 2012. View Article : Google Scholar
|
|
14
|
Wheeler JB, Ikonomidis JS and Jones JA:
Connective tissue disorders and cardiovascular complications: the
indomitable role of transforming growth factor-beta signaling. Adv
Exp Med Biol. 802:107–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Briones-Orta MA, Tecalco-Cruz AC,
Sosa-Garrocho M, et al: Inhibitory Smad7: emerging roles in health
and disease. Curr Mol Pharmacol. 4:141–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moustakas Aristidis and Heldin Paraskevi:
TGFβ and matrix-regulated epithelial to mesenchymal transition.
Biochim Biophys Acta. 1840:2621–2634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Dai C, Li Y, et al: Wnt/beta-catenin
signaling promotes renal interstitial fibrosis. J Am Soc Nephrol.
20:765–776. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou B, Liu Y, Kahn M, et al: Interactions
between β-catenin and transforming growth factor-β signaling
pathways mediate epithelial-mesenchymal transition and are
dependent on the transcriptional co-activator cAMP-response
element-binding protein (CREB)-binding protein (CBP). J Biol Chem.
287:7026–7038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Akhmetshina A, Palumbo K, Dees C, et al:
Activation of canonical Wnt signaling is required for
TGF-β-mediated fibrosis. Nat Commun. 3:7352012. View Article : Google Scholar
|
|
20
|
Ledbetter S, Kurtzberg L, Doyle S, et al:
Renal fibrosis in mice treated with human recombinant transforming
growth factor-beta2. Kidney Int. 58:2367–2376. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson JR, Nishioka M, Chakir J, et al:
IL-22 contributes to TGF-β1-mediated epithelial-mesenchymal
transition in asthmatic bronchial epithelial cells. Respir Res.
14:1182013. View Article : Google Scholar
|
|
22
|
MacDonald BT, Tamai K and He X:
Wnt/β-catenin signaling. Components, mechanisms, and diseases. Dev
Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hill TP, Taketo MM, Birchmeier W and
Hartmann C: Multiple roles of mesenchymal beta-catenin during
murine limb patterning. Development. 133:1219–1229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang RR, Oyajobi BO, Harris SE, et al:
Wnt/β-catenin signaling activates bone morphogenetic protein 2
expression in osteoblasts. Bone. 52:145–156. 2013. View Article : Google Scholar :
|
|
25
|
Johnson RC, Leopold JA and Loscalzo J:
Vascular calcification: pathobiological mechanisms and clinical
implications. Circ Res. 99:1044–1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia ZH, Wang SG, Tang JH, et al: Does
stone formation in genetic hypercalciuric rat kidney tissue share
similarities with bone formation? Urology. 83:509.e7–509.e17. 2014.
View Article : Google Scholar
|
|
27
|
Liern M, Bohorquez M and Vallejo G:
Treatment of idiopathic hypercalciuria and its impact on associated
diseases. Arch Argent Pediatr. 111:110–114. 2013.PubMed/NCBI
|
|
28
|
González-Vázquez A, Planell JA and Engel
E: Extracellular calcium and CaSR drive osteoinduction in
mesenchymal stromal cells. Acta Biomater. 10:2824–2833. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang H, Curinga G and Giachelli CM:
Elevated extracellular calcium levels induce smooth muscle cell
matrix mineralization in vitro. Kidney Int. 66:2293–2299. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Collin-Osdoby P: Regulation of vascular
calcification by osteoclast regulatory factors RANKL and
osteoprotegerin. Circ Res. 95:1046–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang W, Tu C, Pratt S, et al:
Extracellular Ca(2+)-sensing receptors modulate matrix production
and mineralization in chondrogenic RCJ3.1C5.18 cells.
Endocrinology. 143:1467–1474. 2002. View Article : Google Scholar : PubMed/NCBI
|