Introduction
Ovarian cancer is a malignant tumor derived from
epithelial and germ cells (1). In
humans, ovarian cancer is the second most prevalent gynecological
malignancy and has the highest mortality rate among them (2). According to the US Centers for
Disease Control and Prevention, there are >22,000 novel cases of
ovarian cancer and ~14,000 mortalities each year in the United
States (3). Advanced surgical
methods and chemotherapeutic agents are available for the treatment
of ovarian cancer; however, early diagnosis only occurs in ~25% of
cases due to the lack of effective screening programs and
non-specific symptoms (4–6).
Studies from the International Collaborative Ovarian
Neoplasm group and the European Organization for Research and
Treatment of Cancer have decided that only patients with stages IA
or IB (non-clear cell histology and well-differentiated (G1) tumor;
according to the International Federation of Gynecology and
Obstetrics guidelines) ovarian cancer may avoid chemotherapy
(7). The majority of patients with
ovarian cancer require chemotherapy to enhance progression-free and
overall survival. Cisplatin and analogous platinum derivatives are
the front-line chemotherapeutic agents used for the management and
treatment of recurrent ovarian cancer (8,9).
The majority of ovarian cancers are initially
responsive to chemotherapy; however, in numerous cases patients
become cisplatin-resistant due to recurrence and metastasis
(10). The mechanism of cisplatin
resistance leading to clinical resistance remains to be elucidated.
Therefore, understanding the molecular dysregulation underlying
chemoresistance in ovarian cancer is essential for developing
successful therapeutic strategies.
Rab25 (also known as CATX8) is a Rab11 guanosine
triphosphatase (GTPase) family member that belongs to the Rab
family. Rab25 was found to contain an unusual amino acid sequence,
WDTAGLE, in its guanosine trisphosphate (GTP)-binding domain
causing it to be constitutively activated (11). GTPase activity modulates the
binding affinities of Rab25, which are critical for its biological
functions, including proliferation, signal transduction, apoptosis,
microtubule organization, recruitment of H+K+
adenosine triphosphatase, transferring receptor recycling and
integrin trafficking (12).
Contrary to the function of other Rab11 GTPases, Rab25
overexpression correlated with the aggressiveness of cancers,
including ovarian and breast cancer (13,14).
Studies have demonstrated that knockdown of RAB25 promoted
autophagy and inhibited cell growth in ovarian cancer cells in
vitro and in vivo (15,16).
The aim of the present study was to investigate the
effect of Rab25 overexpression and phosphoinositide 3-kinase
(PI3K)/AKT signaling on cisplatin resistance in ovarian cancer cell
lines in order to discover a novel strategy for sensitizing cells
to chemotherapeutic agents.
Materials and methods
Cell culture
SKOV-3 and ES-2 ovarian cancer cell lines were
purchased from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). The cells were grown in RPMI
1640 medium (Gibco-BRL, Grand Island, NY, USA) and then
supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin and
10% fetal bovine serum (FBS) (all HyClone, Logan, UT, USA) in a 5%
CO2 atmosphere at 37°C. The cells were routinely
subcultured every three days.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from cells using
the TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
Complementary DNA was synthesized using a reverse transcription
reagent (Promega Corp., Madison, WI, USA). qPCR was performed using
a standard SYBR green PCR kit (Promega Corp.) and PCR-specific
amplification was conducted in an Eppendorf 5331 Real-Time PCR
machine (Eppendorf, Hamburg, Germany). Gene expression was
calculated using the 2−(ΔΔCt) method. The primer
sequences were synthesized by Jie Li Biology (Shanghai, China) as
follows: RAB25 sense, 5′-GCCCTGGACTCTACCAATGTTGA-3′ and antisense,
5′-GCTGTTCTGTCTCTGCTTGGACAC-3′; GAPDH sense,
5′-GCACCGTCAAGGCTGAGAAC-3′ and antisense, 5′-TGGTGAAGACGCCAGTGGA
3′.
Western blot analysis
The cells were lysed on ice with RIPA lysis buffer
[150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM
Tris (pH 7.9), 10 mM NaF, PMSF and 1X protease inhibitors], and
protein concentrations were measured using the Bicinchoninic Acid
Protein Assay reagent (Pierce Biotechnology, Inc., Rockford, IL,
USA). Protein extracts (40 μg) were subjected to SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (Invitrogen Life
Technologies). The mouse anti-human p-AktThr308 antibody (1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) was used as the
primary antibody, and the horseradish peroxidase-labeled goat
anti-mouse immunoglobulin G (1:2,000; Cell Signaling Technology,
Inc.) was used as the secondary antibody. The β-actin antibody
(1:5,000; Sigma, St. Louis, MO, USA) was used as an internal
control. The bands were detected using an enhanced
chemiluminescence kit (GE Healthcare, Little Chalfont, UK) and
visualized using the ChemiDoc XRS system (Bio-Rad Laboratories,
Hercules, CA, USA). Multi Gauge V3.2 software (Fujifilm, Kanagawa,
Japan) was used to quantitatively determinate the gray level of
each band (absorbance measured at 570 nm) and the objective
band/internal band ratio.
Small interfering RNA (siRNA)
transfection
The human Rab25-specific siRNA (siRab25) and control
siRNA (siCon) were purchased from Dharmacon, Inc. (Lafayette, CO,
USA). DharmaFCET 1 reagent (Dharmacon, Inc.) was used to transfect
siRNAs, according to the manufacturer’s instructions. The Rab25
siRNA sense sequence used was 5′-TCCCTCTGGCTGCAGAAGT-3′.
Cell viability assay
Cells were plated into 96-well microplates
(104 cells/well) and incubated with siRab25, siCon,
LY294002 (PI3K/AKT inhibitor; Sigma) or dimethyl sulfoxide
(control; Sigma) for 24 h. In order to investigate the inhibitory
effect of cisplatin in cells, a series of cisplatin concentrations
(1, 5, 10, 50 and 100 μM; Sigma) was added to the ovarian cancer
cells. Cell viability was monitored using an MTT assay (Sigma) as
previously described (17).
Absorbance was measured at 570 nm using a Bio-Rad MicroPlate
Reader, model 450 (Bio-Rad Laboratories). Experiments were
performed in triplicate.
Cell cycle analysis
Cells were synchronized in G1-phase by serum
starvation for 12 h. Flow cytometric (FCM; BD FACSCalibur;
Becton-Dickinson, San Jose, CA, USA) analysis was used to determine
the cell cycle phases of cells. In brief, cells were washed with
4°C phosphate-buffered saline (PBS) and fixed with 70% cold ethanol
(HyClone, Logan, UT, USA) at 4°C overnight. The fixed cells were
then collected, washed with PBS and stained with propidium iodide
(PI; Sigma) in the presence of RNAase (Sigma). The phase of cell
cycle was analyzed using ModFit software, version 3.2 (Verity
Software House, Topsham, ME, USA).
Apoptosis assay
To evaluate cell apoptosis, an Annexin V-fluorescein
isothiocyanate (FITC) Apoptosis kit (Cell Signaling Technology,
Inc.) was used. In brief, cells were harvested and washed with 4°C
PBS. Cells were then resuspended in binding buffer and then
incubated with Annexin V-FITC and PI buffers (Invitrogen Life
Technologies) for 15 min at 4°C in the dark. Annexin V-FITC and PI
signals were then detected using flow cytometry.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. Statistical analyses were performed using Student’s
t-test. SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was
performed for statistical analysis P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Rab25 expression and PI3K/AKT pathway
activity are increased in cisplatin-resistant cell lines
The cisplatin-resistant SKOV-3 and the
cisplatin-sensitive ES-2 ovarian cancer cell lines were used to
investigate the role of Rab25 in cisplatin resistance in ovarian
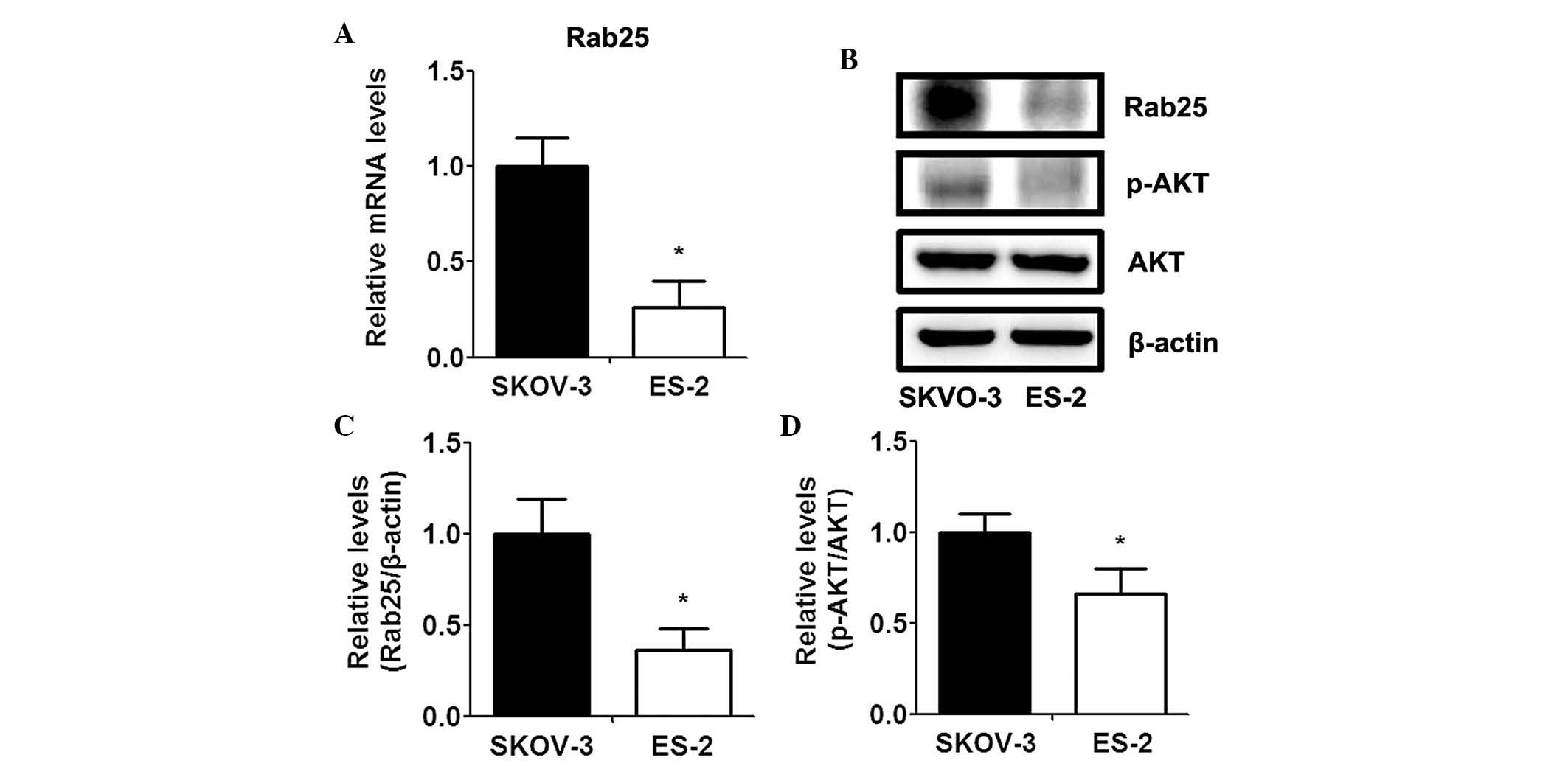
cancer. SKOV-3 cells were found to overexpress Rab25 messenger RNA
(mRNA), whereas decreased expression of Rab25 mRNA was observed in
the ES-2 cells (Fig. 1A). In
addition, western blot analysis revealed that Rab25 protein
expression levels were significantly elevated in SKOV-3 cells
compared to those of ES-2 cells (Fig.
1B and C). Furthermore, AKT activity was markedly increased in
the SKOV-3 cell line compared to that in the ES-2 cell line
(Fig. 1B and D).
Cisplatin-resistant cell line lacks G1
cell cycle arrest following cisplatin treatment
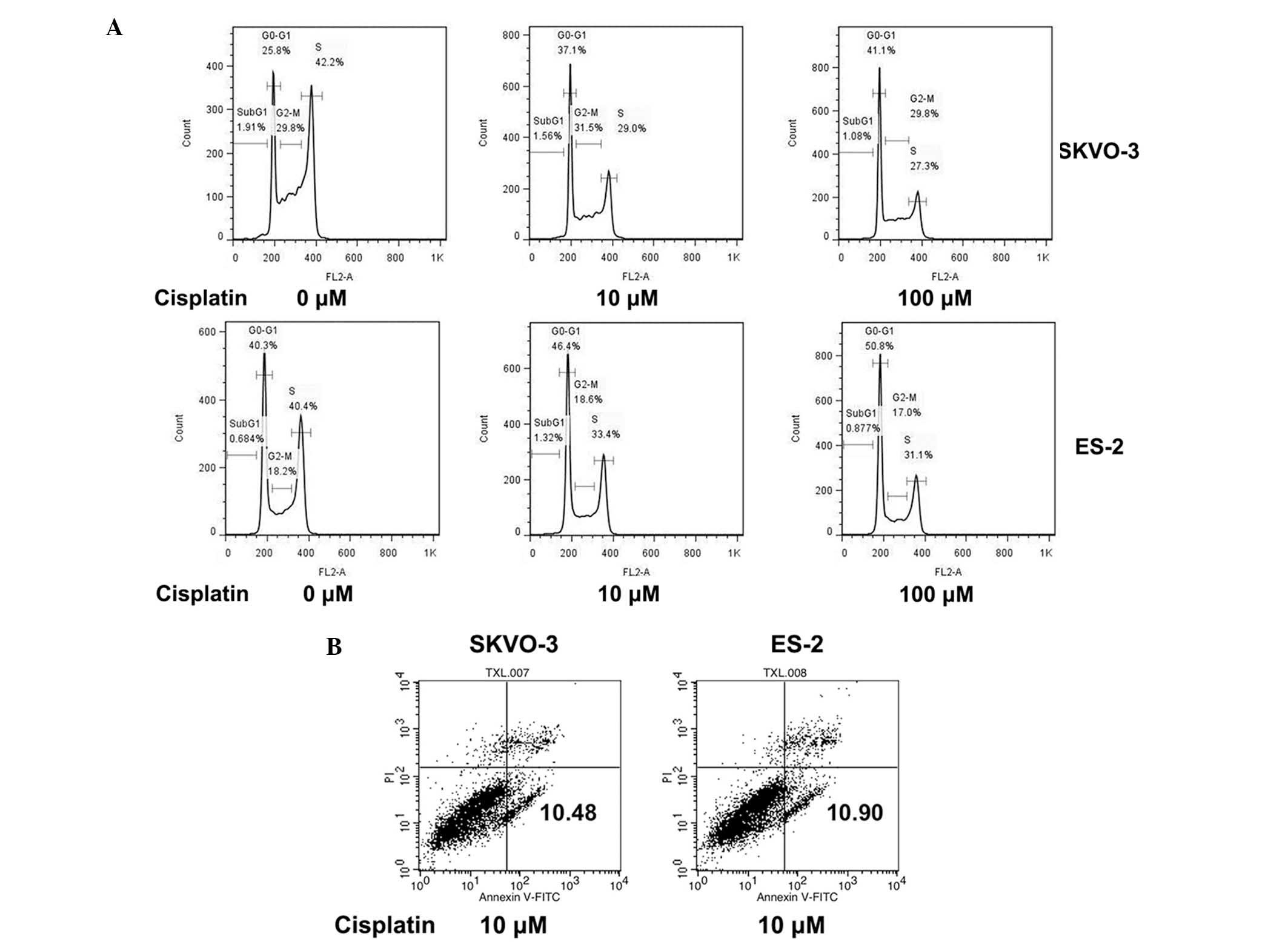
In order to further elucidate the mechanism of
cisplatin resistance in different cells, the cell cycle and
apoptotic rate were analyzed. Following treatment with cisplatin
for 2 h, there was a significant dose-dependent increase in the
percentage of ES-2 cells in G1-phase (Fig. 2A; Table I). However, SKOV-3 cells did not
respond to cisplatin treatment and the number of cells in G1-phase
was not significantly altered (Fig.
2A; Table I). The apoptotic
rates of the two cell lines showed no significant difference from
each other (Fig. 2B). These data
indicated that deficiency in G1-phase cell cycle arrest was induced
by cisplatin rather than apoptosis and therefore, the lack of
SKOV-3 cells in G1-pase may be due to cisplatin resistance.
 | Table ICisplatin-induced G1-phase arrest in
SKVO 3 cells is lower than that in ES-2 cells. |
Table I
Cisplatin-induced G1-phase arrest in
SKVO 3 cells is lower than that in ES-2 cells.
| SKVO-3 | ES-2 |
|---|
|
|
|
|---|
| Phase/Cisplatin | 0 μM | 10 μM | 100 μM | 0 μM | 10 μM | 100 μM |
|---|
| SubG1 (%) | 1.88±0.2 | 1.55±0.35 | 1.11±0.76 | 1.51±1.30 | 16.7±1.81 | 1.75±0.35 |
| G0/G1 (%) | 26.7±2.03a | 37.3±0.9a | 40.7±1.69a | 39.7±2.56 | 46.7±3.91 | 51.3±1.45 |
| S (%) | 41.9±1.95 | 28.0±3.9 | 30.5±2.19 | 38.7±0.61 | 29.6±1.88 | 31.1±2.16 |
| G2/M (%) | 31.4±2.74 | 31.4±4.67 | 30.2±1.07 | 19.5±1.49 | 18.4±1.87 | 17.5±0.83 |
Inhibition of the PI3K/AKT pathway
downregulates Rab25 expression levels
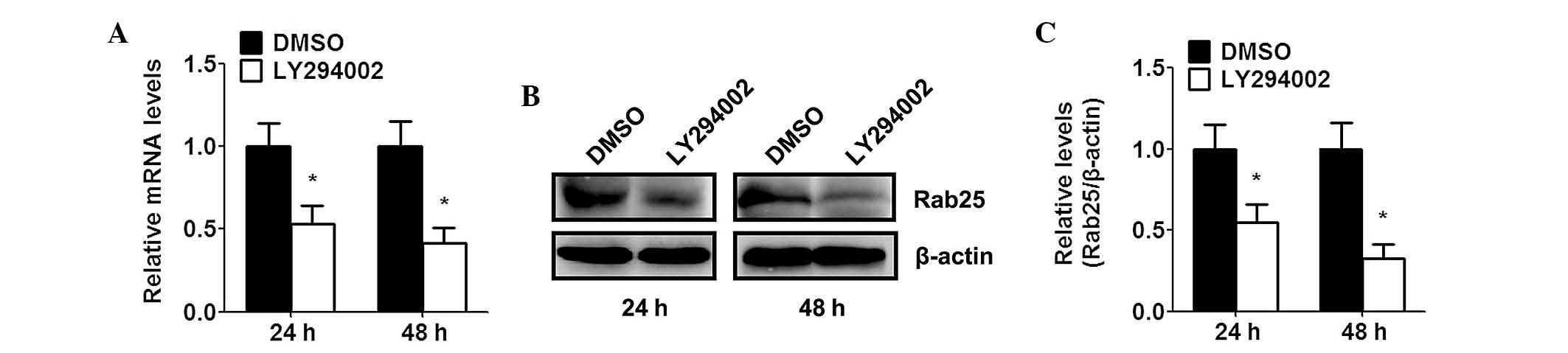
In order to investigate whether Rab25 was associated
with the PI3K/AKT pathway, SKOV-3 cells were treated with the
PI3K/AKT inhibitor LY294002. Following treatment with 10 μM
LY294002 for 24 and 48 h, Rab25 mRNA expression in SKOV-3 cells was
decreased by ~46.7 and 58.4%, respectively (Fig. 3A). Subsequently, Rab25 protein
expression levels were determined using western blot analysis. The
results demonstrated that LY294002 treatment markedly suppressed
Rab25 protein expression; this therefore indicated that inhibition
of the PI3K/AKT pathway downregulated Rab25 protein expression
(Fig. 3B and C).
Knockdown of Rab25 or inhibition of the
PI3K/AKT pathway increases the sensitivity of SKOV-3 cells to
cisplatin
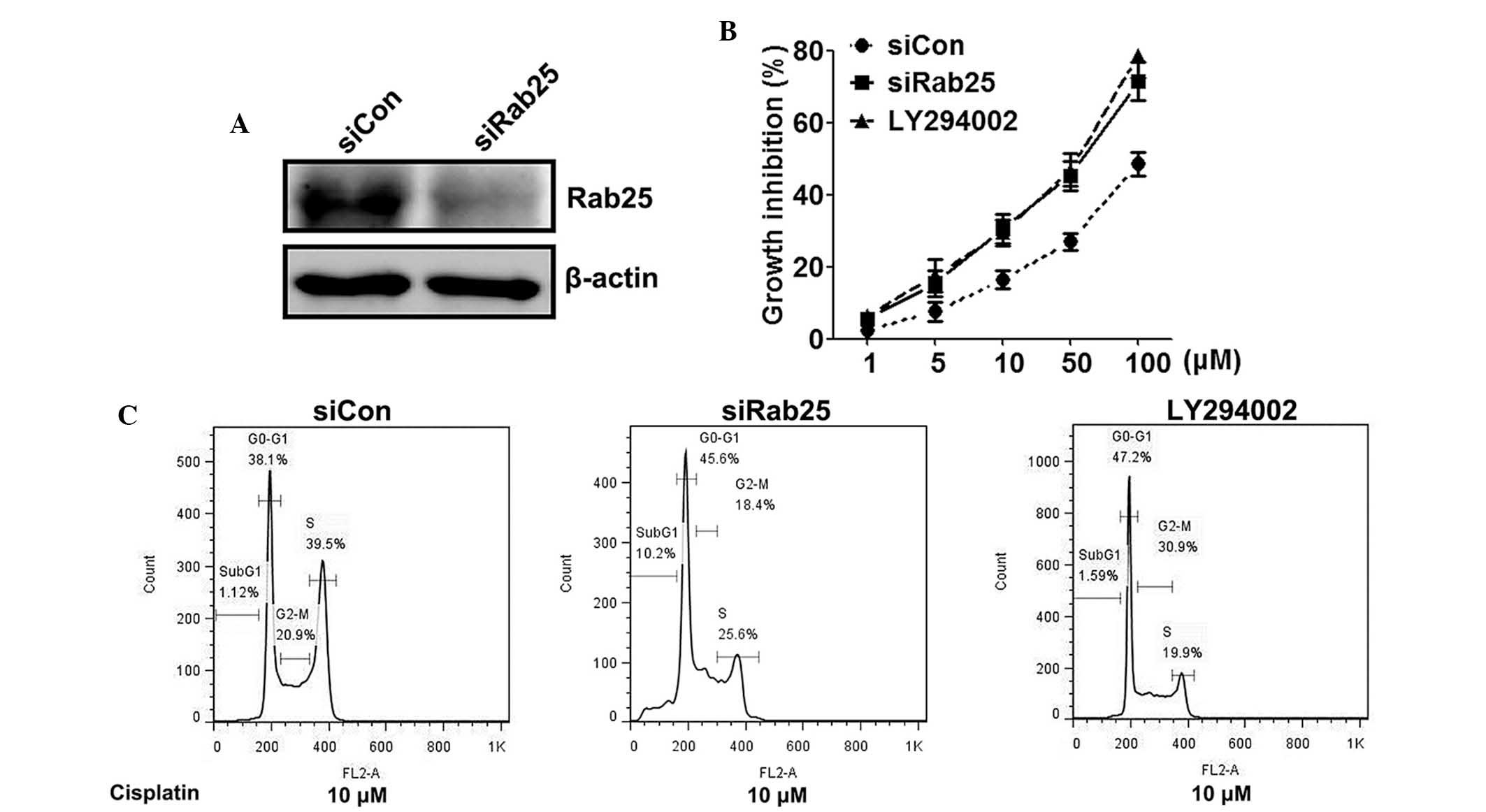
In the present study, a Rab25-specific siRNA was
used to block the expression of ubiquitously-produced Rab25 in
SKOV-3 cells. Western blot analysis revealed that protein levels of
Rab25 were significantly decreased (by 70.2%) following siRab25
transfection as compared with those of the control groups (Fig. 4A). In order to determine whether
Rab25 or the PI3K/AKT pathway were involved in the decreased
chemosensitivity of cisplatin-resistant cells, the cell growth
inhibiting effect of siRab25 or PI3K/AKT signaling inhibitors in
combination with cisplatin was measured. An MTT assay demonstrated
that siRab25 as well as LY294002 decreased the IC50
values of cisplatin on SKOV-3 cells (Fig. 4B). In addition, Rab25 gene
silencing or LY294002 treatment increased the G1-phase cell cycle
arrest induced by cisplatin (Fig.
3C). In conclusion, these results indicated that elevation of
Rab25, via activation of the PI3K/AKT pathway, may be the mechanism
for cisplatin resistance of ovarian cancer cells.
Discussion
Rab25, a Rab11 subfamily protein, was previously
reported to be expressed in all eukaryotes, where it shared a
conserved mechanism of regulation (12). Rab25 has been suggested to have a
comparable function to that of Rab11; in addition, Rab25 was
reported to be spatially and functionally associated with the
regulation of apical-to-basolateral transcytosis in polarized
epithelial cells, indicating that Rab25 was an important regulator
of polarized cell surface composition (18). Rab25 was shown to enhance the
invasive ability of cells due to its epithelial cell polarity
modulatory characteristic, therefore suggesting that Rab25
dysregulation may have a role in tumorigenesis (14). Previous studies have demonstrated
that Rab25 controlled tumor progression, aggressiveness and
potentially chemosensitivity (13,19–21);
furthermore, Rab25 was amplified at the DNA level and overexpressed
at the RNA level in ovarian cancers (13).
Knockdown of RAB25 promotes autophagy and inhibits
cell growth in ovarian cancer (15). The results of the present study
were concurrent with the hypothesis that Rab25, as an oncogene,
contributed to the aggressiveness of ovarian cancer. In addition,
the present study demonstrated that constitutive overexpression of
Rab25 due to increased activation of PI3K/AKT signaling in SKOV-3
cells resulted in cisplatin resistance, a severe obstacle for the
successful treatment of ovarian cancer. Elucidating the molecular
mechanism underlying cisplatin resistance is critical for improving
sensitivity to chemotherapeutic agents. It is understood that
chemoresistance is due to dysregulation of the balance between the
pathways of cellular survival and apoptosis as well as enhanced
drug clearance, enhanced detoxification and reduced drug efficacy
due to increased DNA repair (22).
A previous study has reported that inhibition of the PI3K/AKT
pathway may enhance chemosensitivity of resistant ovarian cancers,
more prominently in tumors with a high PI3K/AKT activity profile
(10). The present study revealed
that suppression of the PI3K/AKT pathway by LY294002 reduced Rab25
expression, therefore indicating that Rab25 was a key effector
molecule of the PI3K/AKT pathway in cisplatin resistance.
Furthermore, knockdown of Rab25 using siRNAs or inhibition of
PI3K/AKT signaling increased the sensitivity of SKOV-3 cells to
cisplatin via augmentation of G1-phase cell cycle arrest, which was
thought to contribute to the sensitization of cells to
cisplatin.
In conclusion, the results of the present study
confirmed the tumorigenic role of RAB25 in ovarian cancer cells and
suggested a novel role of Rab25 in cisplatin resistance. In
addition, the results demonstrated that inhibition of Rab25 and the
PI3K/AKT pathway sensitized ovarian cancer cells to cisplatin,
providing a potential novel adjuvant therapy in combination with
cisplatin.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81060215).
References
|
1
|
Kim A, Ueda Y, Naka T and Enomoto T:
Therapeutic strategies in epithelial ovarian cancer. J Exp Clin
Cancer Res. 31:142012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo N, Van Gorp T, Parma G, et al:
Ovarian cancer. Crit Rev Oncol Hematol. 60:159–179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wrzeszczynski KO, Varadan V, Byrnes J, et
al: Identification of tumor suppressors and oncogenes from genomic
and epigenetic features in ovarian cancer. PloS One. 6:e285032011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holschneider CH and Berek JS: Ovarian
cancer: epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shih Ie M and Davidson B: Pathogenesis of
ovarian cancer: clues from selected overexpressed genes. Future
Oncol. 5:1641–1657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson AE, Francis JE and Zorbas H:
Population screening and early detection of ovarian cancer in
asymptomatic women. Aust NZ J Obstet Gynaecol. 49:448–450. 2009.
View Article : Google Scholar
|
|
7
|
Trimbos JB, Parmar M, Vergote I, et al;
International Collaborative Ovarian Neoplasm 1 and the European
Organisation for Research and Treatment of Cancer
Collaborators-Adjuvant Chemotherapy in Ovarian Neoplasm.
International collaborative ovarian neoplasm trial 1 and adjuvant
chemotherapy in ovarian neoplasm trial: two parallel randomized
phase III trials of adjuvant chemotherapy in patients with
early-stage ovarian carcinoma. J Natl Cancer Inst. 95:105–112.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aabo K, Adams M, Adnitt P, et al:
Chemotherapy in advanced ovarian cancer: four systematic
meta-analyses of individual patient data from 37 randomized trials.
Advanced ovarian cancer trialists’ group. Br J Cancer.
78:1479–1487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markman M: Optimizing primary chemotherapy
in ovarian cancer. Hematol Oncol Clin North Am. 17:957–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ali AY, Farrand L, Kim JY, et al:
Molecular determinants of ovarian cancer chemoresistance: new
insights into an old conundrum. Ann NY Acad Sci. 1271:58–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mitra S, Cheng KW and Mills GB: Rab25 in
cancer: a brief update. Biochem Soc Trans. 40:1404–1408. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agarwal R, Jurisica I, Mills GB and Cheng
KW: The emerging role of the RAB25 small GTPase in cancer. Traffic.
10:1561–1568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng KW, Lahad JP, Kuo WL, et al: The
RAB25 small GTPase determines aggressiveness of ovarian and breast
cancers. Nat Med. 10:1251–1256. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caswell PT, Spence HJ, Parsons M, et al:
Rab25 associates with alpha5beta1 integrin to promote invasive
migration in 3D microenvironments. Dev Cell. 13:496–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Tao X, Jia L, et al: Knockdown of
RAB25 promotes autophagy and inhibits cell growth in ovarian cancer
cells. Mol Med Rep. 6:1006–1012. 2012.PubMed/NCBI
|
|
16
|
Fan Y, Xin XY, Chen BL and Ma X: Knockdown
of RAB25 expression by RNAi inhibits growth of human epithelial
ovarian cancer cells in vitro and in vivo. Pathology. 38:561–567.
2006. View Article : Google Scholar
|
|
17
|
Wang H, Li H, Zuo M, et al: Lx2-32c, a
novel taxane and its antitumor activities in vitro and in vivo.
Cancer Lett. 268:89–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goldenring JR and Nam KT: Rab25 as a
tumour suppressor in colon carcinogenesis. Br J Cancer. 104:33–36.
2011. View Article : Google Scholar :
|
|
19
|
Cheng KW, Lahad JP, Gray JW and Mills GB:
Emerging role of RAB GTPases in cancer and human disease. Cancer
Res. 65:2516–2519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casanova JE, Wang X, Kumar R, et al:
Association of Rab25 and Rab11a with the apical recycling system of
polarized Madin-Darby canine kidney cells. Mol Biol Cell. 10:47–61.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Kumar R, Navarre J, Casanova JE
and Goldenring JR: Regulation of vesicle trafficking in madin-darby
canine kidney cells by Rab11a and Rab25. J Biol Chem.
275:29138–29146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fraser M, Leung B, Jahani-Asl A, Yan X,
Thompson WE and Tsang BK: Chemoresistance in human ovarian cancer:
the role of apoptotic regulators. Reprod Biol Endocrinol. 1:662003.
View Article : Google Scholar : PubMed/NCBI
|