Introduction
Hepatocellular carcinoma is a major health problem
worldwide and the third largest cause of cancer-associated
mortality in the world annually (1). Metastasis remains a continuing
problem for the management of liver cancer. The multi-step process
of metastasis includes local invasion, intravasation to the lymph
and blood systems, survival in the bloodstream, extravasation from
the microvessels and colonization at a secondary site (2,3).
There is evidence to suggest that epithelial to mesenchymal
transition (EMT) contributes to cancer progression, invasion and
migration in various types of cancer (4,5). EMT
is a cellular process during which epithelial polarized cells
become motile mesenchymal-appearing cells (6). The hallmarks of EMT include loss of
cell-cell adhesion, actin cytoskeleton reorganization and
acquisition of increased migratory characteristics. EMT is
characterized by the downregulation of epithelial differentiation
markers, including E-cadherin and the upregulation of mesenchymal
markers, including vimentin.
EMT can be initiated by external signals, including
fibroblast growth factor, epidermal growth factor (EGF) and
transforming growth factor (TGF)-β1 (7,8).
TGF-β1 is the multifunctional cytokine implicated in different
biological processes by inducing EMT, such as during wound healing,
fibrotic diseases, embryonic development and cancer pathogenesis
(9). Commonly, tumor cells lose
the ability to inhibit the growth activity of TGF-β1.
There is increasing interest in the role of
Traditional Chinese Medicine in maintaining health and treating
disease. Ginsenoside Rg1, is one of most active and abundant
components in ginseng, which has pharmacological effects in the
central nervous system, cardiovascular system and immune system and
also exerts anticancer properties (10–18).
However, the effect of ginsenoside Rg1 on cancer metastasis has not
been investigated. In the present study, the effects of ginsenoside
Rg1 on TGF-β1-induced invasion and migration in liver cancer were
demonstrated and a potential mechanism for these effects was
examined. It was hypothesized that TGF-β1 induces HepG2 cells to
undergo EMT and promotes cell invasion and migration. Ginsenoside
Rg1 may suppress liver cancer invasion and migration through
inhibiting TGF-β1-induced EMT.
Materials and methods
Materials
Ginsenoside Rg1 was obtained from Shanghai
International Port (Group) Co., Ltd. (Shanghai, China)
Sulforhodamine B (SRB), trichloroacetic acid (TCA), acetic acid,
anti-β-actin and dimethyl sulfoxide were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Monoclonal rabbit antibodies
for E-cadherin (1:1,000; #3195) and vimentin (1:1,000; #5741) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Secondary monoclonal rabbit antibodies (1:5,000; PA1-14444) for
western blotting were obtained from Amersham Biosciences Corp.
(Piscataway, NJ, USA). All other reagents were obtained from
Sigma-Aldrich unless stated otherwise.
SRB assay
Cytotoxicity was determined using an SRB assay.
Cells were seeded into 96-well plates and exposed to different
concentrations of ginsenoside Rg1 (50, 100, 200 and 400 μM). After
48 h of incubation, the cells were fixed with TCA for 1 h at 4°C,
air-dried and then stained with 0.4% SRB solution for 30 min at
room temperature. Following staining, the SRB solution was removed
and cells were washed five times with 1% acetic acid. Subsequently,
10 mM Tris base solution (pH 10.5) was added to dissolve the
protein-bound dye and plates were incubated on a plate shaker for
10 min. The OD570 nm was determined using a 96-well
plate reader (MRX; Dynex Technologies, Chantilly, VA, USA).
Wound-healing assay
Cells were cultured in a 6-well plate and incubated
until they reached 80% confluence. Cell monolayers were carefully
wounded by scratching with a 200 μl sterile plastic pipette tip.
Subsequently, cells were washed twice with phosphate-buffered
saline and then replaced with fresh medium without serum. For each
scratch, images were captured at 0 and 24 h using an inverted
microscope (Nikon Eclipse TS100 1064; Nikon, Tokyo, Japan) in the
same field.
Matrigel invasion assay
Invasion of HepG2 cells was performed in a 24-well
transwell unit (8 μM pore size) and was coated with 1 mg/ml
Matrigel matrix as described previously (19). Briefly, cells were placed on the
Matrigel-coated transwell (the upper compartment of the invasion
chamber), and cells were exposed to TGF-β1 (1.25–5.00 ng/ml) for 24
h, or cells were pretreated with ginsenoside Rg1 (50–200 μM) for 48
h in the presence or absence of 5 ng/ml TGF-β1 for 24 h. The cells
were then resuspended in 200 μl serum-free medium and placed in the
upper chambers at 5×104 cells. Conditioned medium (600
μl) was added to the lower compartment of the invasion chamber.
Following incubation at 37°C for 48 h, cells that had invaded the
lower surface of the membrane were fixed with methanol and stained
with hematoxylin and eosin. Random fields were counted by light
microscopy (MF53; Olympus Corporation, Tokyo, Japan).
Western blotting
Cells were harvested and lysed for total cellular
protein extraction and then centrifuged at 2,250 × g for 30 min at
4°C. A DC protein assay kit was used to determine the protein
concentrations (Bio-Rad, Hercules, CA, USA). Total protein (25 μg)
was separated using 8–12% sodium dodecyl sulfate-polyacrylamide
gels and subsequently transferred onto a polyvinylidene difluoride
membrane. Following blocking with skimmed milk, the membranes were
incubated with various primary antibodies, E-cadherin (1:1,000),
vimentin (1:1,000) and β-actin (1:10,000), at 4°C overnight,
respectively. Immunopositive bands were visualized using the
Amersham ECL™ plus western blotting detection kit (GE Healthcare,
Piscataway, NJ, USA).
Statistical analysis
Statistical analysis between groups was performed
using an unpaired Student’s t-test with Sigmaplot 10.0 software
(Jandel Scientific, San Rafael, CA, USA). Data are presented as the
mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
TGF-β1 induces HepG2 cells to undergo
EMT
Initially, the optimum concentrations required for
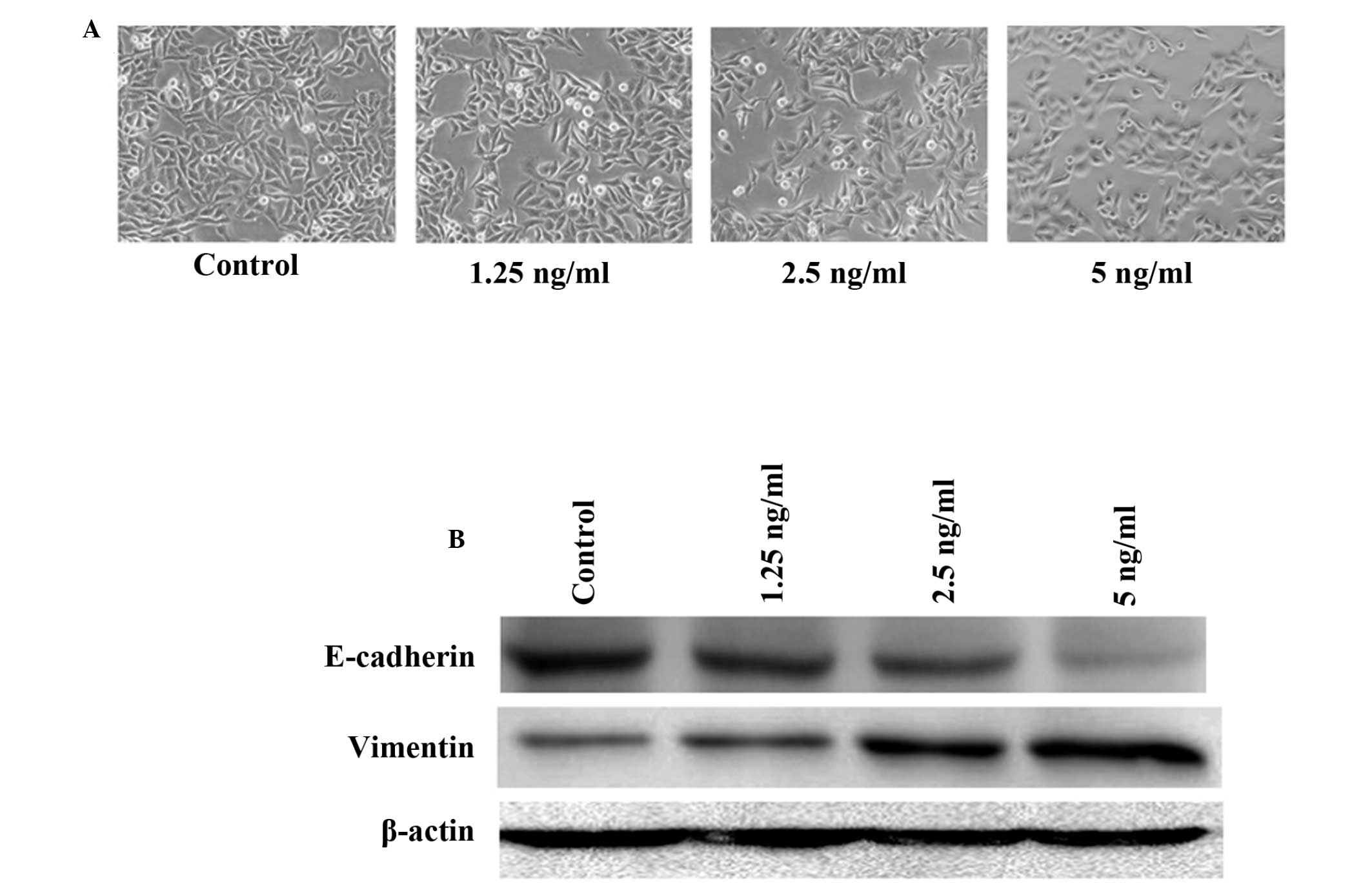
TGF-β1 to initiate EMT in HepG2 cells were ascertained. Cells were
treated with 1.25–5 ng/ml TGF-β1 for 24 h. Fig. 1 shows that the cells acquired a
spindle-like fibroblastic phenotype and reduced their cell-cell
contact when exposed to higher doses of TGF-β1 (5 ng/ml). Since EMT
is closely associated with the loss of E-cadherin expression and
acquisition of vimentin expression, the expression of E-cadherin
and vimentin by western blotting was also assessed. The results
demonstrated that TGF-β1 decreased E-cadherin expression in a
dose-dependent manner, while it significantly induced the
expression of vimentin. The maximal effects of TGF-β1 in initiating
EMT in HepG2 cells were achieved at a concentration of 5 ng/ml,
therefore this concentration was used in subsequent
experiments.
TGF-β1 promotes the invasion and
migration of HepG2 cells
Previous studies have demonstrated that EMT is
involved in cancer invasion and metastasis, which are key processes
in cancer progression and metastasis (20,21).
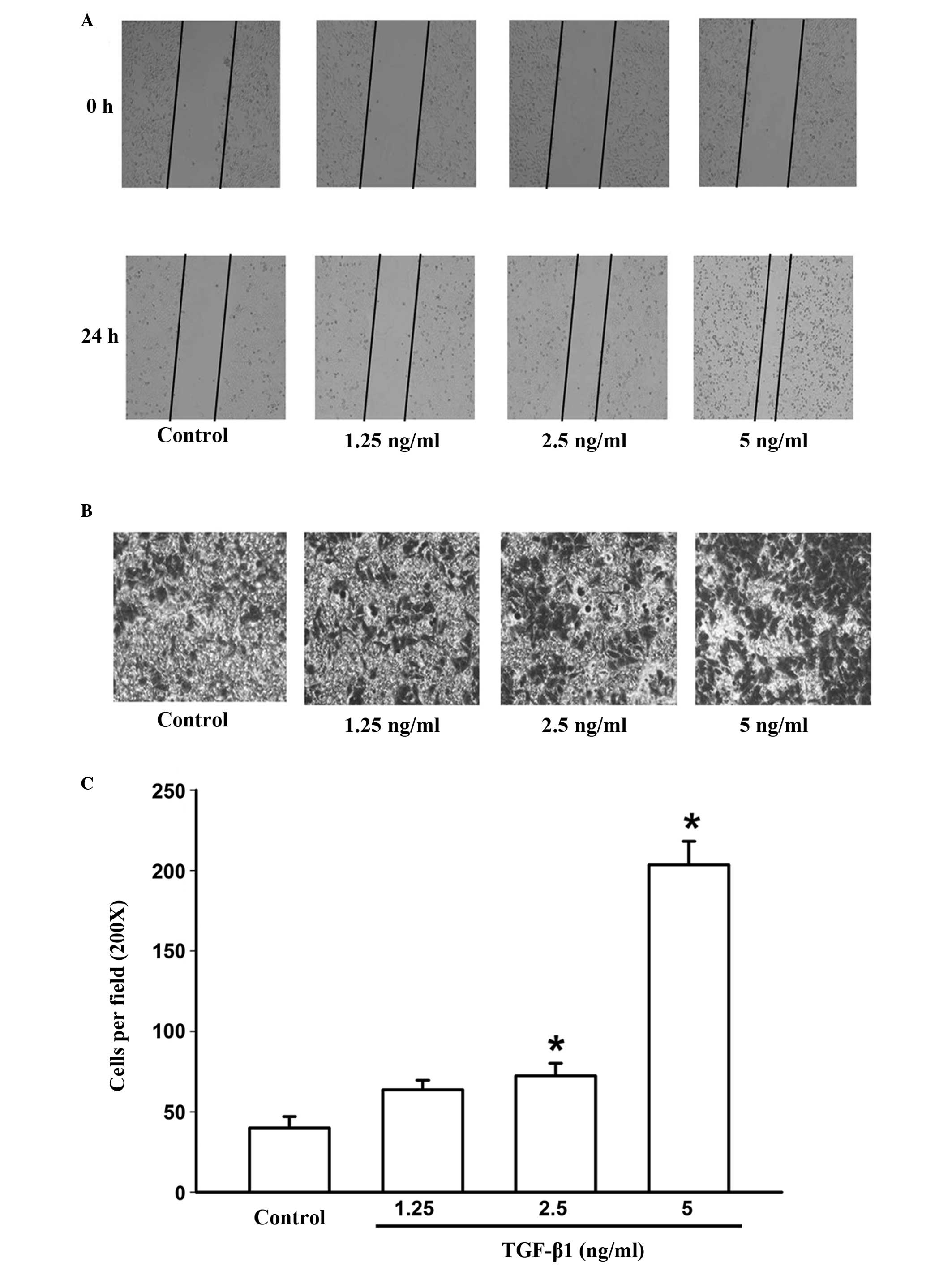
Thus, in the present study, the effect of TGF-β1 on invasion and
migration in HepG2 cells were assessed using transwell and
wound-healing assays. Cells were exposed to different
concentrations of TGF-β1 (1.25–5 ng/ml) for 24 h. The results
demonstrated that TGF-β1 significantly promoted HepG2 cell invasion
and migration compared with the untreated cells (Fig. 2A, B and C).
Ginsenoside Rg1 inhibits TGF-β1-induced
cell invasion and migration
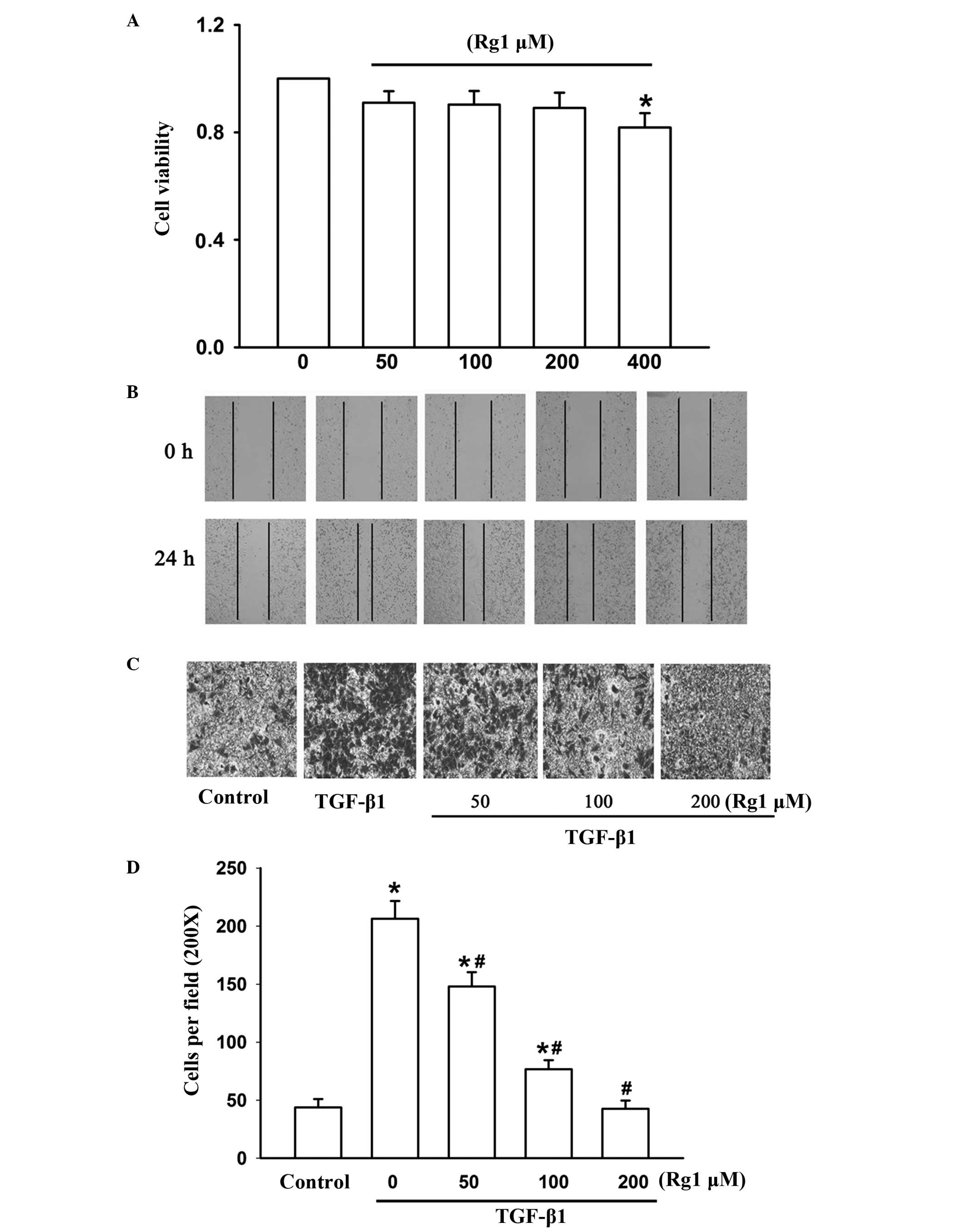
In order to observe the effect of ginsenoside Rg1 on
cell viability in HepG2 cells, cells were treated with increasing
concentrations of ginsenoside Rg1 (50–400 μM) for 48 h and then
assessed using an SRB assay. Ginsenoside Rg1 had no effect on cell
viability up to a concentration of 400 μM. Thus, the non-cytotoxic
concentrations of ginsenoside Rg1, between 50 and 200 μM were used
in subsequent experiments (Fig.
3A).
To investigate the effects of ginsenoside Rg1 on
TGF-β1-induced cell invasion and migration, transwell and
wound-healing assays were performed. Cells were treated with
ginsenoside Rg1 (50–200 μM) for 48 h with or without 5 ng/ml TGF-β1
for 24 h. TGF-β1 significantly increased invasion and migration of
HepG2 cells compared with TGF-β1-untreated control cells, while
ginsenoside Rg1 reversed this effect (Fig. 3B, C and D). These results suggest
that ginsenoside Rg1 may constitute an effective inhibitor of
invasion and migration in HepG2 cells.
Ginsenoside Rg1 regulates morphological
alterations and EMT marker expression during TGF-β1-induced
EMT
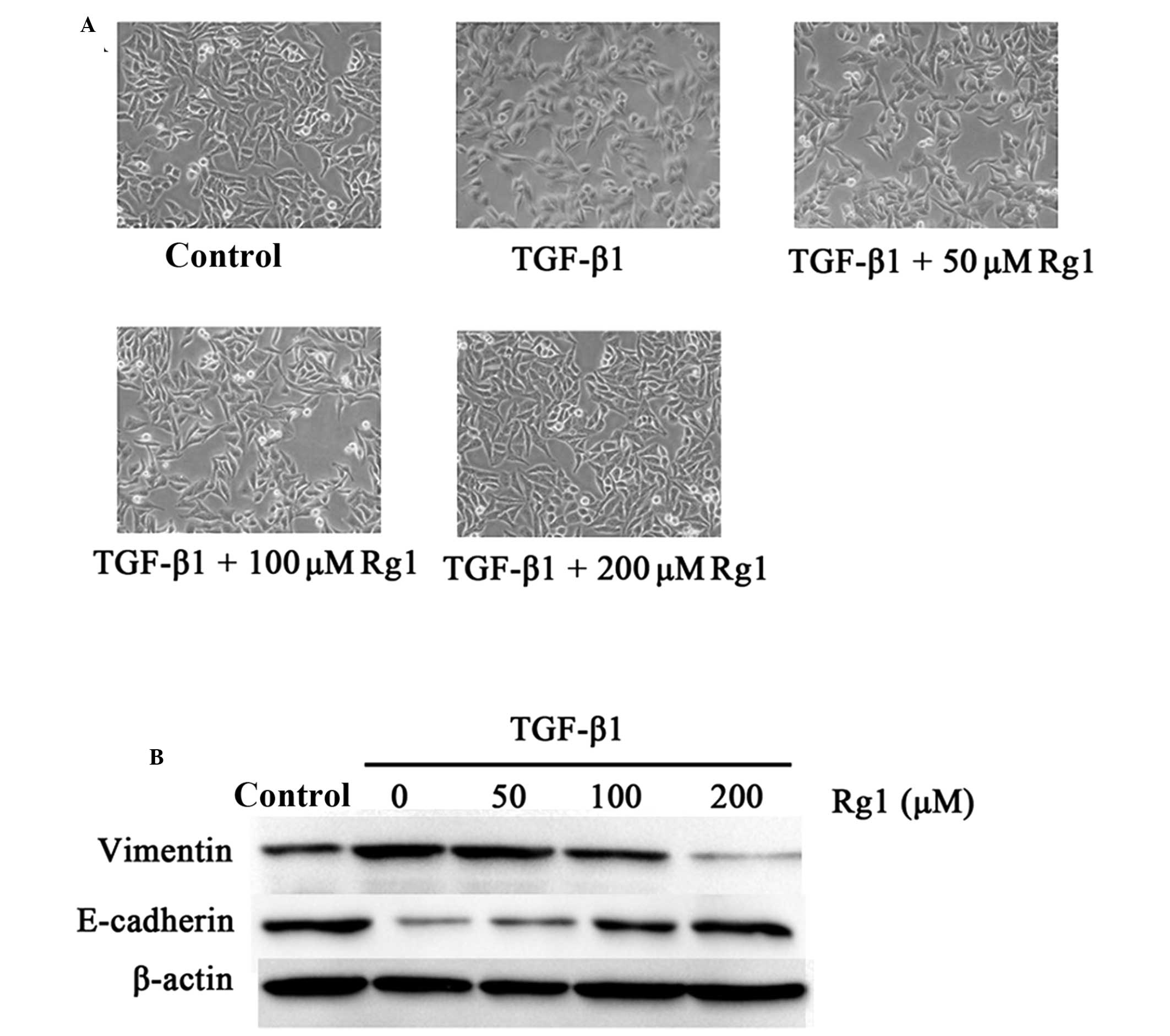
To further clarify whether suppression of
TGF-β1-induced invasion and migration by ginsenoside Rg1 resulted
from regulation of EMT, the expression of EMT marker proteins
vimentin and E-cadherin and alterations in cell morphology were
examined. Fig. 4A shows that HepG2
cells exhibited a mesenchymal phenotype when exposed to TGF-β1 (5
ng/ml), but cells pretreated with ginsenoside Rg1 exhibited a
classical epithelial morphology. The present study also analyzed
the expression of the epithelial phenotype marker E-cadherin and
the mesenchymal phenotype marker vimentin using western blotting.
The results in Fig. 4B demonstrate
that TGF-β1 (5 ng/ml) significantly decreased the expression of
E-cadherin, but induced the expression of vimentin compared with
TGF-β1-untreated control cells, while ginsenoside Rg1 reversed
these effects. These results suggest that ginsenoside Rg1 may
prevent invasion and migration via inhibition of TGF-β1-induced EMT
in HepG2 cells.
Discussion
In the present study, the results demonstrated that
ginsenoside Rg1 significantly suppressed TGF-β1-induced invasion
and migration. It is likely that the suppressive effects of
ginsenoside Rg1 on invasion and metastasis are mediated through the
inhibition of TGF-β1-induced EMT. This indicated that ginsenoside
Rg1 may be a potential inhibitor in preventing the invasion and
migration of human liver cancer.
Tumor metastasis is a multi-stage process beginning
with tumor cell migration and invasion. These proceses require EMT.
During EMT, well-polarized and adhesive epithelial cells lose
polarity and intercellular adhesion, which is mediated by
cadherins. These cells then acquire a highly motile fibroblastoid
or mesenchymal phenotype (22,23).
TGF-β1 is major inducer of EMT and several studies have
demonstrated that TGF-β1 alone or in combination with other growth
factors, including EGF and hepatocyte growth factor is critical in
mediating EMT in various types of malignant tumor (24,25).
Consistent with these studies, the present study found that TGF-β1
induces HepG2 cells to undergo EMT and the cells acquire a
spindle-like fibroblastic phenotype and reduce their cell-cell
contact. TGF-β1 (5 ng/ml) also significantly upregulated the
expression of the mesenchymal marker vimentin, downregulated the
expression of the epithelial marker E-cadherin and promoted
invasion and migration of HepG2 cells.
Previous studies have demonstrated that ginsenoside
Rg1 may exert anti-cancer properties (13,18,26,27),
however, this is the first study, to the best of our knowledge, to
demonstrate that ginsenoside Rg1 may be a potential inhibitor of
invasion and migration. The results of the transwell and
wound-healing assays demonstrated that ginsenoside Rg1 inhibited
TGF-β1-induced cell invasion and migration in HepG2 cells. To
further investigate how ginsenoside Rg1 suppresses TGF-β1-induced
cell invasion and migration, the effect of ginsenoside Rg1 on
TGF-β1-induced EMT was assessed. The results demonstrated that
HepG2 cells exhibit a mesenchymal phenotype when exposed to TGF-β1,
but when exposed to ginsenoside Rg1 this effect was reversed and
the cells exhibited a classical epithelial morphology. Ginsenoside
Rg1 also increased the expression of the epithelial phenotype
marker E-cadherin and repressed the expression of the mesenchymal
phenotype marker vimentin. These results suggest that ginsenoside
Rg1 may prevent invasion and migration via inhibition of
TGF-β1-induced EMT in HepG2 cells.
In conclusion, the present study demonstrated that
ginsenoside Rg1 was able to inhibit liver cancer cell invasion and
migration in vitro by inhibiting TGF-β1-induced EMT. This
indicated that ginsenoside Rg1 may serve as a potential inhibitor
in preventing the invasion and migration of human liver cancer.
Acknowledgements
The present study was supported by the China
Postdoctoral Science Foundation (grant no. 20090461139); the
Foundation of Bengbu Medical College (grant nos. Bykf13A11,
Byycx1329 and 2013Byky1350); the Natural Science Foundation of the
Provincial Education Department of Anhui (grant no. KJ2013A192);
and the National Natural Science Foundation of Anhui (grant no.
1408085MH206).
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang CJ, Chao CH, Xia W, et al: P53
regulates epithelial-mesenchymal transition and stem cell
properties through modulating miRNAs. Nat Cell Biol. 13:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP, Acloque H, Huang RY, et al:
Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
7
|
Lee JM, Dedhar S, Kalluri R, et al: The
epithelial-mesenchymal transition: new insights in signaling,
development, and disease. J Cell Biol. 172:973–981. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: at the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee EJ, Ko E, Lee J, et al: Ginsenoside
Rg1 enhances CD4(+) T-cell activities and modulates Th1/Th2
differentiation. Int Immunopharmacol. 4:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CY, Deng W, Liao XQ, et al: The effects
and mechanism of ginsenoside Rg1 on myocardial remodeling in an
animal model of chronic thromboembolic pulmonary hypertension. Eur
J Med Res. 18:162013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li QF, Shi SL, Liu QR, et al: Anticancer
effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in
osteosarcoma MG-63 cells: nuclear matrix downregulation and
cytoplasmic trafficking of nucleophosmin. Int J Biochem Cell Biol.
40:1918–1929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Cai SZ, Zhou Y, et al: Senescence
as a consequence of ginsenoside rg1 response on k562 human leukemia
cell line. Asian Pac J Cancer Prev. 13:6191–6196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Kou JP and Yu BY: Ginsenoside Rg1
protects against hydrogen peroxide-induced cell death in PC12 cells
via inhibiting NF-κB activation. Neurochem Int. 58:119–125. 2011.
View Article : Google Scholar
|
|
16
|
Qu DF, Yu HJ, Liu Z, et al: Ginsenoside
Rg1 enhances immune response induced by recombinant Toxoplasma
gondii SAG1 antigen. Vet Parasitol. 179:28–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu L, Chen WF and Wong MS: Ginsenoside Rg1
protects dopaminergic neurons in a rat model of Parkinson’s disease
through the IGF-I receptor signalling pathway. Br J Pharmacol.
158:738–748. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li L, Wang Y, Qi B, et al: Suppression of
PMA-induced tumor cell invasion and migration by ginsenoside Rg1
via the inhibition of NF-κB-dependent MMP-9 expression. Oncol Rep.
32:1779–1786. 2014.PubMed/NCBI
|
|
19
|
Ellenrieder V, Hendler SF, Boeck W, et al:
Transforming growth factor beta1 treatment leads to an
epithelial-mesenchymal transdifferentiation of pancreatic cancer
cells requiring extracellular signal-regulated kinase 2 activation.
Cancer Res. 61:4222–4228. 2001.PubMed/NCBI
|
|
20
|
Chen J, Li Q, An Y, et al: CEACAM6 induces
epithelial-mesenchymal transition and mediates invasion and
metastasis in pancreatic cancer. Int J Oncol. 43:877–885.
2013.PubMed/NCBI
|
|
21
|
Creighton CJ, Gibbons DL and Kurie JM: The
role of epithelial-mesenchymal transition programming in invasion
and metastasis: a clinical perspective. Cancer Manag Res.
5:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagai T, Arao T, Furuta K, et al:
Sorafenib inhibits the hepatocyte growth factor-mediated epithelial
mesenchymal transition in hepatocellular carcinoma. Mol Cancer
Ther. 10:169–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin JA, Hong OK, Lee HJ, et al:
Transforming growth factor-β induces epithelial to mesenchymal
transition and suppresses the proliferation and
transdifferentiation of cultured human pancreatic duct cells. J
Cell Biochem. 112:179–188. 2011. View Article : Google Scholar
|
|
26
|
Yang JJ, Lim JY, Huang J, et al: The role
of inherited TPMT and COMT genetic variation in cisplatin-induced
ototoxicity in children with cancer. Clin Pharmacol Ther.
94:252–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Wei Q, Zuo GW, et al: Ginsenoside
Rg1 induces apoptosis through inhibition of the EpoR-mediated
JAK2/STAT5 signalling pathway in the TF-1/Epo human leukemia cell
line. Asian Pac J Cancer Prev. 15:2453–9. 2014. View Article : Google Scholar
|