Introduction
Globally, oral cancer is the sixth most common type
of cancer affecting 3% of the world’s population. There has been an
increase in the rate of occurrence and, in 2007, this rate
increased over 11% in one year (1). Annually, almost 7% of
cancer-associated mortality in males and 4% in females is
attributed to oral cancer (1).
Tobacco use, smoking, alcohol and areca nut chewing are proposed as
possible risk factors for oral cancer (1). Oral cancer can affect any part of the
oral cavity, including the lips, tongue, mouth and throat, which
can be categorized into two types. Oral cavity cancer develops in
the mouth itself, whereas oropharyngeal cancer develops in the
oropharynx, which is located in the throat just behind the mouth.
The most common type of oral cancer is squamous cell carcinoma
(SCC), which affects the tissue lining the oral cavity (2,3).
Despite advances in the treatment of cancer, the
clinical outcome is far from expected and recurs in patients even
following treatment. It has been found that treatment failure, or
minimal residual disease, occurs due to the persistence of cancer
stem cells (CSCs) that evade the treatment regimen (4,5). The
population of CSCs possesses characteristics associated with stem
cells, including self-renewal and exhibits high in vivo
tumorigenicity, differentiation potential as well as multidrug and
apoptotic resistance (6). The
difficulty in eradicating tumor tissue may be due to conventional
treatments targeting the bulk of the tumor cells, but not the CSCs,
which maintain the tumor tissue.
Cells that exclude Hoechst 33342 dye are referred to
as side population (SP) cells. These cells share several
characteristics with CSCs; in particular, they possess the capacity
for tumor initiation, express stem-like genes and demonstrate
resistance to chemotherapeutic drugs. Therefore, the dye exclusion
technique is valuable in assisting in the identification of a
unique population of cells with stem-like characteristics (7). SP cells also overexpress ATPase
binding cassette (ABC) transporters, including ABCB1, also termed
multidrug resistance protein 1 (MDR1), ABCC1 and ABCG2, also termed
breast cancer resistance protein 1, which contribute to multidrug
resistance and also express stem cell surface markers, including
CD133, CD44, CD34, CD29 and CD24. SP cells have also been
identified in several types of solid tumor, including breast cancer
(4), brain tumor (5), glioblastoma (5), gastrointestinal tumor (6), head and neck squamous cell carcinoma
(7) as well as in hepatocellular
cell lines (4) and in primary
cultures from patients with neuroblastoma (8).
Therefore, the sorting of SP cells assists in
identifying cancerous stem cells and its subsequent
characterization substantiates the mechanism underlying oncogenesis
and drug resistance (9).
Consequently, the present study investigated the prevalence of SP
cells using an established oral squamous cell carcinoma (OSCC) cell
line SCC-55, based on Hoechst 33342 efflux. In addition, the
isolated SP cells were characterized for the presence of cell
surface markers, including CD147, CD44 and CD29.
Materials and methods
Cell line and cell culture
The SCC-55 human cell line was obtained from
cancerous growth in the mandibular region (grade three; recurrent
type; American Type Cell Culture, Manasas, VA, USA). The SCC-55
cell lines were cultured in Dulbecco’s modified Eagle’s medium
(DMEM; Gibco-BRL, Carlsbad, CA, USA) with 10% fetal bovine serum
(Gibco-BRL) supplemented with antibiotics and maintained in T-75
flasks at 37°C in a humidified 5% CO2, 95% air
atmosphere. When the SCC-55 cells attained 90% confluence, they
were removed from the culture flask using Trypsin-EDTA (0.25% 53 mM
EDTA; Sigma, St. Louis, MO, USA), washed in phosphate-buffered
saline (PBS), suspended in 10% DMEM and centrifuged at 4,000 × g
for 6 min. The cells were then resuspended in 10% DMEM and cells
were counted using a hemocytometer (Thermo Fisher Scientific,
Waltham, MA, USA). The present study was approved by the ethics
committee of Chongqing Medical University (Chongqing, China).
Study group
The control group consisted of cells + Hoechst 33342
(n=5). The drug treated group consisted of cells + verapamil +
Hoechst 33342 (n=5).
Labeling with Hoechst 33342
The cells in the staining medium (~106
cells/ml of 10% DMEM) were labeled with Hoechst 33342 stock (Sigma)
bis-benzimide (5 μl/ml) with either the dye alone or in combination
with verapamil (0.8 μ/ml). The cells were mixed and placed in a
water bath at 37°C for 90 min. After 90 min, the cells were
centrifuged (1,500 × g for 10 min at 4°C) and resuspended in 500 μl
Hank’s balanced salt solution containing 10 mM
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid. Finally,
the cells were counterstained using propidium iodide (PI; 2 μg/ml)
at 4°C. The cells were filtered through a 50 μm nylon mesh (BD
Biosciences, Franklin Lakes, NJ, USA) to remove clumps of cells
into labeled fluorescence-activated cell sorting (FACS) tubes.
Separate tubes containing 10% DMEM were used for sterile sorting of
the SP cells and the main population cells. The cells were sorted
using a flow cytometer (FACS Aria II; BD Biosciences). The Hoechst
33342 dye was excited at 355 nm and its dual-wavelength
fluorescence was analyzed using a flow cytometer (blue, 450 nm;
red, 675 nm; FACS Aria II; BD Biosciences, Franklin Lakes, NJ,
USA).
In vitro proliferation assay
The sorted SP and non-SP cells were inoculated into
a 96-well plate at 2×106 cells/well and then cultured in
a CO2 incubator. Each group was set up in triplicate.
Cell proliferation was measured every day for 5 days. Each well was
supplemented with Cell Counting kit-8 (CCK-8; Tiangen, Beijing,
China) solution (10 μl) and incubated in a CO2 incubator
for 3 h. The optical density (OD) was determined at 450 nm. These
data were used to calculate the cell growth based on the mean value
of OD450 and the standard deviation values for each
well.
Cell resistance experiment
The obtained SP and non-SP cells were cultured in
96-well plates at a concentration of 1×103 cells/plate.
After 24 h, 5-fluorouracil (5-FU) was added to all cultures to
produce a final concentration of 10 μg/ml. The plates were then
placed in a hatch box for 48 h. Each well was then supplemented
with CCK-8 (10 μl) solution and the plates were incubated for 3 h.
The mean value of OD450 was obtained and is presented as
a graph. Cell resistance was calculated using the following
formula: Cell resistance rate (%) = (experimental group
OD450/control group OD450) × 100.
Immunocytochemistry
The sorted SP cells and main population cells were
seeded in 35 mm culture plates (~100 μl) and incubated for 3 h,
following which 1 ml of medium (10% DMEM) was added. Following
incubation overnight, the cells were rinsed with PBS and fixed in
4% paraformaldehyde in 1X PBS for 5 min at 4°C. The cells were then
washed with 1X PBS and blocked with 1% bovine serum albumin
(BSA)-Tris-buffered saline (TBS) with RNase (10 μl/1,000 μl of 3%
BSA-TBS). After 1 h incubation at room temperature, the cells were
washed with PBS and then incubated with the primary antibodies,
mouse anti-human CD147 monolclonal antibody (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and mouse anti-human CD44
monoclonal antibody (Santa Cruz Biotechnology, Inc.), in 1% BSA-TBS
(1:100; 2 μl/200 μl) prior to incubation overnight at 4°C.
Following washing with 1X PBS, the cells were incubated with a
rabbit anti-mouse polyclonal secondary antibody conjugated with
fluorescein isothiocyanate (1:100 in 1% BSA-TBS; Santa Cruz
Biotechnology, Inc.) at room temperature for 1 h. The cells were
then washed with PBS and PI was added (1 μl/200 μl PBS). The cells
were viewed under a confocal laser scanning microscope (Leica TCS;
Leica, Mannheim, Germany). Image analysis and preparation of
figures was performed using Adobe Photoshop CS4 software (Adobe
Systems, Inc., San Jose, CA, USA).
Biochemistry
For western blot analysis, proteins were extracted
from the SP and non-SP cells and the protein concentration was
determined using a Bradford assay. Following sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transfer onto a
membrane, the gels were treated with the primary antibodies rabbit
anti-human ABCG2, CD147, CD44 and B-cell lymphoma 2 (Bcl-2), the
secondary antibody (goat anti-rabbit IgG with alkaline phosphatase
markers) and a chemiluminescence reagent. Blots were detected and
scanned using a densitometer (Bio-Rad GS-710; Bio-Rad, Hercules,
CA, USA).
Results
FACS analysis of SP cells containing MDR1
using Hoechst 33342
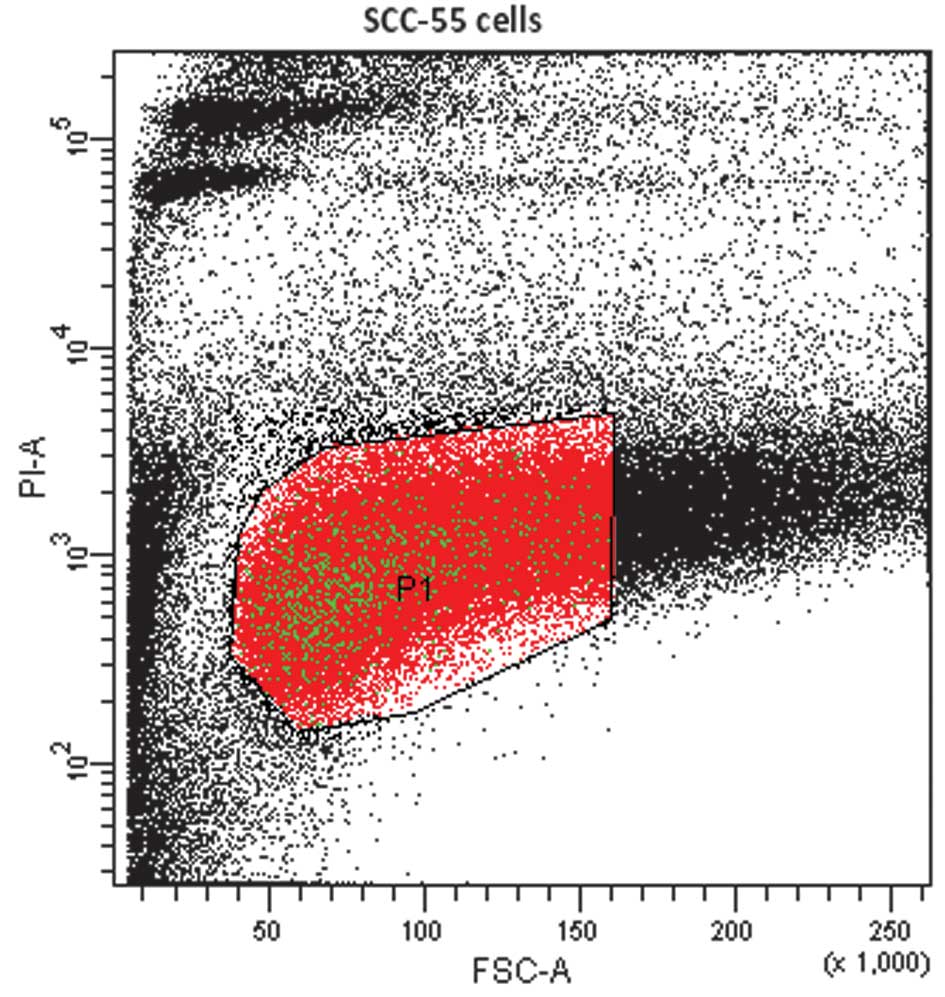
In FACS analysis, cells or particles passing through
the laser beam scatter light. This is detected as forward scatter,
which correlates with cell size, and side scatter, which is
dependent on cell or particle density. The density may include the
number of granules in the cytoplasm or the size of the membrane.
Populations of cells are often determined based on their different
size and density. Live cells were selected against PI as a P1 gated
population. PI was used to exclude dead cells from the sample
(Fig. 1). The SP cells were sorted
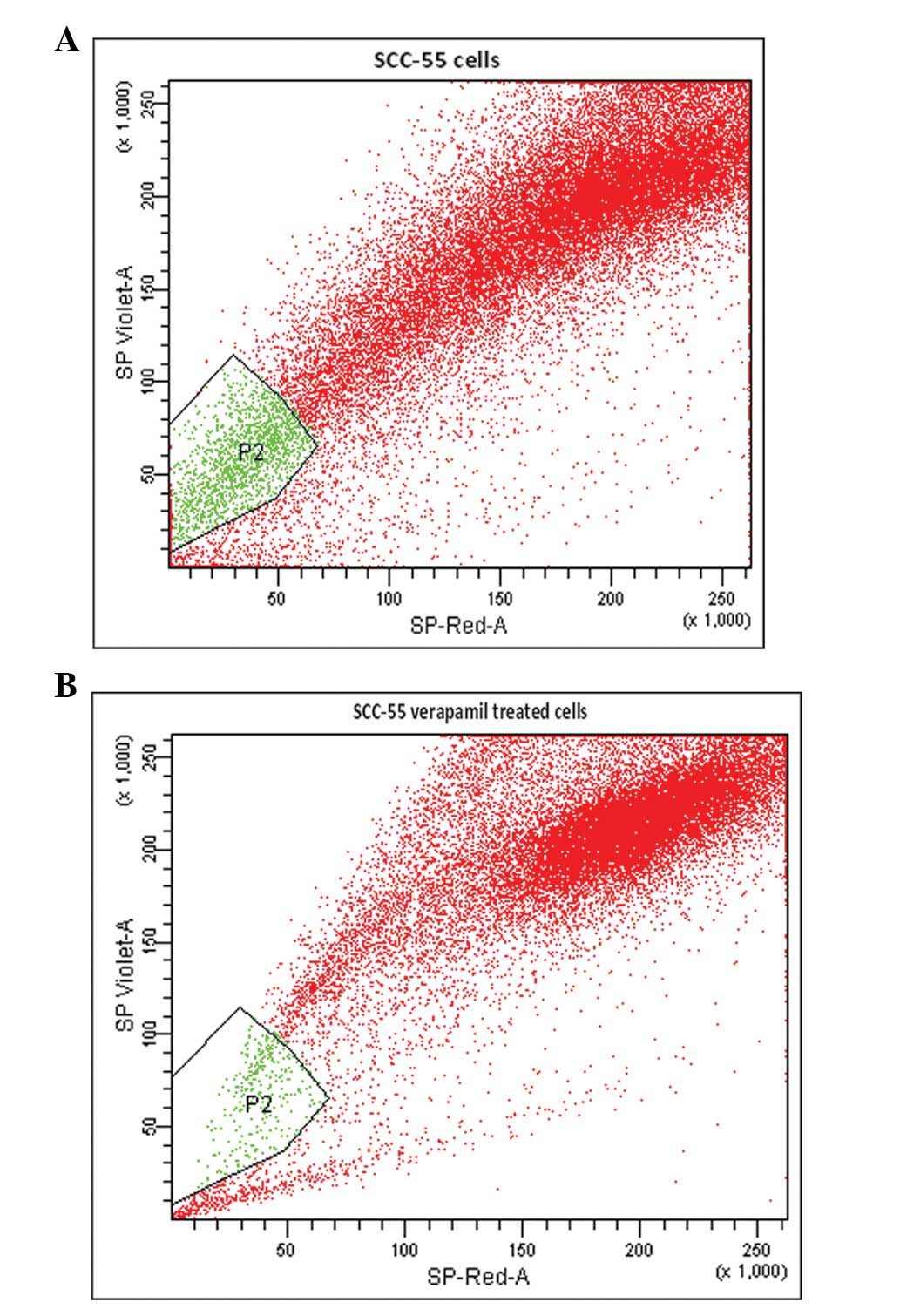
from the gated P1 cells using Hoechst 33342, a DNA binding dye. By
simultaneously monitoring the fluorescence emission of Hoechst
33342 at ~450 nm (SP-Violet) and at 675 nm (SP-Red) following
ultraviolet excitation, a set of cells were observed, which
exhibited low blue and red fluorescence. The gate was set according
to the ability of cells to efflux Hoechst 33342 and to the
sensitivity of the cells to verapamil. In the FACS analysis, the
lower left quadrant was designated SP and was inhibited by
verapamil. The upper right quadrant was designated as MP, in which
Hoechst red was observed and was not inhibited by verapamil
(Fig. 2A and B).
SP cells are a distinct population of P2 gated cells
located towards the SP-violet region of the FACS profile dot plot.
The active exclusion of Hoechst 33342 by SP cells (Fig. 2A) involves the ABC transporter
transmembrane protein MDR1. The number of cells collected (~59,000)
comprised ~55% of the initial cell count. Of the 59×103
cells (P1) analyzed, Hoechst dye was effluxed by 2.8% cells in the
P2 gated region (Fig. 2A). The
verapamil-treated cells of the same cell line were sorted as
described previously with Hoechst 33342. A total of ~53,199 P1
cells were obtained, which was ~44% of the initial cell count.
Following treatment with verapamil, SP cells were reduced from 2.8
to 0.6%. Verapamil is an MDR1 transporter protein inhibitor, which
inhibits the drug efflux action of cells (Fig. 2B). Therefore, treatment with
verapamil significantly reduced the proportion of SP cells.
Characterization of SP cells
The SP and non-SP cells sorted by FACS were subject
to further phenotypic characterization, including cell
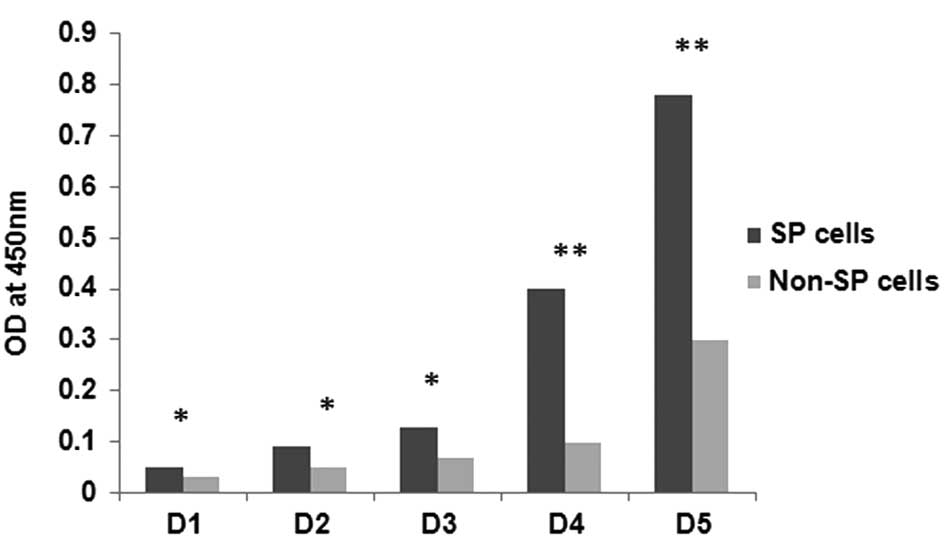
proliferation and cell survival assays. The growth rates of SP and
non-SP cells are shown in Fig. 3.
Rapid proliferation was observed in the SP cells starting from day
(D)3 and became more confluent on D7 (data not shown). However, the
growth rate of the non-SP cells was significantly lower when
compared with the SP cells (Fig.
3). The growth rate of the SP cells was significantly higher
compared with that of the non-SP cells (*P<0.05;
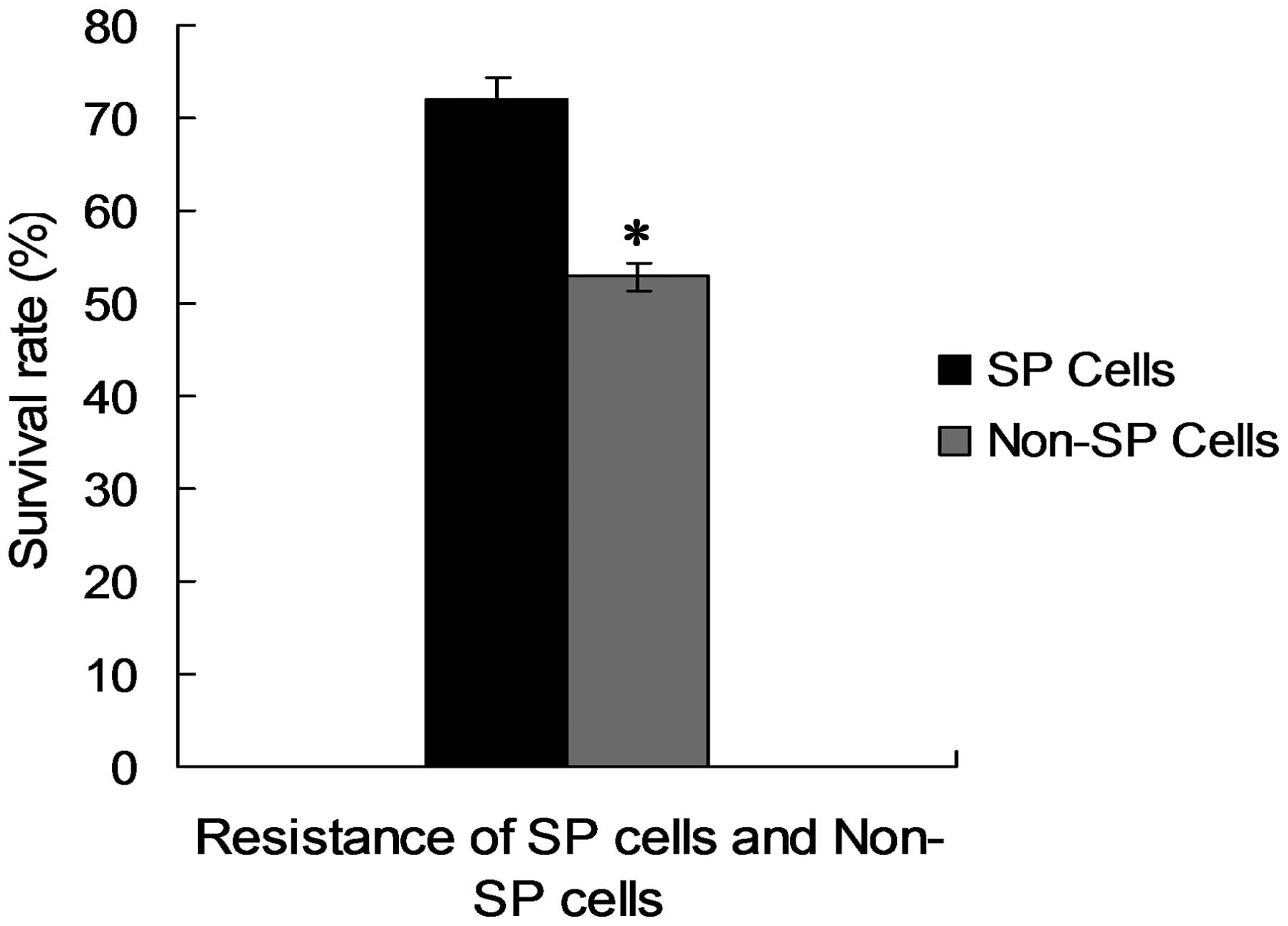
**P<0.01). Following the in vitro
proliferation assays, the survival rates of the SP cells were
analyzed following exposure to 10 μg/ml 5-FU. As shown in Fig. 4, SP cells demonstrated a
significantly higher resistance to 5-FU (73%) compared with the
non-SP cells (52%; *P<0.05).
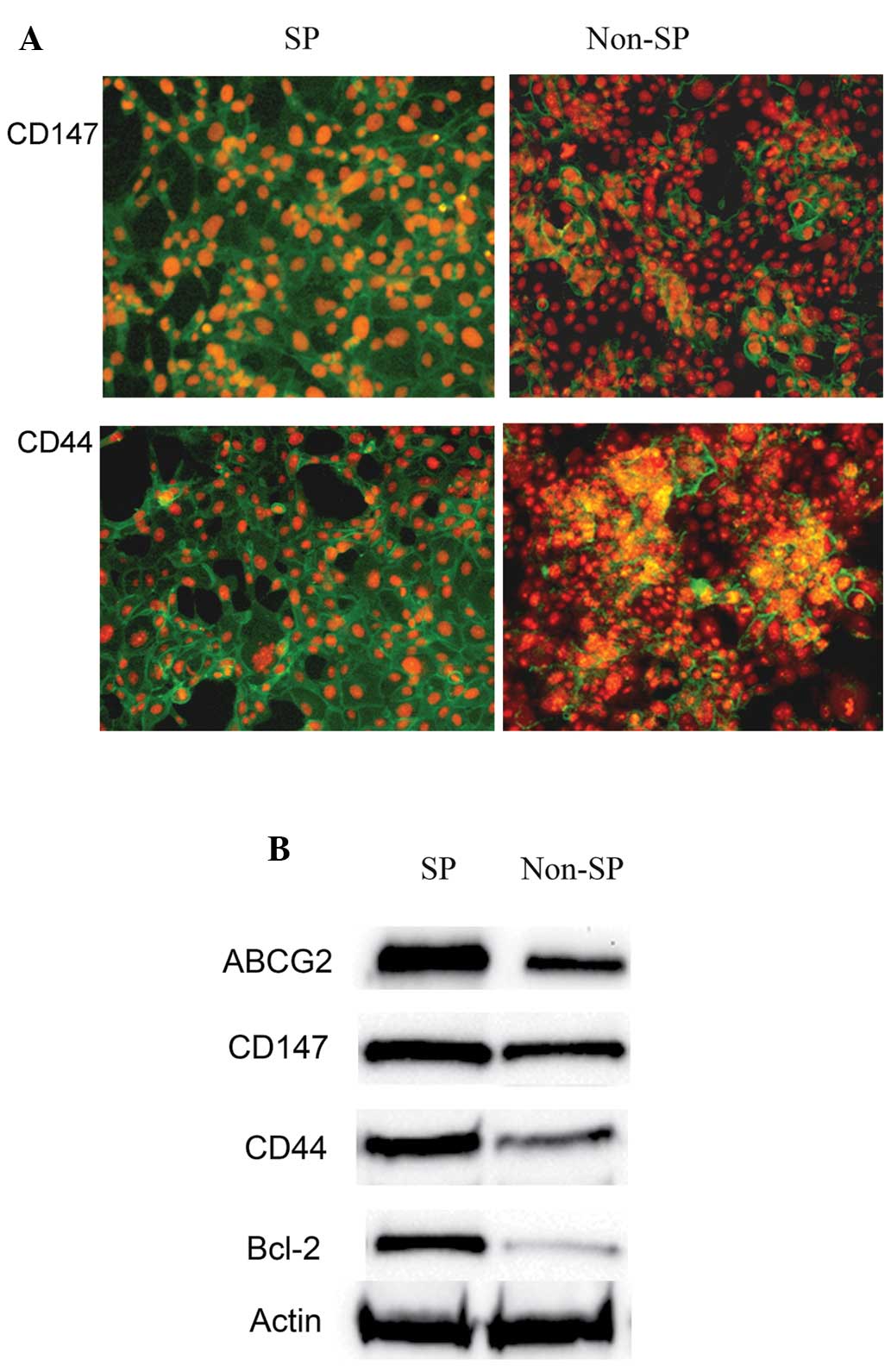
Furthermore, the isolated SP cells were analyzed for
the cell surface markers CD147 and CD44 using antibodies against
them. Notably, analysis using a fluorescence microscope (TCS SP2;
Leica, Mannheim, Germany) revealed that the SP cells were more
positive for the cell surface proteins, including CD147 and CD44
(Fig. 5A) compared with the non-SP
cells. This was further confirmed by western blot analysis. CD147
and CD44 were highly expressed in the SP cells, whereas the levels
of these proteins were reduced in the non-SP cells (Fig. 5B). The results also demonstrated
that the levels of ABCG2 and Bcl-2 expression were higher in the SP
cells compared with the non-SP cells (Fig. 5B).
Therefore, the results of the present study
suggested that the expression of CD147/CD44, ABCG2 and Bcl-2 is
elevated in SP cells and they may act co-operatively as an
important factor in drug resistance and in the marked proliferation
of cancer cells and tumor invasion.
Discussion
Traditional treatments have been developed and
assayed based mainly on their ability to destroy the majority of
the tumor population. However, these treatments may miss CSCs,
which are more resistant to chemotherapeutic drugs. These CSCs are
responsible for tumor growth, metastasis and relapse (10). There is evidence in several
different types of cancer indicating that CSCs survive conventional
anticancer therapies that target only the rapidly dividing cells
(11) and is associated with
treatment failure and tumor recurrence. Therefore, the elimination
of CSCs is an important goal for eradicating types of refractory
cancer and in providing long-term disease-free survival. To enable
this, certain stem cell characteristics can be used to identify
CSCs, one of which is the capacity of cells to extrude dyes,
including Hoechst 33342. Cells that exclude this dye are termed SP
cells (12). SP cells share
characteristics of CSCs, in particular they have increased
tumor-initiating capacity, express stem-like genes and are
resistant to chemotherapeutic drugs. Therefore, the exclusion of
dyes is a valuable technique as it enables the identification of
this unique population of cells with stem-like characteristics.
In the present study, CSC cells from the OSCC SCC-55
cell line were identified and isolated. SP cells were sorted from
the gated P1 cells based on the efflux of Hoechst 33342, a DNA
binding dye, using FACS analysis. The exclusion of Hoechst 33342 by
SP cells is an active process involving MDR1, a member of the ABC
transporter transmembrane proteins. Following treatment with
verapamil, the SP cells were reduced from 2.8 to 0.6% (Fig. 2A and B). Verapamil is an MDR1
transporter protein inhibitor, which inhibits the drug efflux
action of the cells. Its use also provided confirmation of the
presence of the MDR1 protein in the SCC-55 cell line and explained
the chemoresistance and tumor recurrence in the mandibular origin
from which the cell line was established. The multidrug resistance
demonstrated by these cells enabled them to survive the
chemotherapy regimen and resulted in a poor prognosis of the
patients suffering from oral cancer. The sorted SP cells were
further characterized by cell proliferation and cell survival
assays. These cells demonstrated increased proliferation capacity
and resistance to 5-FU (Figs. 3
and 4). The resistance to 5-FU was
most likely facilitated by two major mechanisms, the activation of
ABC transporters, which expel harmful materials from cells and the
suppression of apoptosis. Similar to previous findings in gastric
cancer cell lines (13), the
results of the present study suggested that these processes may
have occurred in the SP cells, where the expression of the ABC
transporter protein, ABCG2, and the anti-apoptotic protein Bcl-2
were significantly higher compared with the non-SP cells.
Collectively, these findings suggested that the chemotherapy
resistance characteristics of SP cells were derived mainly from the
overexpression of the chemotherapy resistant channel protein
ABCG2.
In addition, immunocytochemical analysis was
performed on the sorted SCC-55 SP cells to investigate the presence
of cell surface markers. Using fluorescence microscopy, almost the
entire population of SP cells was more positive to CD147 and CD44
compared with those in the non-SP population (Fig. 5A). Previous studies in different
types of solid tumor also revealed that an increased quantity of
CD147 on the surface of tumor cells induces tumor invasion and
stimulates the production of a number of matrix metalloproteinases
by adjacent stromal cells (14).
Similarly, in head and neck squamous cell carcinoma,
CD44+ cells were enriched with tumorigenic CSCs able to
propagate tumor formation in mice, whereas CD44− cells
were not (15). Taken together,
the findings of the present study suggested that the coexpression
of CD147 and CD44 by SP cells positive to MDR1 in SCC-55 indicate a
role for these cells in tumor growth, metastasis and recurrence in
the mandibular origin from which the recurrent tumor SCC-55 cell
line was established. There may be close interaction between CD147,
CD44 and MDR1 resulting in the marked augmentation of tumor
metastasis, invasion and chemoresistance. Therefore, the
characterization of SP cells assists in designing novel therapeutic
agents, which selectively target CSCs and may improve cancer
therapy by inhibiting ABC transporters. Furthermore, differences
between the signaling pathways in normal cells and CSCs require
elucidation to provide new therapeutic targets, with the eventual
goal of eliminating residual disease and disease recurrence.
References
|
1
|
Ma JL, Zhang L, Brown LM, et al:
Fifteen-year effects of helicobacter pylori, garlic, and vitamin
treatments on gastric cancer incidence and mortality. J Natl Cancer
Inst. 104:488–492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ackerman LV: Verrucous carcinoma of the
oral cavity. Surgery. 23:670–678. 1948.PubMed/NCBI
|
|
3
|
Crissman JD and Zarbo RJ: Dysplasia in
situ carcinoma and progression to invasive squamous cell carcinoma
of the upper aerodigestive tract. Am J Surg Pathol. 13:5–16.
1989.
|
|
4
|
Challen GA and Little MH: A side order of
stem cells: the SP phenotype. Stem Cells. 24:3–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ramachandran C and Melnick SJ: Multidrug
resistance in human tumors molecular diagnosis and clinical
significance. Mol Diagn. 4:81–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ford JM and Hait WN: Pharmacology of drugs
that alter multidrug resistance in cancer. Pharmacol Rev.
42:155–199. 1990.PubMed/NCBI
|
|
7
|
Hirschmann-Jax C, Foster AE, Wulf GG, et
al: A distinct ‘side population’ of cells with high drug efflux
capacity in human tumor cells. Proc Natl Acad USA. 101:14228–14233.
2004. View Article : Google Scholar
|
|
8
|
Robey RW, Shukla S, Finley EM, Oldham RK,
et al: Inhibition of Pgp (ABCB1) and MDR associated protein-1
(ABCC1) mediate transport by the orally administered inhibitor
CBT-1. Biochem Pharmacol. 75:1032–1042. 2008. View Article : Google Scholar
|
|
9
|
Kondo T, Setoguchi T and Taga T:
Persistence of a small subpopulation of cancer stem-like cells in
the C6 glioma cell line. Proc Natl Acad Sci USA. 101:781–786. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gil J, Stembalska A, Pesz KA and Sasiadek
MM: Cancer stem cells: the theory and perspectives in cancer
therapy. App Genet. 49:193–199. 2008. View Article : Google Scholar
|
|
11
|
Komuro H, Saihara R, Shinya M, Takita J,
et al: Identification of side population cells (stem-like cell
population) in pediatric solid tumor cell lines. J Pediatr Surg.
42:2040–2045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho RW and Clarke FM: Recent advances in
cancer stem cells. Curr Opin Genet Dev. 18:48–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li R, Wu X, Wei H and Tian S:
Characterization of side population cells isolated from the gastric
cancer cell line SGC-790. Oncol Lett. 5:877–883. 2013.PubMed/NCBI
|
|
14
|
Guo H, Li R, Zucker S and Toole BP:
EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis,
also binds interstitial collagenase to the tumor cell surface.
Cancer Res. 60:888–891. 2000.PubMed/NCBI
|
|
15
|
Yanamoto S, Kawasaki G, Yoshitomi I,
Iwamoto T, et al: Clinicopathologic significance of EpCAM
expression in squamous cell carcinoma of the tongue and its
possibility as a potential target for tongue cancer gene therapy.
Oral Oncol. 43:869–877. 2007. View Article : Google Scholar : PubMed/NCBI
|