Introduction
Through mutation, tumor cells may express novel
antigens, which are recognized by the immune system. The innate and
acquired immune systems are subsequently activated, resulting in
tumor cell death. However, there are exceptions, in which tumor
cells modify the surface antigens and alter the microenvironment to
evade the immune response (1).
There is substantial evidence that hypoxia is
present in malignant solid tumors and is associated with increased
tumor growth, invasion, metastatic potential and resistance to
therapy (2). Fink et al
(3) indicated that liver cancer
cells exposed to hypoxia were more tolerant to natural killer (NK)
cells compared with those cultivated under normoxic conditions.
Hypoxia-inducible factor-1 (HIF-1) is a
sequence-specific DNA-binding transcription factor, which is
regulated by hypoxia and transcriptionally activates the expression
of several genes (4). HIF-1
consists of HIF-1α and HIF-1β (5).
HIF-1β is stable intracellularly, while HIF-1α is associated with
cellular O2 (6). The
major histocompatibility complex class I chain-related (MIC) gene
has seven family members; however, only the MICA and MICB genes
encode proteins (7). The MICA/B
proteins are located in the cell membrane and act as ligands of the
NK group 2, member D (NKG2D). These proteins are rarely expressed
by normal cells; however, they are expressed in a variety of
malignant diseases (8–10). NKG2D is well-established as a
receptor expressed on NK cells. Following ligand binding, it
transfers the signal downstream to activate the cytotoxic activity
of NK and T-cells (11–13).
Hypoxia in pancreatic cancer widely exists;
therefore, a series of experiments was designed in order to
determine whether pancreatic cancer cells resisted the cytotoxic
effect of NK cells and whether the resistance was associated with
hypoxia. The present study also aimed to elucidate whether the
resistance observed was associated with the expression of NKG2D on
NK cells and the molecule MIC on the pancreatic cancer cell
membrane.
Materials and methods
Materials
Mouse anti-human MICA/B (1:150) and NKG2D (1:100)
monoclonal antibodies were obtained from Santa Cruz Biotechnology,
Inc, (Dallas, TX, USA). Rabbit anti-human HIF-1α polyclonal
antibody was obtained from Boshide Bio, Inc. (Wuhan, China).
Glyceryl trinitrate (GTN) was obtained from Sabex (Boucherville,
QC, Canada), KT5823 and NG-mono-methyl-L-arginine (L-NMMA) were
obtained from Biyuntian (Shanghai, China) and MTT and
8-bromoguanosine cyclic monophosphate (8-Br-cGMP) were obtained
from Sigma-Aldrich (St. Louis, MO, USA).
Patients and samples
Pathological specimens were obtained from 42
patients undergoing surgical resection for pancreatic carcinoma and
nine patients undergoing surgical resection for chronic
pancreatitis at Xiangya Hospital (Changsha, China) between April
2010 and April 2012. In addition, eight normal pancreatic tissue
samples were obtained from the Xiangya Transplant Center (Changsha,
China) or from autopsy. The general sample information for the 59
total samples, graded as previously described (14,15),
is shown in Table I. The study was
approved by the ethics committee of Xiangya Hospital, Central South
University, Changsha, China.
 | Table IExpression of HIF-1α and MICA/B in
pancreatic carcinoma, chronic pancreatitis and normal pancreatic
tissues. |
Table I
Expression of HIF-1α and MICA/B in
pancreatic carcinoma, chronic pancreatitis and normal pancreatic
tissues.
| | HIF-1α | | MIC A/B | |
|---|
| |
| |
| |
|---|
| Sample | n | − | + | ++ | +++ | P-value | 0 | 1 | 2 | P-value |
|---|
| Pancreatic
carcinoma | 42 | 10 | 2 | 12 | 18 | | 4 | 13 | 25 | |
| Chronic
pancreatitis | 9 | 7 | 2 | 0 | 0 | <0.001 | 7 | 2 | 0 | <0.001 |
| Normal pancreas | 8 | 0 | 0 | 0 | 0 | | 7 | 1 | 0 | |
Cells
The PANC-1 cell line, purchased from the Resource
Center of Shanghai Institutes of Biological Sciences (Shanghai,
China), was maintained in a monolayer culture in Dulbecco’s
modified Eagle’s medium supplemented with 20% fetal bovine serum
(Gibco-BRL, Invitrogen Life Technologies, Carlsbad, CA, USA). NK
cells were purchased from the Resource Center of Shanghai
Institutes of Biological Sciences (Shanghai, China). NK cells were
cultured in RPMI-1640 containing 10% fetal bovine serum, penicillin
(100 U/ml) and streptomycin (100 μg/ml). Interleukin (IL)-2 (1000
U/ml) was also added to the medium. Following 72 h of incubation
the NK cells were able to be used as effector cells.
Culture conditions
In order to establish hypoxic conditions, the cells
were placed in airtight chambers, which were flushed with a gaseous
mixture of 4.5% CO2, 95% N2 and 0.5%
O2 with optional addition of GTN (10 nM), 8-Br-cGMP (10
nM) or KT5823 (10 μM). Another group of cells was incubated with
20% O2 and optional addition of L-NMMA (5 μg/ml).
Immunohistochemical analysis
The tumor samples were fixed with 10%
paraformaldehyde (Blue Star, Shanghai, China) and embedded in
paraffin (Behai Chemical Industry Company, Changsha, China).
Sections (4 μm) were then cut from the samples using a Leica RM2135
microtome (Leica Biosystems, Bensheim, Germany) and were adhered to
microscope slides. The sections were dewaxed and washed three times
with phosphate-buffered saline (PBS; Sigma-Aldrich). For
non-specific inhibition, each section was incubated in 10% normal
goat serum (Boster, Wuhan, China) for 30 min at 37°C and then with
primary antibodies, rabbit anti-human polyclonal HIF-1α and the
mouse anti-human monoclonal MIC A/B, overnight at 4°C. The sections
were washed three times with PBS and were incubated with secondary
antibody linked with biotin and then with horseradish peroxidase
(HRP)-marked anti-biotin (Boster) for 30 min at 37°C. Subsequently,
the sections were incubated in freshly prepared diaminobenzidine
(Boster) and subsequently counterstained with hematoxylin (Boster).
The sections were then observed under an optical microscope (CX41;
Olympus Corp., Tokyo, Japan).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was extracted using TRIzol reagent. RT
was performed using 1 μg total RNA and oligo(dT) primers (Sangon
Biotech Co., Ltd, Shanghai, China) which were as follows: homo-MICA
forward, 5′-AGGTACATCTGGATGGTCAG-3′ and reverse,
5′-TTGTCTTCATGGATCTCACA-3′ with an amplified fragment of 232 bp;
homo-MICB forward, 5′-CTTCGTTACAACCT CATGGT-3′ and reverse,
5′-ATATGAGTCAG GGTCCTCCT-3′ with an amplified fragment of 227 bp
and homo-GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCT GTTGCTGTA-3′ with an amplified fragment of 450 bp.
The PCR cycling conditions were as follows: 95°C for 5 min,
followed by 40 cycles of 94°C for 30 sec, annealing at 54°C for 30
sec and 72°C for 30 sec, with a final extension at 72°C for 5 min.
Conditions were then maintained at 4°C.
ELISA analysis
A total of two non-overlapping epitope antibodies
were used for ELISA analysis. The polystyrene board plates (Boster)
were coated with the mouse anti-human monoclonal MIC A/B
immunoglobulin G (IgG) overnight at 4°C, then inhibited with 5%
fetal bovine serum for 2 h at 37°C and washed with PBS.
Subsequently, the cell culture medium samples were added and
incubated for 1 h at 37°C. The plates were then washed with PBS and
incubated with goat anti-mouse IgG antibody linked with HRP for 1 h
at 37°C. TMB chromogenic agent and TMB terminated liquid (Boster)
were added and, following reaction, the absorbance was measured at
450 nm using a DU640 ultraviolet spectrophotometer (Beckman
Coulter, Miami, FL, USA).
Flow cytometric analysis
The cultured cells were collected and resuspended in
PBS at a density of 1×106/ml. Subsequently, antibody
linked with fluorochrome (mouse anti-human monoclonal MIC A/B IgG)
was added and incubated for 30 min at 4°C in the absence of visible
light. Following washing three times with PBS, the cells were
detected by fluorescence-activated cell sorting (FACS) using a
FACSCalibur instrument (BD Biosciences, Franklin Lakes, NJ,
USA).
MTT analysis
The cells were cultured in 96-well plates. MTT (20
μl of 5 mg/ml) was added to each well and the cells were incubated
for 4 h at 37°C. Following incubation, 100 μl MTT solution was
added and the cells were agitated on an orbital shaker for 1 min.
The absorbance was then read at 570 nm using an Elx-800 microplate
reader (Winooski, VT, USA).
Statistical analysis
All statistical analyses were performed using the
SPSS 13.0 statistical software package (SPSS, Inc., Chicago, IL,
USA). Differences between groups were compared using single factor
analysis of variance and Student’s t-test. P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Expression levels of HIF-1α and MICA/B in
pancreatic carcinoma, chronic pancreatitis and normal pancreatic
tissues
As shown in Table
I, protein expression of HIF-1α was observed in 32 out of 42
pancreatic carcinoma samples and 2 out of 9 chronic pancreatitis
samples; however, HIF-1α protein was not detected in the normal
pancreatic samples. In addition, protein expression of MICA/B was
observed in 38 out of 42 pancreatic carcinoma samples and 2 out of
9 chronic pancreatitis samples, but was detected in only one normal
pancreatic tissue sample. The expression levels of HIF-1α and
MICA/B were either positively or negatively correlated to
tumor-node-metastasis (TNM) staging; however, no correlation with
the pathological type was observed (P>0.05; Table II). According to Spearman’s rank
correlation coefficient, the two proteins were negatively
correlated (P<0.001; Table
III). Immunohistochemistry also revealed that, in the
pancreatic carcinoma cells, the expression of MIC on the membrane
was reduced; however, it was detected in the interstitial tissue
(Fig. 1).
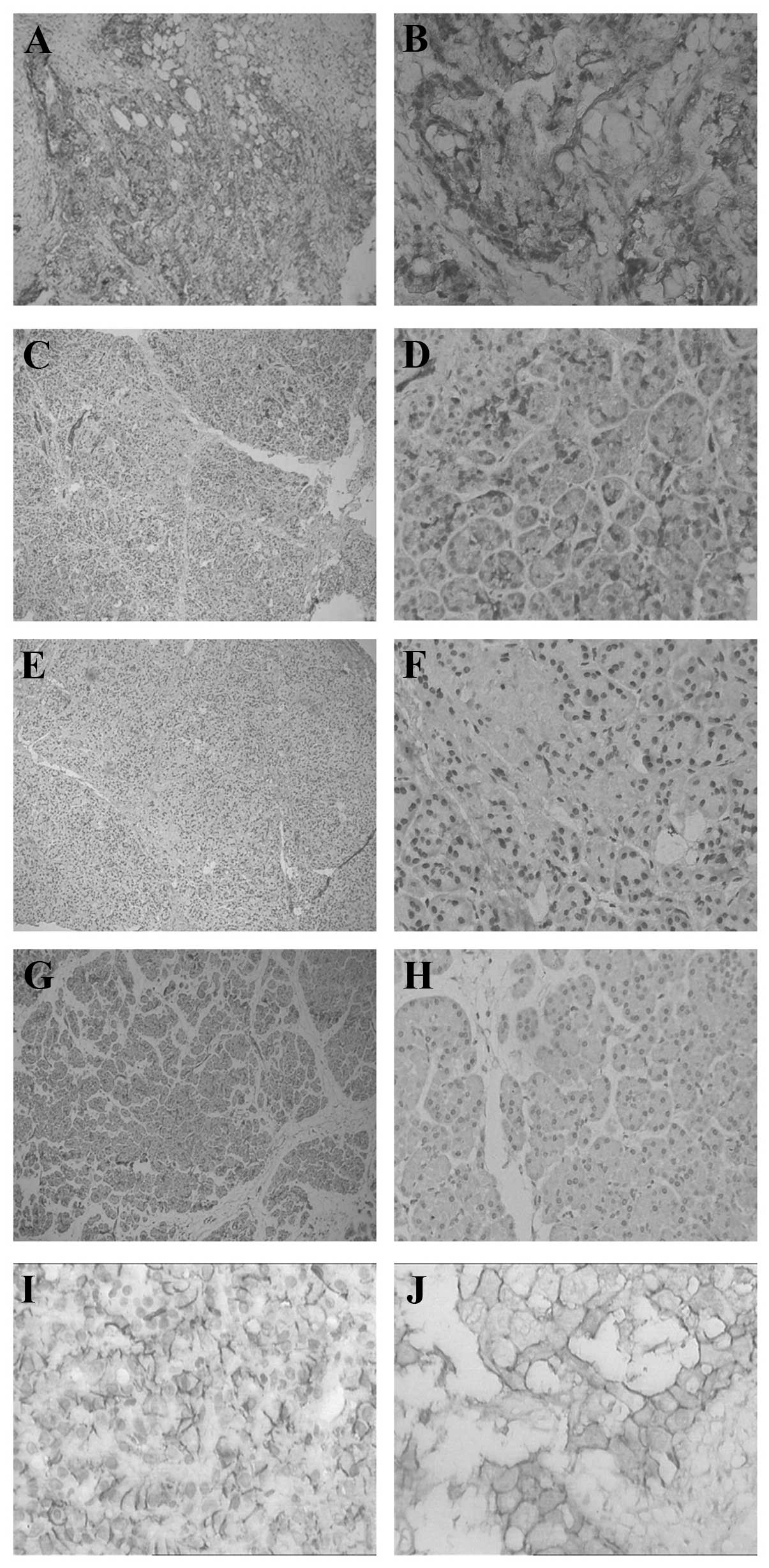 | Figure 1Expression levels of HIF-1α and MIC in
pancreatic carcinoma, chronic pancreatitis and normal pancreatic
tissues. HIF-1α in (A and B) pancreatic carcinoma tissue, (C and D)
chronic pancreatitis tissue, (E and F) chronic pancreatitis tissue,
and (G and H) normal pancreas tissue. (I and J) MIC in pancreatic
carcinoma tissue. Magnification: A, C E G and H, ×100; B, D, F, H
and J, ×400. Dark granules indicate positive staining. HIF-1α,
hypoxia-inducible factor 1α; MIC, major histocompatibility complex
class I chain-related. |
 | Table IIExpression levels of HIF-1α and MICA/B
according to clinicopathological parameters. |
Table II
Expression levels of HIF-1α and MICA/B
according to clinicopathological parameters.
| | HIF-1α | MICA/B |
|---|
| |
|
|
|---|
| Parameter | Cases (n) | Positives (n) | Positive rate
(%) | χ2 | P-value | Low | High | χ2 | P-value |
|---|
| Age |
| <50 | 18 | 14 | 77.8 | 0.025 | >0.05 | 7 | 11 | 0.019 | >0.05 |
| ≥50 | 24 | 18 | 75.0 | 10 | 14 |
| Gender |
| Male | 32 | 24 | 75.0 | 0.010 | >0.05 | 13 | 19 | 0.111 | >0.05 |
| Female | 10 | 8 | 80.0 | 4 | 6 |
| TNM staging |
| I–II | 28 | 16 | 57.1 | 4.025 | <0.05 | 6 | 22 | 10.389 | <0.05 |
| III–IV | 14 | 13 | 92.9 | 11 | 3 |
|
Differentiation |
| High | 12 | 9 | 75.0 | 1.116 | >0.05 | 2 | 10 | 9.141 | <0.05 |
| Medium | 12 | 8 | 66.7 | 3 | 9 |
| Low | 18 | 15 | 88.3 | 12 | 6 |
| Pathological
type |
| Tubular | 32 | 25 | 78.1 | 0.010 | >0.05 | 12 | 20 | 0.111 | >0.05 |
| Papillary | 10 | 7 | 70.0 | 5 | 5 |
| Lymphatic
metastasis |
| Positive | 23 | 21 | 91.3 | 4.693 | <0.05 | 9 | 14 | 0.014 | >0.05 |
| Negative | 19 | 11 | 57.9 | 8 | 11 |
 | Table IIICorrelations between HIF-1α and
MICA/B. |
Table III
Correlations between HIF-1α and
MICA/B.
| MICA/B | | |
|---|
|
| | |
|---|
| HIF-1α | Low (0/1) | High (2) | r | P-value |
|---|
| Low (−/+) | 0 | 12 | −0.522 | <0.001 |
| High (++/+++) | 17 | 13 |
| Total | 17 | 25 | | |
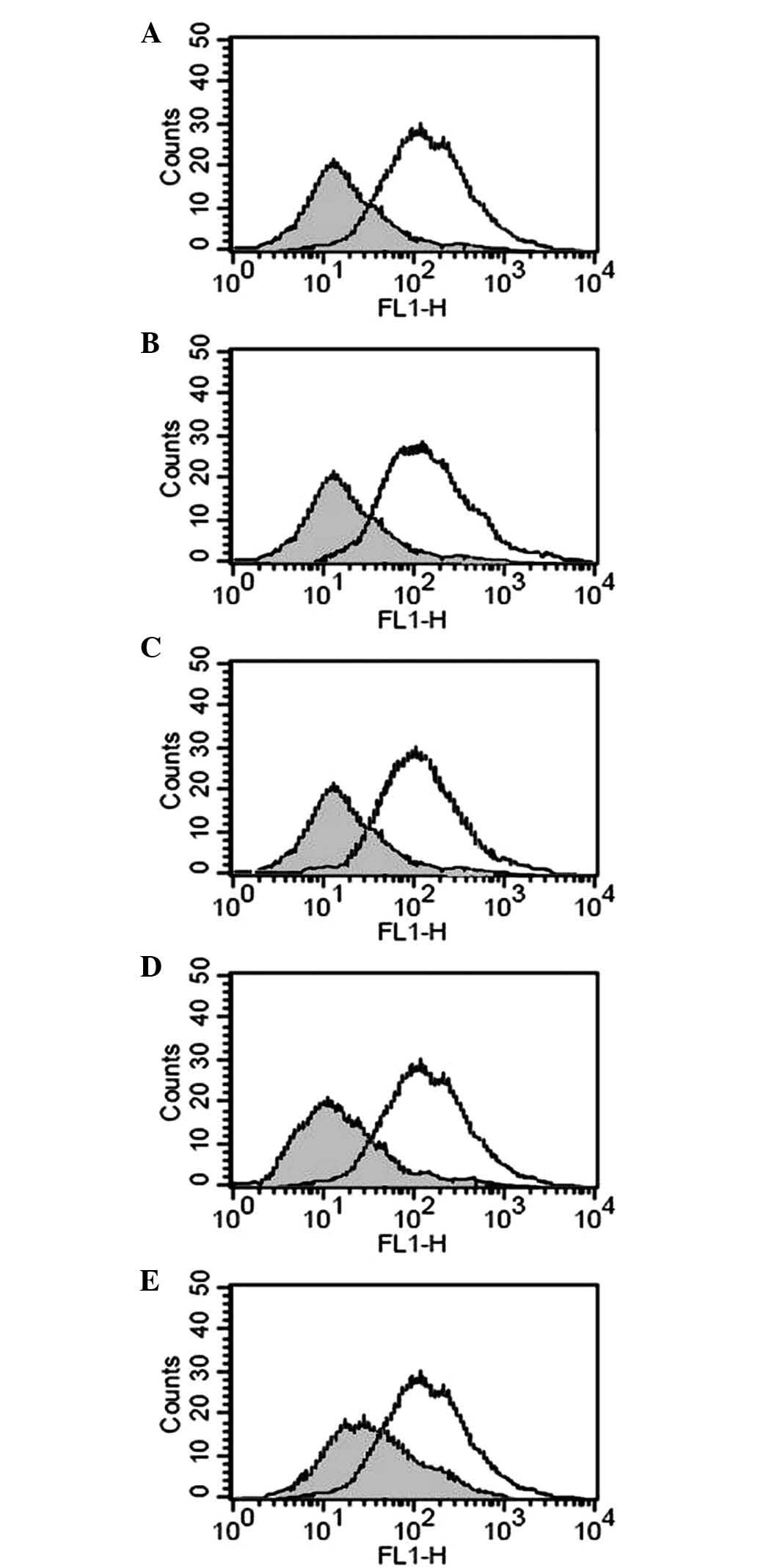
Effects of hypoxia on membrane-bound (m)
and soluble (s)MICA/B
As demonstrated previously, HIF-1α, regulated by
hypoxia, was increased in pancreatic carcinoma tissues, while mMIC
was decreased with accumulation in interstitial tissue (16). To assess whether hypoxia reduced
mMIC and increased sMIC, the PANC-1 pancreatic carcinoma cell line
was cultured in 0.5% O2. The results revealed that the
protein expression of mMIC decreased compared with that in cells
cultured in 20% O2 (Fig.
2). However, no clear differences were detected in the mRNA
expression levels of mMICA/B between the two groups of cells.
Furthermore, the addition of a single dose of either GTN (10 nM) or
8-Br-cGMP (10 nM) to the cells incubated for 24 h in 0.5%
O2 was sufficient to prevent the reduction in mMIC.
Inhibiting nitric oxide (NO) synthesis with the NO synthase
inhibitor L-NMMA (5 μg/ml) or suppressing protein kinase G (PKG)
activity with the PKG inhibitor KT5823 (10 μM) reduced the
expression of mMIC in the cells cultured in 20% O2
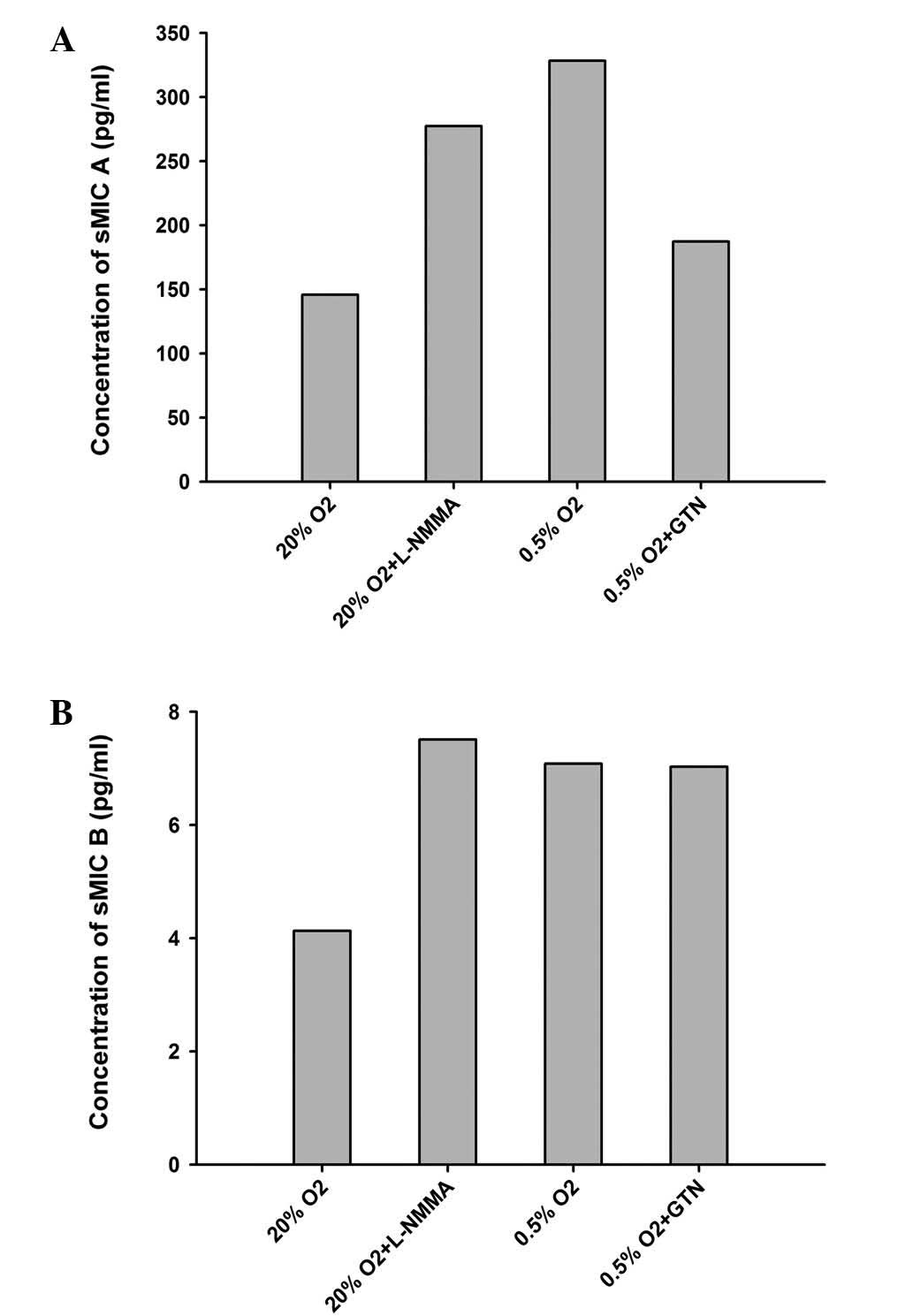
(Fig. 2). By contrast, sMICA
increased ~2-fold (P<0.05) in 0.5% O2, which was
prevented by co-incubation with GTN (10 nM; Fig. 3). Co-incubation with L-NMMA (5
μg/ml) also increased the levels of sMICA (Table IV, Fig. 3). No significant differences were
observed in sMICB (Table IV;
Fig. 3).
 | Table IVExpression of sMICA/B under different
culturing conditions. |
Table IV
Expression of sMICA/B under different
culturing conditions.
|
Conditions/supplements | Absorbance | sMICA (pg/ml) | sMICB (pg/ml) |
|---|
| 20%
O2 | 0.598 | 127.14 | 3.664 |
| 0.776 | 169.92 | 5.219 |
| 0.654 | 140.60 | 3.495 |
| 20% O2 +
L-NMMA | 1.113 | 250.91 | 9.274 |
| 1.429 | 326.86 | 7.077 |
| 1.127 | 254.28 | 6.165 |
| 0.5%
O2 | 1.556 | 357.38 | 5.590 |
| 1.388 | 317.00 | 7.517 |
| 1.360 | 310.27 | 8.125 |
| 0.5% O2
+ GTN | 0.868 | 192.03 | 6.030 |
| 0.796 | 174.73 | 7.348 |
| 0.881 | 195.16 | 7.719 |
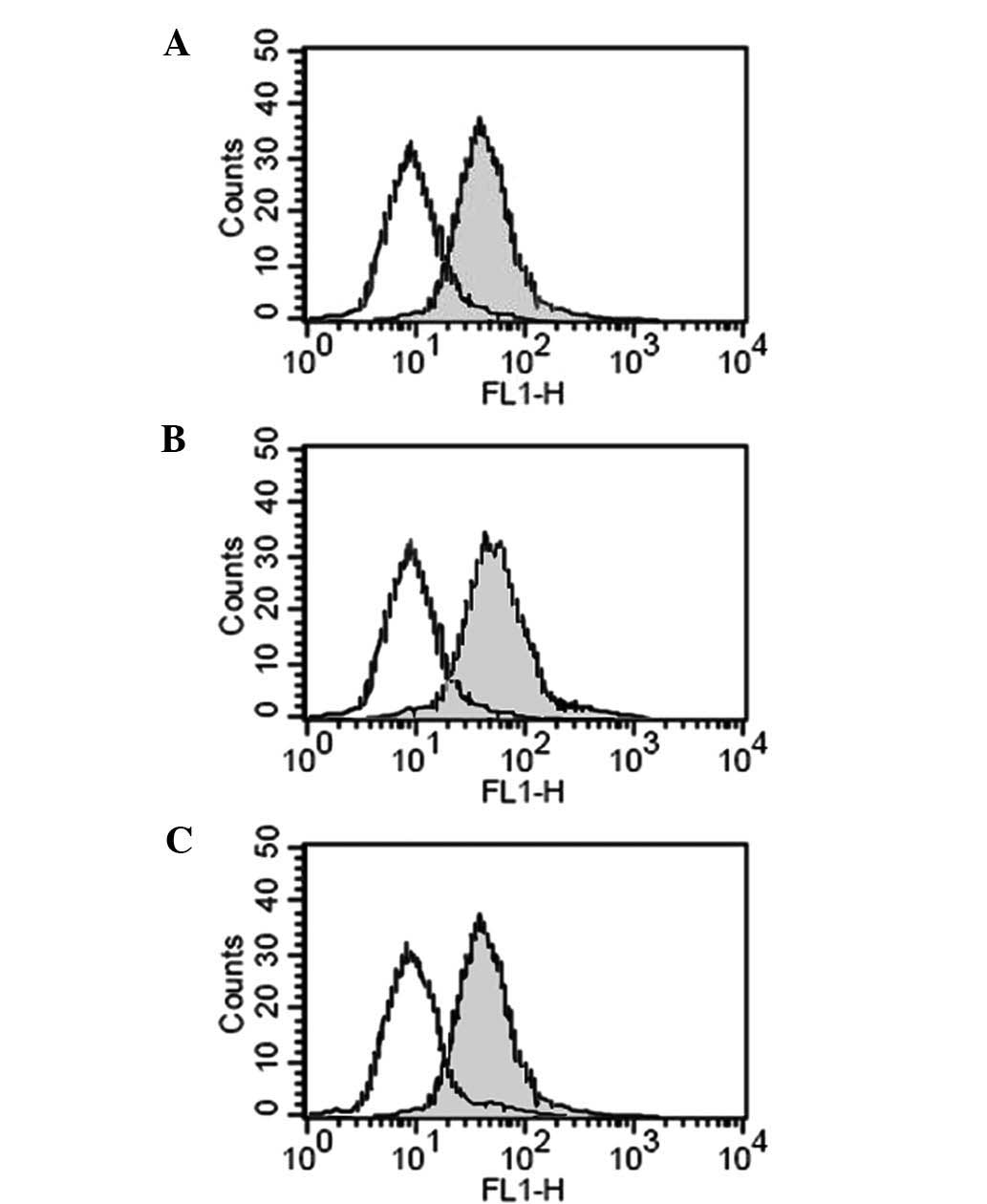
Effect of hypoxia on the protein
expression of NKG2D
To assess the effect of hypoxia on the protein
expression of NKG2D, PANC-1 cells which were pre-incubated in 0.5%
O2 and 20% O2 with or without GTN and L-NMMA
were collected and co-incubated with NK cells. As Fig. 4 shows, compared with the cells
incubated in 20% O2, those cultured in 0.5%
O2 had lower protein expression levels of NKG2D (40.5
vs. 70.3%). The addition of GTN promoted the expression of NKG2D to
62.5%, while L-NMMA downregulated the expression to 48.4%(Fig. 4).
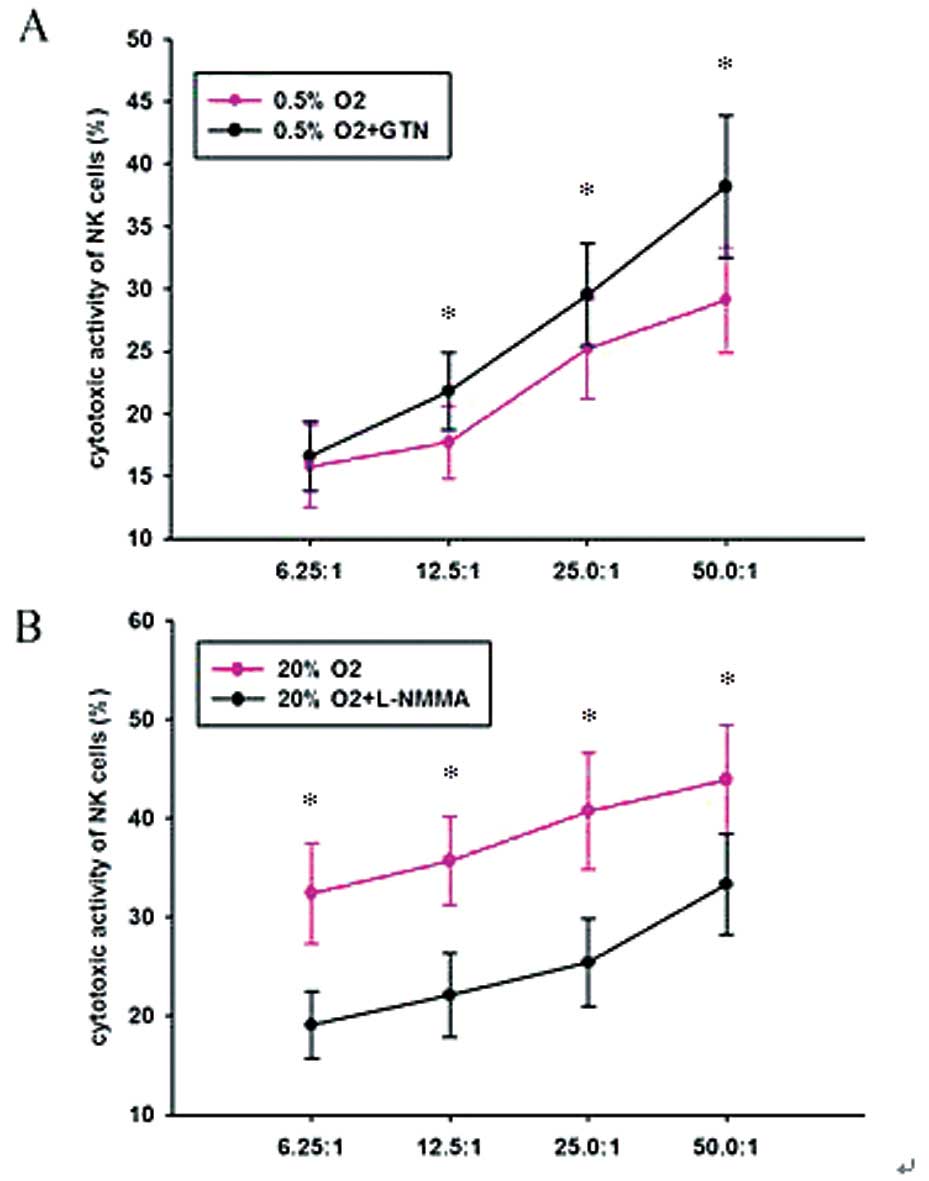
Effect of hypoxia on the cytotoxic
activity of NK cells
To determine the effect of hypoxia on the cytotoxic
activity of NK cells, the PANC-1 cells were pre-cultured under
different conditions and then co-incubated with NK cells in varying
proportions (1:6.25, 1:12.5, 1:25 and 1:50) for 24 h. An MTT assay
revealed that cells cultured in 0.5% O2 were more
tolerant to the NK cells compared with those incubated in 20%
O2. GTN increased the cytotoxic activity and L-NMMA
decreased the cytotoxic activity of the NK cells (Table V; Fig.
5).
 | Table VCytotoxic activity of NK cells on
PANC-1 cells. |
Table V
Cytotoxic activity of NK cells on
PANC-1 cells.
| Pre-incubation
conditions | Cytotoxic activity
of NK cells (%) (NK:PANC-1 cell ratio) |
|---|
|
|---|
| 6.25:1 | 12.5:1 | 25:1 | 50:1 |
|---|
| 20%
O2 | 32.4±5.1 | 35.7±4.5 | 40.7±5.9 | 43.9±5.5 |
| 20% O2 +
L-NMMA | 19.1±3.4 | 22.1±4.2 | 25.4±4.4 | 33.3±5.1 |
| 0.5%
O2 | 15.8±3.3 | 17.7±2.9 | 25.2±4.0 | 29.1±4.2 |
| 0.5% O2
+ GTN | 16.6±2.8 | 21.8±3.1 | 29.5±4.1 | 38.2±5.7 |
Discussion
The present study demonstrated an increase in the
levels of sMIC, resulting in a decrease in the protein expression
of NKG2D, which was key in regulating the hypoxia-mediated immune
evasion of human pancreatic carcinoma cells. Furthermore, the
results of the present study clearly suggested that NO signaling
was essential in this process.
Pathological slides revealed that HIF-1α was highly
expressed in pancreatic carcinoma tissues, which indicated that
hypoxia was present in the pancreatic carcinoma. In addition, MIC
decreased compared with the chronic pancreatitis and normal
pancreatic tissues, suggesting that hypoxia may downregulate
MIC.
To mimic the hypoxic microenvironment experienced by
cancer cells, the present study cultured the PANC-1 cell line in
0.5% O2, with control cells cultured in 20%
O2. The results demonstrated that the expression of
HIF-1α was high and that of MIC was low (data not shown). However,
RT-qPCR revealed no clear differences in the mRNA expression of
MICA/B between cells cultured in 0.5% O2 and 20%
O2, raising the question of whether this was due to
downregulation of MIC translation or detachment of MIC to the
extracellular space. ELISA demonstrated that the level sMIC,
particularly sMICA, increased in the cell culture medium exposed to
0.5% O2, which had a negative effect on the expression
of NKG2D and the cytotoxic activity of NK cells. The results
indicated that hypoxia did not inhibit the gene expression of MIC,
but induced the detachment of MIC from the cell membrane and
downregulated the expression of NKG2D and the cytotoxicity of NK
cells. This resulted in the reduced immunogenicity of cancer cells
and subsequent evasion of the immune system. Matrix
metalloproteinases (MMP) may be important molecules in this
regulation, as it has been reported that MMP-2 and −9 are often
upregulated in tumors (17). Salih
et al (18) investigated
the addition of MMP inhibitor to the medium, which revealed marked
increases in mMIC. Another study confirmed that the hypoxia-induced
detachment of mMIC is regulated by MMPs (19). However, the reason for
downregulation in the expression of NKG2D and NK cytotoxicy remain
to be elucidated. One theory is that the binding of sMIC and NKG2D
causes the internalization of NKG2D. Doubrovina et al
(20) demonstrated that following
binding to soluble MIC, NKG2D is decreasingly located at the
membrane and is increasingly present in the cytoplasm. Groh et
al (21) also observed that
sMIC induced the internalization and degradation of NKG2D on T
cells.
Studies have also demonstrated that hypoxia markedly
inhibits the cellular NO-cGMP-PKG pathway (22–25).
Inhibiting the pathway with drugs has a mimetic effect of hypoxia
(26–29). In the present study, GTN and
8-Be-cGMP were used as NO-cGMP-PKG pathway stimulators, and L-NMMA
and KT5823 were used as inhibitors. Treatment with GTN and
8-Be-cGMP reduced sMICA and promoted the expression of NKG2D and NK
cytotoxicity, whereas treatment with L-NMMA and KT5823 had an
opposite effect. It is understood that the NO-cGMP-PKG pathway
begins with NO synthesis. As a soluble gas molecule, NO passes
through the cell membrane and activates soluble guanylyl cyclase
(sGC), which catalyzes the conversion of GTP to cGMP. cGMP then
acts as a secondary messenger, activating PKG and amplifying NO
signals to downstream effectors (26). According to the results of the
present study, activation of the NO-cGMP-PKG pathway weakened the
hypoxia-mediated immune evasion of pancreatic carcinoma cells.
All the experiments in the present study were
performed in vitro and, as there is greater interest in the
immune response in vivo, subsequent investigation aims to
establish a nude mouse model to study the effect of NO-mimetic
drugs on tumorigenicity.
In conclusion, the results of the present study
indicated that the NO-cGMP-PKG signaling pathway regulated the
immune escape observed in pancreatic cancer. Activation of this
pathway can reverse the immune escape, whereas its inhibition
promoted the immune escape. The hypoxic environment, which the
pancreatic cancer cells existed in, was closely associated with
this signaling pathway. Further research on NO-cGMP-PKG signal
pathway and hypoxia environment will provide novel directions for
the development of immunotherapies for pancreatic cancer.
References
|
1
|
Zhang J, Xie X and Ye D: Adhesion
molecules and tumor immune escape. Shi Yong Zhong Liu Xue Zha Zhi.
19:449–452. 2004.(In Chinese).
|
|
2
|
Ogiso Y, Tomida A, Lei S, et al:
Proteasome inhibition circumvents solid tumor resistance to
topoisomerase II-directed drugs. Cancer Res. 60:2429–2434.
2000.PubMed/NCBI
|
|
3
|
Fink T, Ebbesen P, Koppelhus U and Zachar
V: Natural Killer cell-mediated basal and interferon-enhanced
cytotoxicity against liver cancer cells is significantly impaired
under in vivo oxygen conditions. Scand J Immunol. 58:607–612. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ivan M: HIF-1α targeted for VHL-mediated
destruction by praline hydroxylation: implications for
O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Semenza G: Signal transduction to
hypoxia-inducible factor 1. Biochem Pharmacol. 64:993–998. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ivan M: HIF-1α targeted for VHL-mediated
destruction by praline hydroxylation: implications for
O2 sensing. Science. 292:464–468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Groh V, Rhinehart R, Secrist H, et al:
Broad tumor-associated expression and recognition by tumor-derived
gamma delta T cells of MICA and MICB. Proc Natl Acad Sci USA.
96:6879–6884. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K, Mandai M, Hamanishi J, et al:
Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in
ovarian cancer: high expression of ULBP2 is an indicator of poor
prognosis. Cancer Immunol Immunother. 58:641–652. 2009. View Article : Google Scholar
|
|
9
|
Diefenbach A, Jensen ER, Jamieson AM and
Raulet DH: Rael and H60 ligands of the NKG2D receptor stimulate
tumour immunity. Nature. 413:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groh V, Steinle A, Bauer S and Spies T:
Recognition of stress-induced MHC molecu1es by intestina1
epithelial gammadelta T cel1s. Science. 279:1737–1740. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conejo-Garcia JR, Benencia F, Courreges
MC, et al: A tumor-associated NKG2D immunoreceptor ligand, induces
activation and expansion of effector immune cells. Cancer Biol
Ther. 2:446–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vivier E, Tomasello E and Paul P:
Lymphocyte activation via NKG2D: towards a new paradigm in immune
recognition. Curr Opin Immunot. 14:306–311. 2002. View Article : Google Scholar
|
|
13
|
Holdenrieder S, Stieber P, Peterfi A, et
al: Soluble MICA in malignant diseases. Int Cancer. 118:684–687.
2006. View Article : Google Scholar
|
|
14
|
Li K, Mandai M, Hamanishi J, Matsumura N,
et al: Clinical significance of the NKG2D ligands, MICA/B and ULBP2
in ovarian cancer: high expression of ULBP2 is an indicator of poor
prognosis. Cancer Immunol Immunother. 58:641–652. 2009. View Article : Google Scholar
|
|
15
|
Zhong H, De Marzo AM, Laughner E, et al:
Overexpression of hypoxia-inducible factor 1alpha in common human
cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
16
|
Duan X, Deng L, Chen X, et al: Clinical
significance of the immunostimulatory MHC class I chain-related
molecule A and NKG2D receptor on NK cells in pancreatic cancer. Med
Oncol. 28:466–467. 2011. View Article : Google Scholar
|
|
17
|
Peng TS, Wu JS, Wu HQ, et al: The
expression of matrix metalloproteinase-2,9 and their inhibitors in
osteosarcoma. Journal of Sun Yatsen University (Medical Sciences).
2:132–135. 2003.(In Chinese).
|
|
18
|
Salih HR, Rammensee HG and Steinle A:
Down-regulation of MICA on human tumors by proteolytic shedding. J
Immunol. 169:4098–4102. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siemens DR, Hu N, Sheikhi AK, et al:
Hypoxia increases tumor cell shedding of MHC class I chain-related
molecule: role of nitric oxide. Cancer Res. 68:4746–4753. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doubrovina ES, Doubrovin MM, Vider E, et
al: Evasion from NK cell immunity by MHC class I chain-re1ated
molecules expressing colon adenocarcinoma. J Immunol.
171:6891–6899. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Groh V, Wu J, Yee C and Spies T:
Tumour-derived soluble MIC ligands impair expression of NKG2D and
T-cell activation. Nature. 419:679–680. 2002. View Article : Google Scholar
|
|
22
|
Whorton AR, Simonds DB and Piantadosi CA:
Regulation of nitric oxide synthesis by oxygen in vascular
endothelial cells. Am J Physiol. 272:L1161–L1166. 1997.PubMed/NCBI
|
|
23
|
McCormick CC, Li WP and Calero M: Oxygen
tension limits nitric oxide synthesis by activated macrophages.
Biochem J. 350:709–716. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nathan C and Xie QW: Regulation of
biosynthesis of nitric oxide. J Biol Chem. 269:13725–13728.
1994.PubMed/NCBI
|
|
25
|
Louis CA, Reichner JS, Henry WL Jr, et al:
Distinct arginase isoforms expressed in primary and transformed
macrophages: regulation by oxygen tension. Am J Physiol.
274:R775–R782. 1998.PubMed/NCBI
|
|
26
|
Frederiksen LJ, Sullivan R, Maxwell LR, et
al: Chemosensitization of cancer in vitro and in vivo by nitric
oxide signaling. Clin Cancer Res. 13:2199–2206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Postovit LM, Adams MA, Lash GE, Heaton JP
and Graham CH: Oxygen-mediated regulation of tumour cell
invasiveness: involvement of a nitric oxide signalling pathway.
Biol Chem. 277:35730–35737. 2002. View Article : Google Scholar
|
|
28
|
Postovit LM, Adams MA, Lash GE, Heaton JP
and Graham CH: Nitric oxide-mediated regulation of hypoxia-induced
B16F10 melanoma metastasis. Int J Cancer. 108:47–53. 2004.
View Article : Google Scholar
|
|
29
|
Matthews NE, Adams MA, Maxwell LR, Gofton
TE and Graham CH: Nitric oxide-mediated regulation of
chemosensitivity in cancer cells. Natl Cancer Inst. 93:1879–1885.
2001. View Article : Google Scholar
|