Introduction
Leukemia is a type of malignant cancer of the
hematopoietic stem cells and is a severe threat to human health. At
present, the main therapy for leukemia includes chemotherapy,
allogeneic hematopoietic stem cell transplantation, bone marrow
transplantation, targeted drug therapy and immunotherapy.
Genetic abnormalities have been demonstrated to be
important in leukemogenesis (1).
The AML1/ETO (A/E) fusion gene is a product of the chromosome
translocation t(8;21) (q22; q22), which affects the acute myeloid
leukemia gene 1 (AML1) of chromosome 21 and the eight twenty one
gene (ETO) of chromosome 8. The fusion gene is one of the common
chromosome aberrations in acute myeloid leukemia (AML),
particularly in AML with maturation (M2) (2).
Aggressive cytosine arabinoside-based chemotherapy
is the standard protocol for the treatment of t(8;21) AML. However,
clinical observation has demonstrated that the median survival time
of patients with t(8;21) AML was >2 years, with a 5 year
survival rate of <40% (2). The
development of novel therapies is required in order to further
improve clinical outcome and to provide therapeutic options for
t(8;21) AML patients.
N,N′-di-(m-methylphenyi)-3, 6-dimethyl-1,
4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide (ZGDHu-1) is a novel
oxazine derivative synthesized by Professor Wei-Xiao Hu from the
College of Pharmaceutical Science, Zhejiang University of
Technology (Hangzhou, China) who obtained a Patent of China
(3,4). ZGDHu-1 has been demonstrated to
possess anti-tumor activity (5,6) and
has also been found to inhibit growth and induce apoptosis of
Kasumi-1 cells (7), a cell line of
the t(8;21) (q22;q22) translocation. ZGDHu-1 is also able to
markedly inhibit the cell cycle at the G2/M phase,
however, the underlying mechanisms were not discussed.
The cell cycle includes the mitotic period (M), the
G1 phase, the S phase and the G2 phase. Its
regulation predominantly depends on the regulatory network,
including cyclins, cyclin-dependent kinases (CDKs) and
cyclin-dependent kinase inhibitors (CKIs) (8,9). The
checkpoint of G2/M is important for entrance of cells
into the M phase and is also important in tumor cell resistance
(10). When the cell cycle arrests
at the G2/M phase, the expression levels of the
CDK1/cyclin B1 complex are altered, leading to incomplete mitosis
and ultimately mitotic catastrophe resulting in cell death
(11).
Thus, in the present study, we aimed to investigate
the mechanism by which ZGDHu-1 induces G2/M phase arrest and
apoptosis in Kasumi-1 cells.
Materials and methods
ZGDHu-1
ZGDHu-1 was kindly provided by the Pharmaceutical
Engineering Research Institute, College of Pharmaceutical Science,
Zhejiang University of Technology. It was screened from 14
compounds of 3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine
derivatives. Stock solution (10 mg/ml) was prepared by dissolving
ZGDHU-1 in dimethyl sulfoxide (DMSO; Simga-Aldrich, St. Louis, MO,
USA), then aliquoted and stored at -20°C. For the in vitro
experiment, RPMI-1640 medium (Gibco, Grand Island, NY, USA) was
used to prepare the final working concentration.
Cell culture and drug treatment
Kasumi-1 cells (12), derived from the peripheral blood of
a 7 year old Japanese male who was diagnosed with AML-M2, were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and were maintained in RPMI-1640 with 20% fetal bovine serum
(Gibco). The genetic characteristics of this cell line include a
chromosome t(8;21) (q22;q22) translocation, thus making it a good
research tool for investigating this type of translocation in
leukemia. The cell line was incubated in a 37°C humidified
atmosphere with 5% CO2. Different concentrations of
ZGDHu-1 (50, 100, 200, 500 and 1,000 μg/l) and controls (negative
control and DMSO as solvent control) were added to the Kasumi-1
cells.
Flow cytometric analysis
DNA Prep™ reagent system (Beckman Coulter,
Indianapolis, IN, USA) was used to evaluate cell cycle alterations
in Kasumi-1 cells. The cells were harvested following washing with
phosphate-buffered saline (PBS; DingGuo Biotechnology Co., Ltd.,
Beijing, China). DNA Prep LPR (50 μl; Beckman Coulter) was added
for 1 min and then 150 μl DNA Prep stain was added to the cells.
Following gentle agitation, the cells were incubated for 5 min at
room temperature, the results were detected using
fluorescence-activated cell sorting (FACS) using a Coulter Epics XL
flow cytometer (Beckman Coulter) and the proportion of cells at
each stage of the cell cycle was determined.
To elucidate the underlying mechanisms of apoptosis,
the expression of certain apoptosis-associated proteins were
analyzed using FACS. To detect Apo 2.7, collected cells were
permeabilized for 20 min at 4°C with 100 μg/ml digitonin and then
phycoerythrin-labeled Apo 2.7 mouse monoclonal (mAb) immunoglobulin
G antibody (IM2088U; Beckman Coulter) was added for 15 min.
Collected cells were stained with propidium iodide
(PI, 10 μg/ml) and rhodamine 123 (Rh123, 10 μg/ml; Calbiochem, San
Diego, CA, USA) to detect the mitochondrial transmembrane
potentials using FACS analysis.
Dihydrorhodamine 123 (DHR123; Sigma-Aldrich) was
used to detect the ROS levels of collected cells (13). Following being washed in PBS, 150
μl 10 μM DHR123 was added to the cells. Subsequently, the cells
were incubated at 37°C for 30 min. FACS was used to measure the
changes in median fluorescence intensity (MFI).
IntraPrep permeabilization reagent (Beckman Coulter)
was used to detect the expression of the following intracellular
proteins: B-cell lymphoma 2 (Bcl-2), Bcl-2-associated death
promoter (Bad), Bcl-2-associated X protein (Bax) and cyclin B1. A
total of 50 μl fixation reagent was added to the collected cells
and then incubated for 15 min at room temperature. Following being
washed with PBS, 50 μl permeabilization reagent was added. After 5
min incubation, specific antibodies, including Bcl-2 (BD
Biosciences, San Jose, CA, USA), Bad (Biovision, Mountain View, CA,
USA), Bax (BD Biosciences) and mouse mAb cyclin B1 (1:2,000; #4135;
Cell Signaling Technology, Inc., Beverly, MA, USA) were added to
the cells. The cells were incubated for 15 min in the dark at room
temperature and then FACS was used to determine the results.
Western blot analysis
Following incubation with different concentrations
of ZGDHU-1, Kasumi-1 cells were lysed and proteins were extracted
and quantitated using a bicinchoninic protein assay kit (DingGuo
Biotechnology Co., Ltd.). The proteins were loaded into wells of an
8 or 12% SDS-PAGE, electrophoresed and transferred onto a
nitrocellulose membrane (DingGuo Biotechnology Co., Ltd.). The
membrane was incubated with the appropriate primary antibody and
then washed and incubated with horseradish peroxidase-conjugated
secondary antibody (Cell Signaling Technology, Inc.). Detection was
performed using a western blotting luminol reagent (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat no. sc-2048). The
following antibodies were used: Rabbit mAb caspase-3 (1:1,000;
#9665), rabbit polyclonal (pAb) cleaved caspase-3 (1:1,000; #9661),
rabbit mAb poly ADP ribose polymerase (PARP; 1:1,000; #9532),
rabbit pAb NF-κB (1:1,000; #3034), rabbit pAb inhibitor of κB
(IκB)-α (1:1,000; #9242), rabbit pAb Bax (1:1,000; #2774), rabbit
mAb Bad (1:1,000; #9239), rabbit mAb AML1 (1:1,000; #4336), rabbit
pAb ETO (1:1,000; #4498), mouse mAb cyclin B1 (1:2,000; #4135),
mouse mAb cdc2 (1:2,000; #9116), rabbit mAb cdc25C (1:1,000;
#4688), rabbit mAb phospho-cdc25C (Ser216) (1:1,000; #4901), rabbit
pAB Chk1 (1:1,000; #2345), rabbit mAb phospho-Chk1 (Ser345)
(1:1,000; #2348), mouse mAb Chk2 (1:1,000; #3440), mouse mAb
phospho-p53 (Ser15)(1;1,000; #9286), rabbit pAb phospho-p53 (Ser20)
(1:1,000; #9287), rabbit mAb p27 (1:1,000;#3688), which were all
purchased from Cell Signaling Technology, Inc., as well as mouse
mAb Bcl-2 (1:250; sc-7382), mouse mAb β-actin (1:1,000; sc-47778)
and rabbit pAb p53 (1:1,000; sc-6243), which were purchased from
Santa Cruz Biotechnology, Inc.
Treatment with CHIR-124, a selective CHK1
inhibitor
In a separate experiment, 200 nM CHIR-124 (14,15),
a selective CHK1 inhibitor, was incubated with Kasumi-1 cells for 2
h and then 200 μg/l ZDGHU-1 for 48 h. Cell cycle stages were
analyzed by FACS to observe differences.
Statistical analysis
All statistical calculations were performed using
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA). Results are
expressed as the mean ± standard deviation. The differences between
treated and control groups were analyzed using a one-way analysis
of variance or χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
ZGDHu-1 induces Kasumi-1 cell apoptosis
through the activation of caspase-3 and the mitochondrial apoptosis
pathway
The caspase family is important in the apoptotic
signaling pathway (16). In the
present study, alterations in caspase-3, cleaved caspase-3 (the
active part of caspase-3) and PARP (the substrate of caspase-3)
were assessed following treatment with different concentrations of
ZGDHu-1 for 48 h in Kasumi-1 cells using western blotting.
Caspase-3 decreased and cleaved caspase-3 markedly increased in a
dose-dependent manner. The cleaved fragments of PARP (89 kDa) were
also easily observed, which suggested that caspase-3 was activated
following ZGDHu-1 treatment (Fig.
1A).
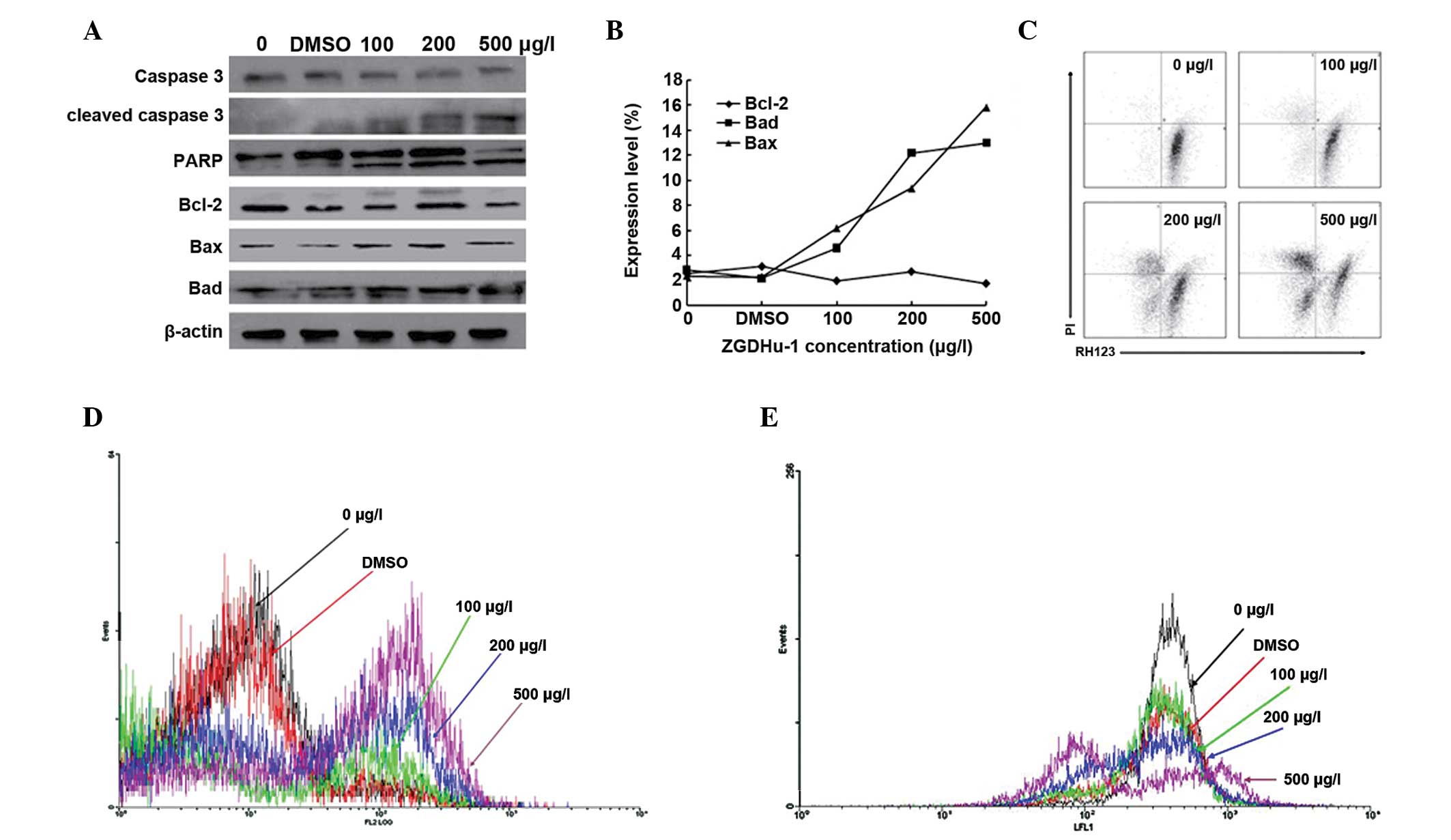 | Figure 1ZGDHu-1 induces Kasumi-1 cell
apoptosis through the activation of caspase-3 and mitochondrial
apoptosis pathway (A) Effect of ZGDHu-1 on caspase-3, cleaved
caspase-3, PARP, Bcl-2, Bax and Bad. There was a negative control
with no drugs and a DMSO control. β-actin was used as a loading
control. (B) Expression levels of Bcl-2, Bax and Bad were detected
by fluorescence activated cell sorting. (C) Kasumi-1 cells stained
with propidium iodide and Rh123 were detected by fluorescence
activated cell sorting. (D) Results of Apo 2.7 following treatment
with ZGDHU-1 in Kasumi-1 cells. Black indicates the negative
control, red indicates the DMSO control, green indicates 100 μg/l
ZGDHU-1, blue indicates 200 μg/l ZGDHU-1 and brown indicates 500
μg/l ZGDHU-1. (E) ROS production was induced in Kasumi-1 cells.
Rh123, the product of DHR123 and ROS, was measured by flow
cytometry. Black indicates the negative control, red indicates the
DMSO control, green indicates 100 μg/l ZGDHU-1, blue indicates 200
μg/l ZGDHU-1 and brown indicates 500 μg/l ZGDHU-1. ZGDHu-1,
N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; Bad,
Bcl-2-associated death promoter; ROS, reactive oxygen species;
DMSO, dimethyl sulfoxide; PARP, poly ADP ribose polymerase; Rh123,
rhodamine 123. |
FACS and western blotting were used to detect
alterations in the expression of Bcl-2, Bad and Bax following
treatment with different concentrations of ZGDHu-1 for 48 h in
Kasumi-1 cells. ZGDHu-1 treatment led to an upregulation of Bad and
Bax, however, Bcl-2 was not altered (Fig. 1A and B)
In order to evaluate the effects on the
mitochondrial signaling pathway, FACS was used to detect
alterations in mitochondrial membrane protein (Apo 2.7) (17) and Δψm, which reflects the integrity
of the mitochondrial membrane. The Kasumi-1 cells were
double-stained with PI and Rh123 (18), which was proportional to Δψm.
Following treatment with different concentrations of ZGDHu-1
(control, DMSO control, 100, 200 and 500 μg/l), the MFI was
significantly decreased between 40.4±1.6, 39.1±2.2, 33.3±2.6,
30.3±2.4 and 27.7±1.9 (P<0.05) in PI negative and Rh123 positive
cells (Fig. 1C). In addition, the
expression of Apo 2.7 was increased significantly between 5.35±0.4,
5.30±0.3, 9.73±1.4, 36.90±1.6 and 54.40±1.8% (P<0.05; Fig. 1D).
ZGDHu-1 induces ROS production in
Kasumi-1 cells
Overproduction of ROS may cause oxidative stress,
which is a major factor leading to apoptosis. In order to
investigate whether ROS accumulation had occurred, DHR123, one of
the most widely used ROS probes for intracellular measurement and
analysis, was used. DHR123 is not fluorescent until oxidized by ROS
to the highly fluorescent product rhodamine 123, therefore, MFI was
measured using flow cytometry. The MFI was increased to 26.6±1.2,
25±1.4, 24.2±1.0, 23.7±1.6 and 38.3±2.1 (P<0.05) at a
concentration of 500 μg/l (Fig.
1E).
ZGDHu-1 downregulates the expression of
NF-κB and upregulates the expression of IκB
Following being incubated with different
concentrations of ZGDHu-1 (0, 100, 200 and 500 μg/l), the
expression levels of NF-κB and IκB were detected using western
blotting. The result suggested that IκB levels were induced by
ZGDHu-1 to suppress the function of NF-κB and inhibit the growth of
Kasumi-1 cells (Fig. 2A).
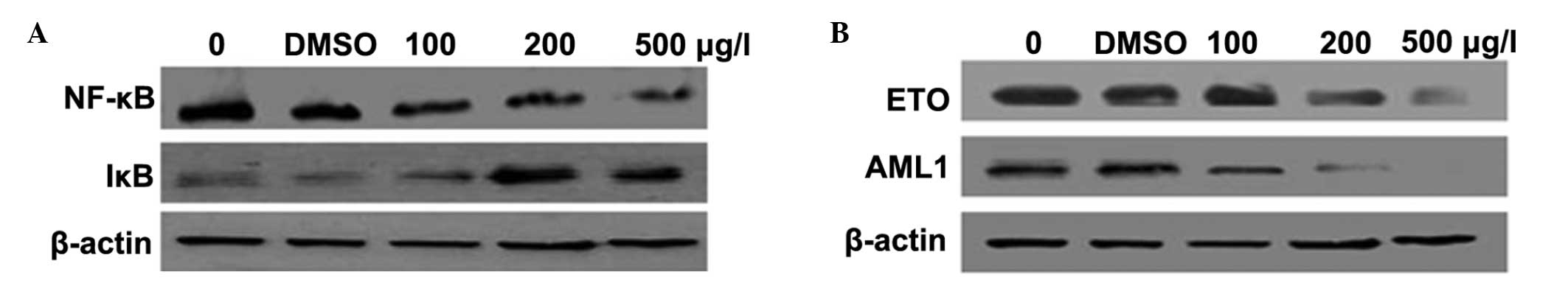 | Figure 2ZGDHu-1 downregulates the expression
of NF-κB and upregulates the expression of IκB, causing degradation
of the A/E fusion protein (A) Effect of ZGDHu-1 on NF-κB and IκB.
There was a negative control with no drugs and a DMSO control.
β-actin was used as a loading control. (B) Protein levels of the
A/E fusion gene following treatment with ZGDHu-1. Western blot
analysis was used to detect changes in these genes. β-actin was
used as a loading control. ZGDHu-1,
N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide;
NF-κB, nuclear factor-κB; DMSO, dimethyl sulfoxide; AE,
AML1/ETO. |
Effect of ZGDHu-1 on the A/E fusion gene
of Kasumi-1 cells at the mRNA and protein level
The A/E fusion gene is a vital feature of Kasumi-1
cells and it also has a major role in the development of AML.
Therefore, in the present study, alterations in the expression of
this fusion gene following treatment with different concentrations
of ZGDHu-1 were investigated. The result demonstrated that A/E
fusion levels did not change at the mRNA level (data not shown),
but AML and ETO genes were degraded by ZGDHU-1 (Fig. 2B).
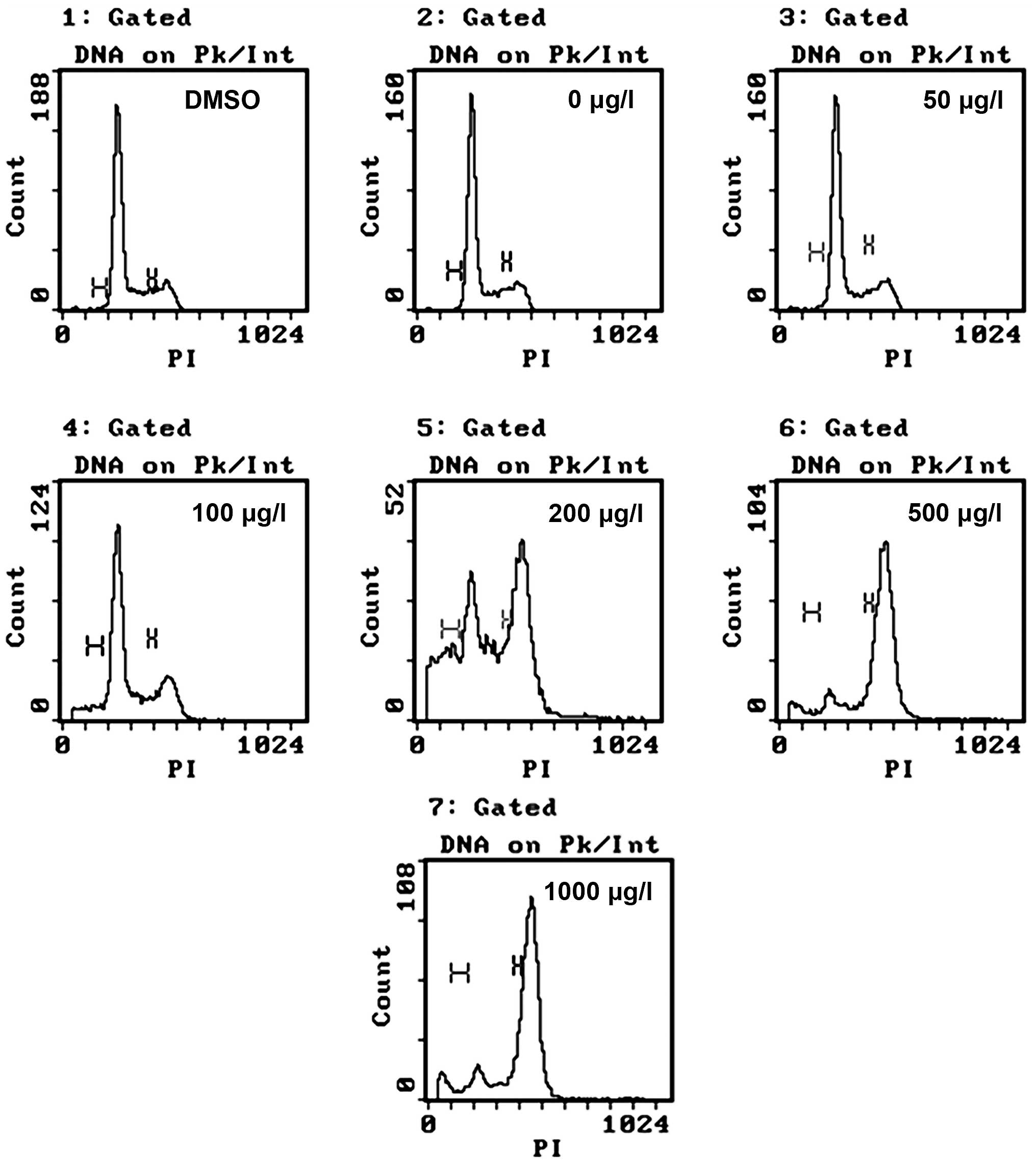
ZGDHu-1 induces G2/M arrest in
Kasumi-1 cells
Following incubation with different concentrations
of ZGDHu-1 (50, 100, 200, 500 and 1,000 μg/l) and controls
(negative control and DMSO control) for 48 h, cell cycle stages
were analyzed using FACS (Fig. 3).
The present results demonstrated that cells in the G2/M
phase significantly accumulated in a concentration-dependent manner
(between 6.4±1.5, 13.4±1.3, 40.1±1.4, 81.2±1.4 and 79.9±1.4%).
Based on this observation, it was hypothesized that the inhibition
and apoptotic effects of Kasumi-1 cells may operate through the
disturbance of the cell cycle check point.
Activation of CHK1 and p53 induces
G2/M arrest in Kasumi-1 cells
To further investigate the molecular mechanism for
ZGDHu-1-induced G2/M arrest in Kasumi-1 cells, the
protein levels of cyclin B1 were analyzed using FACS. Additionally,
certain CDKs and CKIs were measured using western blotting.
The combination of cyclin B1 and cdc2 is an
important step for eukaryotic cells entering into mitosis (19). The present result implied that the
protein level of cdc2 (Fig. 4A)
and cyclin B1 (Fig. 4A, D and E)
were markedly decreased in a concentration- and time-dependent
manner, which inhibited the number of Kasumi-1 cells entering
mitosis.
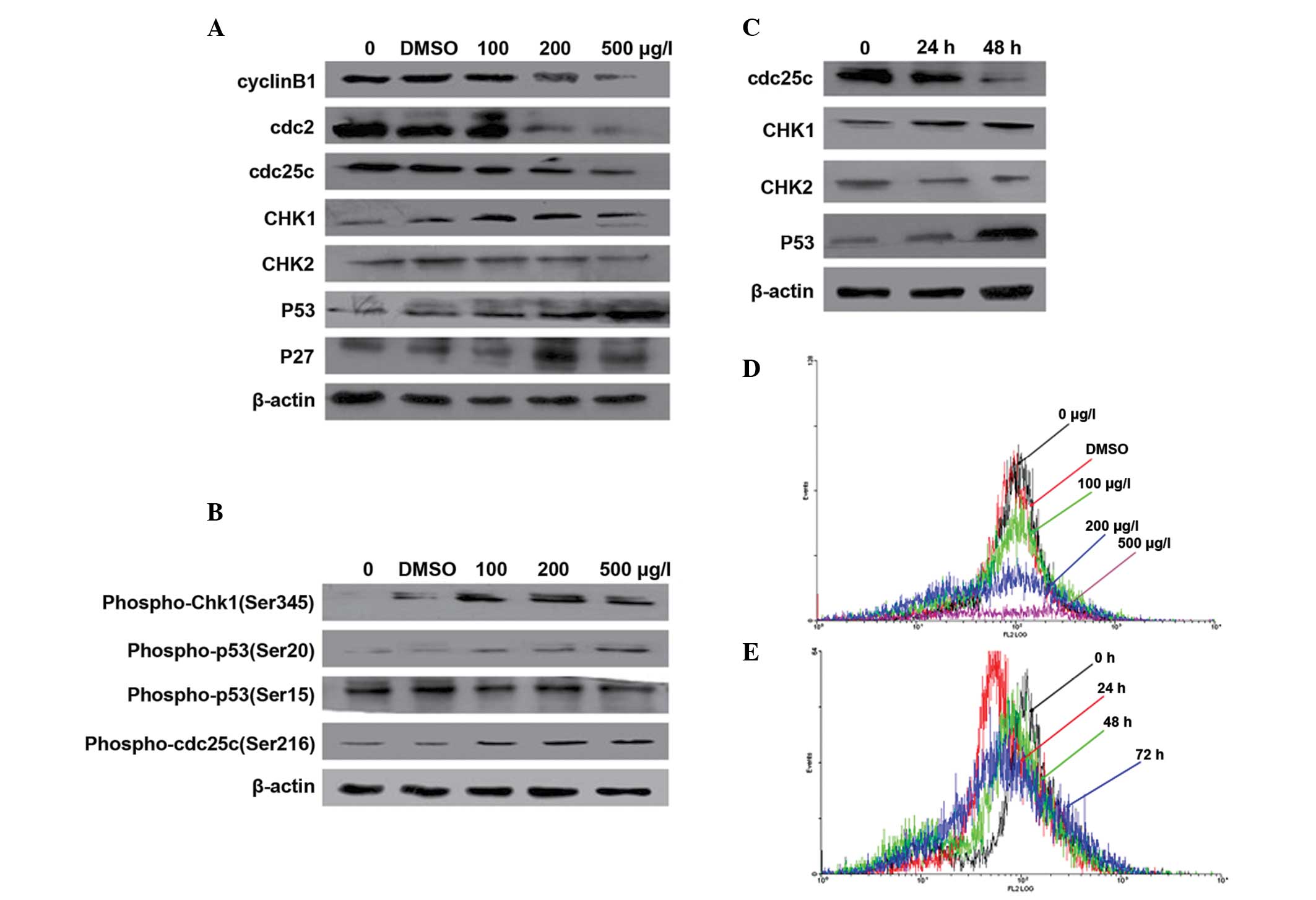 | Figure 4Expression changes in
G2/M-associated CDKs and CKIs. (A) Western blot analysis
of the level of G2/M cell cycle control protein. Cyclin
B1, cdc2, cdc25c, CHK1, CHK2, p53 and p27 were treated with
different concentrations of ZGDHU-1 and controls for 48 h. β-actin
was used as a loading control. (B) Western blot analysis of the
level of phospho-cdc25c at Ser216, phospho-Chk1 at Ser345,
phospho-p53 at Ser20 and Ser15. (C) Western blot analysis of
cdc25c, CHK1, CHK2, p53 at 200 μg/l ZGDHU-1 with different
cultivating times. (D) Expression of cycin B1 with different
concentrations of ZGDHU-1 and controls for 48 h using fluorescence
activated cell sorting. The black line indicates the negative
control, red line indicates the DMSO control, green line indicates
100 μg/l ZGDHU-1, blue line indicates 200 μg/l ZGDHU-1 and the
brown line indicates 500 μg/l ZGDHU-1. (E) Expression of cycin B1
at 200 μg/l ZGDHU-1 with different cultivating times using
fluorescence activated cell sorting. Black indicates 0 h, red
indicates 24 h, green indicates 48 h and blue indicates 72 h.
ZGDHu-1,
N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide;
CDK, cyclin-dependent kinase; CKI, cyclin-dependent kinase
inhibitor; cdc, cell division ccontrol; DMSO, dimethyl sulfoxide;
CHK, checkpoint kinase. |
There are also additional CDKs and CKIs, which have
important roles in G2/M arrest. Cdc25c is a protein
phosphatase responsible for dephosphorylating and activating cdc2,
while phosphorylation at Ser216 is DNA damage dependent at the
G2/M checkpoint. Therefore, the expression of cdc25c and
phospho-cdc25c at Ser216 was detected and the result revealed that
the level of cdc25c was decreased in a concentration- and
time-dependent manner, while phospho-cdc25c was increased in a
concentration-dependent manner (Fig.
4A–C).
CHK1 and CHK2 are important in DNA damage check
point control (20,21), therefore, their protein levels were
evaluated in order to determine through which pathway ZGDHu-1
induced G2/M arrest in Kasumi-1 cells. The expression of
CHK1 was significantly increased in a concentration- and
time-dependent manner, while no difference was identified in the
expression of CHK2 and phospho-CHK1 (Ser245), which exhibited
similar results compared with CHK1 (Fig. 4A–C).
p53, a well-known tumor suppressor, has various
roles in the cellular response to damage information (22). In the present study, p53 was
activated in a time- and concentration-dependent manner when
treated with ZGDHu-1 (Fig. 4A–C).
Phospho-p53 at Ser20 site was also upregulated via CHK1, while no
difference was identified at the Ser15 site (Fig. 4B). P27, a CKI, was also upregulated
(Fig. 4A).
CHIR-124 reduces the proportion of cells
in the G2/M phase and alters the expression of certain
CDKs
To confirm the role of CHK1 in ZGDHu-1-mediated
G2/M arrest, Kasumi-1 cells were treated with 200 nM of
the CHK1 inhibitor prior to ZGDHu-1 treatment. As shown in Fig. 5, the proportion of cells in the
G2/M phase reduced from 40.1±1.4 to 8.6±1.2%
(P<0.05), while cells in the G0/1 phase
increased.
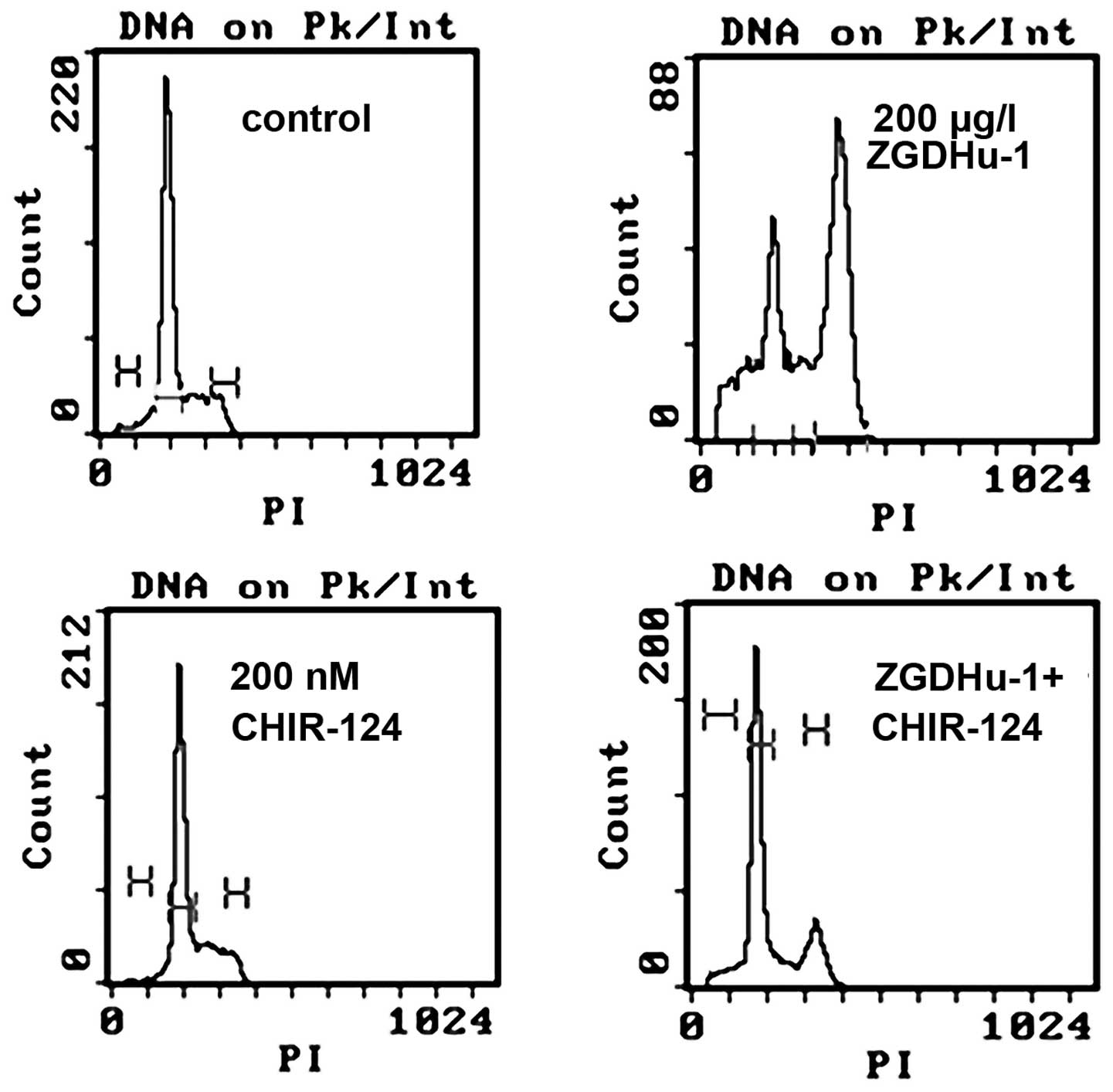 | Figure 5Effects of the CHK1 inhibitor on
ZGDHu-1-treated Kasumi-1 cells. The CHIR-124 inhibitor reduced cell
cycle arrest in ZGDHu-1-treated cells. Fluorescence-activated cell
sorting was used to analyze the cell cycle of Kasumi-1 cells, which
were treated with ZGDHu-1 or CHIR-124 alone or with both. ZGDHu-1,
N,N′-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide;
CHK, checkpoint kinase; PI, propidium iodide. |
Discussion
Our previous study revealed that ZGDHu-1 may inhibit
the growth of Kasumi-1 cells in a time- and dose-dependent manner
in vitro, however, the underlying mechanisms have not been
discussed. In the present study, ZGDHu-1 induced apoptosis through
the activation of caspase-3. Caspases are a family of cytosolic
aspartate-specific cysteine proteases, which are involved in the
initiation and execution of apoptosis. They are expressed as latent
zymogens and are activated by an autoproteolytic mechanism or by
processing by other proteases. Within the caspase family, caspase-3
is a key enzyme and it was demonstrated to be activated following
treatment with ZGDHu-1 in leukemia cells (23). Overall, the present results
demonstrate that ZGDHu-1 may induce Kasumi-1 cell apoptosis.
Furthermore, ZGDHu-1 may arrest the cell cycle at the
G2/M phase of leukemia cells when the concentration of
ZGDHu-1 was 100 μg/l.
NF-κB is a protein complex that controls the
transcription of DNA, while IκB is an inhibitory factor (24); activation of the NF-κB signaling
pathway is initiated by the signal-induced degradation of IκB
proteins. With the degradation of IκB, the NF-κB complex is then
translocated to the nucleus where it can ‘turn on’ the expression
of specific genes that have DNA-binding sites for NF-κB.
Furthermore, the present data revealed that ZGDHu-1 may upregulate
the expression of IκB and downregulate the expression of NF-κB to
inhibit the growth of Kasumi-1 cells. The A/E fusion gene is a
vital characteristic of Kasumi-1 cells and it also has a major role
in the biology of this type of leukemia. In the present study, it
was revealed that ZGDHu-1 may significantly decrease the protein
level of this fusion gene, suggesting that ZGDHU-1 may effectively
inhibit the development of this leukemia partly through this
mechanism.
There are two major pathways able to induce
apoptosis (25), which are
classified as the extracellular (extrinsic inducers) or
intracellular (intrinsic inducers) pathway. Mitochondrial
regulation is a vital part of the intracellular pathway. The
changes in mitochondrial membrane protein (Apo 2.7) and Δψm
suggested that the integrity of the mitochondrial membrane was
destroyed. There was also ROS accumulation in the Kasumi-1 cells.
The changes in Bcl-2, Bad and Bax determined via FACS and western
blotting confirmed that ZGDHu-1 induced apoptosis through the
mitochondrial pathway (26).
The cell cycle is a series of events that take place
in a cell leading to its division and duplication. It includes the
mitotic period and interphase; interphase may be further subdivided
into three phases, which include the G1 phase, S phase
and G2 phase. In the present study, ZGDHu-1 induced
G2/M phase arrest in Kasumi-1 cells in a
concentration-dependent manner.
Numerous proteins and kinases are involved in the
process of the cell cycle. The protein levels of cdc2 and cyclin B1
were markedly decreased in a concentration-dependent manner,
leading to the obstruction of mitotic entry in Kasumi-1 cells. The
level of cdc25c was also decreased; cdc25c is a protein phosphatase
responsible for dephosphorylating and activating cdc2, while
phosphorylation at Ser216 is DNA damage dependent at the
G2/M checkpoint. The present results demonstrated that
phospho-cdc25c was increased to inhibit the combination of cdc2 and
cyclin B1. p53 was activated and p27, as CKIs, were upregulated to
induce G2/M arrest.
In order to elucidate whether CHK1 is important in
ZGDHu-1-induced cell cycle arrest in Kasumi-1 cells, western
blotting was used to detect the protein level of CHK1 and p-CHK1,
which revealed an increased concentration of this protein. In
addition, a type of CHK1 inhibitor was added prior to ZGDHu-1
administration. Following being incubated with ZGDHu-1, it was
revealed that the proportion of cells in the G2/M
reduced, while the number of cells in the G0/1 phase
increased.
In conclusion, the present study demonstrated that
ZGDHu-1 was able to inhibit the proliferation and induce the
apoptosis of Kasumi-1 cells. Notably, this compound was able to
arrest the cell cycle at the G2/M phase. CHK1 kinase was
found to be important in these activities. The present results
suggested that ZGDHu-1 may be a potential drug to treat leukemia in
the future.
Acknowledgements
This study was supported by a fund from the Zhejiang
Province Health Bureau (grant no. 2012KYA015) and key platform
funded projects from the Zhejiang Province Health Bureau (grant no.
2013ZDA005). The authors would like to thank Dr Jin Ling Liu for
his assistance in the preparation of the manuscript.
References
|
1
|
Kelly LM and Gilliland DG: Genetics of
myeloid leukemias. Annu Rev Genomics Hum Genet. 3:179–198. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferrara F and Del Vecchio L: Acute myeloid
leukemia with t(8;21)/AML1/ETO: a distinct biological and clinical
entity. Haematologica. 87:306–319. 2002.PubMed/NCBI
|
|
3
|
Rao GW and Hu WX: Synthesis, X-ray
crystallographic analysis, and antitumor activity of
1-acyl-3,6-disubstituted phenyl-1,4-dihydro-1,2,4,5-tetrazines.
Bioorg Med Chem Lett. 15:3174–3176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao GW and Hu WX: Synthesis, structure
analysis, and antitumor activity of
3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg
Med Chem Lett. 16:3702–3705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou YL, Hu WX, Lü YP, Qiu LN, Wang WS,
Yang ZY, Liu JD and Rao GW: Effect of ZGDHu-1 on proliferation and
apoptosis of A549 cells in vitro and antitumor activity in vivo.
Yao Xue Xue Bao. 42:26–34. 2007.(In Chinese). PubMed/NCBI
|
|
6
|
Zhou YL, Lü YP, Hu WX, Qiu LN, Wang WS,
Liu JD and Wu JG: ZGDHu-1-inducing apoptosis of SHI-1 leukemia
cells and its molecular mechanism. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 15:483–489. 2007.(In Chinese). PubMed/NCBI
|
|
7
|
Zhou YL, Chen LC and Lu YP: Inhibition
effects of ZGDHu-1 on proteasome in Kasumi-1 cells. Zhonghua Xue Ye
Xue Za Zhi. 33:61–63. 2012.(In Chinese). PubMed/NCBI
|
|
8
|
Graña X and Reddy EP: Cell cycle control
in mammalian cells: role of cyclins, cyclin dependent kinases
(CDKs), growth suppressor genes and cyclin-dependent kinase
inhibitors (CKIs). Oncogene. 11:211–219. 1995.PubMed/NCBI
|
|
9
|
Murray AW: Recycling the cell cycle:
cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen T, Stephens PA, Middleton FK and
Curtin NJ: Targeting the S and G2 checkpoint to treat cancer. Drug
Discov Today. 17:194–202. 2012. View Article : Google Scholar
|
|
11
|
Wu ZZ, Chien CM, Yang SH, Lin YH, Hu XW,
Lu YJ, Wu MJ and Lin SR: Induction of G2/M phase arrest and
apoptosis by a novel enediyne derivative, THDA, in chronic myeloid
leukemia (K562) cells. Mol Cell Biochem. 292:99–105. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larizza L, Magnani I and Beghini A: The
Kasumi-1 cell line: a t(8;21)-kit mutant model for acute myeloid
leukemia. Leuk Lymphoma. 46:247–255. 2005. View Article : Google Scholar
|
|
13
|
Boulton S, Anderson A, Swalwell H,
Henderson JR, Manning P and Birch-Machin MA: Implications of using
the fluorescent probes, dihydrorhodamine 123 and
2′,7′-dichlorodihydrofluorescein diacetate, for the detection of
UVA-induced reactive oxygen species. Free Radic Res. 45:139–146.
2011. View Article : Google Scholar
|
|
14
|
Tao Y, Leteur C, Yang C, Zhang P, Castedo
M, Pierré A, Golsteyn RM, Bourhis J, Kroemer G and Deutsch E:
Radiosensitization by Chir-124, a selective CHK1 inhibitor: effects
of p53 and cell cycle checkpoints. Cell Cycle. 8:1196–1205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tse AN, Rendahl KG, Sheikh T, Cheema H,
Aardalen K, Embry M, Ma S, Moler EJ, Ni ZJ, Lopes de Menezes DE,
Hibner B, Gesner TG and Schwartz GK: CHIR-124, a novel potent
inhibitor of Chk1, potentiates the cytotoxicity of topoisomerase I
poisons in vitro and in vivo. Clin Cancer Res. 13:591–602. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Favaloro B, Allocati N, Graziano V, Di
Ilio C and De Laurenzi V: Role of apoptosis in disease. Aging
(Albany NY). 4:330–349. 2012.
|
|
17
|
Koester SK, Roth P, Mikulka WR, Schlossman
SF, Zhang C and Bolton WE: Monitoring early cellular responses in
apoptosis is aided by the mitochondrial membrane protein-specific
monoclonal antibody APO2.7. Cytometry. 29:306–312. 1997. View Article : Google Scholar
|
|
18
|
Chazotte B: Labeling mitochondria with
rhodamine 123. Cold Spring Harb Protoc. 2011.892–894.
2011.PubMed/NCBI
|
|
19
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen MS, Ryan CE and Piwnica-Worms H: Chk1
kinase negatively regulates mitotic function of Cdc25A phosphatase
through 14-3-3 binding. Mol Cell Biol. 23:7488–7497. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CH and Chung JH: The hCds1 (Chk2)-FHA
domain is essential for a chain of phosphorylation events on hCds1
that is induced by ionizing radiation. J Biol Chem.
276:30537–30541. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CY, Oliner JD, Zhan Q, Fornace AJ Jr,
Vogelstein B and Kastan MB: Interactions between p53 and MDM2 in a
mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA.
91:2684–2688. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Snigdha S, Smith ED, Prieto GA, et al:
Caspase-3 activation as a bifurcation point between plasticity and
cell death. Neurosci Bull. 28:14–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abraham E: NF-kappaB activation. Crit Care
Med. 28:N100–N104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halestrap AP, Doran E, Gillespie JP and
O’Toole A: Mitochondria and cell death. Biochem Soc Trans.
28:170–177. 2000.PubMed/NCBI
|
|
26
|
Zhai D, Jin C, Huang Z, Satterthwait AC
and Reed JC: Differential regulation of Bax and Bak by
anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem.
283:9580–9586. 2008. View Article : Google Scholar : PubMed/NCBI
|