Introduction
Renal cell carcinoma (RCC), which has been suggested
to originate from the renal tubules and collecting tube epithelial
cells, accounts for 85% of malignant kidney neoplasms and ~2% of
all human malignancies (1,2). RCC is a pathologically heterogeneous
disease, which can be classified into clear, papillary, granular,
spindle and mixed cell subtypes based on certain cytoplasmic
features (3). RCC morbidity
increases by 2% annually and mortality has reached ~100,000
cases/year worldwide (4).
Approximately 30% of patients with RCC develop metastatic disease,
most frequently in the lungs, bones or brain (5). A clinical study confirmed that
osteolysis represented 30% of the total metastatic disease cases
associated with RCC (6). The
incidence rate of bone tissue metastasis was higher in autopsy data
from patients with RCC (5). The
prognosis of RCC patients is influenced by a variety of factors,
including tumor size, invasion, metastasis, histological type and
nuclear grade (7); the five-year
survival rate of patients with RCC was 90% for stage I, 51% for
stage II, 22% for stage III and 4.6% for stage IV (8). Therefore, the elucidation of the key
factors and molecular mechanisms underlying the metastasis of RCC
to bone is required. A previous study by our group established an
ACHN cell subline (ACHN-BO5) with high potential of bone metastasis
compared with that of ACHN cells in animals in order to aid the
elucidation of the underlying molecular mechanisms (9). In this subline, the pathological
karyokinesis was significantly increased, which indicated that the
malignant phenotype of the ACHN subline was higher than that of the
parental ACHN cells. Following five passages of in vitro
culture, the subline was named ‘ACHN-BO5’. Subsequently, the gene
alterations responsible for the high potential of bone metastasis
were investigated using a complementary DNA (cDNA) microarray
analysis to compare ACHN-BO5 cells with the parental ACHN cells.
Alterations in the expression of enhancer of zeste homolog 2 (EZH2)
and matrix metalloproteinase-2 (MMP2) were detected in ACHN-BO5
cells. EZH2 is involved in maintaining the transcriptional
repressive state in cells and mutation of EZH2 causes Weaver
syndrome (10), a congenital
disorder associated with rapid growth beginning in the prenatal
period, resulting in a characteristic facial appearance and certain
skeletal features (11). In
addition, studies have demonstrated that altered EZH2 expression
promotes human cancer development (12–16).
Proteins of the MMP family degrade or break down the extracellular
matrix during normal physiological processes, including embryonic
development and tissue remodeling, but also have a significant role
in tumor metastasis (17). By
contrast, tissue inhibitor of metalloproteinase-2 (TIMP2) is a
natural matrix metalloproteinase inhibitor. Therefore, these
proteins may have a role in mediating the metastasis of RCC to
bone. In the present study, the expression of EZH2, MMP2 and TIMP2
were evaluated in RCC tissue specimens with or without bone
metastasis and in ACHN-BO5 and ACHN cells to elucidate the
correlation between their expression and metastasis in bone.
Methylation-specific PCR analysis of TIMP2 promoter methylation was
also performed. The results of the present study may aid the
elucidation of the mechanisms underlying RCC metastasis and provide
potential therapeutic targets for the prevention or treatment of
RCC metastasis.
Materials and methods
Cell lines and culture
The human renal carcinoma cell line ACHN was
obtained from the China Center of Type Culture Collection (Wuhan,
China). The ACHN-B05 cell line was a subline of ACHN with high
potential of bone metastasis, established in a previous study by
our group (9). These cell lines
were cultured in Dulbecco’s modified Eagle’s medium (DMEM;
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen Life Technologies) at 37°C
in a humidified incubator with 5% CO2.
Tissue specimens and
immunohistochemistry
Primary renal cancer biopsy specimens (n=25) and
biopsies of renal cancer that had metastasized to bone tissues
(n=13) were obtained from The Tongji Hospital affiliated with
Huazhong University of Science and Technology (Wuhan, China)
between March 2010 and April 2013. The normal control renal biopsy
specimens were obtained from adjacent normal tissues. The present
study was approved by the ethics committee of Tongji Hospital
affiliated with Huazhong University of Science and Technology.
Written informed consent was obtained from all patients or their
family. All tissues were fixed in 4% paraformaldehyde solution
(Boster Biological Technology, Ltd., Wuhan, China) for 20 min at
room temperature and embedded into paraffin using a routine tissue
process (18). Tissue sections
(4-μM thick) were prepared from the paraffin blocks and mounted
onto glass slides. For immunohistochemical analysis, tissue
sections were deparaffinized and rehydrated in water. The sections
were heated in a pressure cooker (121°C, 4 min) in a citric acid
buffer (Boster Biological Technology, Ltd.) for antigen retrieval
and then incubated with 3%
H2O2/phosphate-buffered saline (PBS; Boster
Biological Technology, Ltd.) at room temperature for 30 min to
block potential peroxidase activity. Following incubation with 20%
normal serum (Boster Biological Technology, Ltd.) for 30 min, the
sections were further incubated with primary antibodies: Mouse
monoclonal immunoglobulin G1 (IgG1) anti-MMP2
antibody at a dilution of 1:800 (sc-13594; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), goat polyclonal IgG
anti-TIMP2 antibody at a dilution of 1:600 (sc-6835; Santa Cruz
Biotechnology Inc.) or an goat polyclonal IgG anti-EZH2 antibody at
a dilution of 1:100 (E6906; Sigma-Aldrich, St. Louis, MO, USA)
overnight at 4°C. The following day, the sections were washed three
times with PBS and subsequently incubated with the secondary
antibodies (anti-mouse IgG and anti-goat IgG horseradish
peroxidase; Boster Biological Technology, Ltd.) for 1 h at 37°C. A
color reaction was performed using 3,3′-diaminobenzidine (Boster
Biological Technology, Ltd.) as the chromogen. Diluted Sav-HRP
conjugates were applied to the sections on the slides and incubated
in a humidified chamber at room temperature for 30 min (protected
from the light). Slides were washed with PBS twice, for 5 min each.
DAB substrate solution (freshly made just before use: 0.05% DAB -
0.015% H2O2 in PBS) was applied to the
sections on the slides to reveal the color of antibody staining.
The stained tissue sections were independently reviewed and scored
under an Olympus CKX31 inverted microscope (Olympus Corp., Tokyo,
Japan) by two investigators. The statistical results were analyzed
using Sigmaplot 11.0 software (Systat Software, Inc., Chicago, IL,
USA).
DNA extraction and methylation-specific
PCR (MSP)
Genomic DNA was extracted from ACHN and ACHN-BO5
cell lines using a Genomic DNA extraction kit (Boehringer,
Mannheim, Germany) according to the manufacturer’s instructions.
DNA concentration, purity and integrity were measured using a
spectrophotometer (Gilford 250; Gilford Instrument Laboratories,
Inc., Oberlin, OH, USA) and gel electrophoresis. For MSP, genomic
DNA samples (2 μg) were denatured with sodium hydroxide chemically
modified with sodium bisulfite (Boster Biological Technology,
Ltd.). An MSP primer for the amplification of the TIMP2 promoter
was designed using Methprime software (http://www.urogene.org/methprimer/index1.html). The
methylation primers of the TIMP2 promoter were as follows: Forward,
5′-TTTTATTGTAGGAAAGGTCGA-3′ and reverse,
5′-GAAATCATAAAACAACGCGTA-3′, which amplified a 159-bp PCR product.
The demethylation primers of the TIMP2 promoter used were: Forward,
5′-GAAGGAATATTTTATTGTAGGAAAGGTT-3′ and reverse,
5′-TATAACACAAAATCATAAAACAACACATA-3′, which amplified a 176-bp PCR
product. PCR amplification occurred in a final volume of 25 μl
containing: 2.5 μl PCR buffer, 2 μl MgCl2, 2.5 μl
deoxynucleotide triphosphate mixture, 1 μl of each primer, 5 μl
modified DNA template, 10.85 μl sterilized deionized water and 0.15
μl Taq enzyme (Takara Bio, Inc., Otsu, Japan). The PCR conditions
were set at a pre-denaturation temperature of 94°C for 5 min
followed by 45 cycles of 94°C for 30 sec, 60°C (methylated) or 55°C
(demethylated) for 45 sec, 75°C for 40 sec and a final extension at
73°C for 5 min. Genomic DNA chemically modified with sss1
methylation enzyme (New England Biolabs, Shanghai, China) and
bisulfite salts (Sigma-Aldrich) was used as a positive control.
Deionized water was used as a negative control. PCR products were
isolated using agarose gel electrophoresis and imaged under an
ultraviolet lamp (XX15B, Spectronics Corp., Westbury, NY, USA).
RNA isolation and semi-quantitative
RT-PCR
The expression levels of EZH2, MMP2 and TIMP2 mRNA
were evaluated using RT-PCR analysis. Briefly, total cellular RNA
was isolated from cells using TRIzol reagent (Invitrogen Life
Technologies) and reverse-transcribed to cDNA using the First
Strand cDNA Synthesis kit (Rever Tra Ace-α; ToYoBo Co., Ltd.,
Osaka, Japan) according to the manufacturer’s instructions. The
primer sequences of genes and fragment sizes are indicated in
Table I. The PCR conditions were
as follows: Pre-denaturation at 94°C for 5 min and 32 cycles of
94°C for 45 sec; 52.5°C, 56°C and 54°C as the annealing temperature
for 1 min, 72°C for 90 sec and a final extension at 72°C for 10
min. The PCR product was subsequently sequenced and identified by
gel electrophoresis.
 | Table IPrimers for PCR amplification of gene
expression. |
Table I
Primers for PCR amplification of gene
expression.
| Gene | Sequences | Size of PCR
products (bp) |
|---|
| EZH2 |
5′-GTGGAGAGATTATTTCTCAAGATG-3′ | |
|
5′-CCGACATACTTCAGGGCATCAGCC-3′ | 289 |
| MMP2 |
5′-GAGAACCAAAGTCTGAAGAG-3′ | |
|
5′-GGAGTGAGAATGCTGATTAG-3′ | 207 |
| TIMP2 | 5′-CCTCGGCCTTTC
CTGCAAT-3′ | |
|
5′-TATCTACACGGCCCCCTCCT-3′ | 89 |
| GAPDH |
5′-GAAGGTGAAGGTCGGAGTC-3′ | |
|
5′-GAAGATGGTGATGGGATTTC-3′ | 226 |
Protein extraction and western blot
analysis
Cells were collected and lysed with a pre-cooled
cell lysis buffer (Boster Biological Technology, Ltd.) containing
100 mM Tris-HCl, 500 mM EDTA, 20 mM NaCl, and 10% SDS. The protein
concentration was determined using a Bicinchoninic Acid Assay kit
(Sigma-Aldrich, Irvine, Scotland). Briefly, an equal amount of
protein sample was separated by SDS-PAGE and transferred onto a
polyvinylidene fluoride membrane (A-FIT Biosciences, Beijing,
China). For western blot analysis, the membranes were incubated in
5% skimmed milk/PBS at room temperature for 1 h and then further
incubated with anti-MMP2 antibody at a dilution of 1:800,
anti-TIMP2 antibody at a dilution of 1:600 or anti-EZH2 antibody at
a dilution of 1:100 at 4°C overnight. The following day, the
membranes were washed three times with PBS-Tween-20 and incubated
with a horseradish peroxidase-conjugated secondary antibody at a
dilution of 1:7,500 at room temperature for 2 h. Immunoreactive
proteins were visualized using enhanced chemiluminescence (Pierce
Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer’s instructions and exposed to x-ray films (Kodak,
Rochester, NY, USA). The expression levels of these proteins were
normalized to an internal control, GAPDH.
Statistical analysis
Values are presented as the mean ± standard
deviation of three independent experiments. All statistical
analyses were performed using SPSS 11.0 software (SPSS, Inc.,
Chicago, IL, USA). The expression levels of EZH2, MMP2, TIMP2 mRNA
and protein in ACHN and ACHN-B05 cells were compared using one-way
analysis of variance. Differences in TIMP2 promoter methylation
were analyzed using a χ2 test. A Pearson’s correlation
test was used to analyze the associations between different groups.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Differential expression of EZH2, MMP2 and
TIMP2 proteins in tissues of patients with RCC as well as ACHN and
ACHN-B05 cells
In the present study, the expression of EZH2, MMP2
and TIMP2 proteins in tissues from patients with RCC with and
without bone metastasis were evaluated by immunohistochemical
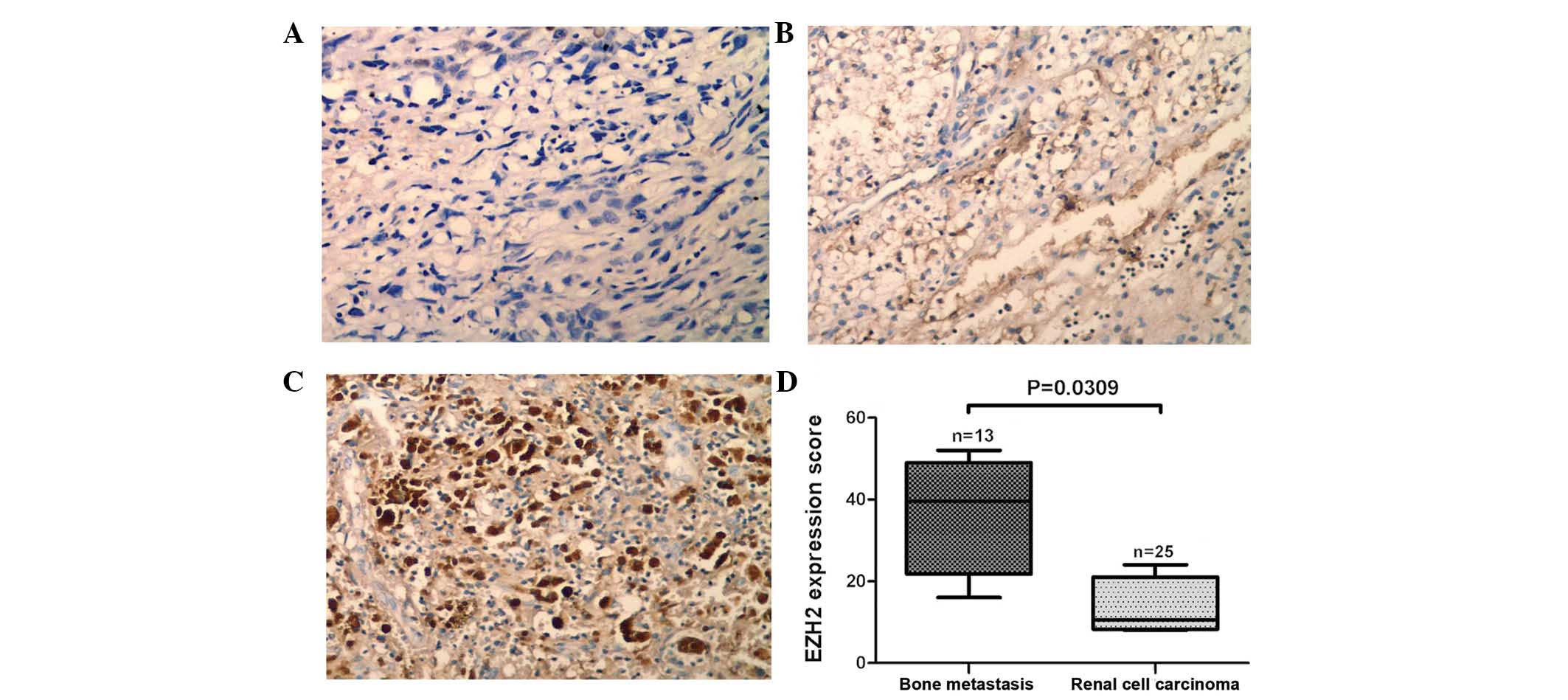
analysis. The expression of EZH2 protein was higher in tissues from
patients with RCC that had metastasized to the bone than in tissues
of patients with RCC without metastasis (P=0.031; Fig. 1A–D). Analogously, expression levels
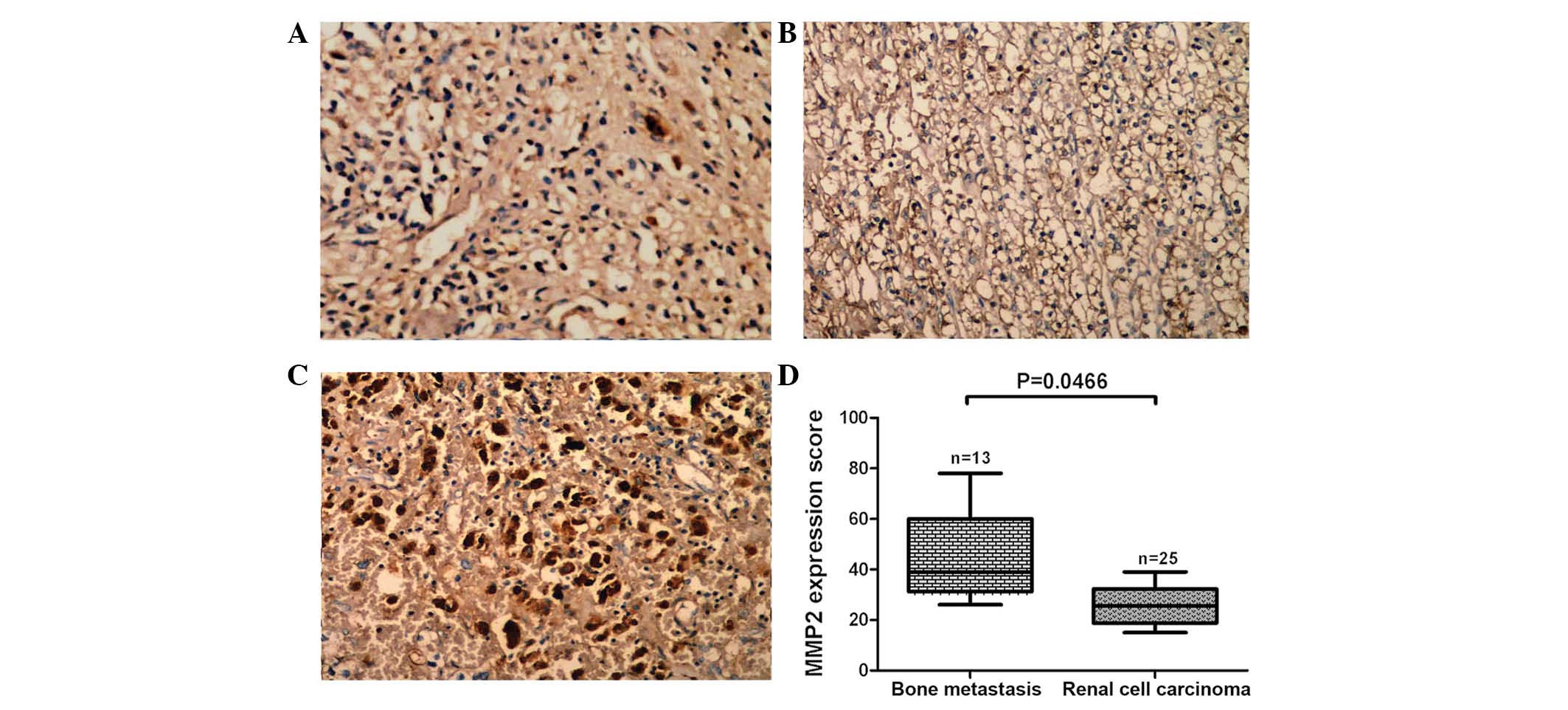
of MMP2 protein were also higher in tissues from patients where RCC
had metastatized than those in patients with RCC without metastasis
(P=0.047; Fig. 2A–D). By contrast,
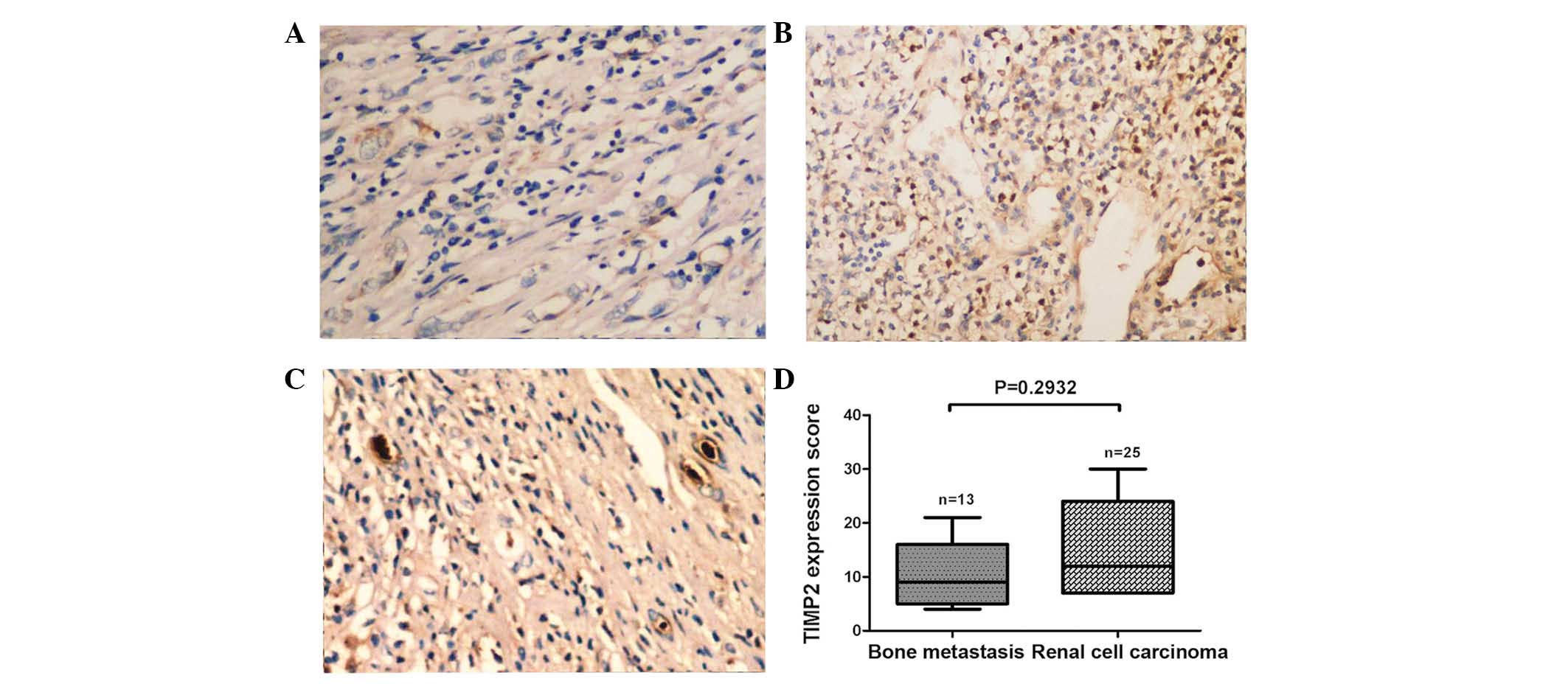
there were no significant differences in the expression of TIMP2
protein between the tissue types (P=0.2932; Fig. 3A–D). Furthermore, the expression of
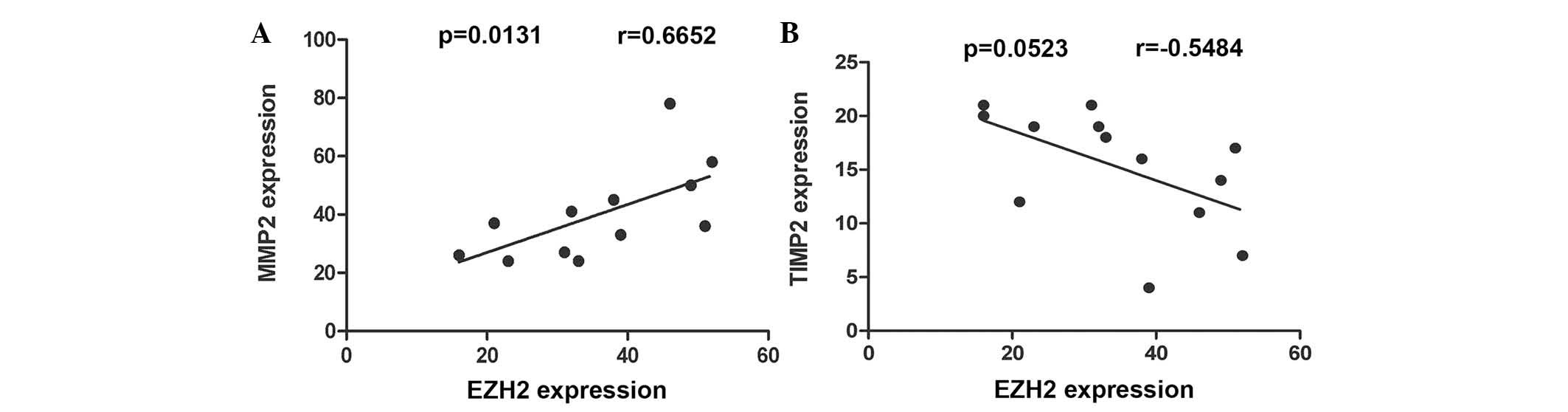
EZH2 and MMP2 proteins were found to be correlated (r=0.6652;
P=0.0131; Fig. 4A), whereas there
was no significant correlation between EZH2 and TIMP2 protein
expression (r=−0.5484; P=0.0523; Fig.
4B).
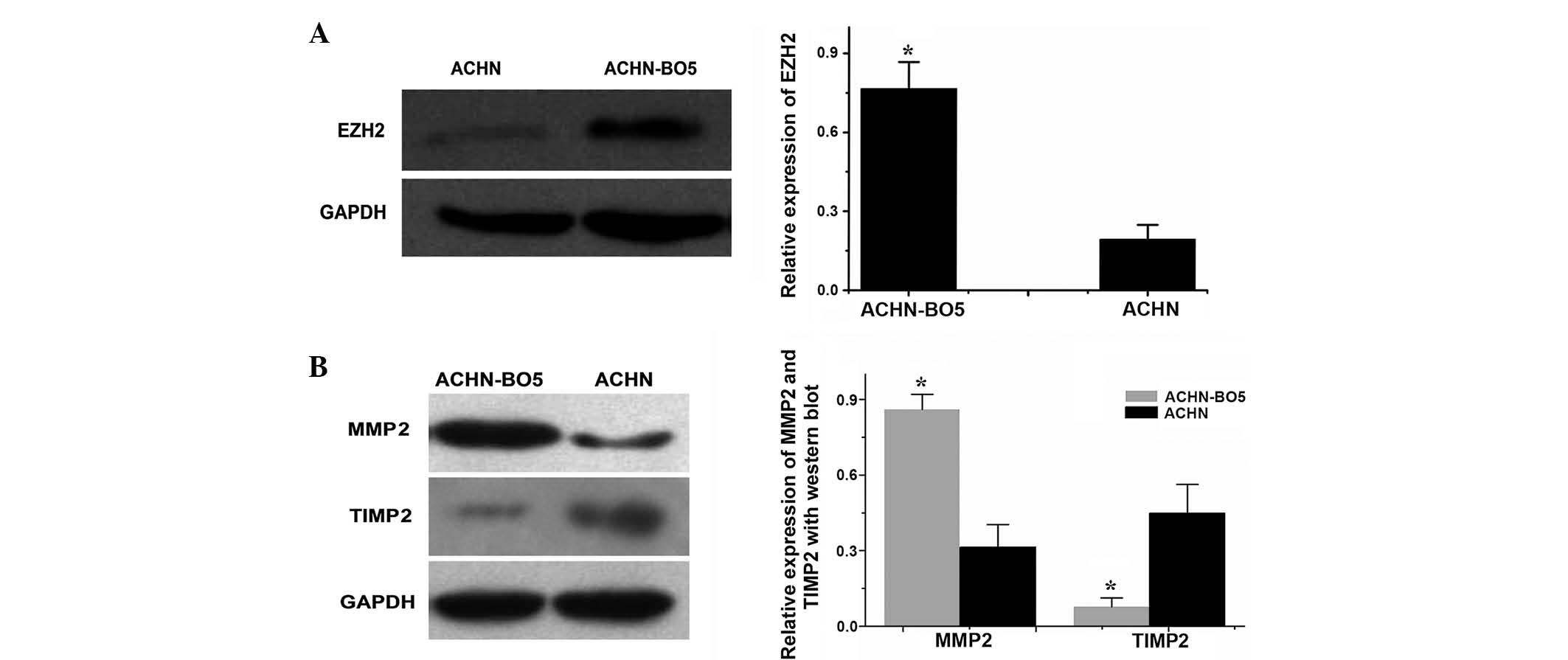
Western blot analysis was used to investigate the
expression levels of EZH2 protein in ACHN and ACHN-BO5 cells. EZH2
protein expression levels were demonstrated to be higher in
ACHN-BO5 cells, a sub-line of ACHN with a higher potential for
metastasis to the bone, than those in the parental ACHN cells
(P<0.05), suggesting that EZH2 protein may be involved in
mediating the metastasis of RCC to bone (Fig. 5A). In addition, the expression
levels of MMP2 protein were higher in ACHN-BO5 cells than those in
the parental ACHN cells (P<0.05). By contrast, TIMP2 protein
expression levels were lower in ACHN-BO5 cells than those in the
parental ACHN cells (P<0.05; Fig.
5B).
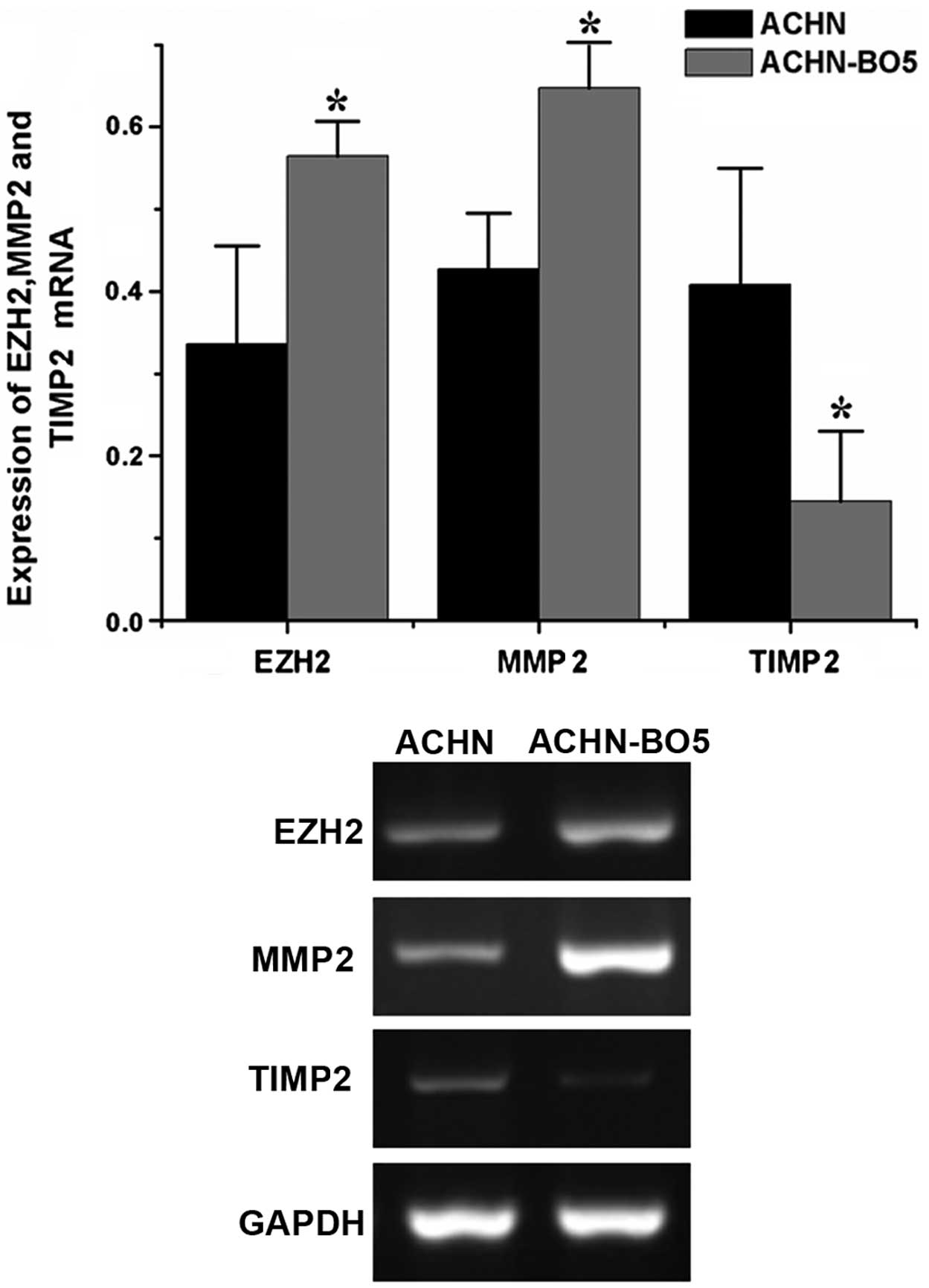
EZH2, MMP2 and TIMP2 mRNA expression
levels differ between ACHN and ACHN-B05 cells
RT-PCR analysis was performed in order to evaluate
whether the altered expression of these three proteins occurred at
the transcriptional level in the ACHN and ACHN-BO5 cell lines. The
expression levels of EZH2 and MMP2 mRNA were higher in ACHN-BO5
cells than those in ACHN cells, whereas the expression levels of
TIMP2 mRNA were lower in ACHN-BO5 cells than those in ACHN cells
(Fig. 6).
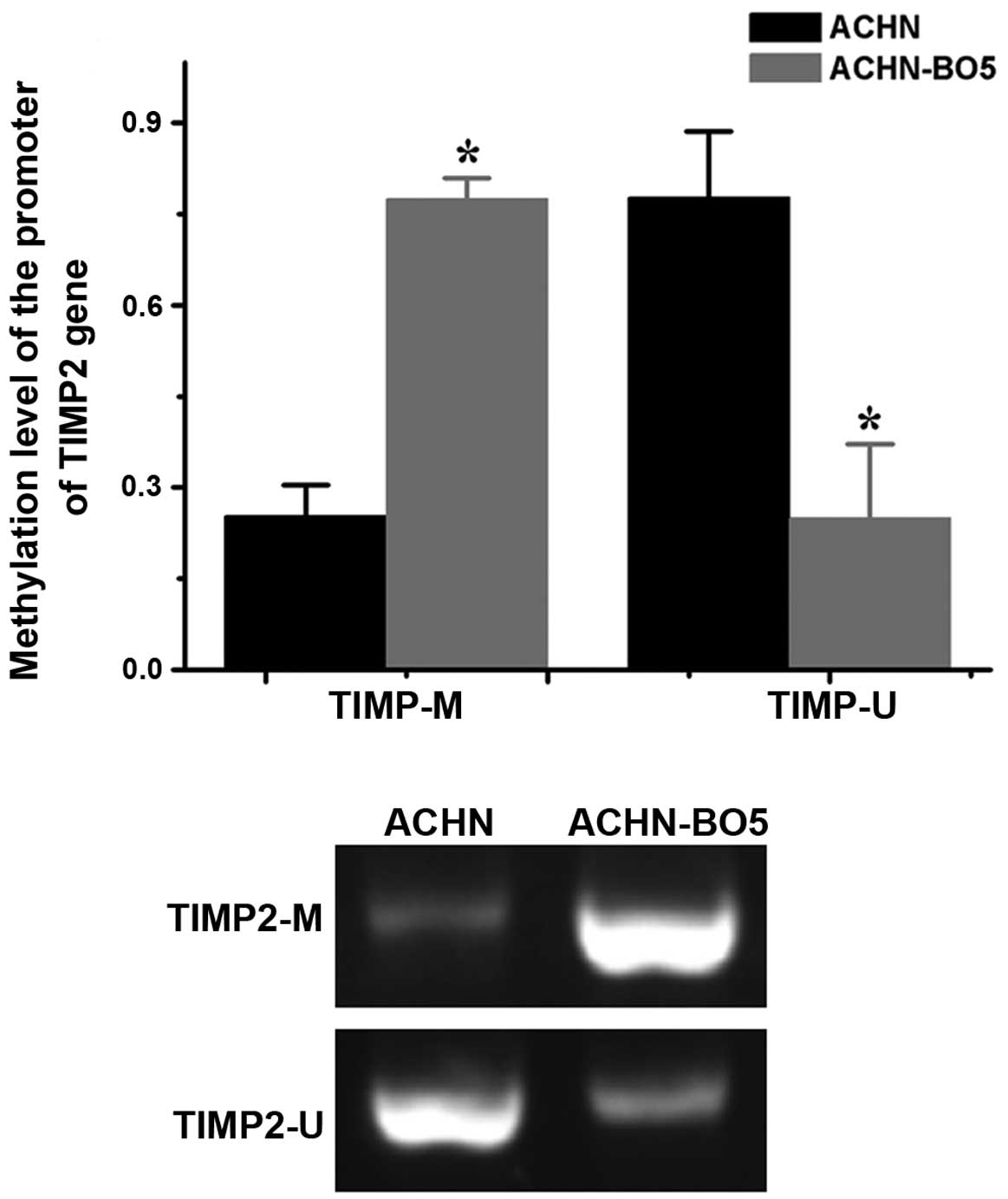
TIMP2 is more highly methylated in
ACHN-BO5 cells
The potential mechanism underlying the downregulated
expression of TIMP2 mRNA in ACHN-BO5 cells was assessed using MSP.
The TIMP2 promoter was more highly methylated in ACHN-BO5 cells
than in ACHN cells (Fig. 7).
Discussion
Cancer metastasis is a complex process, where cancer
cells migrate from their site of origin and invade other parts of
the body via the bloodstream, lymphatic system or direct extension
(19). Molecularly, tumor cells
gain gene transcription capabilities and express various proteins
and enzymes in order to degrade extracellular matrix proteins and
invade adjacent tissues (20).
However, the distant metastasis of various types of human cancer
indicates preferences for certain organs; therefore, certain types
of cancer tend to spread to particular organs and tissues (19). For example, breast cancer
preferentially metastasizes to bone and lung tissue (21). RCC frequently metastasizes to the
lungs, bone or brain, whereas the brain is most commonly the
distant site of metastasis of melanoma (22). Therefore, a study of organ-specific
RCC metastasis may aid in the prevention of RCC progression. In a
previous study by our group, an RCC cell line (ACHN-BO5) with high
potential for metastasis to the bone was generated (9). These cells exhibited a significantly
enhanced invasion and proliferation capacity in vitro,
compared to that of the parental ACHN cells. The present study
investigated whether cell adhesion molecules, including EZH2, MMP2
and TIMP2, contributed to alterations in the phenotype of tumor
cells. Expression levels of EZH2 and MMP2 mRNA and protein were
higher in ACHN-BO5 cells than those in ACHN cells, whereas the
expression of TIMP2 mRNA and protein was lower in ACHN-BO5 cells
than that in ACHN cells. MSP data indicated that the downregulated
expression of TIMP2 may be due to the methylation of the
TIMP2 promoter. Furthermore, the expression of these
proteins was evaluated in tissue specimens from patients with RCC
that had metastasized to the bone and patients with RCC without
metastasis. The results confirmed those of the in vitro
investigations, indicating that the expression of EZH2 and MMP2
protein was higher in tissues from patients with RCC that had
metastasized to the bone than that in tissues from renal cancer
patients without metastasis; and that there was no significant
difference in the expression of TIMP2 protein between the two types
of tissue.
The epigenetic modification enzyme, EZH2, has a
homologous structure to the Drosophila E(z) gene, and was
identified in the 1990s (23).
Cardoso et al (24)
demonstrated that the EZH2 protein was a key member of the polycomb
group gene family and was able to modulate cell proliferation and
the signaling pathway via the suvar3–9, enhancer of zeste, trtharax
domain. The EZH2 protein is involved in mediating the development,
metastasis, invasiveness and prognosis of various types of human
cancer (23–32). Therefore, EZH2 is of interest in
basic and clinical studies of cancer. Several studies have
demonstrated that EZH2 protein is highly expressed in RCC tissues
(33–36), the level of which is associated
with RCC dedifferentiation, suggesting that the EZH2 protein may
contribute to RCC progression and metastasis. More recently,
studies have indicated that EZH2 protein is highly expressed in
prostate cancer tissues, which are also associated with a high rate
of metastasis to the bone (16,37,38).
Knockdown of EZH2 expression in metastatic bone tumors leads to
atrophy of the metastatic bone foci and a reduction in or end to
bone destruction (39), suggesting
that EZH2 protein has a role in mediating metastasis to the
bone.
MMPs, including MMP2, are required for degradation
of the extracellular matrix and are specifically inhibited by the
TIMPs. Therefore, these two families of proteins have significant
roles in tumor metastasis. Numerous studies have confirmed that
alterations in the balance of MMPs/TIMPs may lead to the metastasis
of human cancers to the bone (40–43).
Further studies have demonstrated that a downregulation of TIMP2
expression was associated with methylation of the TIMP2 promoter
(44–50). Of note, EZH2 contains histone
methyltransferase activity, which is able to silence genes via the
methylation of H3 histone lysine 27 (51). It was therefore hypothesized that
overexpression of EZH2 protein may lead to epigenetic silencing of
TIMP2 expression. The results of the present study confirmed that
there was an enhanced level of TIMP2 promoter methylation in
ACHN-BO5 cells compared to that in ACHN cells. However, the results
of the ex vivo investigations did not demonstrate an
association between EZH2 expression and reduced TIMP2 protein,
which does not support the hypothesis that EZH2 protein may be able
to cause epigenetic silencing of TIMP2 expression. Due to the small
sample size in the present study, further investigations are
required in order to confirm this hypothesis.
Further studies are required to elucidate the
molecular mechanisms underlying how the expression of EZH2, MMP2
and TIMP2 is altered in RCC tissues or cells with high potential
for bone metastasis.
Acknowledgements
The present study was part of the programs on The
Natural Science Foundation funded through The Science and
Technology Department of Hubei Province, Wuhan, China (no.
2008CDB168) and The Science Foundation funded through The Health
Department of Hubei Province, Wuhan, China (no. 2010JX5B03). This
study was also part of the programs on The Nature Science
Foundation funded by The Huazhong University of Science and
Technology (2013QN200). The authors would like to thank all study
participants and Medjaden Bioscience Limited (Hong Kong,
China) for assisting in the preparation of this manuscript.
References
|
1
|
Kosary C and McLaughlin J: Kidney and
renal pelvis. Cancer Statistics Review: 1973–1990. Miller BA, Ries
LAG, Hankey BE, et al: National Institutes Of Health; Bethesda: pp.
x1–x22. 1993
|
|
2
|
Fleming S and Lewi H: Collecting duct
carcinoma of the kidney. Histopathology. 10:1131–1141. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thoenes W, Störkel S and Rumpelt H:
Histopathology and classification of renal cell tumors (adenomas,
oncocytomas and carcinomas). The basic cytological and
histopathological elements and their use for diagnostics. Pathol
Res Pract. 181:125–143. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pisani P, Parkin D and Ferlay J: Estimates
of the worldwide mortality from eighteen major cancers in 1985.
Implications for prevention and projections of future burden. Int J
Cancer. 55:891–903. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ritchie A and Chisholm G: The natural
history of renal carcinoma. Semin Oncol. 10:390–400.
1983.PubMed/NCBI
|
|
6
|
Wood SL and Brown JE: Skeletal metastasis
in renal cell carcinoma: current and future management options.
Cancer Treat Rev. 38:284–291. 2012. View Article : Google Scholar
|
|
7
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erdoğan F, Demirel A and Polat O:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Int J Clin Pract. 58:333–336. 2004. View Article : Google Scholar
|
|
9
|
Wang J, Chen A, Yang C, Zeng H, Qi J and
Guo FJ: A bone-seeking clone exhibits different biological
properties from the ACHN parental human renal cell carcinoma in
vivo and in vitro. Oncol Rep. 27:1104–1110. 2012.
|
|
10
|
Gibson WT, Hood RL, Zhan SH, et al; FORGE
Canada Consortium. Mutations in EZH2 cause Weaver syndrome. Am J
Hum Genet. 90:110–118. 2012. View Article : Google Scholar :
|
|
11
|
Weaver DD, Graham CB, Thomas I and Smith
DW: A new overgrowth syndrome with accelerated skeletal maturation,
unusual facies, and camptodactyly. J Pediatr. 84:547–552. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kleer CG, Cao Q, Varambally S, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kehinde EO, Maghrebi M and Anim JT: The
importance of determining the aggressiveness of prostate cancer
using serum and tissue molecular markers. Can J Urol. 15:3967–3974.
2008.PubMed/NCBI
|
|
14
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Varambally S, Cao Q, Mani RS, et al:
Genomic loss of microRNA-101 leads to overexpression of histone
methyltransferase EZH2 in cancer. Science. 322:1695–1699. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen L, Cui J, Liang S, Pang Y and Liu P:
Update of research on the role of EZH2 in cancer progression. Onco
Targets Ther. 6:321–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: an
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen DX and Massagué J: Genetic
determinants of cancer metastasis. Nat Rev Genet. 8:341–352. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong GS and Rustgi AK: Matricellular
proteins: priming the tumour microenvironment for cancer
development and metastasis. Br J Cancer. 108:755–761. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drabsch Y and ten Dijke P: TGF-β signaling
in breast cancer cell invasion and bone metastasis. J Mammary Gland
Biol Neoplasia. 16:97–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zlotnik A, Burkhardt AM and Homey B:
Homeostatic chemokine receptors and organ-specific metastasis. Nat
Rev Immunol. 11:597–606. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Rossier C and Antonarakis SE:
Cloning of a human homolog of the Drosophila enhancer of zeste gene
(EZH2) that maps to chromosome 21q22.2. Genomics. 38:30–37. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardoso C, Timsit S, Villard L,
Khrestchatisky M, Fontès M and Colleaux L: Specific interaction
between the XNP/ATR-X gene product and the SET domain of the human
EZH2 protein. Hum Mol Genet. 7:679–684. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heinen A, Tzekova N, Graffmann N, et al:
Histone methyltransferase enhancer of zeste homolog 2 regulates
Schwann cell differentiation. Glia. 60:1696–1708. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia H, Yu CH, Zhang Y, et al: EZH2
silencing with RNAi enhances irradiation-induced inhibition of
human lung cancer growth in vitro and in vivo. Oncol Lett.
4:135–140. 2012.PubMed/NCBI
|
|
27
|
Cho HM, Jeon HS, Lee SY, et al:
microRNA-101 inhibits lung cancer invasion through the regulation
of enhancer of zeste homolog 2. Exp Ther Med. 2:963–967. 2011.
|
|
28
|
Kikuchi J, Takashina T, Kinoshita I, et
al: Epigenetic therapy with 3-deazaneplanocin A, an inhibitor of
the histone methyltransferase EZH2, inhibits growth of non-small
cell lung cancer cells. Lung Cancer. 78:138–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marchesi I, Fiorentino FP, Rizzolio F,
Giordano A and Bagella L: The ablation of EZH2 uncovers its crucial
role in rhabdomyosarcoma formation. Cell Cycle. 11:3828–3836. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou J, Roh JW, Bandyopadhyay S, et al:
Overexpression of enhancer of zeste homolog 2 (EZH2) and focal
adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol
Oncol. 128:344–348. 2013. View Article : Google Scholar
|
|
31
|
Wan L, Li X, Shen H and Bai X:
Quantitative analysis of EZH2 expression and its correlations with
lung cancer patients’ clinical pathological characteristics. Clin
Transl Oncol. 15:132–138. 2013. View Article : Google Scholar
|
|
32
|
Zhang R, Wang R, Chang H, et al:
Downregulation of Ezh2 expression by RNA interference induces cell
cycle arrest in the G0/G1 phase and apoptosis in U87 human glioma
cells. Oncol Rep. 28:2278–2284. 2012.PubMed/NCBI
|
|
33
|
Wagener N, Macher-Goeppinger S, Pritsch M,
et al: Enhancer of zeste homolog 2 (EZH2) expression is an
independent prognostic factor in renal cell carcinoma. BMC Cancer.
10:5242010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wagener N, Holland D, Bulkescher J, et al:
The enhancer of zeste homolog 2 gene contributes to cell
proliferation and apoptosis resistance in renal cell carcinoma
cells. Int J Cancer. 123:1545–1550. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hinz S, Weikert S, Magheli A, et al:
Expression profile of the polycomb group protein enhancer of Zeste
homologue 2 and its prognostic relevance in renal cell carcinoma. J
Urol. 182:2920–2925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakurai T, Bilim VN, Ugolkov AV, et al:
The enhancer of zeste homolog 2 (EZH2), a potential therapeutic
target, is regulated by miR-101 in renal cancer cells. Biochem
Biophys Res Commun. 422:607–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li K, Chen MK, Situ J, et al: Role of
co-expression of c-Myc, EZH2 and p27 in prognosis of prostate
cancer patients after surgery. Chin Med J (Engl). 126:82–87.
2013.
|
|
38
|
Yang YA and Yu J: EZH2, an epigenetic
driver of prostate cancer. Protein Cell. 4:331–341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Takeshita F, Minakuchi Y, Nagahara S, et
al: Efficient delivery of small interfering RNA to bone-metastatic
tumors by using atelocollagen in vivo. Proc Natl Acad Sci USA.
102:12177–12182. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Voorzanger-Rousselot N, Juillet F, Mareau
E, Zimmermann J, Kalebic T and Garnero P: Association of 12 serum
biochemical markers of angiogenesis, tumour invasion and bone
turnover with bone metastases from breast cancer: a crossectional
and longitudinal evaluation. Br J Cancer. 95:506–514. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Andela VB, Gordon AH, Zotalis G, et al:
NFkappaB: a pivotal transcription factor in prostate cancer
metastasis to bone. Clin Orthop Relat Res. (415 Suppl): S75–S85.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lhoták S, Elavathil LJ, Vukmirović-Popović
S, Duivenvoorden WC, Tozer RG and Singh G: Immunolocalization of
matrix metalloproteinases and their inhibitors in clinical
specimens of bone metastasis from breast carcinoma. Clin Exp
Metastasis. 18:463–470. 2000. View Article : Google Scholar
|
|
43
|
Yoneda T, Sasaki A, Dunstan C, et al:
Inhibition of osteolytic bone metastasis of breast cancer by
combined treatment with the bisphosphonate ibandronate and tissue
inhibitor of the matrix metalloproteinase-2. J Clin Invest.
99:2509–2517. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsu CH, Peng KL, Kang ML, et al: TET1
suppresses cancer invasion by activating the tissue inhibitors of
metalloproteinases. Cell Rep. 2:568–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shin YJ and Kim JH: The role of EZH2 in
the regulation of the activity of matrix metalloproteinases in
prostate cancer cells. PloS One. 7:e303932012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fornari F, Milazzo M, Chieco P, et al: In
hepatocellular carcinoma miR-519d is upregulated by p53 and DNA
hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J
Pathol. 227:275–285. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Matsusaka K, Kaneda A, Nagae G, et al:
Classification of Epstein-Barr virus-positive gastric cancers by
definition of DNA methylation epigenotypes. Cancer Res.
71:7187–7197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hervouet E, Vallette FM and Cartron PF:
Dnmt3/transcription factor interactions as crucial players in
targeted DNA methylation. Epigenetics. 4:487–499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Martinez R, Schackert G and Esteller M:
Hypermethylation of the proapoptotic gene TMS1/ASC: prognostic
importance in glioblastoma multiforme. J Neurooncol. 82:133–139.
2007. View Article : Google Scholar
|
|
50
|
Chu LC, Eberhart CG, Grossman SA and
Herman JG: Epigenetic silencing of multiple genes in primary CNS
lymphoma. Int J Cancer. 119:2487–2491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Crea F, Fornaro L, Bocci G, et al: EZH2
inhibition: targeting the crossroad of tumor invasion and
angiogenesis. Cancer Metastasis Rev. 31:753–761. 2012. View Article : Google Scholar : PubMed/NCBI
|