Introduction
End-stage renal disease (ESRD) is the terminal stage
of irreversible chronic kidney disease (CKD), leading to a
devastating condition that necessitates patient dependency on
life-long treatments including dialysis, or renal transplantation
(1,2). The number of patients with CKD has
been reported to be increasing at an annual rate of ~7%, and the
human and economic impacts of ESRD to family members, the medical
community and society is vast (3,4). The
majority of patients with CKD are diagnosed prior to reaching
end-stage renal failure; however, there are no effective treatment
options that can effectively halt the progressive decline in renal
function. An improved understanding of the pathophysiology of CKD
may lead to the development of novel treatment options to stabilize
renal function.
The pathogenesis of CKD is characterized by a
progressive loss of kidney function, and the relentless
accumulation and deposition of extracellular matrix (ECM),
resulting in widespread renal fibrosis (5,6).
Histologically, the risk for ESRD and the rate of excretory
function loss is positively correlated with the degree of tubular
atrophy and interstitial fibrosis (7). Chronic tubulointerstitial hypoxia,
one of numerous possible initial insults, has been shown to trigger
tubulointerstitial pathology (8,9).
Previous research has demonstrated that hypoxia has a critical role
in tubulointerstitial injury prior to microvasculature structural
damage (8), suggesting pathogenic
implications of hypoxia in the early stages of kidney disease. An
important mechanism underlying the development of renal
interstitial fibrosis is the epithelial-to-mesenchymal transition
(EMT), a process in which the tubular epithelial cells lose their
epithelial phenotype and acquire new phenotypical features,
characteristic of mesenchymal cells, including the expression of
mesenchymal markers and the acquisition of mobility (10). It has been observed, that under
hypoxic conditions, myofibroblasts, which are transdifferentiated
from tubular cells, are capable of migrating to the tubular
interstitium, leading to renal fibrogenesis (11). Previous studies have demonstrated
the importance of transcriptional responses to hypoxia, including
the hypoxia-inducible factor (HIF-1), in mediating EMT (12,13);
however, the exact mechanisms underlying EMT induced by hypoxia
remain largely unknown.
Adrenomedullin (ADM) is a vascular-derived peptide
that was initially purified from a phaeochromocytoma (14) but has also been shown to be
expressed in other body tissues, suggesting that it possesses
numerous biological functions. In addition to being produced by
both stromal (i.e., endothelial, vascular smooth muscle, myocardial
and central nervous system) and tumor cells, as an
autocrine/paracrine factor, ADM has been shown to be produced
during the differentiation of macrophages in response to
pro-inflammatory stimuli and hypoxia (15,16).
ADM is also implicated in renal fibrogenesis, and the expression of
ADM and its receptor, in rat kidney interstitial cells, has been
shown to inhibit cell proliferation and mRNA expression levels of
ECM proteins (17). Nagae et
al (18) showed that
overexpression of exogenous ADM, in the ureteral-obstructed kidney,
prevented tubulointerstitial fibrosis and cell proliferation. ADM
signaling, however, has not yet been extensively explored during
EMT, particularly with respect to its induction in response to
hypoxia.
EMT is a process aggravated by chronic
tubulointerstitial hypoxia, a condition that is believed to be
associated with ADM production. Therefore, the aim of the present
study was to verify whether ADM signaling was activated during EMT,
and whether regulation of ADM could affect the development of EMT
in human proximal tubular epithelial cells.
Materials and methods
Antibodies and reagents
A primary anti-ADM antibody was obtained from Abcam
(Cambridge, MA, USA), and the anti-HIF-1α, anti-E-cadherin,
anti-tight junction protein-1 (ZO-1), anti-vimentin and
anti-α-smooth muscle actin (α-SMA) antibodies were purchased from
Santa Cruz Biotechnology Inc., (Dallas, TX, USA). The antibodies
against total extracellular signal-regulated kinase (ERK),
phospho-ERK (Thr202/Tyr204), and β-actin were purchased from Cell
Signaling Technology Inc., (Danvers, MA, USA). Human ADM and ADM
22–52 (an ADM receptor antagonist), and apigenin (an activator of
ERK) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture and treatment
The HK-2 and HKC human proximal tubular epithelial
cells (American Type Culture Collection, Manassas, VA, USA) were
cultured in Dulbecco’s modified Eagle’s medium/Ham’s F12
(Invitrogen Life Technologies, Carlsbad, CA, USA), supplemented
with 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, 36
ng/ml hydrocortisone, 4 pg/ml tri-iodo-L-thyronine, 10 ng/ml
epidermal growth factor, 50 U/ml penicillin, 50 μg/ml streptomycin,
and 2 mM glutamine (all Invitrogen Life Technologies, Carlsbad, CA,
USA). The HK-2 and HKC cells from passages 6–16 were used in the
following experiments. The cells were starved of serum overnight,
prior to experimentation, to avoid the effects of serum-induced
signaling. The cells were maintained at 37°C in a humidified
atmosphere, under normoxic (21% O2) or hypoxic (1%
O2) conditions for 12, 24 or 48 h with 95% N2
and 5% CO2. In some experiments, the cells were exposed
to normoxia or hypoxia in the absence or presence of the following
compounds: 0.1 or 1.0 μM ADM; 5 nM ADM22-52; and 100 μM
apigenin.
Gene silencing in the HK-2 cell line
using ADM-small hairpin (sh)RNA
The HK-2 cells from the sixth passage were seeded in
6-well plates (1×105 cells/well) and allowed to adhere
for 24 h, prior to transfection. The commercial ADM shRNA plasmid
was purchased from Santa Cruz (Santa Cruz Biotechnology, Dallas,
Texas, USA). The plasmid is based on a pool of 3 target-specific
lentiviral vector plasmids, each encoding 19–25 nt (plus hairpin)
shRNAs designed to knockdown human ADM gene expression. Each
plasmid contains a puromycin resistance gene and a copGFP gene for
the selection of cells stably expressing shRNA. The shRNAs were
transfected using specifically designed reagent for shRNA
transfection, including Santa Cruz Biotechnology’s shRNA Plasmid
Transfection Reagent (sc-108061, 0.2 ml) and shRNA Plasmid
Transfection Medium (sc-108062, 20 ml) according to manufacturer’s
protocol. Then the cells were incubated for 8 h at 37°C in a 95%
air/5% CO2 incubator. In addition, the cells were
transfected with a non-targeting negative-control shRNA (NC-shRNA)
(Santa Cruz Biotechnology, Dallas, Texas, USA) to monitor and
optimize transfection efficiency. At 72 h post-transfection,
puromycin (7.0 μg/ml) was added to the culture medium for selection
and further characterization. The transfection efficiency of the
HK-2 cells expressing ADM-shRNA (copGFP) was assessed using flow
cytometry. In brief, transfected cell were fixed in cold 70%
ethanol, washed twice and resuspended in cold phosphate-buffered
saline buffer. The cell suspensions were analyzed on an FC500/MPL
flow cytometer (Beckman Coulter, Brea, CA), using the FlowJo
software (Tree Star, Ashland, OR, USA). GFP was detected on the
FITC channel using a 488 nm laser, and the transfection efficiency
was no less than 85%.. The cells were cultured for 48 h following
transfection and then exposed to hypoxia for 12–48 h.
Cell lysis and western blot analysis
The treated HK-2 and HKC cells were harvested and
lysed for 30 min on ice using lysis buffer (50 mM Tris–HCl, pH 7.4;
1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA;
1 mM PMSF; 1 μg/ml each aprotinin, leupetin, and pepstatin; 1 mM
Na3VO4; 1 mM NaF). The samples were then
centrifuged at 14,000 × g for 10 min at 4°C, and the protein
concentrations of the cell lysates were determined using the
Bradford method. The proteins (50 μg/lane) were loaded onto 10%
SDS-containing polyacrylamide gels and were separated by SDS-PAGE,
prior to a transfer to polyvinylidene fluoride membranes
(Millipore, Bedford, MA, USA). The membranes were blocked with 5%
milk in Tris-buffered saline (TBS) with Tween® 20 (20 mM
Tris-HCl, 150 mM NaCl, and 0.1% Tween® 20) for 1 h at
room temperature. Subsequently, the membranes were incubated
overnight at 4°C with the primary antibodies, in blocking buffer
containing 5% milk, at the dilutions specified in the
manufacturer’s instructions. Following extensive washing in TBS,
the membranes were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz, Dallas,
Texas, USA), for 1 h at room temperature in 5% nonfat milk
dissolved in TBS. The membranes were visualized using a
chemiluminescent substrate and developed following exposure to
Kodak X-OMAT film (Kodak, Rochester, NY, USA). The immunoreactive
protein bands were quantified by densitometry using QuantityOne
Image Analysis Software (Bio-Rad Laboratories, Hercules, CA, USA)
and were normalized to the β-actin values.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from treated cells using the
RNAiso Plus kit (Takara Biotechnology Co., Ltd., Dalian, China). A
total of 1 μg RNA was used for first-strand cDNA synthesis using
the PrimeScript reverse trasncription kit reagents (Takara
Biotechnology Co., Ltd.). Quantification was performed using the
iCycler iQ™ Sequence Detection system (Bio-Rad, USA), and the SYBR
Green Premix kit (Takara Biotechnology Co., Ltd.) was used as the
fluorophore. The following oligonucleotides were used as primers:
ADM forward, 5′-ACTTGGCAGATCACTCTCTTAGCA-3′, and reverse,
5′-ATCAGGGCGACGGAAACC-3′; β-actin forward,
5′-ATCCTGCCAGTAGCATATGC-3′, and reverse,
5′-ACCGGGTTGGTTTTGATCTG-3′. For each primer pair, the annealing
temperature was optimized using a gradient PCR reaction. The
expression levels of each target mRNA, relative to the β-actin mRNA
expression levels, were calculated from the cycle threshold (Ct)
using the 2−ΔΔCt formula. For each sample, the
experiment was repeated in triplicate, using RNA from three
separate culture wells.
Statistical analysis
All experimental data are expressed as the means ±
standard error of the mean. The significant differences were
evaluated using a one- or two-way analysis of variance, followed by
multiple comparisons tests. Statistical analyses were performed
using the commercially available software package SPSS version 13
(SPSS, Inc., Chicago, IL, USA). A P<0.05 was considered to
indicate a statistically significant difference.
Results
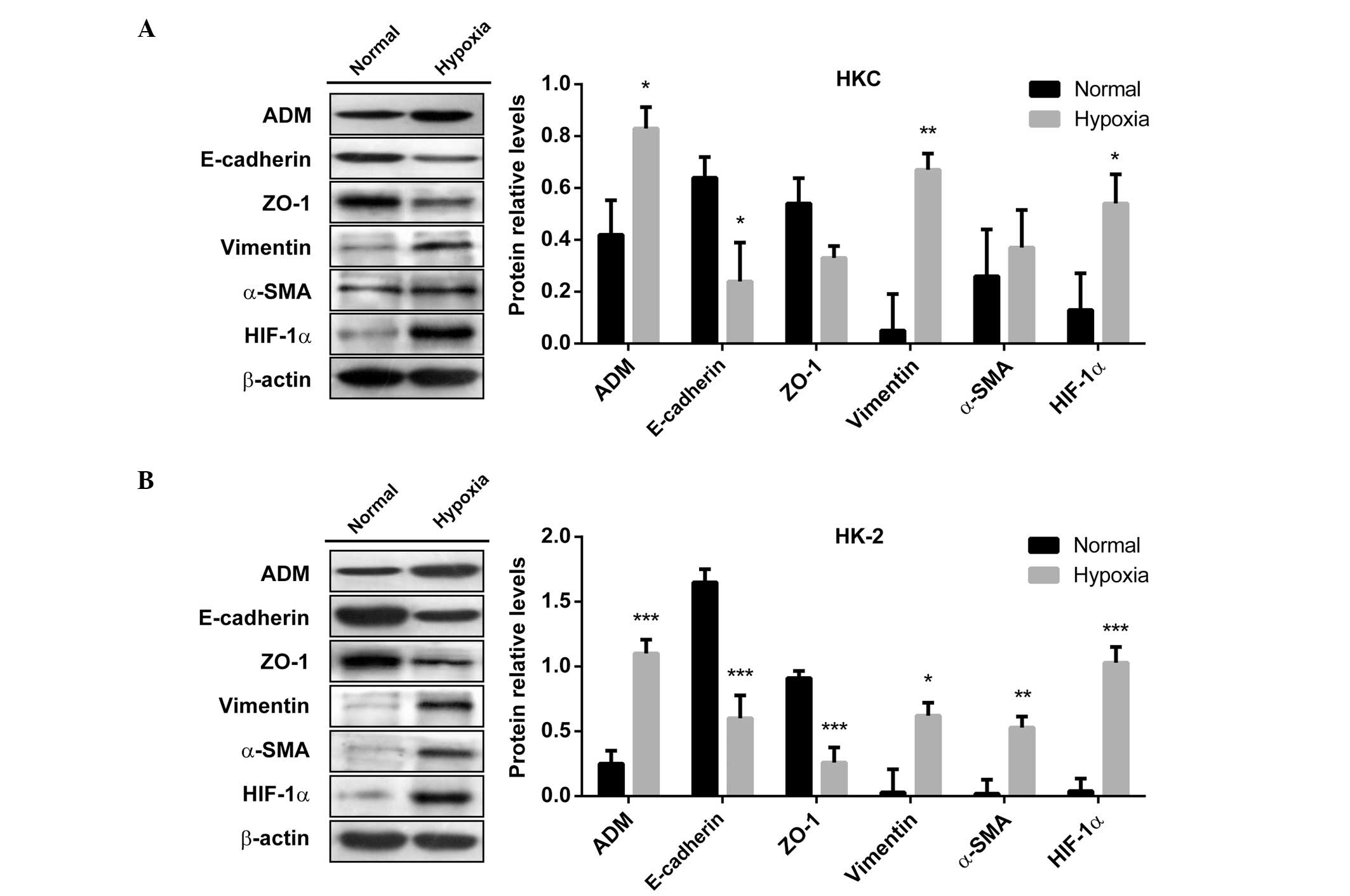
Hypoxia induces EMT in human tubular
epithelial cells and increases the expression of ADM at the
transcriptional level
The HK-2 and HKC cells, cultured under chronic
hypoxia for 48 h, exhibited a downregulation in the expression
levels of the epithelial markers, E-cadherin and ZO-1, and an
upregulation in the expression levels of the mesenchymal markers,
vimentin and α-SMA, as compared with the cells cultured under
normoxia (Fig. 1). The expression
levels of the hypoxia-regulated molecule HIF-1α were also
determined to confirm the hypoxic response. These results suggest
that the epithelial cells underwent EMT, with a more
fibroblast-like cell type, and indicate that hypoxia may promote
EMT, one of the major mechanisms that mediates renal fibrosis.
Furthermore, ADM expression levels were shown to be increased in
HKC and HK-2 cells under hypoxic conditions, and was negatively
correlated to the expression levels of E-cadherin and ZO-1.
ADM mRNA expression was evaluated in the HK-2 and
HKC cells, by qPCR. The mRNA expression of β-actin was used as an
internal control, and the ratio of ADM/β-actin was used to
determine the relative mRNA expression levels of ADM. As shown in
Fig. 2, the relative expression
levels of ADM mRNA, in HKC and HK-2 cells, were significantly
affected by both the time the cells were exposed to hypoxia
(Ftime (3, 24) = 52.43, P<0.0001) and the type of
cell line (Fcell lines (1, 24) = 30.90, P<0.0001);
these two factors also showed significant interactions (Ftime
× cell lines (3, 24) = 8.57, P<0.0005). A post hoc
analysis showed that the mRNA expression levels of ADM
significantly increased in both HKC and HK-2 cells, as a function
of the level of hypoxia. Between the two cell lines, the HK-2 cells
appeared more sensitive to hypoxic stress, as compared with the HKC
cells (P<0.0001, at 48 h of hypoxia). These results suggest that
expression of ADM was significantly enhanced in a time-dependent
manner in human tubular cell lines under hypoxic conditions.
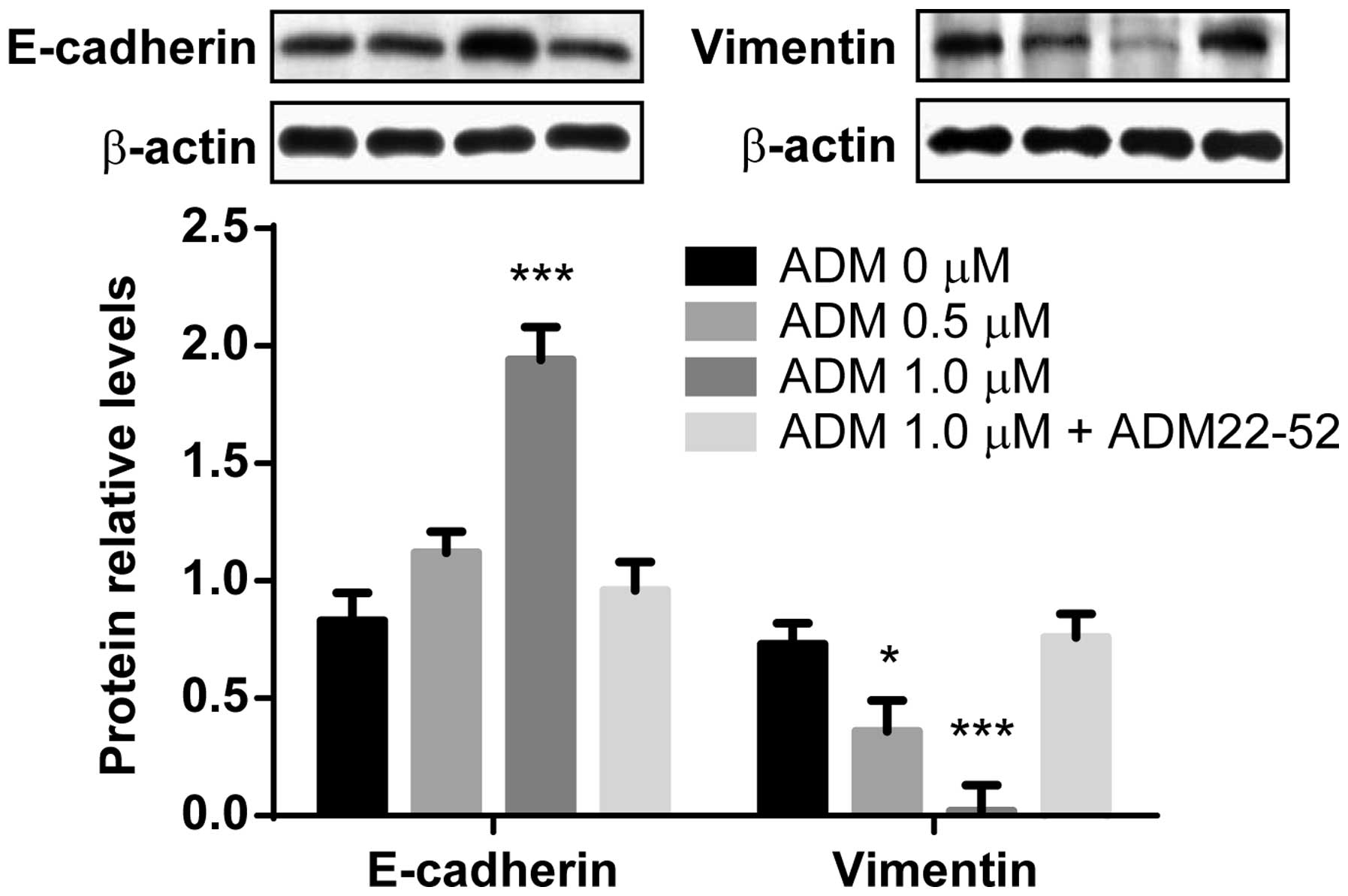
Hypoxia-induced EMT is prevented by
exogenous administration of ADM
ADM was shown to be upregulated in
hypoxia-stimulated HK-2 and HKC cells, therefore it was assessed as
to whether exogenous ADM was capable of ameliorating
hypoxia-induced EMT. HK-2 cells were exposed to hypoxia for 48 h in
the presence of 0, 0.1 or 1.0 μM ADM. As shown in Fig. 3, the expression levels of
E-cadherin were significantly increased in response to 1.0 μM ADM,
as compared with the control cells not treated with ADM
(P<0.0001); however, this upregulation did not occur when the
ADM peptide inhibitor ADM 22–52 was co-administered with 1.0 μM
ADM. Furthermore, the protein expression levels of vimentin were
significantly decreased by ADM, in a dose-dependent manner
(P<0.05 and P<0.0001, respectively); however, this
downregulation did not occur when ADM was co-administered with ADM
22–52. These results suggest that exogenous ADM administration
ameliorated hypoxia-induced EMT.
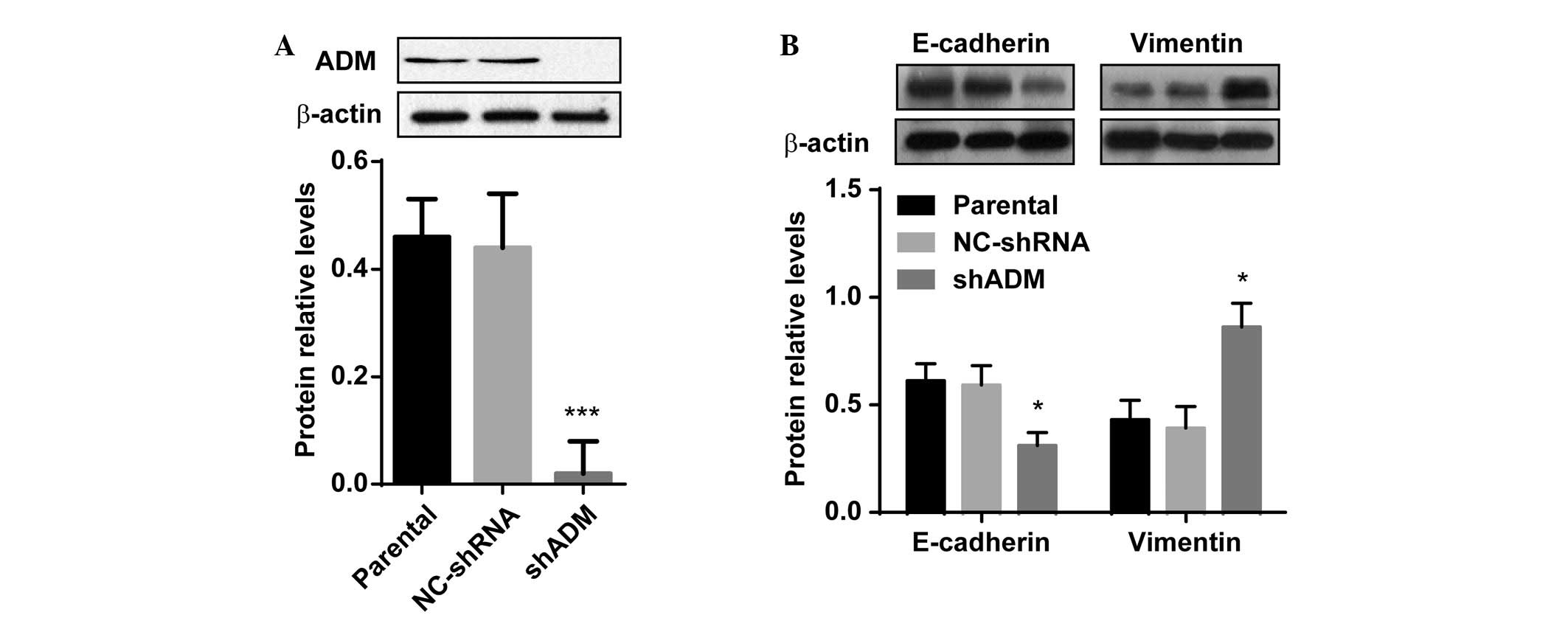
ADM knockdown accelerates EMT in human
tubular epithelial cells
To further elucidate the effects of ADM on
hypoxia-induced EMT in human tubular epithelial cells, a stable
cell line was constructed, in which ADM expression was successfully
knocked down. Because HK-2 cells appeared to have increased
sensitivity to hypoxic stress, lentiviral ADM-shRNA was transfected
into the HK-2 cells. Following the transfection, western blot
analysis revealed that ADM protein expression levels were markedly
decreased in the HK-2 cells, as compared with the parental cells
(Fig. 4A, P<0.01). The
expression levels of ADM were not different between the
vector-control and untransfected parental HK-2 cells. Following 48
h exposure to hypoxia, the HK-2 cells that stably expressed
ADM-shRNA exhibited significantly lower expression levels of
E-cadherin, as compared with the untransfected cells (Fig. 4B, P<0.05). There was no
difference in the vimentin protein expression levels between the
parental and vector-control cells, under hypoxic conditions;
however, vimentin expression levels were markedly upregulated in
the hypoxic shRNA-ADM-transfected cells (P<0.05). These results
indicate that ADM knockdown may accelerate EMT in tubular
epithelial cells, and that this effect may be partly mediated by
hypoxia-induced EMT processes.
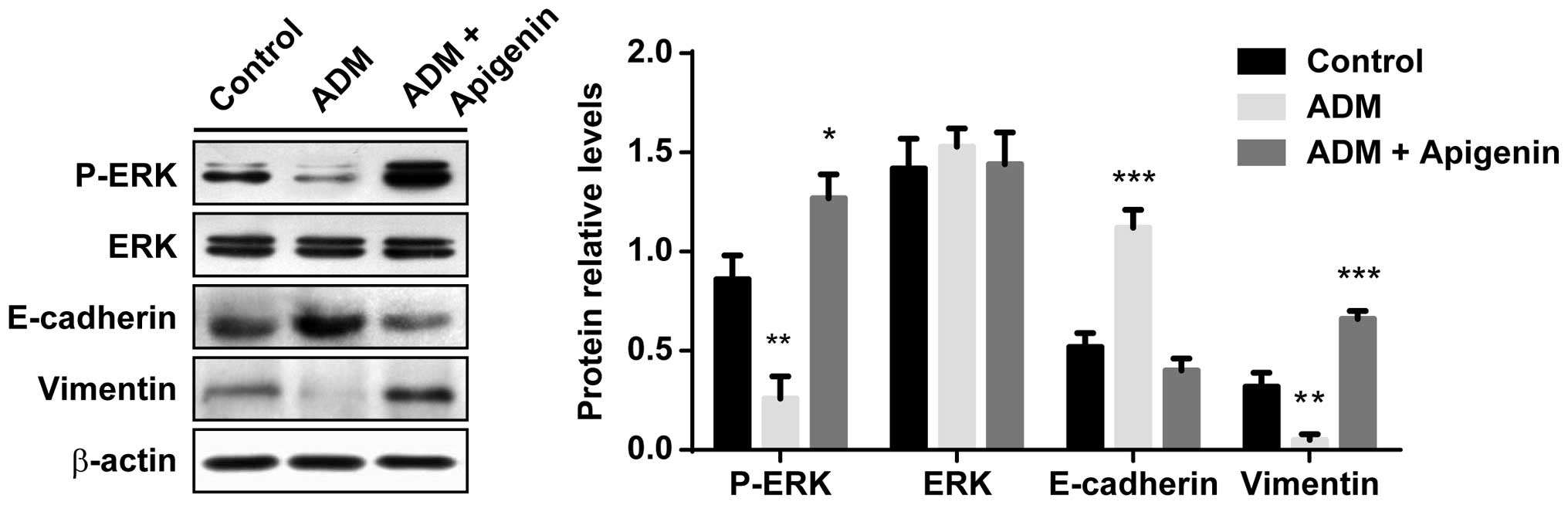
Effects of hypoxia-induced ADM on
ameliorating EMT are regulated by the inhibition of the activation
of ERK
It was investigated whether the upregulated
expression of ADM ameliorated hypoxia-induced EMT, through
inhibiting the activation of ERK. The HK-2 cells were exposed to
hypoxia for 48 h in the presence of 1.0 μM ADM, with or without
apigenin (100 μM). As shown in Fig.
5, the phosphorylation of ERK was significantly decreased in
the ADM-treated cells, as compared with the control cells
(P<0.01). However, when the HK-2 cells were treated with
exogenous ADM in combination with apigenin, the levels of
phospho-ERK were significantly elevated (P<0.05). Conversely,
total ERK expression levels were not affected by either ADM nor
apigenin treatment (F (2, 15) = 0.1833, P=0.83).
E-cadherin expression levels were significantly increased by 1.0 μM
ADM (P<0.0001), as compared with the control cells not treated
with ADM; however, 100 μM apigenin blocked the ADM-induced
upregulation of E-cadherin. The expression levels of vimentin were
significantly decreased by ADM (P<0.01); however, this
downregulation was completely prevented by treatment with ADM in
combination with apigenin (P<0.0001). These results suggest that
the effects of hypoxia-induced ADM on the amelioration of EMT may
be regulated by inhibition of the activation of ERK, and this
effect can be effectively prevented by the administration of
activators of ERK.
Discussion
It has previously been reported that a hypoxic
environment is associated with the induction of EMT in renal
fibrogenesis (19). A previous
study demonstrated that overexpression of exogenous ADM in the
ureteral-obstructed kidney prevented tubulointerstitial fibrosis
and cell proliferation (18). In
the present study, ADM signaling was shown to be active in human
tubular epithelial cells, under hypoxic conditions. Overexpression
of ADM significantly increased the expression levels of the
epithelial markers and decreased the expression levels of the
mesenchymal markers; whereas the knockdown of ADM expression
suppressed the epithelial phenotype. ADM protected the HK-2 and HKC
cells from EMT, via the inhibition of the activation of ERK, and
the ADM-mediated protection was prevented by administration of an
ADM peptide inhibitor. These data provide an explanation for the
protective effects of ADM on renal fibrogenesis, through the
suppression of EMT.
Chronic hypoxia is a principal contributing factor
associated with end-stage renal failure. Previous experimental
evidence has shown that the proximal tubular epithelial cells adapt
to hypoxic environments through the transcriptional activation of
HIF-1 (20). This activation leads
to alterations in the composition of the secreted proteins, which
are released primarily into the basolateral compartment of the
kidney. These secreted proteins favor the recruitment of
inflammatory cells into the renal interstitium and contribute to
fibrogenic reactions (21).
Furthermore, it has been suggested that in certain circumstances,
tubular expression of antifibrotic cytokines protects the proximal
tubular epithelial cells from apoptosis, and reduces the
interstitial collagen accumulation (22). The present study demonstrated the
hypoxia-mediated induction of ADM expression in human tubular
epithelial cells.
It has previously been observed that hypoxia can
increase the expression levels of both ADM mRNA and secreted
protein, in various of tissues (23,24).
These increases are at least partly mediated by the activation of
HIF-1 (25). Gene expression
profiling and a systems biology analysis previously identified the
activation of intracellular vascular endothelial growth factor
signaling and hypoxia-response pathways in patients with
progressive proteinuric glomerulopathiesl; these pathways are
associated with the upregulation of HIF-1α and numerous HIF target
genes (26). In addition, the
expression of ADM and its receptor has been shown in renal tubular
cell lines (27). The results of
the present study suggested that ADM expression is higher in human
proximal tubular epithelial cells under hypoxia, as compared with
the cells cultured under normoxia, and the increased expression of
ADM may protect the kidney against ischemic injury. Shah et
al (28) previously reported
that ADM, combined with ADM binding protein-1, can prevent and/or
minimize damage in a rat model of renal ischemia and reperfusion
injury.
Previous studies have shown that EMT, a process by
which differentiated epithelial cells undergo a phenotypic
conversion that results in the production of matrix-producing
fibroblasts and myofibroblasts, has a critical role in the
development of renal interstitial fibrosis. Chronic hypoxia may
affect the development of renal fibrogenesis in CKD, through EMT.
The morphology of tubular epithelial cells and interstitial
myofibroblasts are markedly different, and the phenotypically
altered cells are located in tissue compartments that are separated
by the tubular basement membranes within the kidney. Gene
expression profiling, using microarray technology, has identified
numerous genes with altered expression patterns during tubular EMT
(29,30). Previously, Sun et al
(13) showed that under hypoxic
conditions, HIF-1α induced the overexpression of Twist, a basic
helix-loop-helix transcription factor, that lead to the promotion
of EMT in human tubular epithelial cells. The results of the
present study suggested that overexpression of ADM protected
tubular epithelial cells from converting to interstitial
fibroblasts, as indicated by ADM-induced increased expression
levels of the epithelial markers (E-cadherin and ZO-1) and
decreased expression levels of the mesenchymal markers vimentin and
α-SMA, during hypoxia. E-cadherin is associated with the actin
filament network through catenins, a family of intracellular
adhesive junction proteins (31).
The importance of E-cadherin in the development of normal
epithelium, has been established using knockout mice, in which its
role in embryonic epitheliogenesis during kidney development was
demonstrated (32). Furthermore,
the expression levels of ZO-1, a component of the tight junctions
between epithelial cells, were found to be suppressed during EMT in
previous studies (33,34). Such alterations may consequently
result in the destabilization of the structural integrity of the
renal epithelium, and induce the cells to lose their epithelial
adhesion properties and polarity. The reorganization of the actin
cytoskeleton and the induction of α-SMA may provide a structural
foundation, not only for defining the morphology of the transformed
cells, but also to explain their ability to migrate, invade, and
acquire the capacity for contractility. In addition to the actin
cytoskeleton, cytoplasmic intermediate filaments also undergo
conversion from an epithelial type of cytokeratin to the
mesenchymal vimentin (34–36).
Tubular epithelial cells can acquire a mesenchymal
phenotype, through the process of EMT. This allows for an enhanced
migratory capacity, enabling the cells to shift from the renal
tubular microenvironment, into the interstitium and avoid
undergoing apoptosis. Previous findings have suggested that EMT may
be reversible in tubular epithelial cells; however, this is
dependent on the surviving cells to repopulate the injured tubules
with functional epithelia (37).
The major regulators of renal epithelial cell plasticity are bone
morphogenic protein-7 (BMP-7) and transforming growth factor
(TGF)-β1. TGF-β1 is known to be associated with the induction of
EMT in renal tubular epithelial cells (10), whereas BMP-7 has been shown to
oppose TGF-β1-induced EMT and reverse chronic renal injury
(33). Previous studies have
suggested that numerous mitogenic signaling components; such as,
protein kinase C, mitogen activated protein kinase kinase and
oxidant, are associated with the regulation of TGF-β1 (38), and that activation of ERK is
involved in renal fibrosis following kidney damage (39). In the present study, ERK activity
was shown to be markedly inhibited, following exogenous
administration of ADM, in proximal tubular epithelial cells. The
effects of ADM on EMT were effectively prevented when an activator
of ERK, apigenin, was co-administered with ADM. These observations
imply that ADM may suppress EMT through the inhibition of ERK
signaling in human tubular epithelial cells.
In conclusion, the present study demonstrated that
ADM signaling is induced by hypoxia and is functionally active in
human proximal tubular epithelial cells. Furthermore, ADM was shown
to inhibit hypoxia-induced EMT, which has a critical role in the
promotion of renal fibrogenesis. Therefore, the selective
upregulation of ADM signaling may be therapeutically efficacious in
patients with CKD.
References
|
1
|
Klahr S and Morrissey J: Progression of
chronic renal disease. Am J Kidney Dis. 41:S3–S7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Owen WF Jr: Patterns of care for patients
with chronic kidney disease in the United States: dying for
improvement. J Am Soc Nephrol. 14:S76–S80. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coresh J, Selvin E, Stevens LA, et al:
Prevalence of chronic kidney disease in the United States. JAMA.
298:2038–2047. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Centers for Disease Control and
Prevention. Prevalence of chronic kidney disease and associated
risk factors - United States, 1999–2004. MMWR Morb Mortal Wkly Rep.
56:161–165. 2007.
|
|
5
|
Liu Y: Renal fibrosis: new insights into
the pathogenesis and therapeutics. Kidney Int. 69:213–217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strutz F and Zeisberg M: Renal fibroblasts
and myofibroblasts in chronic kidney disease. J Am Soc Nephrol.
17:2992–2998. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nath KA: Tubulointerstitial changes as a
major determinant in the progression of renal damage. Am J Kidney
Dis. 20:1–17. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nangaku M: Chronic hypoxia and
tubulointerstitial injury: a final common pathway to end-stage
renal failure. J Am Soc Nephrol. 17:17–25. 2006. View Article : Google Scholar
|
|
9
|
Nangaku M and Eckardt KU: Hypoxia and the
HIF system in kidney disease. J Mol Med (Berl). 85:1325–1330. 2007.
View Article : Google Scholar
|
|
10
|
Zeisberg M and Kalluri R: The role of
epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med
(Berl). 82:175–181. 2004. View Article : Google Scholar
|
|
11
|
Manotham K, Tanaka T, Matsumoto M, et al:
Transdifferentiation of cultured tubular cells induced by hypoxia.
Kidney Int. 65:871–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Higgins DF, Kimura K, Bernhardt WM, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesenchymal transition. J Clin Invest. 117:3810–3820.
2007.PubMed/NCBI
|
|
13
|
Sun S, Ning X, Zhang Y, et al:
Hypoxia-inducible factor-1alpha induces Twist expression in tubular
epithelial cells subjected to hypoxia, leading to
epithelial-to-mesenchymal transition. Kidney Int. 75:1278–1287.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kitamura K, Kangawa K, Kawamoto M, et al:
Adrenomedullin: a novel hypotensive peptide isolated from human
pheochromocytoma. Biochem Biophys Res Commun. 192:553–560. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinson JP, Kapas S and Smith DM:
Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev.
21:138–167. 2000.PubMed/NCBI
|
|
16
|
Chen L, Qiu JH, Zhang LL and Luo XD:
Adrenomedullin promotes human endothelial cell proliferation via
HIF-1α. Mol Cell Biochem. 365:263–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eto Y, Shimosawa T, Nitta K, Nihei H and
Maruyama N: Interaction between adrenomedullin and angiotensin II
in DNA synthesis and extracellular matrix accumulation in cultured
rat kidney interstitial cells. Clin Exp Nephrol. 6:7–12. 2002.
View Article : Google Scholar
|
|
18
|
Nagae T, Mori K, Mukoyama M, et al:
Adrenomedullin inhibits connective tissue growth factor expression,
extracellular signal-regulated kinase activation and renal
fibrosis. Kidney Int. 74:70–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Norman JT, Clark IM and Garcia PL: Hypoxia
promotes fibrogenesis in human renal fibroblasts. Kidney Int.
58:2351–2366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leonard MO, Cottell DC, Godson C, Brady HR
and Taylor CT: The role of HIF-1 alpha in transcriptional
regulation of the proximal tubular epithelial cell response to
hypoxia. J Biol Chem. 278:40296–40304. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rudnicki M, Eder S, Perco P, et al: Gene
expression profiles of human proximal tubular epithelial cells in
proteinuric nephropathies. Kidney Int. 71:325–335. 2007. View Article : Google Scholar
|
|
22
|
Wang S, de Caestecker M, Kopp J, Mitu G,
Lapage J and Hirschberg R: Renal bone morphogenetic protein-7
protects against diabetic nephropathy. J Am Soc Nephrol.
17:2504–2512. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ogita T, Hashimoto E, Yamasaki M, et al:
Hypoxic induction of adrenomedullin in cultured human umbilical
vein endothelial cells. J Hypertens. 19:603–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oehler MK, Norbury C, Hague S, Rees MC and
Bicknell R: Adrenomedullin inhibits hypoxic cell death by
upregulation of Bcl-2 in endometrial cancer cells: a possible
promotion mechanism for tumour growth. Oncogene. 20:2937–2945.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nguyen SV and Claycomb WC: Hypoxia
regulates the expression of the adrenomedullin and HIF-1 genes in
cultured HL-1 cardiomyocytes. Biochem Biophys Res Commun.
265:382–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rudnicki M, Perco P, Enrich J, et al:
Hypoxia response and VEGF-A expression in human proximal tubular
epithelial cells in stable and progressive renal disease. Lab
Invest. 89:337–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato K, Imai T, Iwashina M, Marumo F and
Hirata Y: Secretion of adrenomedullin by renal tubular cell lines.
Nephron. 78:9–14. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shah KG, Rajan D, Jacob A, et al:
Attenuation of renal ischemia and reperfusion injury by human
adrenomedullin and its binding protein. J Surg Res. 163:110–117.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zavadil J, Bitzer M, Liang D, et al:
Genetic programs of epithelial cell plasticity directed by
transforming growth factor-beta. Proc Natl Acad Sci USA.
98:6686–6691. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jechlinger M, Grunert S, Tamir IH, et al:
Expression profiling of epithelial plasticity in tumor progression.
Oncogene. 22:7155–7169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bajpai S, Feng Y, Wirtz D and Longmore GD:
β-Catenin serves as a clutch between low and high intercellular
E-cadherin bond strengths. Biophys J. 105:2289–2300. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Horster MF, Braun GS and Huber SM:
Embryonic renal epithelia: induction, nephrogenesis, and cell
differentiation. Physiol Rev. 79:1157–1191. 1999.PubMed/NCBI
|
|
33
|
Zeisberg M, Hanai J, Sugimoto H, et al:
BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal
transition and reverses chronic renal injury. Nat Med. 9:964–968.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okada H, Danoff TM, Kalluri R and Neilson
EG: Early role of Fsp1 in epithelial-mesenchymal transformation. Am
J Physiol. 273:F563–F574. 1997.PubMed/NCBI
|
|
35
|
Yang J and Liu Y: Dissection of key events
in tubular epithelial to myofibroblast transition and its
implications in renal interstitial fibrosis. Am J Pathol.
159:1465–1475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Strutz F, Zeisberg M, Ziyadeh FN, et al:
Role of basic fibroblast growth factor-2 in epithelial-mesenchymal
transformation. Kidney Int. 61:1714–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keller C, Kroening S, Zuehlke J, Kunath F,
Krueger B and Goppelt-Struebe M: Distinct mesenchymal alterations
in N-cadherin and E-cadherin positive primary renal epithelial
cells. PLoS One. 7(8): e435842012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Grewal JS, Mukhin YV, Garnovskaya MN,
Raymond JR and Greene EL: Serotonin 5-HT2A receptor induces
TGF-beta1 expression in mesangial cells via ERK: proliferative and
fibrotic signals. Am J Physiol. 276:F922–F930. 1999.PubMed/NCBI
|
|
39
|
Pat B, Yang T, Kong C, Watters D, Johnson
DW and Gobe G: Activation of ERK in renal fibrosis after unilateral
ureteral obstruction: modulation by antioxidants. Kidney In.
67:931–943. 2005. View Article : Google Scholar
|