Introduction
Glioblastoma is the most lethal type of primary
adult brain tumor. It has a poor prognosis as it diffusely
infiltrates other regions of the brain, which makes total surgical
resection difficult (1–3). Hence, the majority of patients
succumb to the disease within two years of diagnosis, in spite of
current multidisciplinary therapies, which include surgery,
radiotherapy and chemotherapy. The ultimate aim of chemotherapy is
the successful elimination of cancer cells through apoptosis
(4,5). Apoptosis is an important process that
controls the growth and development of organisms, and the
perturbation of apoptosis is considered to be a promising strategy
for the prevention and treatment of gliomas (6).
The endoplasmic reticulum (ER) is an important
organelle involved in the secretory pathway, which possesses a
vital role in membrane protein biosynthesis, protein folding and
protein modification (7,8). However, homeostasis in the lumen of
the ER is perturbed under a variety of toxic insults, including
hypoxia, failure of protein synthesis, protein misfolding, and
Ca2+ overload, which may result in a state of ER stress
(9,10). The unfolded protein response (UPR)
is induced to relieve this stress in eukaryotic cells in an attempt
to restore and maintain normal ER homeostasis and function
(11). If the UPR is unable to
correct the balance of ER stress, the cellular apoptotic machinery
may be triggered, ultimately leading to cell death. A major
proapoptotic transcription factor induced during ER stress is CHOP,
which is one of the main mediators of the apoptotic machinery
(12,13). ER stress has been shown to occur in
cancer cells in vivo and the ER stress response has been
hypothesized to be a potential pathway that can be
pharmacologically exploited to induce apoptosis in gliomas
(14–17).
Fatsioside A, a novel baccharane-type triterpenoid
glycoside, is extracted from the fruits of Fatsia japonica.
Fatsioside A exerts growth inhibition, cell cycle arrest, and
apoptosis in C6 rat glioma cells and U251 human glioma cells
(18,19). Hence, Fatsioside A is a promising
candidate for adjunctive therapy against human gliomas through
activation of cell death. However to the best of our knowledge, no
detailed studies have been reported on its action on U87MG glioma
cells to date and its exact mechanisms are yet to be
elucidated.
In the current study, the changes in growth of U87MG
cells in response to Fatsioside A treatment were evaluated and the
possible underlying molecular mechanisms were explored. The results
of this study may be useful in identifying potential candidates for
the targeted therapeutic intervention of glioma.
Materials and methods
Materials
Fatsioside A was obtained from the College of
Pharmaceutical Sciences at Zhejiang University (Hangzhou, China).
Fatsioside A was dissolved in 0.8 mM dimethylsulfoxide (Sigma, St.
Louis, MO, USA) and diluted with fresh medium to obtain the desired
concentration. Fetal bovine serum (FBS) and
3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Cell Signaling Technology, Inc. (Beverly, MA,
USA). Mouse monoclonal antibodies specific for eIF2α, p-eIF2α, PERK
and p-PERK, as well as rabbit polyclonal antibodies for cleaved
caspase-4, CHOP and β-actin were purchased from Sigma. Secondary
antibodies were obtained from Santa Cruz Biotechnology Inc., (Santa
Cruz, CA, USA).
Cell culture
U87MG cells were provided by the College of
Pharmaceutical Sciences at Zhejiang University (Hangzhou, China)
and were cultured at 37°C with 5% CO2 in Dulbecco’s
modified Eagle’s medium (Sigma), supplemented with 10% FBS, 100
U/ml penicillin and 100 U/ml streptomycin (Sigma). When cells
reached 80% confluence they were split into three plates.
Experiments were performed once cells reached 50–60%
confluence.
MTT assay
An MTT assay was employed to examine the effects of
Fatsioside A on the proliferation of glioma cells. Briefly, the
cells were seeded into 96-well plates at a density of
5×103 cells/well in 200 μl medium. Subsequently, the
cells in the wells were treated with various concentrations of
Fatsioside A and cultured for 24 or 48 h. At the end of the
culture, MTT solution (0.5 mg/ml in 20 μl phosphate-buffered
saline; PBS) was added to each well and incubated for 4 h at 37°C.
An ELISA instrument (Multiscan FC; Thermo Scientific, Waltham, MA,
USA) was used to measure the absorbance of each well at a
wavelength of 570 nm. Data were calculated from three independent
experiments.
Flow cytometry
Flow cytometry was used to analyze the effects of
Fatsioside A on the apoptosis of glioma cells. Briefly, U87MG cells
were incubated with Fatsioside A at various concentrations for 24
h, and then the cells were harvested and washed twice with ice-cold
PBS. Apoptotic cells were determined by Annexin V-fluorescein
isothiocyanate/propidium iodide (FITC/PI; Beyotime, Shanghai,
China) double staining in a binding buffer (Beyotime) using flow
cytometry (FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA).
All experiments were performed in triplicate.
Transfection with small interfering RNA
(siRNA)
Cells were seeded at a density of 2×105
cells per well into 6-well plates 24 h prior to transfection with
opti-MEM and Lipofectamine 2000 (Sigma). The cells were transfected
with CHOP-specific siRNA or nontargeting siRNA using Lipofectamine
2000 (Sigma) according to the manufacturer’s instructions. Four
hours later, the opti-MEM was completely replaced by medium and the
cells were incubated for an additional 48 h. Following the
incubation, the cells were treated with various concentrations of
Fatsioside A for 24 h and then they were used for subsequent
experiments. The siRNAs were purchased from GenePharma (Shanghai,
China) with the following sequences: CHOP-specific siRNA,
5′-AAGAACCA GCAGAGGUCACAA-3′; si-control, 5′-GAGCGCUAGACA
AUGAAG-3′.
Western blot analysis
Whole cellular protein was extracted from the U87MG
cells and prepared with lysis buffer for western blotting. Briefly,
the cells were lysed in radioimmunoprecipitation assay buffer
(Sigma) for 30 min on ice. Protein levels were quantified using the
Lowry method. Equivalent amounts of protein (30 μg per lane) were
separated by 5–12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and then transferred to nitrocellulose blotting
membranes (Cell Signaling Technology). The membranes were blocked
in Tris-buffered saline with Tween 20 (Cell Signaling Technology)
containing 5% non-fat dry milk (w/v) for 2 h. Subsequently, the
membranes were incubated overnight with primary antibodies at a
1:1,000 dilution at 4°C, followed by treatment with the
corresponding horseradish peroxidase-conjugated secondary
antibodies at room temperature for 2 h. Protein bands were
visualized using chemiluminescence detection (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation of three independent experiments. The difference between
two mean values was evaluated using the Student’s t-test and
P<0.05 was considered to indicate a statistically significant
difference. The statistical analyses were performed using the SPSS
software, version 19.0 (IBM, Armonk, New York, USA).
Results
Fatsioside A inhibits the growth of U87MG
cells and induces apoptosis
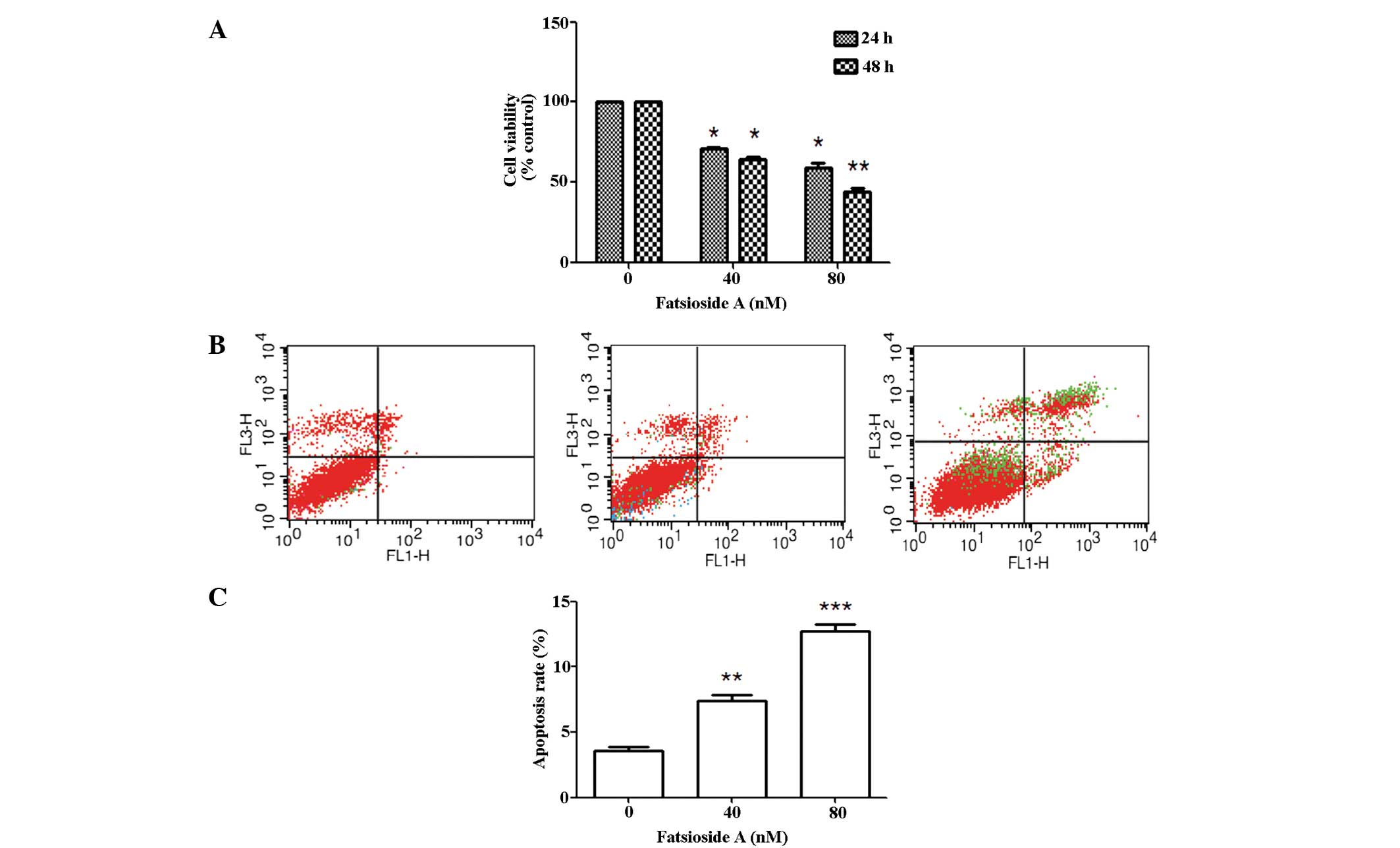
The MTT assay revealed that Fatsioside A treatment
markedly inhibited the growth of U87MG cells in a concentration-
dependent manner; in addition, a time-dependent inhibition in cell
viability was observed at 80 nM. Fatsioside A treatment that at 24
h. (Fig. 1A). Furthermore, Annexin
V-FITC/PI double staining was applied to examine whether the
Fatsioside A-induced reduction of the viability of glioma cells
occurred via apoptosis. The flow cytometry results showed a marked
concentration-dependent increase in the apoptotic rate of cells
treated with 40 nM (apoptosis rate, 6.7%) and 80 nM (apoptosis
rate, 11.9%) Fatsioside A compared with the control cells, which
were not treated with Fatsioside A (apoptosis rate, 3.4%) (Fig. 1B and C).
ER stress-associated apoptosis is
involved in Fatsioside A-induced cell death
A number of studies have demonstrated that ER
stress-associated apoptosis may be involved in cell death induced
by anti-tumor drugs (20,21). Therefore, to determine whether
Fatsioside A induces ER stress in U87MG cells, the cells were
exposed to various concentrations of Fatsioside A for 24 h, and
then western blot analysis was used to identify ER stress markers.
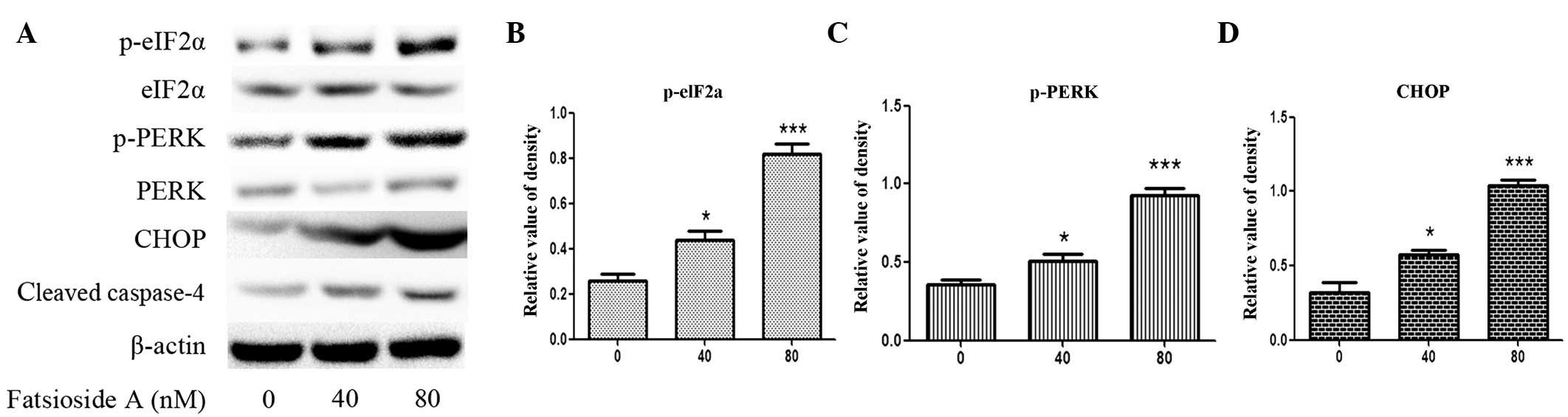
The results revealed that Fatsioside A significantly increases the
phosphorylation of eIF2α and PERK as well as the expression levels
of CHOP, all of which are ER stress markers, in a
concentration-dependent manner (Fig.
2A–D). A number of studies have demonstrated that caspase-4,
which is an ER-resident caspase, is activated during ER stress to
complete the execution of ER stress-induced apoptosis. Western blot
analysis showed that Fatsioside A markedly accelerated the cleavage
of caspase-4 in a concentration-dependent manner in U87MG cells
(Fig. 2A). Altogether, these
results indicate that ER stress-associated apoptosis is involved in
Fatsioside A-induced cell death in U87MG cells.
CHOP is a vital mediator in ER
stress-mediated apoptosis induced by Fatsioside A
Previous studies have demonstrated that CHOP, as a
major proapoptotic transcription factor induced during ER stress,
is one of the main mediators in the apoptotic machinery (22–24).
In order to confirm the role of ER stress in apoptosis induced by
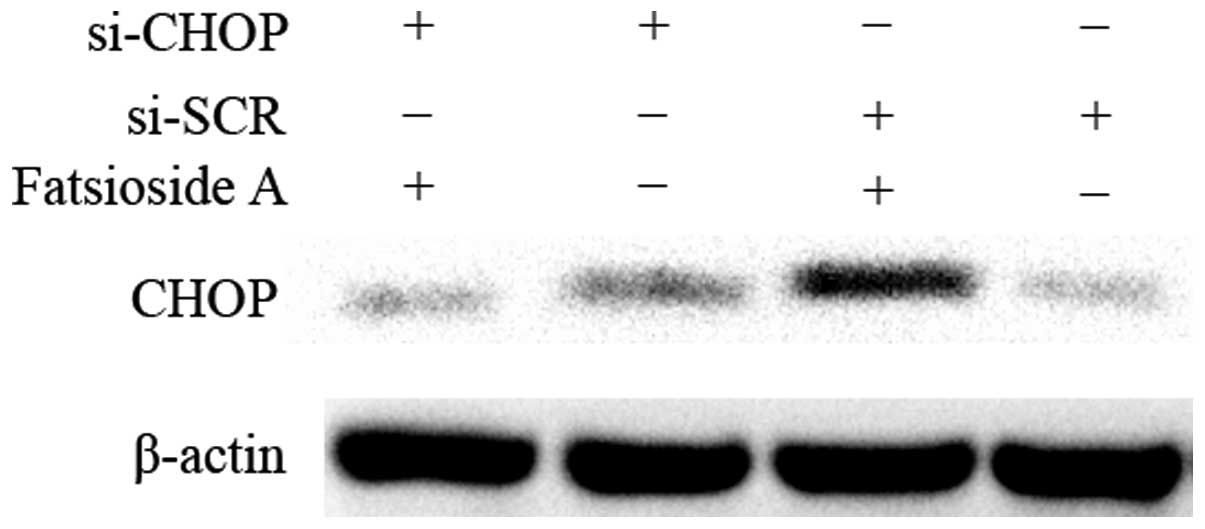
Fatsioside A in U87MG cells, CHOP-specific siRNA was applied to
downregulate the expression of CHOP. Western blot analysis revealed
that CHOP-specific siRNA successfully downregulated the increased
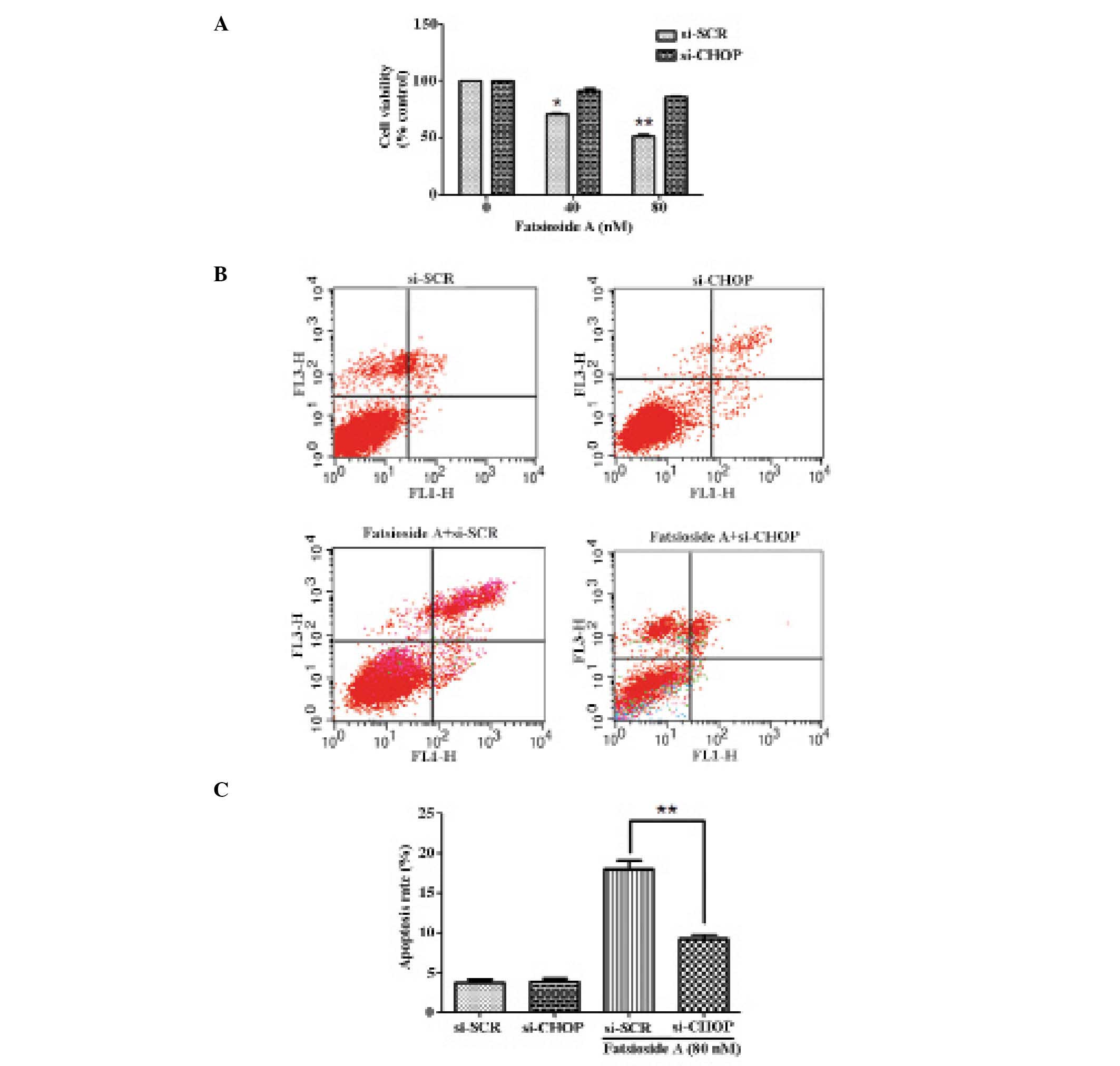
protein expression levels of CHOP induced by Fatsioside A (Fig. 3). The MTT assay showed that the
knockdown of CHOP significantly reduced the cytotoxicity of
Fatsioside A to U87MG cells (Fig.
4A). Following a 24-h exposure to 80 nM Fatsioside A, the
average apoptotic percentage of U87MG cells was reduced from 17.6%
in cells transfected with scrambled siRNA to 9.7% in cells
transfected with CHOP-specific siRNA (P<0.05) (Fig. 4B and C). These results indicate
that CHOP is a vital mediator in ER stress-mediated apoptosis
induced by Fatsioside A.
Discussion
Malignant gliomas are one of the most lethal types
of tumor in humans and current anti-glioma therapies fail to obtain
positive effects on these glioma cells (25,26).
As the glioblastomas are resistant to apoptosis, patients with
glioblastomas usually have a very poor prognosis (27–30).
Thus, the development of novel therapies to target the inherent
apoptosis-resistant phenotype of malignant gliomas is
necessary.
The results of the present study revealed that
Fatsioside A markedly inhibited the growth of U87MG cells and that
this effect is exerted by inducing ER stress-mediated apoptosis.
Furthermore, it was determined that CHOP is a vital mediator in the
ER stress-mediated apoptosis induced by Fatsioside A.
In agreement with previous studies (18), the current study revealed that
Fatsioside A significantly inhibits the proliferation of U87MG
cells in a concentration- and time-dependent manner. One of the
novel findings of the present study was the confirmation that
Fatsioside A-induced reduction of the viability of glioma cells
occurred via apoptosis, which was determined through the
application of Annexin V-FITC/PI double staining. Apoptosis is a
physiological phenomenon. The significance of apoptosis is to
remove senescent and dysfunctional cells, such as activated T cells
(31,32). Deregulation of apoptosis is
associated with the pathogenesis of a number of disorders,
including tumor cell growth (6,33,34).
Thus, one of the predominant strategies to treat tumors is the
induction of the apoptosis of tumor cells. The results of the
current study indicate that Fatsioside A disturbs the inherent
apoptosis-resistant ability of glioma cells.
Previous studies have demonstrated that ER stress is
an important factor during tumorigenesis in response to oxidative
stress, nutrient starvation and other metabolic dysregulations of
cells (35,36). Numerous current anticancer
therapies have been devised to induce ER stress in order to
stimulate its pro-apoptotic function or block its pro-survival
function. The results of the current study revealed that Fatsioside
A induced ER stress in glioma cells, including increased
phosphorylation of PERK and eIF2α, in addition to increased
expression levels of CHOP. Furthermore, the upregulation of CHOP
accelerates the cleavage of caspase-4, thus leading to cell
apoptosis (37). Downregulated
CHOP protects cells from the lethal consequences of ER stress
(38).
In conclusion, the current study determined that
treatment with siRNA-targeting CHOP afforded U87MG cells an
increased resistance to Fatsioside A-induced apoptosis. In summary,
the results of the current study demonstrate the ability of
Fatsioside A to activate the key proteins of ER stress in addition
to the ER-associated apoptotic proteins, CHOP and caspase-4. These
data preliminarily indicate that Fatsioside A induces ER-mediated
apoptosis in U87MG cells.
However, further studies are required to identify
the underlying mechanisms of Fatsioside A in glioma therapy. For
example, previous studies have shown that there is a crosstalk
among the processes of apoptosis, autophagy and ER stress (39,40).
Thus, further studies are required to determine whether autophagy
is involved in ER-mediated apoptosis induced by Fatsioside A.
Additionally, since ER stress is involved in pro-death and
pro-survival mechanisms in glioma cells, further studies are
required to define the optimal concentration of Fatsioside A to
modulate ER stress for cancer treatment.
Acknowledgements
The authors thank Dr Li of the Department of
Pharmacology and Neurology, Emory University (Atlanta, GA, USA) for
the critical reading and modification of the manuscript.
References
|
1
|
Behin A, Hoang-Xuan K, Carpentier AF and
Delattre JY: Primary brain tumours in adults. Lancet. 361:323–331.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruna A, Darken RS, Rojo F, et al: High
TGFbeta-Smad activity confers poor prognosis in glioma patients and
promotes cell proliferation depending on the methylation of the
PDGF-B gene. Cancer Cell. 11:147–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furnari FB, Fenton T, Bachoo RM, et al:
Malignant astrocytic glioma: genetics, biology, and paths to
treatment. Genes Dev. 21:2683–2710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hannun YA: Apoptosis and the dilemma of
cancer chemotherapy. Blood. 89:1845–1853. 1997.PubMed/NCBI
|
|
5
|
El-Aneed A: Current strategies in cancer
gene therapy. Eur J Pharmacol. 498:1–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ellgaard L and Helenius A: Quality control
in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 4:181–191.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sitia R and Braakman I: Quality control in
the endoplasmic reticulum protein factory. Nature. 426:891–894.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang K and Kaufman RJ: From
endoplasmic-reticulum stress to the inflammatory response. Nature.
454:455–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malhotra JD and Kaufman RJ: The
endoplasmic reticulum and the unfolded protein response. Semin Cell
Dev Biol. 18:716–731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar
|
|
13
|
Marciniak SJ, Yun CY, Oyadomari S, et al:
CHOP induces death by promoting protein synthesis and oxidation in
the stressed endoplasmic reticulum. Genes Dev. 18:3066–3077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salazar M, Carracedo A, Salanueva IJ, et
al: Cannabinoid action induces autophagy-mediated cell death
through stimulation of ER stress in human glioma cells. J Clin
Invest. 119:1359–1372. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carracedo A, Gironella M, Lorente M, et
al: Cannabinoids induce apoptosis of pancreatic tumor cells via
endoplasmic reticulum stress-related genes. Cancer Res.
66:6748–6755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pyrko P, Kardosh A, Wang W, Xiong W,
Schönthal AH and Chen TC: HIV-1 protease inhibitors nelfinavir and
atazanavir induce malignant glioma death by triggering endoplasmic
reticulum stress. Cancer Res. 67:10920–10928. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tiwari M, Kumar A, Sinha RA, et al:
Mechanism of 4-HPR-induced apoptosis in glioma cells: evidences
suggesting role of mitochondrial-mediated pathway and endoplasmic
reticulum stress. Carcinogenesis. 27:2047–2058. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu S, Ye X, Xin W, Xu K, Lian XY and Zhang
Z: Fatsioside A, a rare baccharane-type glycoside inhibiting the
growth of glioma cells from the fruits of Fatsia japonica. Planta
Med. 80:315–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kemertelidze É, Kemoklidze Z, Dekanosidze
G and Bereznyakova A: Isolation and pharmacological
characterization of triterpenoid glycosides from Fatsia japonica
cultivated in Georgia. Pharm Chem J. 35:429–432. 2001. View Article : Google Scholar
|
|
20
|
Lin SS, Huang HP, Yang JS, et al: DNA
damage and endoplasmic reticulum stress mediated curcumin-induced
cell cycle arrest and apoptosis in human lung carcinoma A-549 cells
through the activation caspases cascade- and
mitochondrial-dependent pathway. Cancer Lett. 272:77–90. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu JJ, Chen SM, Zhang XW, Ding J and Meng
LH: The anti-cancer activity of dihydroartemisinin is associated
with induction of iron-dependent endoplasmic reticulum stress in
colorectal carcinoma HCT116 cells. Invest New Drugs. 29:1276–1283.
2011. View Article : Google Scholar
|
|
22
|
Tabas I and Ron D: Integrating the
mechanisms of apoptosis induced by endoplasmic reticulum stress.
Nat Cell Biol. 13:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Puthalakath H, O’Reilly LA, Gunn P, et al:
ER stress triggers apoptosis by activating BH3-only protein Bim.
Cell. 129:1337–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oyadomari S, Araki E and Mori M:
Endoplasmic reticulum stress-mediated apoptosis in pancreatic
beta-cells. Apoptosis. 7:335–345. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pulkkanen KJ and Yla-Herttuala S: Gene
therapy for malignant glioma: current clinical status. Mol Ther.
12:585–598. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okada H, Lieberman FS, Edington HD, et al:
Autologous glioma cell vaccine admixed with interleukin-4 gene
transfected fibroblasts in the treatment of recurrent glioblastoma:
preliminary observations in a patient with a favorable response to
therapy. J Neurooncol. 64:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lefranc F, Brotchi J and Kiss R: Possible
future issues in the treatment of glioblastomas: special emphasis
on cell migration and the resistance of migrating glioblastoma
cells to apoptosis. J Clin Oncol. 23:2411–2422. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lefranc F, Facchini V and Kiss R:
Proautophagic drugs: a novel means to combat apoptosis-resistant
cancers, with a special emphasis on glioblastomas. Oncologist.
12:1395–1403. 2007. View Article : Google Scholar
|
|
29
|
Chan JA, Krichevsky AM and Kosik KS:
MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells.
Cancer Res. 65:6029–6033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagane M, Levitzki A, Gazit A, Cavenee WK
and Huang HJ: Drug resistance of human glioblastoma cells conferred
by a tumor-specific mutant epidermal growth factor receptor through
modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad
Sci USA. 95:5724–5729. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Savill J, Dransfield I, Gregory C and
Haslett C: A blast from the past: clearance of apoptotic cells
regulates immune responses. Nat Rev Immunol. 2:965–975. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hartwell LH and Kastan MB: Cell cycle
control and cancer. Science. 266:1821–1828. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bedi A, Pasricha PJ, Akhtar AJ, et al:
Inhibition of apoptosis during development of colorectal cancer.
Cancer Res. 55:1811–1816. 1995.PubMed/NCBI
|
|
34
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye J and Koumenis C: ATF4, an ER stress
and hypoxia-inducible transcription factor and its potential role
in hypoxia tolerance and tumorigenesis. Curr Mol Med. 9:411–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koumenis C: ER stress, hypoxia tolerance
and tumor progression. Curr Mol Med. 6:55–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hitomi J, Katayama T, Eguchi Y, et al:
Involvement of caspase-4 in endoplasmic reticulum stress-induced
apoptosis and Abeta-induced cell death. J Cell Biol. 165:347–356.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen S, Zhang Y, Wang Z, Liu R and Gong X:
Bufalin induces the interplay between apoptosis and autophagy in
glioma cells through endoplasmic reticulum stress. Int J Biol Sci.
10:212–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ogata M, Hino S, Saito A, et al: Autophagy
is activated for cell survival after endoplasmic reticulum stress.
Mol Cell Biol. 26:9220–9231. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Høyer-Hansen M and Jäättelä M: Connecting
endoplasmic reticulum stress to autophagy by unfolded protein
response and calcium. Cell Death Differ. 14:1576–1582. 2007.
View Article : Google Scholar : PubMed/NCBI
|