Introduction
Hypoxia-inducible factor-1α (HIF-1α) is a critical
transcription factor that mediates cellular responses to hypoxia
(1,2). By regulating a series of genes
involved in angiogenesis, anaerobic energy metabolism and
inflammation, HIF-1α is crucial in driving tumor progression and
metastasis (3,4). Consistent with these reports,
increased HIF-1α levels have been observed in various types of
solid tumor (5). Moreover,
elevated levels of HIF-1α protein are usually associated with poor
prognosis and treatment-resistance in cancer patients (5). Since tumor progression and metastasis
rely heavily on HIF-1α signaling, this pathway has become an
attractive target for therapy. In the past decades, several
small-molecule inhibitors of the HIF-1α pathway have been explored
as potential therapeutic agents for tumors, including the heat
shock protein 90 inhibitor 17-allyl-aminogeldanamycin, the
topoisomerase I inhibitor topotecan and the thioredoxin inhibitor
pleurotin (6). Agents such as
camptothecin-11 and SN38 (7-ethyl-10-hydroxy-camptothecin) inhibit
tumor angiogenesis, growth and metastasis by decreasing HIF-1α
levels and inhibiting the expression of HIF-1α-modulated genes
(7), including vascular
endothelial growth factor receptor 2 (VEGFR2). Notably, endostatin,
a potent endogenous inhibitor of angiogenesis, may also repress
HIF-1α and HIF-1α-regulated gene expression (8). However, the molecular mechanism by
which endostatin suppresses HIF-1α expression remains
uncharacterized.
Endostatin, a 183-amino acid C-terminal proteolytic
fragment of collagen XVIII, is a potent endogenous tumor
angiogenesis inhibitor (9). It has
been well documented that endostatin impairs angiogenesis and tumor
progression by inhibiting the proliferation, migration and tube
formation of endothelial cells (10). A number of studies reported that
endostatin exerts its functions extracellularly (11,12).
Notably, endostatin may be internalized and may translocate into
the nucleus (13). Shi et
al (13) reported that
activated endothelial cells express high levels of nucleolin, which
may associate with endostatin and mediate its internalization. In
addition, the internalized endostatin interrupted angiogenesis and
tumor growth by inhibiting the phosphorylation of nucleolin.
Besides nucleolin, the caveolae/lipid rafts and clathrin-coated
pits were also crucial in endostatin internalization (14). Notably, cholesterol sequestration
by nystatin increased the internalization and activity of
endostatin in the endothelium, which was positively correlated with
its antiangiogenic efficacy (14).
More recently, Song et al (15) reported that endostatin
internalization by endothelial cells was mediated by the integrin
α5β1-nucleolin-uPAR receptor complex. Following the internalization
of endostatin from the cell membrane to the cytoplasm, nucleolin
and importin α1β1 mediate endostatin nuclear translocation
(15). However, the detailed
mechanism, particularly the contribution of the
nuclear-translocated endostatin to HIF-1α signaling, has not been
identified.
In the present study, nuclear-translocated
endostatin was shown to inhibit HIF-1α expression, which is
mediated by importin α1β1 and nucleolin. The nuclear-translocated
endostatin disrupts the association of CREB-binding protein
(CBP)/p300 with HIF-1α by impairing Zn(II) homeostasis. In
conclusion, these results reveal how the nuclear-translocated
endostatin downregulates HIF-1α expression in endothelial cells and
also provides a novel explanation for the broad-spectrum
anti-angiogenic activity of endostatin.
Materials and methods
Reagents
Antibodies against HIF-2α, GAPDH and actin were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The anti-VEGFR2 antibody was obtained from Abcam (Cambridge,
MA, USA). Antibodies against HIF-1α, CBP and Oct-4 were obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Horseradish peroxidase-linked goat anti-mouse and goat anti-rabbit
IgG antibody for immunoprecipitation and immunoblotting analysis,
fluorescein isothiocyanate-linked goat anti-mouse and goat
anti-rabbit IgG antibody for immunofluorescence and confocal
microscopy were purchased from Jackson ImmunoResearch (Newmarket,
UK). Small interfering (si)RNAs were obtained from Gene Pharma
(Shanghai, China). Endostatin and N-4 endostatin (Δ2-5 endostatin)
were obtained from Protgen Ltd. (Beijing, China).
Plasmids and construction
Wild type mouse nucleolin (mNCLwt;
NM_005381) was subcloned into the pCMV6-AN-GFP vector (Origene,
Rockville, MD, USA) and NLS-deficient mutant nucleolin
(mNCLmut) was constructed using the Quick Change
Mutagenesis kit (Stratagene, Santa Clara, CA, USA). All the
plasmids were purified from Escherichia coli using PowerPrep
Plasmid Purification kits (Origene, Rockville, MD, USA).
Cell transfection
The HUVEC cells in 24-well plates were transfected
with the mNCLwt or the mNCLmut plasmid using
a TurboFect reagent (Thermo Fisher Scientific, Waltham, MA, USA),
then co-transfected twice (at 0 and 24 h) with 200 pmol scramble
siRNA (or siRNA pool) or nucleolin siRNA.
Apo and holo sample preparation
Apo and holo samples (lab stock) preperation was
performed as previously described (16) Briefly, HUVECs were incubated with
bovine serum albumin, apo-endostatin (non-zinc-binding),
BSA+ZnCl2 or holo-endostatin (zinc-binding) for 12 h,
all of which were lab stock.
Cell culture and RNAi
CRL-1730 human umbilical vein endothelial cells
(HUVECs) were obtained from the American Type Culture Collection
(Manassas, VA, USA) and cultured with 5% CO2 in
endothelial cell medium (Sciencell, Carlsbad, CA, USA) as
previously described (17). For
RNAi, oligofection of siRNA duplexes was performed using
Oligofectamine (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s instructions. In brief, HUVECs were
transfected twice (at 0 and 24 h) with 200 pmol of scramble siRNA
or importin α1, importin β1 or nucleolin siRNAs. Following 24 h,
the mRNA expression levels of target genes in transfected cells
were detected by quantitative polymerase chain reaction (qPCR). The
oligonucleotide sequences were as follows: importin α1,
CGUUGUACCAGAAACUACC; importin β1, UCGGUUAUAUUUGCCAAGA; and
nucleolin, GGCAAAGCAUUGGUAGCAA.
Luciferase assay
HUVECs were plated in 12-well plates at
4×105 cells/well and transfected with pGL3 (empty
vector) or pGL3-HIF-1α luciferase plasmid (lab stock) (15) using TurboFect (Thermo Fisher
Scientific Inc., Waltham, MA, USA). Following 24 h,
mNCLwt and NLS deficient mNCLmut were
ectopically expressed in HUVECs cotransfected with si-nucleolin
siRNA (avoiding the interference of endogenous nucleolin). The
luciferase activity was measured in triplicate using the Bright-Glo
Luciferase assay system (Promega Corporation, Madison, WI,
USA).
qPCR
HUVECs were incubated with bovine serum albumin
(BSA), apo-endostatin (non-zinc-binding), BSA+ZnCl2 or
holo-endostatin (zinc-binding) for 12 h. Total RNA was isolated
using an RNeasy kit (Qiagen, Hilden, Germany) and reverse
transcribed using the First Strand cDNA Synthesis kit (Thermo
Fisher Scientific Inc.). qPCR amplification was performed using the
SYBR-Green qPCR Master mix kit (Stratagene, Santa Clara, CA, USA).
The primers used were as follows: Forward: 5′-CGTTCC
TTCGATCAGTTGTC-3′ and reverse: 5′-TCAGTG GTGGCAGTGGTAGT-3′ HIF-1α;
forward: 5′-CAAGCT ACTCAAGCTGCCAG-3′ and reverse: 5′-CAACAG
AGAAATGAATGCTG-3′ for importin α1; forward: 5′-CAA
GGCACAATATCAGC-3′ and reverse: 5′-GCAGTC AGAATCTCATTGG-3′ importin
β1; forward: 5′-CCTTCT GAGGACATTCCAAGACA-3′ and reverse: 5′-ACGGTA
TTGCCCTTGAAATGTT-3′ for nucleolin; and forward:
5′-CGGCTACCACATCCAAGGAA and reverse: 5′-ACC ACCCTGTTGCTGTAGCC-3′
for GAPDH. Independent experiments were performed in
triplicates.
Immunofluorescence and confocal
microscopy
HUVECs were fixed with 4% paraformaldehyde,
permeabilized with phosphate-buffered saline (PBS) containing 0.2%
Triton X-100, washed twice with PBS and blocked with PBS containing
10% normal goat serum for 15 min. Cells were stained with primary
antibodies for 2 h, washed three times with PBS for 15 min and
incubated with fluorescein isothiocyanate FITC-linked anti-mouse
and anti-rabbit IgG antibodies for 30 min at room temperature.
Nuclei were stained with DAPI (Beyotime Institute of Biotechnology,
Haimen, China). All immunofluorescence images were analyzed with a
Nikon A1 laser scanning confocal microscope (63x/1.49 NA oil
objective) and NIS-Elements AR software (Nikon Corporation, Tokyo,
Japan).
Immunoprecipitation and immunoblotting
analysis
Immunoprecipitation and immunoblotting assays were
performed as previously described (15) and experiments were repeated at
least twice.
Statistical analysis
For statistical analysis, the data are expressed as
the mean ± standard deviation and compared using Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
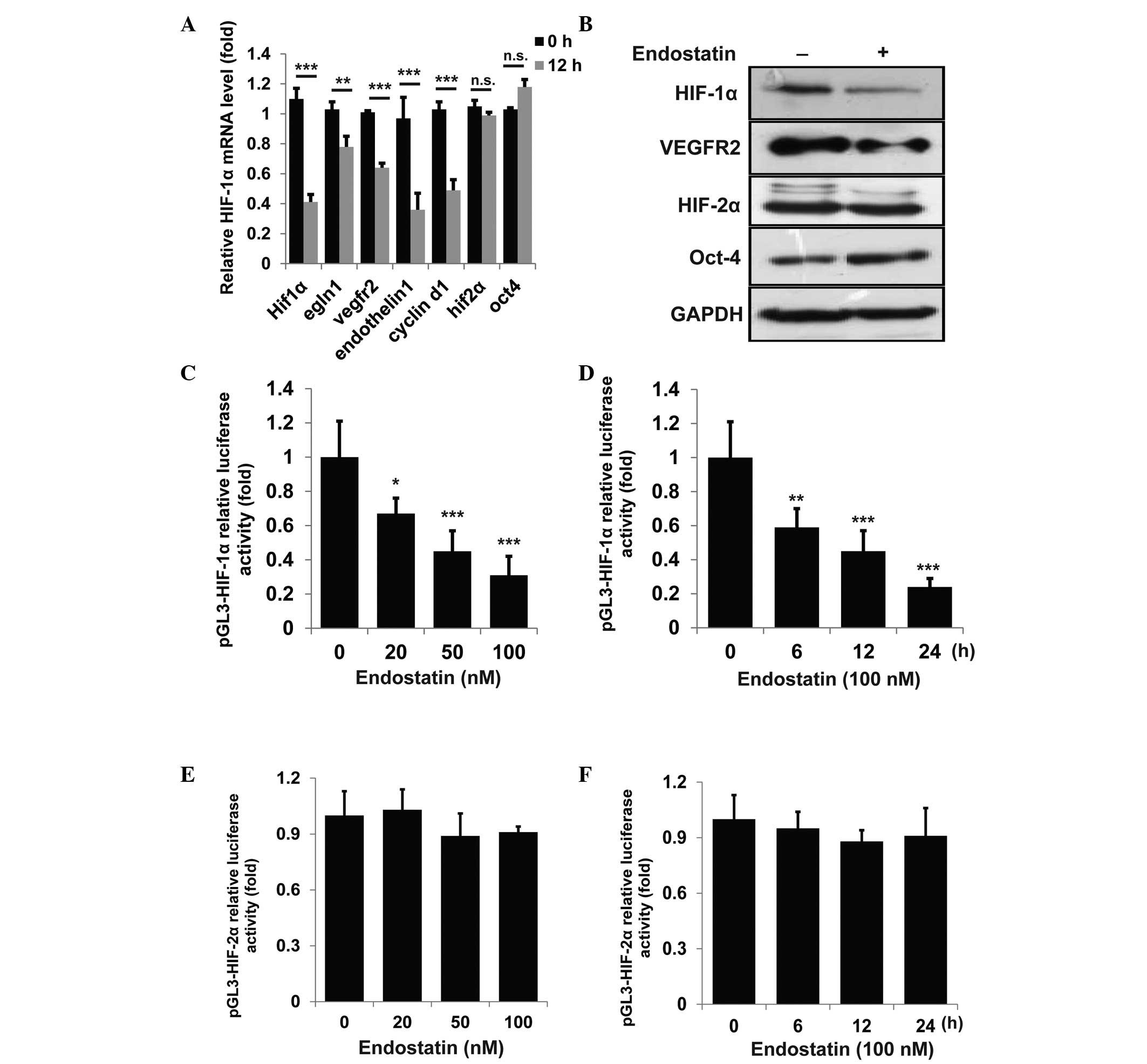
Endostatin suppresses HIF-1α
expression
Abdollahi et al (8) have reported that the impact of
endostatin on genetic expression is widespread in 12% of the
genome. Notably, a number of hypoxia-associated genes are
significantly downregulated by endostatin. Since HIF-1 signaling
has been well-documented as an angiogenic inducer (18–20),
the endostatin-induced repression of several HIF-1 or
HIF-2-associated genes were examined by qPCR. As shown in Fig. 1A, the expression of all HIF-1α
associated genes in HUVECs, including hif-1α, egln1,
vegfr2, endothilin-1 and cyclin D1 was
significantly suppressed by endostatin, while the HIF-2α-associated
genes, including hif-2α and oct-4, were not affected.
The protein levels of HIF-1α, VEGFR2, HIF-2α and Oct-4 were further
detected. Consistently, the expression of HIF-1α and VEGFR2, but
not HIF-2α and Oct-4, were suppressed by endostatin (Fig. 1B). To further confirm whether
endostatin may directly repress the transcriptional activity of
HIF-1α, HIF-1α-luciferase activity was detected following
endostatin treatment. As shown in Fig.
1C and D, endostatin suppressed HIF-1α-luciferase activity in a
time- and dose-dependent manner, while having little effect on
HIF-2α-luciferase activity (Fig. 1E
and F). These results indicate that endostatin inhibits HIF-1α
expression at the transcriptional level.
Downregulation of HIF-1α is modulated by
nuclear-translocated endostatin
A number of studies have reported that nuclear
translocation is essential for the antitumor effects of endostatin
(13,15). As treatment of human umbilical vein
endothelial cells (HUVECs) with endostatin for 30 min leads to
endostatin in cell nucleus (15),
endostatin in the cell nucleus was defined as nuclear-tanslocated
endostatin. Downregulation of HIF-1α was hypothesized to be
modulated by nuclear-translocated endostatin. The importin
α1β1/nucleolin complex has been observed to mediate endostatin
nuclear translocation (15). In
the current study, siRNAs were used to block importin-dependent
translocation. The results showed that siRNAs that targeted
importin α1 or β1 significantly eliminated the endostatin-mediated
suppression of HIF-1α expression (Fig.
2A and B). In addition, endostatin exhibited little effect on
HIF-1α-luciferase activity upon knock-down of importin α1 or β1
(Fig. 2C). To further verify that
the downregulation of HIF-1α was modulated by the
nuclear-tanslocated endostatin, wild-type mouse nucleolin
(mNCLwt) and NLS-deficient mutant nucleolin
(mNCLmut) were ectopically expressed in HUVECs
co-transfected with si-nucleolin siRNA. Compared with
mNCLwt, the ectopic mNCLmut in HUVECs
significantly attenuated endostatin-mediated suppression of the
HIF-1α mRNA level (Fig. 2D). These
observations demonstrate that nuclear-translocated endostatin is
critical in HIF-1α suppression.
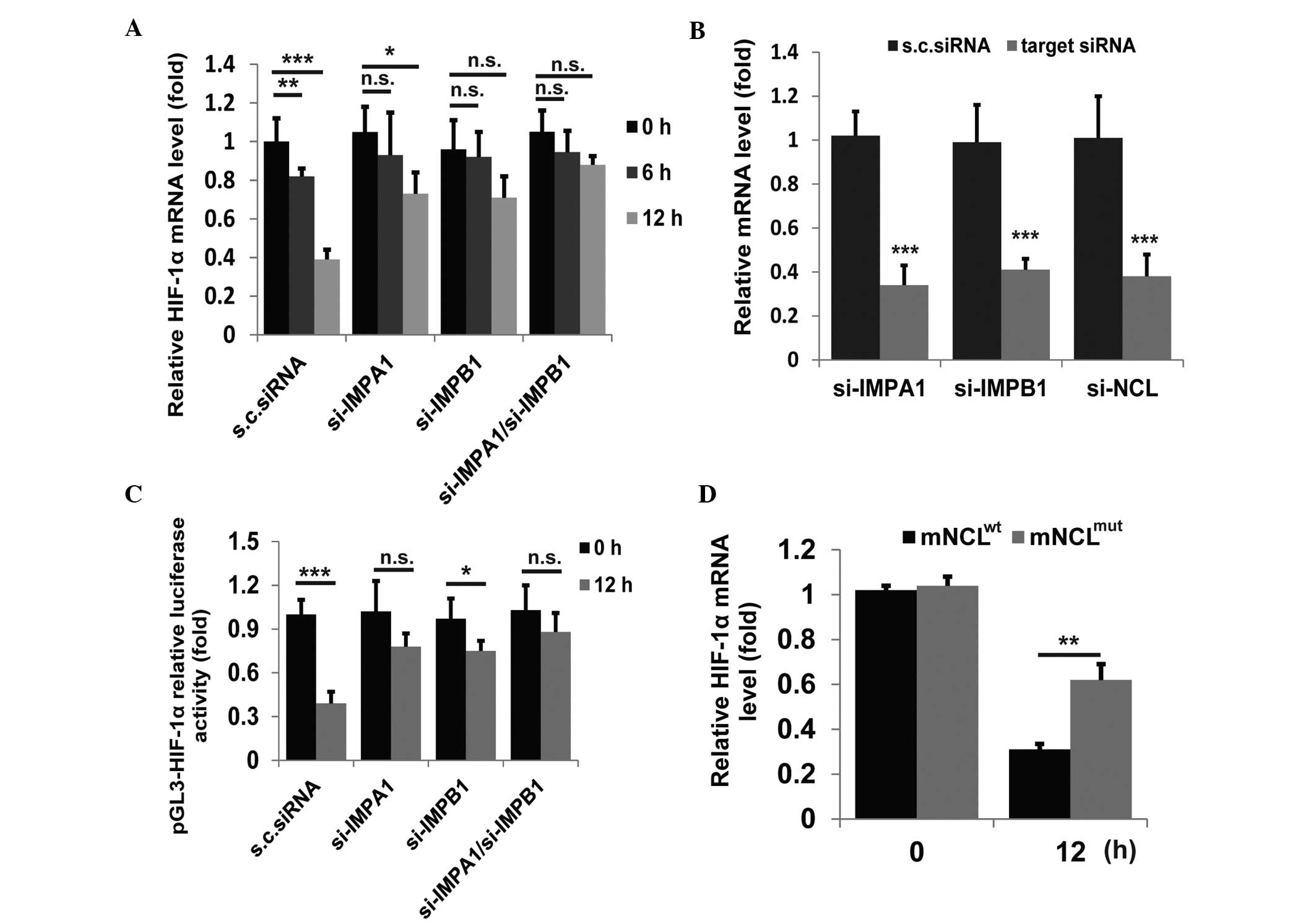 | Figure 2Effect of nuclear-translocated
endostatin on HIF-1α expression. (A) The relative mRNA level (fold)
of HIF-1α in HUVECs transfected with siRNA targeting indicated
genes or scrambled siRNA, detected by qPCR. (B) HUVECs were
transfected twice with indicated siRNAs and analyzed following 24
h. The knock-down efficiencies of targeted genes were detected by
qPCR. (C) HUVECs were transfected with siRNA targeting indicated
genes or scrambled siRNA and the pGL3-HIF-1α relative luciferase
activity (fold) in cell lysates was measured. (D) The relative mRNA
level (fold) of HIF-1α in HUVECs co-transfected with siRNA
targeting human nucleolin and the vector encoding mNCLwt
or mNCLmut, detected by qPCR. Data are shown as the mean
±standard deviation. All experiments were repeated at least twice.
*P<0.05, **P<0.01 and
***P<0.005, vs. control. HIF-1α, hypoxia-inducible
factor-1α; HUVECs, human umbilical vein endothelial cells; qPCR,
quantitative polymerase chain reaction; mNCLwt, mouse
wildtype nucleolin; mNCLmut, NLS-mutant nucleolin;
IMPA1, importin α1; IMPB1, importin β1. |
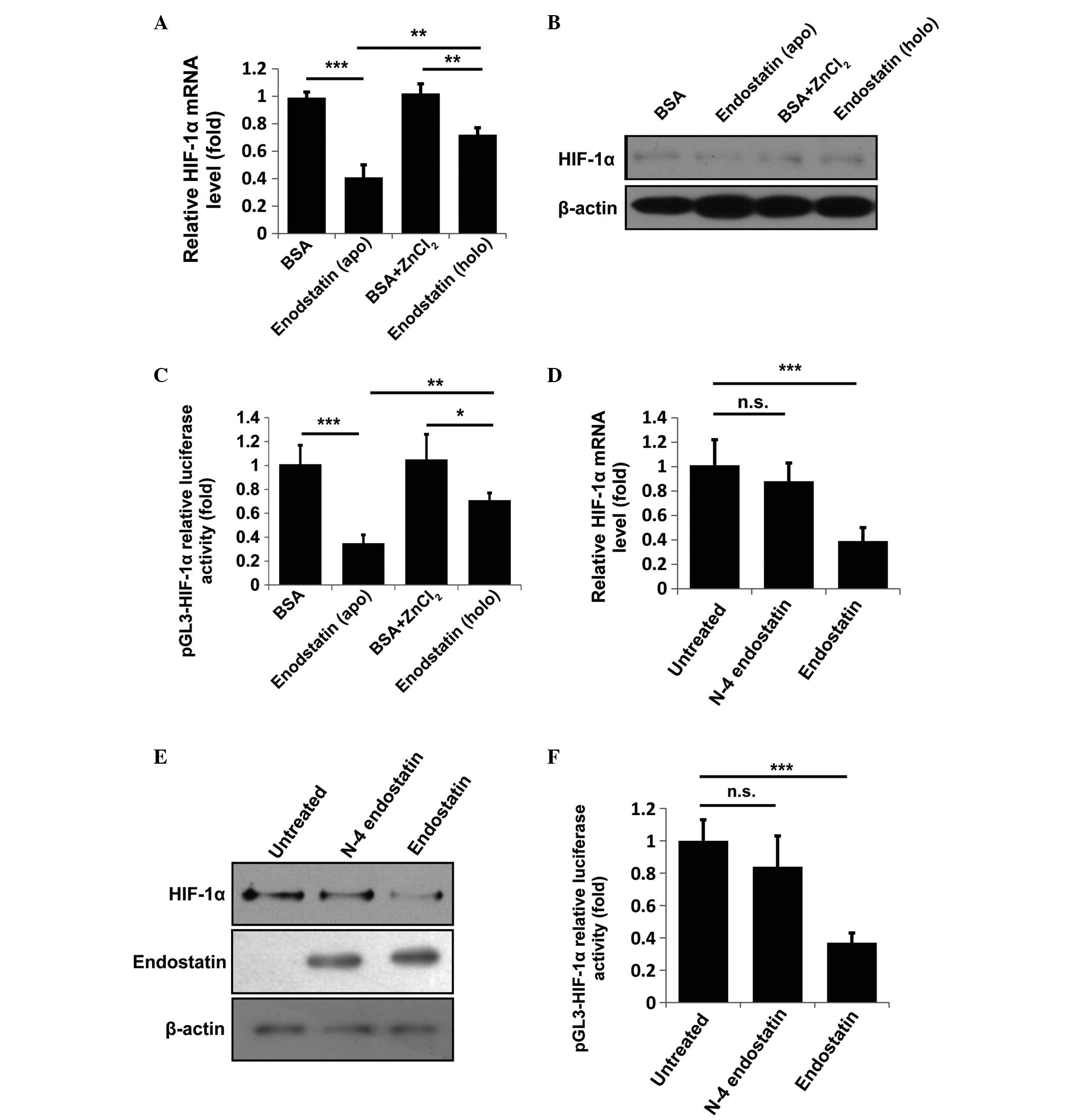
Zn(II)-binding capacity is indispensable
for endostatin-mediated HIF-1α inactivation
The crystal structure of endostatin shows that
endostatin is a Zn(II)-dependent protein (21) and other studies have reported that
its Zn(II)-binding capacity is responsible for its anti-angiogenic
activity (16,22). Moreover, the Zn(II) dissociation
constant of endostatin was measured to be 6.7 nM (16), suggesting a marked Zn(II)-binding
capacity. Thus, Zn(II) homeostasis in the nucleus was hypothesized
to be regulated by the nuclear-translocated endostatin. To verify
this hypothesis, HUVECs were incubated with BSA, apo-endostatin
(non-zinc-binding), BSA+ZnCl2 or holo-endostatin
(zinc-binding) for 12 h. The cells were collected and analyzed, as
shown in Fig. 3A–C. Compared with
apo-endostatin, holo-endostatin had a reduced ability to suppress
the mRNA and protein levels of HIF-1α and marginally downregulated
the HIF-1α-luciferase activity. Previously, Fu and Luo (22) reported that the N-terminal 2–5
amino acid residues (HSHR) of endostatin were critical for its zinc
binding. N-4 endostatin (Δ2-5 endostatin) was used to further
confirm this observation. Consistently, N-4 endostatin treatment
had little effect on HIF-1α expression and HIF-1α-luciferase
activity (Fig. 3D–F). In addition,
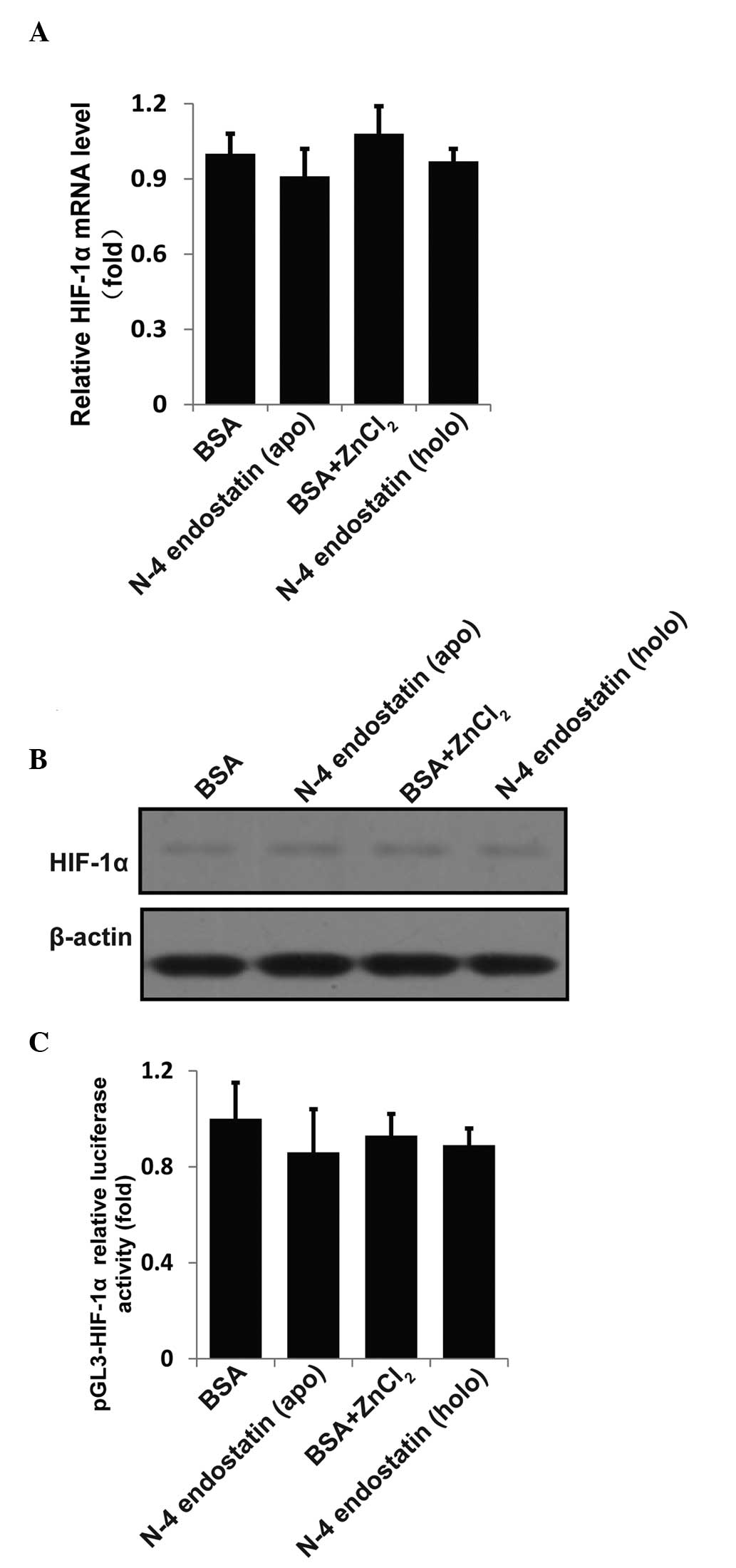
no significant differences among BSA, apo-N-4 endostatin,
BSA+ZnCl2 or holo-N-4 endostatin groups in regulating
HIF-1α expression or HIF-1α-luciferase activity were identified
(Fig. 4A–C). These results
indicate that the competition for Zn(II) by nuclear-translocated
endostatin results in the transcriptional inactivation of
HIF-1α.
Nuclear-translocated endostatin disrupts
the interaction between CBP/p300 and HIF-1α by competing for
Zn(II)
In this study, the inhibitory effect of endostatin
on HIF-1α expression was investigated. Although HIF-1α itself is
not a Zn(II)-binding protein, it activates the transcription of
adaptive genes by recruiting a well-known co-activator, CBP/p300 in
a Zn(II)-dependent manner (23).
As a result of a reduction in free Zn(II) induced by
nuclear-translocated endostatin, the interaction between HIF-1α and
CBP/p300 may be interrupted and the HIF-1α-mediated transcription
is likely to be further disturbed. Since HIF-1α is a self-regulated
gene, the disrupted association of CBP/p300 with HIF-1α may also be
caused by endostatin-induced HIF-1α suppression. To exclude this
possibility, HUVECs were treated with endostatin for the indicated
times and mRNA were detected by qPCR. As shown in Fig. 5A, endostatin treatment for 3 h
showed little effect on HIF-1α mRNA. Therefore, HUVECs were treated
with BSA, apo-endostatin, BSA+ZnCl2 or holo-endostatin
for 3 h and the cell immunofluorescence imaging was captured by
confocal microscopy. As shown in Fig.
5B and C, compared with apo-endostatin, holo-endostatin only
marginally interfered with the co-localization of CBP/p300 and
HIF-1α. To further confirm this result, HUVECs were treated as
described in Fig. 4B and the
interaction between HIF-1α and CBP/p300 was evaluated by
immunoprecipitation assay. Consistently, holo-endostatin treatment
exhibited a marginal effect on the association of CBP/p300 with
HIF-1α compared with the apo-endostatin group (Fig. 5D). These findings demonstrate that
competition for Zn(II) by nuclear-translocated endostatin disrupts
the interaction between CBP/p300 and HIF-1α.
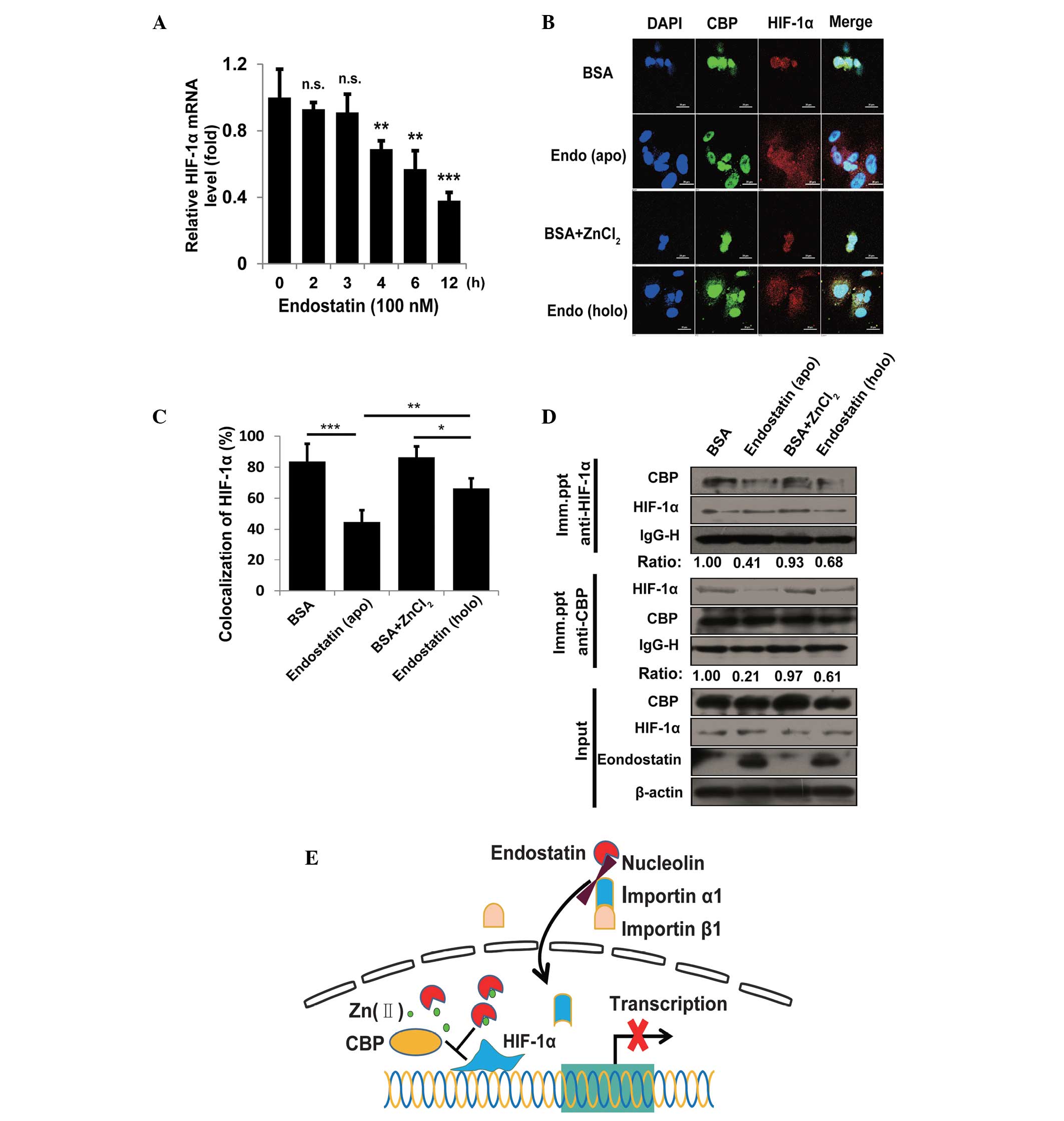 | Figure 5Effect of nuclear-translocated
endostatin on the interaction between CBP/p300 and HIF-1α. (A)
HUVECs were treated with endostatin (100 nM) for the indicated
times and the relative mRNA level of HIF-1α (fold) was detected by
qPCR. (B) Immunofluorescence staining of CBP/p300 (green) and
HIF-1α (red) in HUVECs treated with BSA, apo-endostatin,
BSA+ZnCl2 and holo-endostatin. Digital images were
captured by the Nikon A1 fluorescence microscope using 63x/1.49 NA
oil objectives. Images were captured with NIS-Elements AR 3.0
software. Nuclei (blue) were stained by DAPI. Scale bar, 20 μm. (C)
The levels of colocalization (Fig.
4B) were quantified by calculating the percentage of red pixels
(HIF-1α) that colocalized with light blue pixels (green, blue
merged) from eight random fields per well of three experiments. (D)
Following incubation with BSA, apo-endostatin, BSA+ZnCl2
and holo-endostatin, cells were lysed and immunoprecipitated with
agarose-conjugated anti-HIF-1α and anti-CBP to detect the binding
pattern between HIF-1α/CBP. The indicated proteins were detected by
immunoblotting. (E) Model for the nuclear-translocated endostatin
effect on HIF-1α inactivation: Nuclear-translocated endostatin
mediated by nucleolin and importin α1β1 disrupts the interaction
between CBP/p300 and HIF-1α though interfering with Zn(II)
homeostasis and then governs the HIF-1α signaling at the
transcriptional level. Data are presented as the mean ± standard
deviation. All experiments were repeated at least twice.
*P<0.05, **P<0.01 and
***P<0.005, vs. control. CBP, CREB-binding protein;
HIF-1α, hypoxia-inducible factor-1α; HUVECs, human umbilical vein
endothelial cells; qPCR, quantitative polymerase chain reaction;
BSA, bovine serum albumin. |
Discussion
Nuclear-translocated endostatin has been shown to
downregulate the transcriptional activity of HIF-1α by disrupting
the interaction between CBP/p300 and HIF-1α. Thus, a working model,
based on the present results, is proposed as shown in Fig. 5E i.e., nuclear-translocated
endostatin mediated by nucleolin and importin α1β1 disrupts the
interaction between CBP/p300 and HIF-1α by competing for Zn(II) and
then governs the HIF-1α signaling at transcriptional level.
Endostatin is a well-documented endogenous inhibitor
of angiogenesis (9). Although the
structure, function and molecular mechanism of endostatin have been
extensively investigated, several controversial observations
remain. It was unclear why P. pastoris-expressed endostatin
was failed at phase II in the USA, whereas endostar, an
N-terminal-modified endostatin expressed by E. coli, was
approved by the State Food and Drug Administration (24). To determine the cause of this,
P. pastoris- and E. coli-expressed endostatins were
investigated. Notably, ~93% of P. pastoris-expressed
endostatin was observed in the truncated form, which lost its
zinc-binding capacity, leading to reduced stability and lowered
anti-angiogenic capacity. Endostatin expressed by E. coli
was shown to have an intact molecular structure with full antitumor
activity (22). Therefore, E.
coli-expressed endostatin was used in the current study.
Increasing evidence demonstrated that endostatin
inhibited endothelial cell proliferation, migration and tube
formation by blocking a number of well-known pathways associated
with angiogenesis, including HIF-1α, nuclear factor (NF)-κB,
activator protein 1 and Stats (8).
In the current study, nuclear-translocated endostatin was observed
to downregulate HIF-1α activation at the transcriptional level.
Since HIF-1α expression was partly regulated in an NF-κB-dependent
manner, endostatin inhibition of the NF-κB pathway may augment the
inhibition of HIF-1α. In addition, VEGFR2, a downstream gene of
HIF-1α, was also downregulated by endostatin (Fig. 1A and B), which was consistent with
previous studies (8). The
aforementioned mentioned results provide substantial evidence that
endostatin downregulates the HIF-1α pathway in endothelial
cells.
Exclusive internalization of endostatin in
endothelial cells has been observed and was demonstrated to exhibit
an integral role in inhibiting angiogenesis and tumor growth
(13–15). More recently, Song et al
(15) reported that endostatin was
transported into the nucleus by the importin α1β1/nucleolin
complex. Similarly, in the current study, the nuclear-translocation
of endostatin mediated by the importin α1β1/nucleolin complex was
shown to be critical for the regulation of HIF-1α transcription
(Fig. 2A–E). Notably, endostatin
is a Zn(II)-binding protein and the Zn(II)-binding site consists of
three histidine residues (His1, His3 and His11) and an aspartic
acid residue (Asp76) at the N-terminus (16,21).
Neither double mutation in H1/3A nor site mutation in His11 or
Asp76 significantly impaired its anti-angiogenic activity,
suggesting that the Zn(II)-binding capacity is central in the
bioactivity of endostatin. In addition, N-4 endostatin exhibited a
reduced zinc-binding capacity (22), which led to decreased stability and
impaired antitumor capacity of endostatin, also indicating that the
Zn(II)-binding capacity was indispensable for its function.
Moreover, Song et al (15)
proposed that nuclear-translocated endostatin may block a number of
well-known pathways associated with angiogenesis. Consistently, the
current results have shown that nuclear-translocated endostatin
impairs the interaction between CBP/p300 and HIF-1α through the
competition for Zn(II), which results in downregulation of HIF-1α
expression. Based on the current studies, these observations
provide solid evidence to support the proposed working model
(Fig. 5E). In conclusion, the
present study shows that nuclear-translocated endostatin disrupts
the interaction between CBP/p300 and HIF-1α through the competition
for Zn(II), which leads to the transcriptional inactivation of
HIF-1α. This study identifies a novel molecular mechanism by which
nuclear-translocated endostatin inhibits HIF-1α expression and
provides a novel explanation for understanding the contribution of
Zn(II) to the bioactivity of endostatin.
Acknowledgements
This study was supported in part by grants from the
General Programs of the National Natural Science Foundation of
China (grant nos. 81171998, 81171999 and 81071742), the PhD
Programs Foundation for New Teachers of Ministry of Education of
China (grant no. 20110002120039), the National Science and
Technology Major Project (grant nos. 2009ZX09103-703 and
2009ZX09306-002) and Major Scientific and Technological Special
Project for ‘significant new drugs creation’ (grant nos.
2011ZX09101-001-08 and 2009ZX09102-243). The authors would like to
thank Miss Bipo Sun for her contribution as the laboratory manager,
Dr Nan Song for critical reading of the manuscript and to all the
Luo lab members for the insightful discussions.
References
|
1
|
Ke Q and Costa M: Hypoxia-inducible
factor-1 (HIF-1). Mol Pharmacol. 70:1469–1480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung KW, Ng HM, Tang MK, Wong CC, Wong RN
and Wong AS: Ginsenoside-Rg1 mediates a hypoxia-independent
upregulation of hypoxia-inducible factor-1α to promote
angiogenesis. Angiogenesis. 14:515–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liao D, Corle C, Seagroves TN and Johnson
RS: Hypoxia-inducible factor-1alpha is a key regulator of
metastasis in a transgenic model of cancer initiation and
progression. Cancer Res. 67:563–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Frolova O, Samudio I, Benito JM, et al:
Regulation of HIF-1α signaling and chemoresistance in acute
lymphocytic leukemia under hypoxic conditions of the bone marrow
microenvironment. Cancer Biol Ther. 13:858–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan HE, Lo J and Johnson RS: HIF-1 alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onnis B, Rapisarda A and Melillo G:
Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med.
13:2780–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sapra P, Kraft P, Pastorino F, et al:
Potent and sustained inhibition of HIF-1α and downstream genes by a
polyethyleneglycol-SN38 conjugate, EZN-2208, results in
anti-angiogenic effects. Angiogenesis. 14:245–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abdollahi A, Hahnfeldt P, Maercker C, et
al: Endostatin’s antiangiogenic signaling network. Mol Cell.
13:649–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O’Reilly MS, Boehm T, Shing Y, et al:
Endostatin: an endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar
|
|
10
|
Abdollahi A, Hlatky L and Huber PE:
Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat.
8:59–74. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
MacDonald NJ, Shivers WY, Narum DL, et al:
Endostatin binds tropomyosin. A potential modulator of the
antitumor activity of endostatin. J Biol Chem. 276:25190–25196.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karumanchi SA, Jha V, Ramchandran R, et
al: Cell surface glypicans are low-affinity endostatin receptors.
Mol Cell. 7:811–822. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi H, Huang Y, Zhou H, et al: Nucleolin
is a receptor that mediates antiangiogenic and antitumor activity
of endostatin. Blood. 110:2899–2906. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Wang S, Lu X, Zhang H, Fu Y and
Luo Y: Cholesterol sequestration by nystatin enhances the uptake
and activity of endostatin in endothelium via regulating distinct
endocytic pathways. Blood. 117:6392–6403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song N, Ding Y, Zhuo W, et al: The nuclear
translocation of endostatin is mediated by its receptor nucleolin
in endothelial cells. Angiogenesis. 15:697–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Q, Fu Y, Zhou H, He Y and Luo Y:
Contributions of Zn(II)-binding to the structural stability of
endostatin. FEBS Lett. 581:3027–3032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song N, Huang Y, Shi H, et al:
Overexpression of platelet-derived growth factor-BB increases tumor
pericyte content via stromal-derived factor-1alpha/CXCR4 axis.
Cancer Res. 69:6057–6064. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toffoli S, Roegiers A, Feron O, et al:
Intermittent hypoxia is an angiogenic inducer for endothelial
cells: role of HIF-1. Angiogenesis. 12:47–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Calvani M, Rapisarda A, Uranchimeg B,
Shoemaker RH and Melillo G: Hypoxic induction of an
HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in
human endothelial cells. Blood. 107:2705–2712. 2006. View Article : Google Scholar
|
|
20
|
Chen MC, Lee CF, Huang WH and Chou TC:
Magnolol suppresses hypoxia-induced angiogenesis via inhibition of
HIF-1α/VEGF signaling pathway in human bladder cancer cells.
Biochem Pharmacol. 85:1278–1287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding YH, Javaherian K, Lo KM, et al:
Zinc-dependent dimers observed in crystals of human endostatin.
Proc Natl Acad Sci USA. 95:10443–10448. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu Y and Luo Y: The N-terminal integrity
is critical for the stability and biological functions of
endostatin. Biochemistry. 49:6420–6429. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nyborg JK and Peersen OB: That zincing
feeling: the effects of EDTA on the behaviour of zinc-binding
transcriptional regulators. Biochem J. 381:e3–e4. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fu Y, Tang H, Huang Y, Song N and Luo Y:
Unraveling the mysteries of endostatin. IUBMB Life. 61:613–626.
2009. View
Article : Google Scholar : PubMed/NCBI
|