Introduction
Ischemia-reperfusion (I/R) injury was first
described in 1968 (1). The
influence of I/R on various tissues has been widely discussed,
since various organs may be affected during traumatic,
reconstructive and transplant surgeries (2,3). I/R
injury consists of two consecutive components, which comprise
ischemia, a breakdown of blood perfusion and reperfusion, where the
nutrient blood supply is restored. Ischemia leads to a lack of
oxygen within cells of the affected organs, resulting in the
conversion of the cellular metabolism to an anaerobic state. This
results in lactate accumulation, depletion of cellular adenosine
triphosphate, increased production of reactive oxygen species (ROS)
and dysfunction of membrane transport systems (4,5).
Recent clinical and experimental studies have demonstrated that
paradoxically, the major damage of I/R injury occurs during the
reperfusion period (6,7). Reperfusion initiates complex
reactions which lead to the induction of leukocyte accumulation,
micro-vascular barrier dysfunction, edema formation, and the
release of inflammatory cytokines and complement activation
(8,9). The parenchymal damage of I/R injury
occurs due to leukocytes being carried to the affected area and the
release of inflammatory factors in response to the tissue damage
caused by ischemia. The reperfusion reintroduces oxygen that can
cause damage to cellular proteins, DNA and the plasma membrane, and
results in an increase in release of free radicals, which initiates
apoptosis. Leukocytes may additionally act on the capillaries,
causing obstruction and leading to increased ischemia (4,5,10).
Numerous organs may be clinically involved in I/R
injury. The intestine, kidney and skeletal muscle are the three
most affected, in their function, by I/R insult. Studies
investigating treatment options for I/R injury are limited in
animal studies and are rarely conducted in the clinical setting due
to the limited understanding of the molecular mechanisms of I/R
injury (2). The mechanisms of I/R
injury are challenging to study since numerous interacting
bioactivities are presented at different time-points. During the
shock status, the individual organs suffer from I/R insult,
respectively, and receive the toxin from the other organs during
reperfusion. Simplification of the method for mechanical study of
I/R is important. Tracing the biological changes during I/R at the
genomic level is one method that can be employed. There currently
are limited reports that have used this approach, and only few
pro-inflammatory genes have been identified following I/R insult.
These genes include upregulated S100A4, complement
C4, ADAM2, HO-1, UCP-2 and
TMSB4X, and downregulated GLUL, CYP2A6 and
CYP2d9 in a renal model; upregulated MRP2 and
PGP in an intestinal model, and upregulated IGF-1 and
p27Kp1 in a skeletal muscle model (11,12).
These studies have been limited to individual or small groups of
genes, which restrict the exploration of the entire mechanism.
There have been no studies, to the best of our knowledge, comparing
the genomic changes between different organs under the same I/R
insult. In the present study, a kidney, intestine and skeletal
muscle model of I/R was used to investigate the genomic changes
using a DNA microarray approach, with the aim to identify target
genes involved in I/R injury.
Materials and methods
Animals and experimental groups
A total number of 45 male inbred Lewis rats aged
8–12 weeks with a body weight of 270–330 g were purchased from the
National Laboratory Animal Center (Taipei, Taiwan) and used for I/R
experiments. All experiments were approved by the Chang Gung
Memorial Animal Research and Ethic Committee (Tao-Yuan, Taiwan).
During surgery, all animals were placed under a heat lamp to
prevent a decrease in body temperature, and during ischemia and
reperfusion the exposed organs were covered with normal saline wet
gauzes to maintain normal moisture levels. General anesthesia was
induced by an intraperitoneal injection of urethane (15 mg/kg).
Rats were assigned to three different study groups, and ischemia
and reperfusion injury was studied individually in the kidneys,
intestine, and skeletal muscle. Animals in the first group (group
I, n=5) were sham operated and served as controls. Animals in the
second group (group II, n=5) were subjected to 60 min of vascular
occlusion. Animals in the third group (group III, n=5) were
subjected to 60 min of ischemia followed by 60 min of reperfusion.
According to the literature, 60 min of ischemia and reperfusion
were considered appropriate to study early changes in gene
expression following I/R injury (2–5,7,23–25).
Establishment of kidney I/R injury
Briefly, the abdomen was opened through a midline
incision, and the pedicles of both kidneys were located and freed
from surrounding tissue. The left renal artery and vein was clamped
with a single microvascular clamp and ischemia was macroscopically
verified by a change in color of the kidney to pale blue. For
reperfusion studies, the renal clamp was removed and
reestablishment of blood flow was again monitored
macroscopically.
Establishment of intestinal I/R
injury
To study the effects of experimental ischemia on
gene expression within intestinal tissue, rats were laparotomized
through a midline incision. Briefly, the superior mesenteric artery
(SMA) and the supplied intestine were identified and the superior
mesenteric vessels were freed from the surrounding tissue. The SMA
and superior mesenteric vein (SMV) were occluded with a single
vascular clamp for 60 min and ischemia was verified macroscopically
by observing the color change of the intestinal segment to a dark
pale color. For reperfusion, the clamp of the superior mesenteric
vessels was removed and biopsies were taken after 60 min.
Establishment of skeletal muscle I/R
injury
The rat hind-limb vascular occlusion model was used
to study the impact of ischemia and reperfusion in the skeletal
muscle. Briefly, an incision in the inner side of the hind leg,
from the inguinal ligament to the tendon calcaneus insertion, was
made. Other than the femoral vessels, all of the muscles, tendons,
nerves and vessels were dissected and the femur head was dislocated
from the acetabulum. Next, the femoral artery and vein were clamped
with a single vascular clamp. For reperfusion, the clamp occluding
the femoral vessel was removed to regain of the blood supply to the
distal limb was monitored macroscopically.
Organ tissue collection and RNA
preparation
At the endpoint of the study, organs subjected to
ischemia and reperfusion were harvested under terminal anesthesia.
The organs were carefully removed, gently rolled on cotton swabs
and irrigated with normal saline to remove the adjacent tissue and
excess blood. The organs were then blotted dry, weighed and
shock-frozen in liquid nitrogen for storage and subsequent RNA
extraction.
The tissue was homogenized and total RNA isolated
using TRIzol™ reagent (Gibco-BRL, Carlsbad, CA, USA) according to
the manufacturer’s instructions. Subsequently, two
phenol/chloroform extractions were performed, followed by a DNAse
digestion. Total RNA from the organs of individual rats of each
experimental group was pooled and poly A+ RNA (mRNA)
isolated with oligo (dT) cellulose columns (Gibco-BRL). Both total
RNA and poly A+ RNA concentrations were determined
spectrophotometrically at A260 and all samples were
checked by formaldehyde gel electrophoresis.
Microarray experiment
The samples were prepared for microarray analysis
according to the Nimblegen gene expression analysis protocol (Roche
Diagnostics, Manheim, Germany). Double-stranded (ds) cDNA from 10
μg of total RNA was synthesized using the SuperScriptTM
Double-Stranded cDNA Synthesis kit (Invitrogen Life Technologies,
Carlsbad, CA, USA). The cDNA was treated with RNase and the total
RNA was purified using phenol/chloroform/isoamyl alcohol (25:24:1
v/v) and precipitated by adding 16 μl of 7.5 M ammonium acetate, 7
μl glycogen (5 mg/ml stock solution), 326 μl ice-cold absolute
ethanol. The resulting pellet was washed with 500 μl ethanol (80%)
and dissolved in 20 μl water. Gel electrophoresis was used to
verify successful dscDNA synthesis, which was confirmed by the
presence of a smeared band of 500–2,000 bp. The reactions were
labeled with Cy3–9mer primers using a Nimblegen One-Cola DNA
Labeling kit, followed by precipitation using NaCl and isopropanol.
The precipitate was resuspended in 25 μl distilled water.
Microarray hybridization and data
analysis
Microarray hybridization was combined with 4 μg cDNA
from each of the samples. A NimbleGen Hybridization kit (NimbleGen
Systems; Roche) was used for the hybridization reaction according
to the manufacturer’s instructions. The hybridization reaction was
performed in a MAUI Hybridization system (BioMicro®
Systems, Inc., Salt Lake City, UT, USA). Following hybridization,
the array was washed and dried according to the NimbleGen Washing
kit (NimbleGen Systems; Roche) protocol. The array image was
acquired using an Axon GenePix 4000B (Axon Instruments, Inc., Union
City, CA, USA) laser scanner at a 5-μm resolution and the intensity
data were extracted using the NimbleScan software (NimbleGen
Systems; Roche). The data was further examined using NexuExp
software (BioDiscovery, El Segundo, CA, USA). Gene expression
changes that were greater or less than two-fold as compared with
the control group, and with a P<0.01, were considered to
indicate a statistically significant difference in the expressed
genes between the samples.
Quantitative polymerase chain reaction
(qPCR)
SYBR® Green qPCR primers were designed
using Beacon Designer software version 2 (PREMIER Biosoft
International, Palo Alto, CA, USA) with the following sequences:
forward, 5′-AGTCGTGGGAAGAGGGAACT-3′, and reverse,
5′-CCCTGGAAGTTGTTCATGCT-3′ for adrenomedullin (Adm);
forward, 5′-ACAGAGCATGACCCTGAACC-3′, and reverse,
5′-CCGTTGCTGGACTGGATTAT-3′ for Jun; forward,
5′-CAAGACAAAAGCGTGGTTGA-3′, and reverse, 5′-TCTTCCTGAGTCCCTCCTGA-3′
for Junb; forward, 5′-AATGGAGGTGATGGCAGACA-3′, and reverse,
5′-GAGCAACCCACAGAGTACCT-3′ for c-FBJ osteosarcoma
(c-Fos); forward, 5′-GGGTCACTGGTGTTTGAGGA-3′, and
reverse, 5′-CCTCGGCTTTTGTGATGGAC-3′ for activating transcription
factor 3 (Atf3) and forward, 5′-CTCAGCCAATTGTCCCAACC-3′, and
reverse, 5′-AGGTAAGCAAGGCAGATGGT-3′ for dual specificity
phosphatase 1 (Dusp1) genes,. SYBR Green reactions were
performed using the SYBR Green Supermix (BioRad, Hercules, CA,
USA). The qPCR reactions were then performed using the BioRad
iCycler iQ Real-Time Detection system (BioRad). The cycling
conditions were as follows; 3 min at 95°C, 15 sec at 95°C and 45
sec at 55°C for 45 cycles. The relative expression levels of
Adm, Jun, Junb, c-fos, Atf3 and
Dusp1 were analyzed using the iCycle iQ system software and
presented as a ratio to the expression of the housekeeping gene,
tubulin. Each sample was replicated twice from three independent
sets of RNA preparations.
Statistical analysis
All values are expressed the mean + standard
deviation. The results of the gene expression levels across the
different groups were analyzed by analysis of variance with
post-hoc comparison using Kruskal-Wallis test. A
P<0.05 was considered to indicate a statistically significant
difference. The statistical analysis was performed using SPSS 17.0
(SPSS Inc., Chicago, IL, USA).
Results
Gene expression profiling in I/R
models
The microarray compared the expression profile of
>21486 genes, using the Nexus Expression™ analysis software
(BioDiscovery). Each organ had a different number of genes that
were differentially expressed during the I/R condition (Table I). As compared with the sham
operation group, in the intestinal model, there were 76 genes
upregulated and 429 genes downregulated in the ischemia-only group
(group II) and 172 genes upregulated and 416 genes downregulated in
the I/R group (group III). In the renal model, there were 903 genes
upregulated and 1351 genes downregulated in the ischemia only group
and 467 genes upregulated and 437 genes downregulated in the I/R
group. In the skeletal muscle model, there were 2658 genes
upregulated and 1972 genes downregulated in the ischemia only group
and 3932 genes upregulated and 4203 genes down-regulated in I/R
group (Table I).
 | Table ITotal number of up- and downregulated
genes in the kidney, intestine and skeletal muscle. |
Table I
Total number of up- and downregulated
genes in the kidney, intestine and skeletal muscle.
| Kidney | Intestine | Skeletal
muscle |
|---|
|
|
|
|
|---|
| No. of genes | Is | I/R | Is | I/R | Is | I/R |
|---|
| Upregulated
genes | 903 | 467 | 76 | 172 | 2658 | 3932 |
| Downregulated
genes | 1351 | 437 | 429 | 416 | 1972 | 4203 |
Comparisons of the gene expression
profiling in different organ models
The details of the up- and downregulated genes were
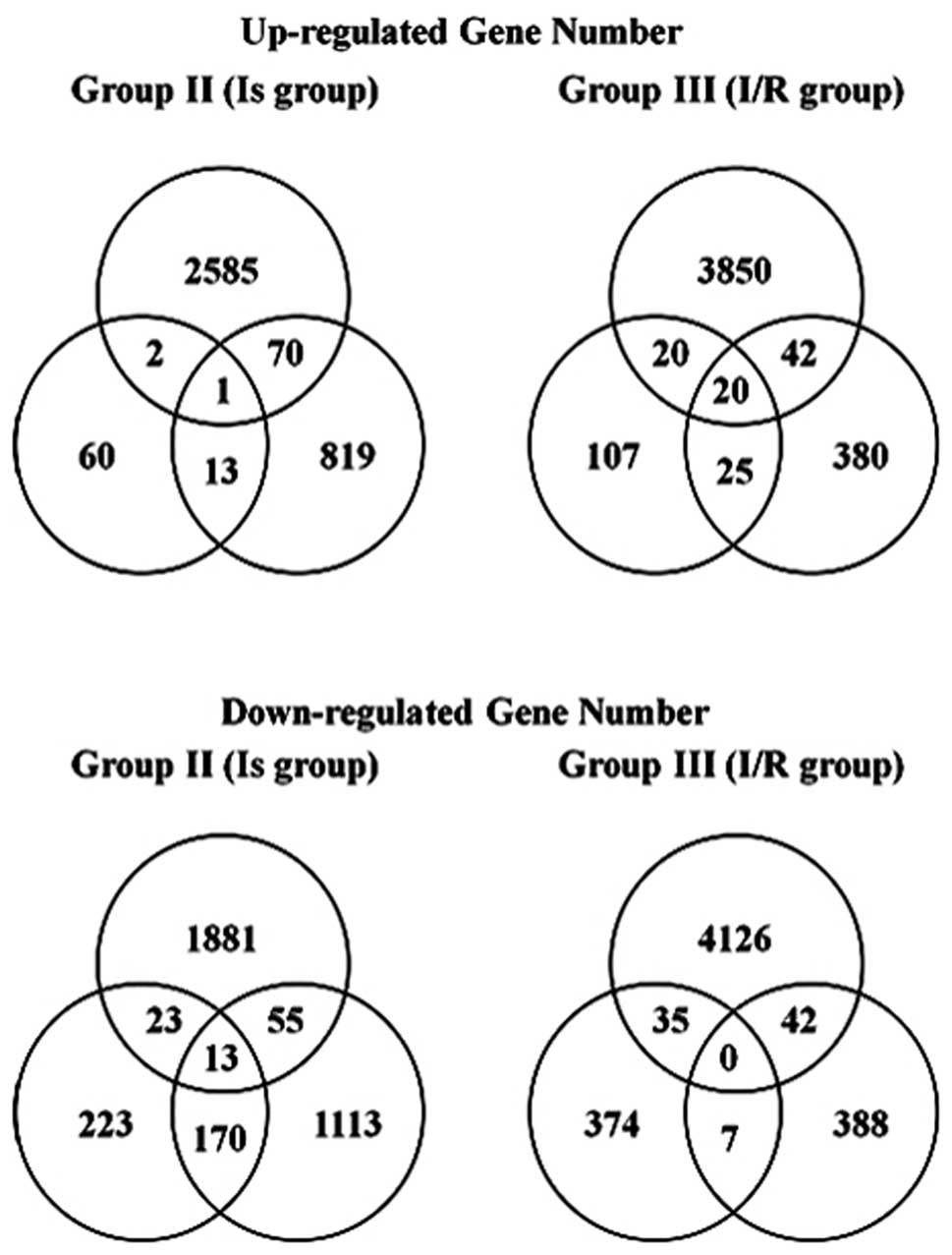
markedly different between the organs. Fig. 1 shows the Venn diagram of the genes
that were differentially up- and downregulated in the Is and I/R
groups in all three models. As for the common up- or downregulated
genes, one and 13 gene probe sets were up- and downregulated in the
Is group, respectively; 20 gene probe sets were upregulated in the
I/R group and no genes were downregulated in the I/R group. The
details of the common up and downregulated genes are shown in
Table II.
 | Table IICommon up- and downregulated genes in
each group. |
Table II
Common up- and downregulated genes in
each group.
| A, Commonly
upregulated genes in the Is group |
|---|
|
|---|
| Probes | Name | Gene symbol | Chromosome | Intestine log
ratioa | Kidney log
ratioa | Muscle log
ratioa |
|---|
| Transcription
factor | | | | | | |
| NM_001024781 | SRY-box containing
gene 18 | Sox18 | 3 | 1.2144 | 1.5284 | 1.0687 |
|
| B, Commonly
downregulated genes in the Is group |
|
| Probes | Name | Gene symbol | Chromosome | Intestine log
ratioa | Kidney log
ratioa | Muscle log
ratioa |
|
| Apoptosis | | | | | | |
| AF517560 | Caspase 9 | Casp9 | 5 | −1.0343 | −1.1199 | −1.1743 |
| Signaling
pathway | | | | | | |
| NM_144730 | GATA binding
protein 4 | Gata4 | 15 | −1.2419 | −1.1162 | −1.0620 |
| NM_024400 | A disintegrin-like
and metallopeptidase with thrombospondin type 1 motif, 1 | Adamts1 | 11 | −1.0472 | −1.1230 | −1.7934 |
| NM_001000131 | Olfactory receptor
50 | Olr50 | 1 | −1.4110 | −2.0005 | −1.1353 |
| Adhesion
molecules | | | | | | |
| NM_012702 | Carcinoembryonic
antigen-related cell adhesion molecule 3 | Ceacam3 | 1 | −1.1057 | −1.8840 | −1.0048 |
| Protein coding | | | | | | |
| NM_001037518 | Defensin beta
23 | Defb23 | 3 | −1.0558 | −1.4811 | −1.0821 |
| XM_575765 | Similar to
suppressor of initiator codon mutations, related sequence 1 | RGD1560994 | 5 | −1.0367 | −1.3214 | −1.2535 |
| XM_001053867 | Hypothetical
protein LOC679650 | LOC679650 | 4 | −1.2825 | −1.3383 | −1.7832 |
| XM_001058313 | Hypothetical
protein LOC680675 | LOC680675 | 2 | −1.1665 | −1.8205 | −1.3372 |
| XM_001066721 | Hypothetical
protein LOC688387 | LOC688387 | 15 | −1.3056 | −1.5512 | −2.0187 |
| XM_001071268 | Hypothetical
protein LOC689585 | LOC689585 | 14 | −1.2310 | −1.2955 | −3.3694 |
| XM_001075138 | Hypothetical
protein LOC690663 | LOC690663 | 7 | −1.0482 | −1.3571 | −1.8585 |
| XM_001079793 | Hypothetical
protein LOC691833 | LOC691833 | 7 | −1.2318 | −2.5800 | −1.1789 |
|
| C, Commonly
upregulated genes in the I/R group |
|
| Probes | Name | Gene symbol | Chromosome | Intestine log
ratioa | Kidney log
ratioa | Muscle log
ratioa |
|
| Toll-like receptor
signaling pathway | | | | | | |
| MAPK pathway | | | | | | |
| BC078738 | Jun oncogene | Jun | 5 | 1.5935 | 2.2445 | 4.1868 |
| BC078903 | Activating
transcription factor 3 | Atf3 | 13 | 2.8074 | 3.3245 | 4.4505 |
| NM_012715 | Adrenomedullin | Adm | 1 | 1.0172 | 1.5631 | 1.6412 |
| NM_021836 | Jun-B oncogene | Junb | 19 | 1.8383 | 1.2752 | 3.8498 |
| NM_022197 | FBJ osteosarcoma
oncogene | Fos | 6 | 2.1100 | 3.9940 | 2.1579 |
| NM_053769 | Dual specificity
phosphatase 1 | Dusp1 | 10 | 1.9596 | 1.1450 | 2.7656 |
| NF-κB pathway | | | | | | |
| XM_221537 | Nfkbiz | Nfkbiz | 11 | 1.3469 | 1.8728 | 3.9178 |
| NM_017259 | B-cell
translocation gene 2 | Btg2 | 13 | 1.1585 | 1.1407 | 1.6365 |
| NM_022542 | Ras homolog gene
family, member B | Rhob | 6 | 1.9195 | 1.2385 | 2.9469 |
| L25925 |
Cyclooxygenase-2 | Cox2 | 13 | 2.7905 | 2.1123 | 1.0614 |
| Cell proliferation
and differentiation | | | | | | |
| BC070878 | Polo-like kinase 2
(Drosophila) | Plk2 | 2 | 1.6628 | 1.8470 | 1.7820 |
| NM_031642 | Kruppel-like factor
6 | Klf6 | 17 | 1.1219 | 1.6330 | 1.6238 |
| NM_024388 | Nuclear receptor
subfamily 4 | Nr4a1 | 7 | 2.2865 | 1.6597 | 4.9402 |
| Protein
binding | | | | | | |
| NM_001003401 | Ectodermal-neural
cortex 1 | Enc1 | 2 | 1.7737 | 1.2063 | 3.1094 |
| NM_001009541 | Immediate early
response 2 | Ier2 | 19 | 1.6128 | 2.0729 | 1.8945 |
| Cytokine | | | | | | |
| NM_031512 | Interleukin 1
beta | IL-1β | 3 | 1.0116 | 1.0882 | 2.1743 |
| NM_053565 | Suppressor of
cytokine signaling 3 | Socs3 | 10 | 1.3094 | 1.2761 | 1.4714 |
| NM_012945 | Heparin-binding
EGF-like growth factor | Hbegf | 18 | 2.8253 | 1.5989 | 2.3193 |
| Circulation and
coagulation | | | | | | |
| NM_173141 | Tissue factor
pathway inhibitor 2 | Tfpi2 | 4 | 1.0111 | 1.3111 | 2.7850 |
| NM_001003403 | Vascular early
response gene protein | Verge | 4 | 1.0461 | 1.1412 | 3.5718 |
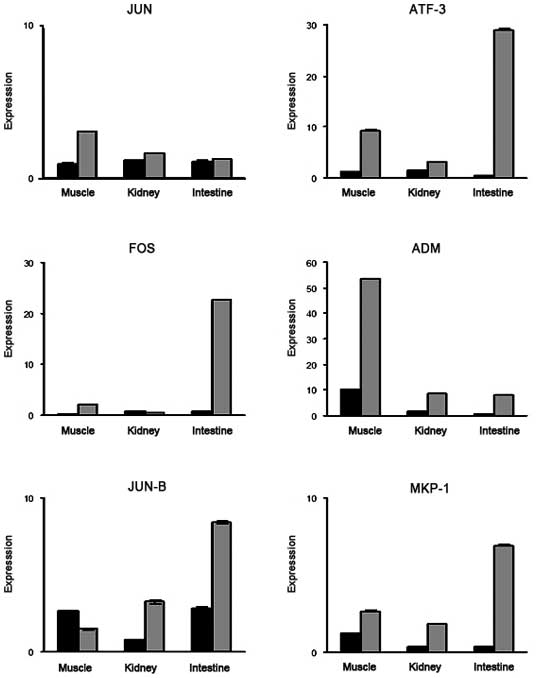
Validation of target gene expression in
the I/R injury model using qPCR
In order to confirm the validity of the microarray
findings with regard to the genes up- or downregulated in common in
all three organ models, the same RNA samples of the three organs
used for the microarrays were subjected to qPCR. Primers were
selected for six representative genes associated with the MAPK
pathway, including Atf3 (GenBank: NM_012912, BC078903),
Jun (GenBank: BC078738), Jun b (GenBank: NM_021836),
c-Fos (GenBank: NM_022197), Dusp1 (GenBank:
NM_053769) and Adm (GenBank: NM_012715). The results of the
qPCR expression are shown in Fig.
2. The majority of the qPCR results confirmed the upregulated
gene expression. Selected gene expression status in three different
organs was additionally examined by qPCR. The expression levels of
each gene in each organ detected by qPCR and microarray experiments
were comparable.
Discussion
The detailed mechanisms of I/R injury in individual
organs have not been fully elucidated due to the molecular
complexity of the condition. The present study used a single organ
model and gene expression profiling method to identify specific
molecules that may be important in I/R injury at an early ischemia
and reperfusion time-point. After 1 h ischemia, there was only one
commonly upregulated gene (Sox18; NM_001024781) and 13
downregulated genes. Overexpression of Sox18 in blood
vascular endothelial cells was previously reported to induce
angiogenesis and lymphangiogenesis, which is associated with the
ischemic response of organs. Sox18 therefore has the
potential be an organ-ischemic marker (13). Of the 13 common downregulated
genes, Gata4 (NM_144730) is a downstream gene of the MAPK
pathway and its downregulation may represent the inactive status of
the extracellular signal-regulated protein kinase (ERK) 1/2
pathway, which corresponded to the inactivity of nuclear factor
kappa-light-chain-enhancer of activated B-cells (NFκB) and
activator protein (AP)-1 at this time-point (14). Ischemic insult also induces
apoptosis and angiogenesis in order to respond to the hypoxic
status, thus the downregulation of the adversely effected genes,
including Casp9 (AF517560), Adamts1 (NM_024400) and
Ceacam3 (NM_012702) are expected (15–17).
After 1 h reperfusion, additional biological
activities were present, in which the interacting functions
increased the biological complexity. There were 20 commonly
upregulated gene probe sets in the I/R group. The majority of genes
were not significantly upregulated during the initial 1 h of
ischemia. Among these genes, several were involved in the MAPK and
NFκB pathways. These two pathways may serve as the common pathways
between the three organs at this time-point and modulate the
biochemical response towards I/R injury (18,19).
Six genes were identified that were involved in the
MAPK signaling pathway. Four of these were associated with the
heterodimeric protein AP-1, Jun, Atf3, Jun b, and Fos. AP-1 is one
of the end targets of the MAPK signaling pathway, and is considered
to mediate I/R-induced gene expression since numerous subunit genes
are known to mediate either proliferation, differentiation, or
apoptosis (Jun family predominant) by altering the expression
levels of cytokines, neurotransmitters, and other intercellular
signaling molecules (20,21). AP-1 is additionally known to
function in the process of T-cell activation, which is a key
process in transplant immunology (22). In addition, AP-1 activates numerous
downstream genes which are implicated in organ damage (23,24).
AP-1 consists of three major subfamilies, including Jun, Fos, and
Atf (25). In the early phase
following I/R stress, the high expression levels of Jun and Atf
activate the JNK and P38 pathways, promoting apoptosis. The high
expression levels of Jun and Fos activate the ERK1/2 pathway to
promote cellular proliferation (26). The data from the present study
showed that there was a higher expression of Jun,
Junb and Fos, but no significant difference in the
expression of Atf3. This expression pattern was compatible
with the previously described theories of apoptosis (27). Atf3, however, was found to
be a common gene with higher expression (26). Atf3 is a stress-inducible
gene that encodes a member of the ATF/cyclic adenosine
monophosphate response element binding protein family of
transcription factors (28).
Atf3 mRNA was observed to increase in expression within 2 h
following exposure of cells to stress signals, and therefore,
Aft3 is a suitable candidate for further analysis in I/R
injury.
The MAPK pathway may additionally be mediated during
I/R injury by higher expression levels of Dusp1 and Adm, which
downregulate the MAPK pathway. Dusp1 is an oxidative
stress-inducible gene that acts as a negative regulator of the JNK
and p38 pathways (29). Adm
selectively inhibits the JNK pathway, therefore the two genes may
act in opposition to AP-1 (30).
The adjustment of their expression may facilitate a reduction in
I/R injury.
The NFκB pathway is another important pathway that
responds to I/R injury at this time-point. Ischemic insult
activates NFκB-inducing kinase, which degrades IκB kinase and
releases NFκB. NFκB then translocates to the nucleus to induce
bioactivities including promotion of transcription and activation
of adhesion molecules, cytokines and maturing of B cells (31). According to the presented database,
the upregulation of Rhob (NM_022542) may repress NFκB
signaling by inhibiting dissociation and subsequent degradation of
IκB, therefore further diminishing the downstream inflammatory
response. Two genes were additionally identified to modulate
B-cells. Btg2 (NM_017259), the p53-transcriptional target,
is an anti-proliferative B-cell translocation gene. Over-expression
of Btg2 has a protective role, inducing B-cell depletion,
which can further reduce the inflammatory response. Conversely,
Nfkbiz (GenBank: XM_221537) activates B-cell proliferation
and differentiation to enhance the inflammatory response (32). The present study additionally
identified prostaglandin-endoperoxide synthase 2 (Cox2;
GenBank: L25925, NM_017232) to be upregulated in the three organ
models. Cox2 is an enzyme that catalyzes the initial step of
the synthesis of inflammatory prostaglandins from arachidonic acid.
The upregulation of Cox2 can activate the NFκB pathway and
perform additional downstream bioactivities (33).
The cytokines and adhesion molecules triggered by
different signaling pathways function to initiate the inflammatory
response towards I/R insult. According to the presented database,
only interleukin 1β (IL-1β; NM_031512) was identified to be
upregulated in all three organ models. However, Hbegf
(GenBank: NM_012945) and Socs3 (GenBank: NM_053565) were two
genes identified that act as a negative controller, eliciting
protective effects against cytokine and adhesion molecules, and
diminishing the inflammatory response.
Other genes were identified in the present study
that have not been previously associated with I/R injury, however
may be functional in the I/R response. These genes included
Verge (GenBank: NM_001003403) and Tfpi2 (GenBank:
NM_173141), which were noted to be associated with angiogenesis and
capillary endothelial and microcirculation dysfunction, as well as
Plk2 (GenBank: NM_031821, BC070878), Klf6 (NM_031642)
and Nr4a1 (NM_024388), which are involved in the G1 phase of
the cell cycle and can promote cellular proliferation and prevent
apoptosis (34). The schematic
diagram in Fig. 3 illustrates the
proposed complex mechanisms of I/R conditions.
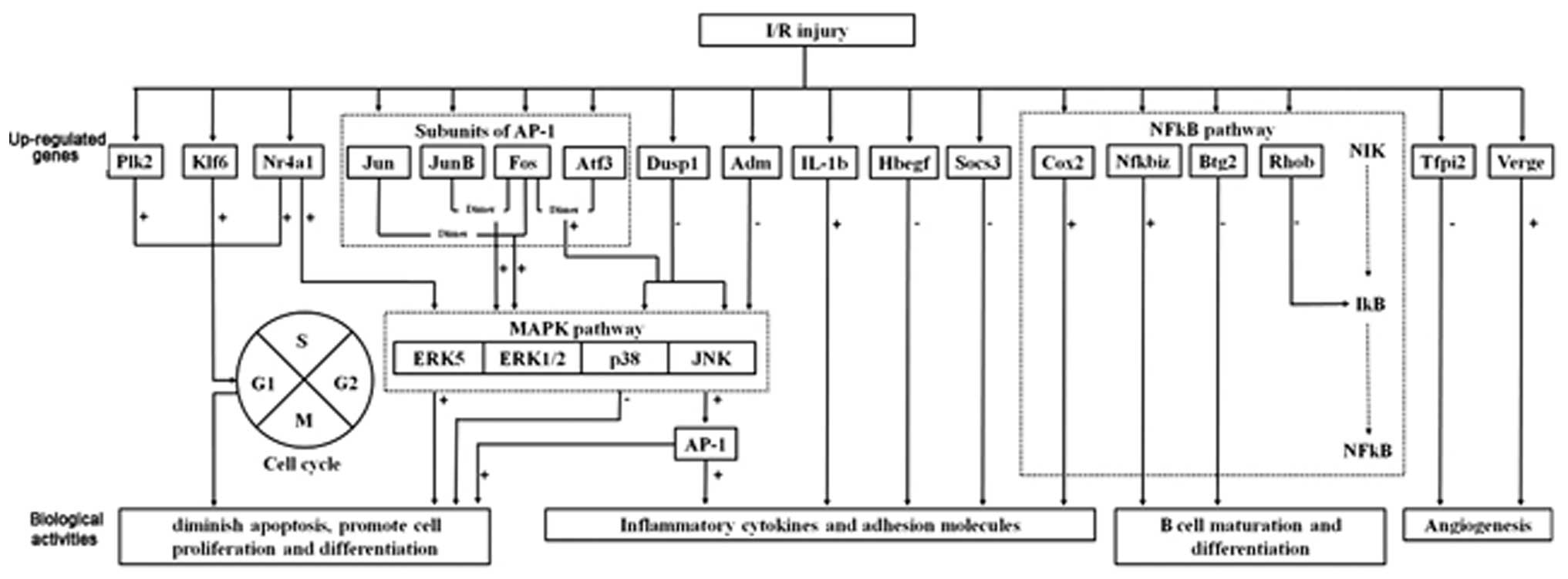 | Figure 3Schematic diagram illustrating the
proposed complex mechanisms of I/R conditions. For MAPK pathway
modulation, the selective inhibition of the p38 and JNK pathway can
be achieved by enhanced expression of Dusp1 and Adm,
together with inhibition of AP-1. For NFκB pathway
modulation, enhanced expression of Btg2 and Rhob
together with inhibition of Nfkbiz and the downstream target
gene Cox2 can diminish the inflammatory response. The action
towards reducing apoptosis and promotion of cellular proliferation
can be achieved through the upregulation of Klf6 and
Plk2, and inhibition of the p38 pathway. Control of cytokine
and adhesion molecules may be achieved through direct inhibition of
IL-1β and enhanced expression of Hbegf and
Socs3. For modulation of microcirculation, the upregulation
of Verge and downregulation of Tfpi2 may promote
angiogenesis. I/R, ischemia/reperfusion; ATF-3, activating
transcription factor 3; FOS, FBJ osteosarcoma; ADM,
adrenomedullin; MAPK, mitogen-activated protein kinase; NFκB,
nuclear factor κB; Dusp1, dual specificity phosphatase 1;
AP-1, activator protein 1; Btg2, B-cell translocation
gene 2; Rhob, Ras homolog gene family member B; Cox2,
cyclooxygenase 2; Klf6, Kruppel-like factor 6; Plk2,
polo-like kinase 2; IL, interleukin; Hbegf,
heparin-binding EGF-like growth factor; Socs3, suppressor of
cytokine signaling 3; Verge, vascular early response gene
protein; Tfpi2, tissue factor pathway inhibitor 2. |
In the present study, the uniquely affected genes in
the three organ models in both ischemia and reperfusion status were
identified and compared. Among these genes, several were identified
to be associated with the MAPK and NFκB signaling pathways. The
present study focused on only two time-points following I/R insult;
therefore, the kinetic changes of the specific genes require
further investigation. This study provided fundamental information
to the understanding of the key biomechanical changes during I/R
injury.
Acknowledgements
The authors would like to thank the Chang Gung
Memorial Hospital for financial support (nos. CMRPG470041,
CMRPG4B0021, CMRPG4A0101 and CMRPG4A0102), and the Chang Gung
Memorial Hospital Urology Laboratory.
References
|
1
|
Ames A: Cerebral ischemia. II The
no-reflow phenomenon. Am J Pathol. 52:4371968.PubMed/NCBI
|
|
2
|
Hsieh Y-H, Huang S-S, Wei F-C and Hung
L-M: Resveratrol attenuates ischemia - reperfusion-induced
leukocyte - endothelial cell adhesive interactions and prolongs
allograft survival across the MHC barrier. Circ J. 71:423–428.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei W, Wei FC and Hung L-M: Diazoxide
ameliorates microcirculatory disturbances through PKC-dependent
pathway in I/R-injured rat cremaster muscles. J Biomed Sci.
12:521–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kobrin SM: Diabetic nephropathy. Dis Mon.
44:214–234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shihab FS: Cyclosporine nephropathy:
pathophysiology and clinical impact. Semin Nephrol. 16:536–547.
1996.PubMed/NCBI
|
|
6
|
Wu X, Pang ST, Sahlin L, et al: Gene
expression profiling of the effects of castration and estrogen
treatment in the rat uterus. Biol Reprod. 69:1308–1317. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pang ST, Dillner K, Wu X, et al: Gene
expression profiling of androgen deficiency predicts a pathway of
prostate apoptosis that involves genes related to oxidative stress.
Endocrinology. 143:4897–4906. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu MS, Yang CW, Chang CT, Bens M and
Vandewalle A: Cyclosporin increases the density of angiotensin II
subtype 1 (AT1) receptors in mouse medullary thick ascending limb
cells. Nephrol Dial Transplant. 18:1458–1465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu MS, Bens M, Yu HM and Vandewalle A:
Cyclosporine reduces basolateral, but not apical, nitric oxide
secretion in medullary thick ascending limb cells. Transpl Int.
13:S321–S323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mason J: The pathophysiology of Sandimmune
(cyclosporine) in man and animals. Pediatr Nephrol. 4:554–574.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo CC, Chen HM, Chiu CH, Lin JN and Chen
JC: Effect of N(G)-nitro-L-arginine methyl ester on intestinal
permeability following intestinal ischemia-reperfusion injury in a
rat model. Biol Neonate. 80:60–63. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basile DP, Fredrich K, Alausa M, et al:
Identification of persistently altered gene expression in the
kidney after functional recovery from ischemic acute renal failure.
Am J Physiol Renal Physiol. 288:F953–F963. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
François M, Caprini A, Hosking B, et al:
Sox18 induces development of the lymphatic vasculature in mice.
Nature. 456:643–647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang Q, Wiese RJ, Bueno OF, et al: The
transcription factor GATA4 is activated by extracellular
signal-regulated kinase 1-and 2-mediated phosphorylation of serine
105 in cardiomyocytes. Mol Cell Biol. 21:7460–7469. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park M-T, Choi J-A, Kim M-J, et al:
Suppression of extracellular signal-related kinase and activation
of p38 MAPK are two critical events leading to caspase-8- and
mitochondria-mediated cell death in phytosphingosine-treated human
cancer cells. J Biol Chem. 278:50624–50634. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basile DP, Fredrich K, Chelladurai B,
Leonard EC and Parrish AR: Renal ischemia reperfusion inhibits VEGF
expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J
Physiol Renal Physiol. 294:F928–F936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skubitz KM and Skubitz A: Interdependency
of CEACAM-1, -3, -6, and -8 induced human neutrophil adhesion to
endothelial cells. J Transl Med. 6:782008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qi M and Elion EA: MAP kinase pathways. J
Cell Sci. 118:3569–3572. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mullonkal CJ and Toledo-Pereyra LH: Akt in
ischemia and reperfusion. J Invest Surg. 20:195–203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaulian E and Karin M: AP-1 as a
regulator of cell life and death. Nat Cell Biol. 4:E131–136. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Halloran PF: Immunosuppressive drugs for
kidney transplantation. N Engl J Med. 351:2715–2729. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yeh KY, Yeh M, Glass J and Granger DN:
Rapid activation of NF-kappaB and AP-1 and target gene expression
in postischemic rat intestine. Gastroenterology. 118:525–534. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karin M: The regulation of AP-1 activity
by mitogen-activated protein kinases. J Biol Chem. 270:16483–16486.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shima Y, Tajiri T, Taguchi T and Suita S:
Increased expression of c-fos and c-jun in the rat small intestinal
epithelium after ischemia-reperfusion injury: a possible
correlation with the proliferation or apoptosis of intestinal
epithelial cells. J Pediatr Surg. 41:830–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hai T and Hartman MG: The molecular
biology and nomenclature of the activating transcription
factor/cAMP responsive element binding family of transcription
factors: activating transcription factor proteins and homeostasis.
Gene. 273:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ameyar M, Wisniewska M and Weitzman JB: A
role for AP-1 in apoptosis: the case for and against. Biochimie.
85:747–752. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang Y, Chen C-R and Massagué J: A
self-enabling TGFbeta response coupled to stress signaling: Smad
engages stress response factor ATF3 for Id1 repression in
epithelial cells. Mol Cell. 11:915–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Weng Y, Shen F, Li J, Shen Y and Zhang X:
Expression changes of mitogen-activated protein kinase
phosphatase-1 (MKP-1) in myocardium of streptozotocin-induced
diabetic rats. Exp Clin Endocrinol Diabetes. 115:455–460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshimoto T, Fukai N, Sato R, et al:
Antioxidant effect of adrenomedullin on angiotensin II-induced
reactive oxygen species generation in vascular smooth muscle cells.
Endocrinology. 145:3331–3337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Crispín JC, Tedder TF, Dalle Lucca
J and Tsokos GC: B cells contribute to
ischemia/reperfusion-mediated tissue injury. J Autoimmun.
32:195–200. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Sakai T, Sano N and Fukui K:
Nucling mediates apoptosis by inhibiting expression of galectin-3
through interference with nuclear factor kappaB signalling. Biochem
J. 380:31–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Malek HA and Saleh DM: Cyclooxygenase-2
inhibitor celecoxib in a rat model of hindlimb ischemia
reperfusion. Can J Physiol Pharmacol. 87:353–359. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hollander MC, Poola-Kella S and Fornace
AJ: Gadd34 functional domains involved in growth suppression and
apoptosis. Oncogene. 22:3827–3832. 2003. View Article : Google Scholar : PubMed/NCBI
|