Introduction
Oesophageal squamous cell carcinoma (ESCC) is one of
the most common types of malignant tumour, accounting for ~80% of
patients with cancer from developing countries, including Northern
China (1,2). The clinical outcome and prognosis of
patients are poor due to the highly invasive and metastatic nature
of the disease. In previous studies by our group, Twist (3), short chain dehydrogenase/reductase
family 9C, member 7 (4) and bone
morphogenetic protein 7 (5) were
found to have significant roles in the metastasis of ESCC. However,
the precise mechanisms underlying the metastasis of ESCC remain to
be elucidated. Therefore, the identification of potential markers
for the diagnosis and treatment of ESCC metastasis is critical.
p28GANK, which is also known as PSMD10 or gankyrin,
is the product of the p28 gene and a subunit of the regulatory
complex of the human 26S proteosome, with seven ankyrin repeats.
p28GANK regulates the malignant growth of cancer cells by
activating murine double minute 2, leading to the proteasomal
degradation of the tumour suppressor gene p53. A previous study by
our group revealed that overexpression of p28GANK accelerated the
malignant progression of human colorectal and pancreatic cancers
(6,7). The over-expression of p28GANK has
also been shown to accelerate the invasive ability and metastasis
of hepatocellular carcinoma (8),
breast cancer (9), oral cancer
(10) and glioma (11). In addition, p28GANK was
demonstrated to be essential for hypoxia-enhanced metastatic
potential in breast cancer cells (12). The overexpression p28GANK confers
multidrug resistance to liver and gastric cancer cells (13). In ESCC, p28GANK overexpression was
associated with poor prognosis and promoted tumour progression
(14). However, the specific role
of p28GANK in ESCC metastasis remains to be elucidated.
In the present study, the significance of p28GANK
expression in ESCC metastasis was evaluated using quantitative
polymerase chain reaction (qPCR) and immunohistochemical analyses.
Additionally, western blot analysis was performed to assess p28GANK
expression in highly invasive and non-invasive ESCC cells. Finally,
in order to investigate the role of p28GANK in ESCC metastasis,
lentivirus-mediated short interfering RNA (siRNA), targeting
p28GANK was transfected into highly invasive ESCC cells.
Materials and methods
Clinical specimens
For immunohistochemical staining, paraffin-embedded
ESCC tissues from 112 patients (84 males, 28 females), aged 36–72
years (mean, 57.2 years), were obtained between 2008 and 2010 at
the General Hospital of Chengdu Military Command (Chengdu, China).
For qPCR analysis, 52 fresh ESCC tissue samples were obtained, of
which 26 were from patients with metastasis and 26 were from
patients without metastasis. All samples were immediately placed in
liquid nitrogen, in which they were stored prior to use. All
patients received ESCC resections between January 2010 and January
2013 in the Department of Chest Surgery, General Hospital of
Chengdu Military Command, Chengdu, China. These patients did not
undergo chemotherapy or radiotherapy prior to surgical ablations
and written consent for the use of ESCC tissues was obtained from
all patients. The present study was authorised by the Ethical
Committee of the General Hospital of Chengdu Military Command
(Chengdu, China).
ESCC cell lines
ESCC cell lines EC109 and EC9706 were purchased from
the Chinese Academy of Medical Science (Beijing, China) and
maintained in RPMI-1640 medium (Gibco-BRL, Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% foetal
bovine serum (Gibco-BRL) at 37°C in humidified air containing 5%
CO2. Highly and non-invasive ESCC sub-lines were
constructed using repeated Transwell assays as described previously
(4). Following a ten-round
selection and expansion, the highly invasive sub-lines were
established and named EC9706-P and EC109-P, and the non-invasive
sub-lines were named EC109-N and EC9706-N.
qPCR
A qPCR assay was performed to detect p28GANK mRNA
expression in ESCC tissues and highly invasive and non-invasive
cell lines, and the results were calculated as previously described
(15). The primer sequences used
were as follows: p28GANK forward, 5′-TCTTCAAGCCATCCTGTGTG-3′ and
reverse, 5′-TGGTGATGTTGGACT CCTCA-3′; β-actin (internal control)
forward, 5′-ATGATATCGCCGCGCTCGTC-3′ and reverse, 5′-CGCTCGGTGAGG
ATCTTCA-3′ (Huada Gene Biotechnology Co., Ltd., Shenzheng,
Guangdong, China).
Immunohistochemical staining
Immunohistochemical staining was performed as
previously described (8). Briefly,
the paraffin-embedded tissues were deparaffinised, rehydrated,
quenched of endogenous peroxidase activity and blocked with goat
serum (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing,
China). The tissues were incubated with rabbit anti-gankyrin
antibody (1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. Following three washes with phosphate-buffered
saline Tween 20, the tissues were incubated with biotinylated goat
anti-rabbit immunoglobulin G (IgG)/horseradish peroxidase (HRP)
(1;2,000; Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 50
min at room temperature. 3,3-diaminoben-zidine tetrahydrochloride
was used for visualisation. The stained cells were counted, and the
results were scored based on the proportion of stained cells (0%,
0; 1–33%, 1; 34–66%, 2 and 67–100%, 3) and intensity (achromatic,
0; amber, 1; yellow, 2 and brown, 3) of staining. Immunoreactivity
was determined by the combined scores as follows: Negative (−),
0–2; positive (+), 3–6. Ten representative fields for each sample
were counted, using an SZ51 microscope (Olympus Corporation, Tokyo,
Japan).
Western blot analysis
Western blot analyses were performed as previously
described (8). Total protein
lysates were separated by 12% SDS-PAGE and transferred onto
nitrocellulose membranes. The membranes were blocked with 10%
fat-free milk and incubated with rabbit polyclonal anti-p28GANK
(1:100; Santa Cruz Biotechnology, Inc.) and mouse monoclonal
anti-β-actin (1:3,000; Sigma-Aldrich, St. Louis, MO, USA).
Following incubation with monoclonal HRP-coupled goat anti-mouse
IgG (1:2,000; BiosPacific, Inc., Emeryville, CA, USA) and goat
anti-rabbit (1:2,000; BiosPacific, Inc.) secondary antibodies and
enhanced chemiluminescence solution (ECL-kit; Thermo Fisher
Scientific, Waltham, MA, USA) was used to visualise the signals.
The protein expression results were based on the strip area of the
target proteins, relative to the controls.
Lentivirus-mediated siRNA
transfection
The p28GANK siRNA (5′-CTGACCAGGACAGCAGAAC-3′) was
sub-cloned into the PGC-lentiviral system with enhanced green
fluorescent protein (EGFP). The siRNA and control lentiviruses
generated were subsequently transfected into highly invasive
EC9706-P and EC109-P cells, and the transfected cells were named
Con-EC9706-P, Si-EC9706-P, Con-EC109-P and Si-EC109-P,
respectively.
Transwell assays
Transwell assays, including cell migration and
invasion assays, were performed as previously described (4). Once the cells on the upper membrane
surface were removed, the number of cells on the lower surface was
counted under a ×200 visual field using an inverted microscope.
Five representative fields were counted for each cell line using an
SZ51 microscope (Olympus Corporation) and the results presented are
the mean of five representative experiments.
Statistical analysis
The results were analysed using SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered to
indicate a statistically significant difference between values.
Student’s t-test was used to analyse differences in mRNA
expression. The Kruskal-Wallis test was performed to analyse the
potential correlations between p28GANK expression and the clinical
parameters of patients with ESCC. The one-way analysis of variance
test was used to analyse differences in the Transwell assays.
Results
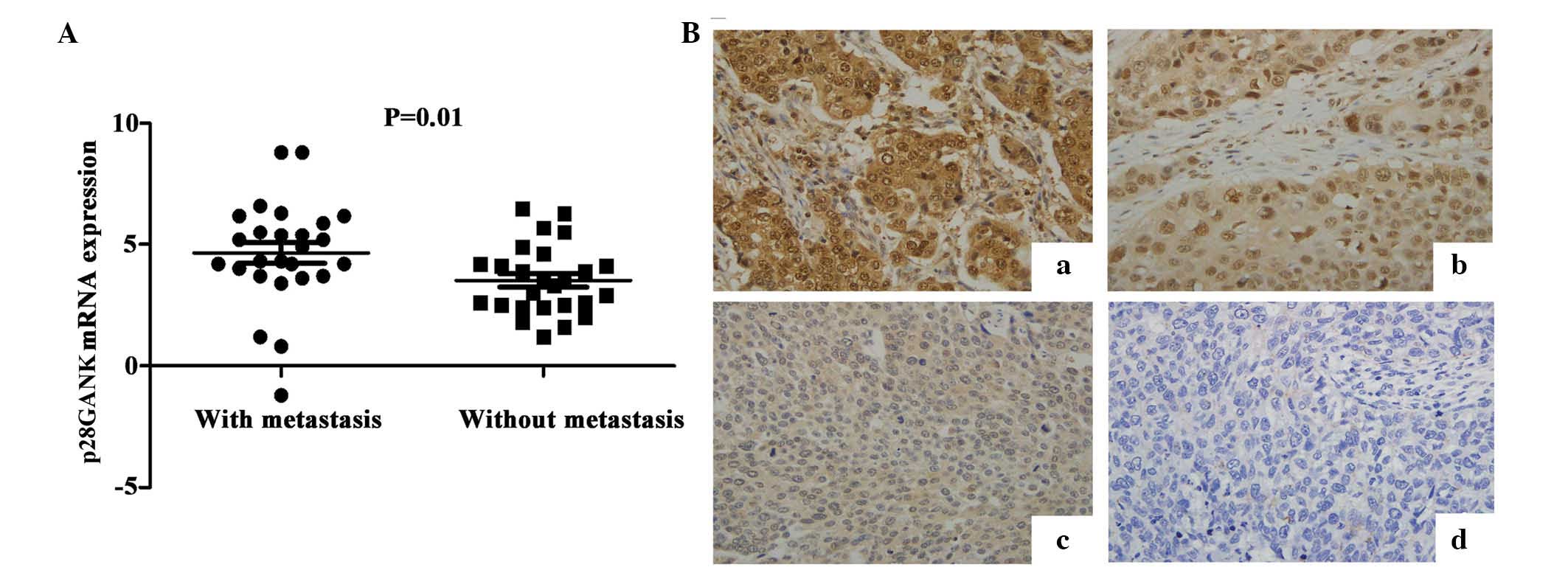
p28GANK is highly expressed in metastatic
ESCC tissues
To investigate the significance of p28GANK
expression in ESCC metastasis, the expression of p28GANK mRNA in 52
ESCC tissues was evaluated using qPCR analysis. The mean expression
levels of p28GANK mRNA in ESCC tissues from patients with
metastasis were significantly higher (4.65±2.16) than those of ESCC
specimens from patients without metastasis (3.52±1.43; P=0.01;
Fig. 1A).
p28GANK expression in ESCC tissues is
correlated with T-stage, lymph node metastasis and lymphatic
invasion
Immunohistochemical staining was performed to assess
the expression of p28GANK protein in ESCC tissues from 112
patients. Positive p28GANK expression was observed in the cytoplasm
and/or nucleus in ESCC tissues (Fig.
1B). The distribution of negative, weakly positive, moderately
positive and strongly positive p28GANK expression were 20.5%
(23/112), 27.7% (31/112), 41.1% (46/112) and 10.7% (12/112),
respectively. Statistical analysis indicated that p28GANK
expression was correlated with ESCC T-stage (P=0.01), lymph node
metastasis (P<0.01) and lymphatic invasion (P<0.01). However,
there was no significant correlation between p28GANK expression and
patient age, gender or level of differentiation (Table I; P>0.05).
 | Table ISignificance of p28GANK expression in
oesophageal squamous cell carcinoma tissues. |
Table I
Significance of p28GANK expression in
oesophageal squamous cell carcinoma tissues.
| Factor | n | p28GANK expression
| P-value |
|---|
| − | ± | + | ++ |
|---|
| Gender | | | | | | 0.51 |
| Female | 28 | 6 | 9 | 11 | 2 | |
| Male | 84 | 17 | 22 | 35 | 10 | |
| Age (years) | | | | | | 0.58 |
| ≤57 | 56 | 12 | 17 | 21 | 6 | |
| >57 | 56 | 11 | 14 | 25 | 6 | |
| T stage | | | | | | 0.01 |
| I | 12 | 5 | 5 | 1 | 1 | |
| II | 46 | 11 | 14 | 17 | 4 | |
| III | 48 | 7 | 12 | 23 | 6 | |
| IV | 6 | 0 | 0 | 5 | 1 | |
| Metastasis | | | | | | <0.01 |
| N0 | 50 | 21 | 18 | 11 | 0 | |
| N1 | 35 | 1 | 6 | 20 | 8 | |
| N2 | 23 | 1 | 5 | 13 | 4 | |
| N3 | 4 | 0 | 2 | 2 | 0 | |
| Differentiation | | | | | | 0.08 |
| Well | 1 | 5 | 9 | 3 | 18 | |
| Moderate | 8 | 10 | 19 | 5 | 42 | |
| Poor | 14 | 16 | 18 | 4 | 52 | |
| Lymphatic
invasion | | | | | | <0.01 |
| Without | 55 | 18 | 21 | 15 | 1 | |
| With | 57 | 5 | 10 | 31 | 11 | |
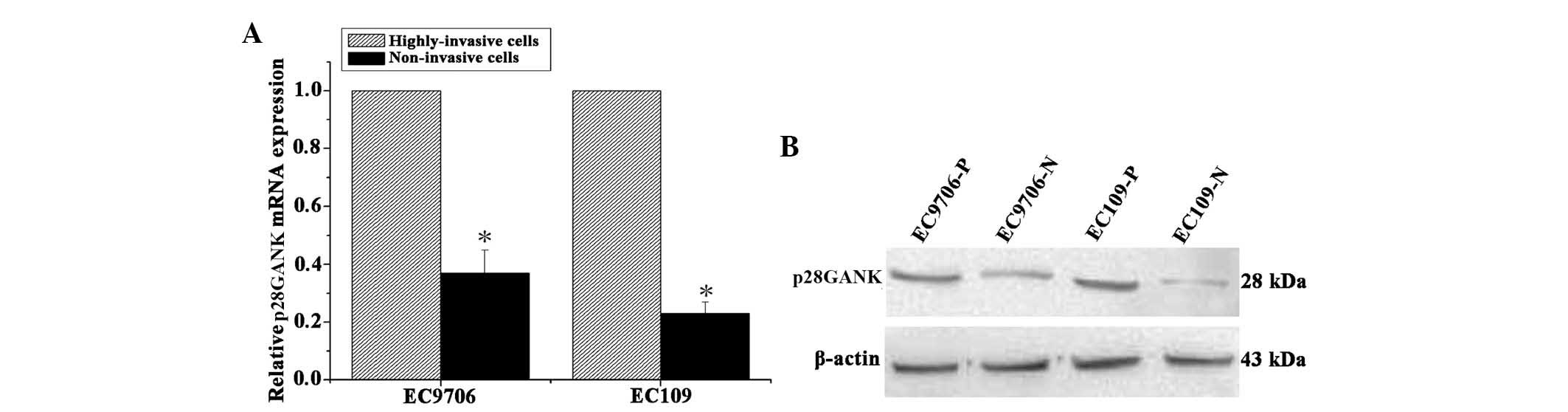
p28GANK expression is increased in highly
invasive ESCC cell lines
The highly invasive and the non-invasive ESCC cell
lines were constructed using repeated Transwell assays, as
described in a previous study by our group (4). qPCR analysis revealed that p28GANK
mRNA expression levels were significantly higher in the highly
invasive ESCC cell lines, EC109-P and EC9706-P, compared with those
in their matched, non-invasive cell lines, EC109-N and EC9706-N
(Fig. 2A). Consistent with the
results of qPCR analysis, western blot analysis indicated that the
expression levels of p28GANK protein were markedly higher in the
highly invasive cell lines compared with those in matched
non-invasive cell lines (Fig. 2B).
These results indicated that p28GANK expression levels were
increased in highly invasive ESCC cells compared with those in
non-invasive ESCC cells.
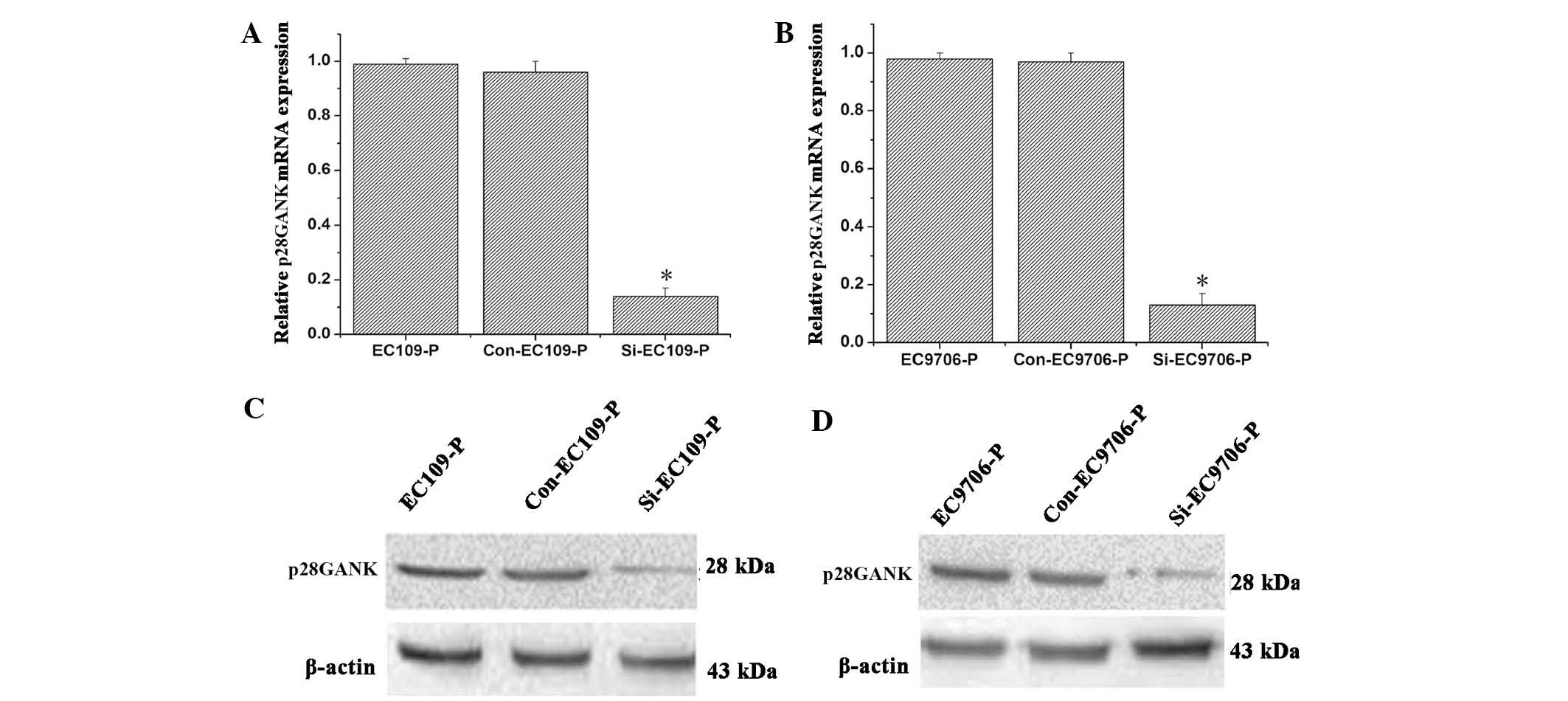
Decreased p28GANK expression suppresses
the migration and invasion of highly invasive ESCC cells
Lentivirus-mediated siRNA, targeting p28GANK was
used to evaluate the effects of p28GANK knockdown on the metastatic
phenotypes of highly invasive ESCC cells. qPCR analysis
demonstrated that lentivirus-mediated knockdown of p28GANK with
siRNA markedly inhibited p28GANK mRNA expression in the EC109-P and
EC9706-P cell lines (Fig. 3A and
B). Consistent with the results of qPCR analysis, western blot
analysis indicated that p28GANK expression levels were markedly
decreased in p28GANK siRNA transfected cells compared with those in
matched controls (Fig. 3C and D).
These results revealed that the lentivirus-mediated siRNA targeting
of p28GANK markedly decreased p28GANK expression in EC109-P and
EC9706-P cells.
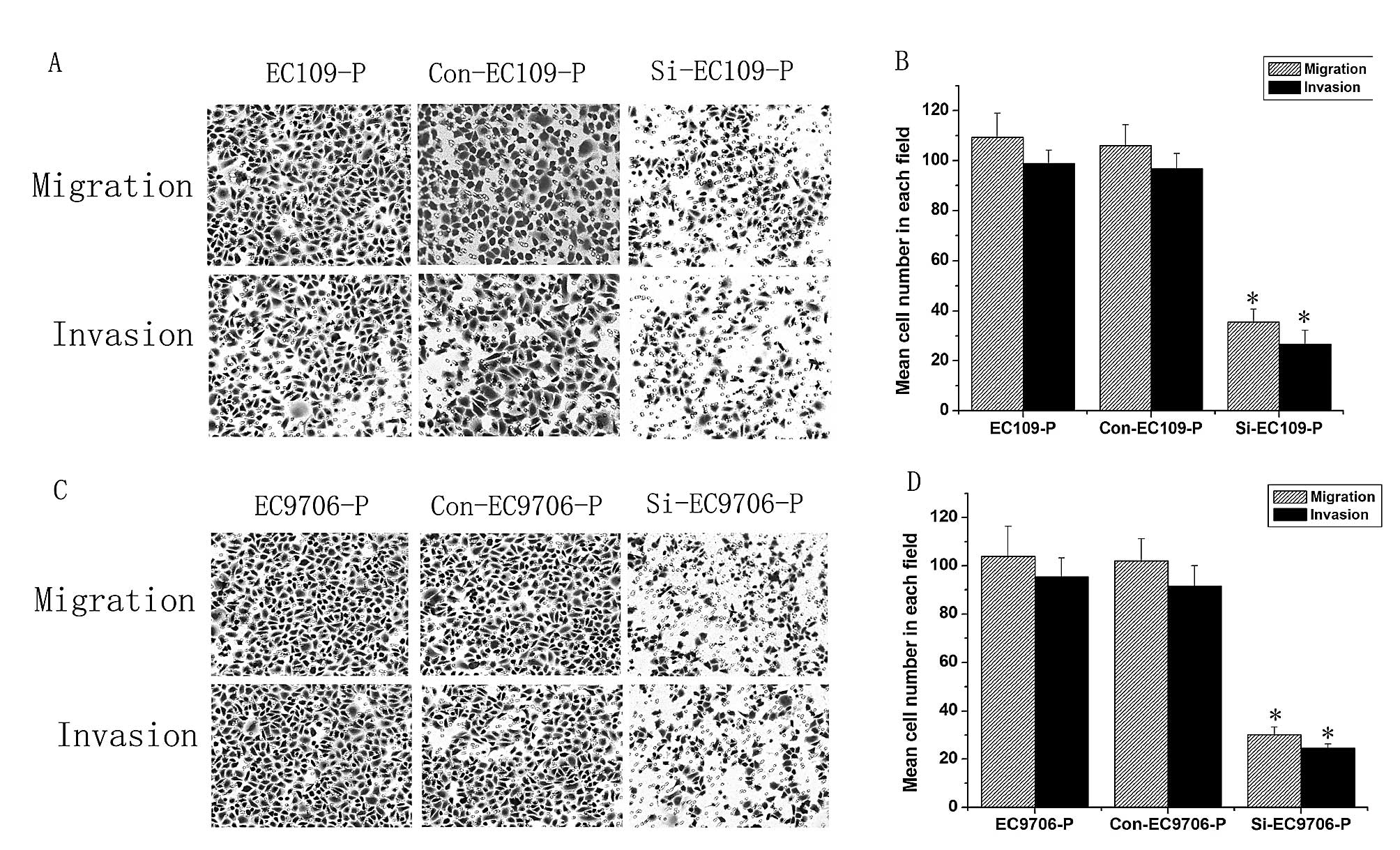
As shown in Fig. 4,
Transwell assays revealed that the migration and invasion abilities
of p28GANK-silenced cells were significantly lower than those of
matched controls, indicating that p28GANK inhibition repressed ESCC
cell metastasis in vitro.
Discussion
P28GANK is a regulator of the 26S proteasome, which
regulates the anti-apoptotic ability of cells by increasing the
proteasome-mediated degradation of p53 and results in the
alteration of p53-dependent gene expression (16). The overexpression of p28GANK
upregulates the phosphorylation of retinoblas-toma 1 (RB1) and
downregulates it, via its RB1-binding motif. This promotes
oncogenic transformation (17) and
degradation of Oct4, and promotes the expansion of
tumour-initiating cells in hepatocarcinogenesis (18). These studies revealed significant
roles for p28GANK in the development of malignant human tumours. In
addition, p28GANK was shown to increase the epithelial-mesenchymal
transition and the motility/invasiveness of hepatocellular
carcinoma cells, a process which is mediated by the activation of
hypoxia-inducible factor 1α signalling and consequently, promotes
TWIST1, vascular endothelial growth factor and metalloproteinase 2
expression (18). Increased
p28GANK expression is correlated with poor prognosis and may have a
significant role in ESCC tumour progression (14). Therefore, p28GANK may be a
potentially important therapeutic target gene in ESCC (14). However, the significance and
specific function of p28GANK in the metastasis of ESCC had remained
to be elucidated.
In the present study, the significance of p28GANK
mRNA expression in the metastasis of ESCC was evaluated. qPCR
analysis indicated that the levels of p28GANK mRNA were
significantly increased in metastatic ESCC tissues compared with
those of non-metastatic ESCC tissues. Furthermore, the results of
the immunohistochemical assay indicated that p28GANK expression was
significantly correlated with T-stage, lymph node metastasis and
lymphatic invasion. These findings were consistent with a previous
study, which indicated that positive p28GANK expression was
correlated with the extent of the primary tumour, lymph node
metastasis and distant lymph node metastasis (14). In addition, the significance of
p28GANK expression in the metastasis of ESCC was confirmed and it
was demonstrated that p28GANK was able to serve as a biomarker for
differentiating ESCC with or without metastatic potential.
In a previous study by our group, highly invasive
and non-invasive cell subpopulations were isolated from established
the ESCC cell lines, EC109 and EC9706-P, using the repeated
Transwell approach described in studies investigating tumour
metastasis (19,20). The two pairs of highly invasive and
non-invasive cell subpopulations that were obtained, were ideal
models for studying ESCC metastasis (4). Therefore, p28GANK expression was
assessed in the highly invasive and non-invasive cell
subpopulations using qPCR and western blot analyses. Consistent
with the results regarding p28GANK expression in ESCC tissues, mRNA
and protein expression levels of p28GANK were markedly higher in
the highly invasive cell lines, EC109-P and EC9706-P, compared with
those of matched, non-invasive cell lines, CE109-N and EC9706-N.
Furthermore, lentivirus-mediated siRNA knockdown of p28GANK
markedly decreased the expression of p28GANK in EC109-P and
EC9706-P cells, and Transwell assays revealed that the migration
and invasion abilities of the p28GANK knockdown cells were
significantly decreased. These results indicated that p28GANK
inhibition suppressed the metastasis of ESCC cells in vitro.
p28GANK promoted the hepatic metastasis of colorectal cancer by
activating the interleukin-8 signalling pathway (21) and controlled stem-cell behaviours
by regulating the expression of stemness factors (22). p28GANK has significant roles in the
metastasis of breast cancer by regulating Ras-related C3 botulinum
toxin substrate 1 activity and may be a potential therapeutic
target for breast cancer metastasis (23). The present study confirmed that
p28GANK was also critical for the metastasis of human ESCC,
although the precise mechanisms underlying these observations
remain to be elucidated.
In conclusion, the present study demonstrated that
the overexpression of p28GANK promoted the metastasis of ESCC in
tissues and cell lines and that p28GANK knockdown significantly
inhibited the metastatic potential of ESCC. This suggested that
p28GANK may function as a biomarker for differentiating ESCC with
or without metastatic potential and may be a potential therapeutic
marker for ESCC metastasis.
Acknowledgments
The present study was supported by a grant from the
National Natural Science Foundations of China (grant no. 81401993,
to Tang S).
References
|
1
|
Yu C, Chen K, Zheng H, et al:
Overexpression of astrocyte elevated gene-1 (AEG-1) is associated
with esophageal squamous cell carcinoma (ESCC) progression and
pathogenesis. Carcinogenesis. 30:894–901. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohebbi M, Mahmoodi M, Wolfe R, et al:
Geographical spread of gastrointestinal tract cancer incidence in
the Caspian Sea region of Iran: spatial analysis of cancer registry
data. BMC Cancer. 8:1372008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong T, Xue Z, Tang S, et al: Nuclear
expression of Twist promotes lymphatic metastasis in esophageal
squamous cell carcinoma. Cancer Biol Ther. 13:606–613. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang S, Gao L, Bi Q, et al: SDR9C7
promotes lymph node metastases in patients with esophageal squamous
cell carcinoma. PLoS One. 8:e521842013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu G, Tang S, Yang J, et al: BMP7
expression in esophageal squamous cell carcinoma and its potential
role in modulating metastasis. Dig Dis Sci. 58:1871–1879. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meng Y, He L, Guo X, et al: Gankyrin
promotes the proliferation of human pancreatic cancer. Cancer Lett.
297:9–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang S, Yang G, Meng Y, et al:
Overexpression of a novel gene gankyrin correlates with the
malignant phenotype of colorectal cancer. Cancer Biol Ther.
9:88–95. 2010. View Article : Google Scholar
|
|
8
|
Fu J, Chen Y, Cao J, et al: p28GANK
overexpression accelerates hepatocellular carcinoma invasiveness
and metastasis via phos-phoinositol 3-kinase/AKT/hypoxia-inducible
factor-1α pathways. Hepatology. 53:181–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhen C, Chen L, Zhao Q, et al: Gankyrin
promotes breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar
|
|
10
|
Li J, Knobloch TJ, Kresty LA, et al:
Gankyrin, a biomarker for epithelial carcinogenesis, is
overexpressed in human oral cancer. Anticancer Res. 31:2683–2692.
2011.PubMed/NCBI
|
|
11
|
Yang Y, Zhang C, Li L, et al: Up-regulated
oncoprotein P28GANK correlates with proliferation and poor
prognosis of human glioma. World J Surg Oncol. 10:1692012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao L, Xie H, Dong L, et al: Gankyrin is
essential for hypoxia enhanced metastatic potential in breast
cancer cells. Mol Med Rep. 9:1032–1036. 2014.
|
|
13
|
Wang G, Rong J, Zhou Z and Duo J: Novel
gene P28GANK confers multidrug resistance by modulating the
expression of MDR-1, Bcl-2 and Bax in osteosarcoma cells. Mol Biol
(Mosk). 44:1010–1017. 2010. View Article : Google Scholar
|
|
14
|
Ortiz CM, Ito T, Tanaka E, et al: Gankyrin
oncoprotein overexpression as a critical factor for tumor growth in
human esophageal squamous cell carcinoma and its clinical
significance. Int J Cancer. 122:325–332. 2008. View Article : Google Scholar
|
|
15
|
Bi Q, Tang S, Xia L, et al: Ectopic
expression of MiR-125a inhibits the proliferation and metastasis of
hepatocellular carcinoma by targeting MMP11 and VEGF. PLoS One.
7:e401692012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higashitsuji H, Higashitsuji H, Itoh K, et
al: The oncoprotein gankyrin binds to MDM2/HDM2, enhancing
ubiquitylation and degradation of p53. Cancer Cell. 8:75–87. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lozano G and Zambetti GP: Gankyrin: an
intriguing name for a novel regulator of p53 and RB. Cancer Cell.
8:3–4. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian YW, Chen Y, Yang W, et al: p28(GANK)
prevents degradation of Oct4 and promotes expansion of
tumor-initiating cells in hepatocarcinogenesis. Gastroenterology.
142:1547–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu YW, Yang PC, Yang SC, et al: Selection
of invasive and metastatic subpopulations from a human lung
adenocarcinoma cell line. Am J Respir Cell Mol Biol. 17:353–360.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tie J, Pan Y, Zhao L, et al: MiR-218
inhibits invasion and metastasis of gastric cancer by targeting the
Robo1 receptor. PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai ZF, Tai Y, Li W, et al: Gankyrin
activates IL-8 to promote hepatic metastasis of colorectal cancer.
Cancer Res. 73:4548–4558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mine H, Sakurai T, Kashida H, et al:
Association of gankyrin and stemness factor expression in human
colorectal cancer. Dig Dis Sci. 58:2337–2344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhen C, Chen L, Zhao Q, et al: Gankyrin
promotes breast cancer cell metastasis by regulating Rac1 activity.
Oncogene. 32:3452–3460. 2013. View Article : Google Scholar
|