Introduction
Telomeres are highly conserved, repetitive
nucleotide sequences at the ends of chromosomes in eukaryotic cells
(1). Human telomeric DNA, which is
comprised of tandem TTAGGG repeats, has a total length of 5–15
kilobases and does not encode a protein (2). The predominant function of telomeres
is to cap the chromosome ends, thereby maintaining chromosomal
stability and the integrity of gene replication by preventing
chromosome degradation, fusion and recombination (3). Consequently, telomeres maintain the
genetic content of chromosomes (4). Telomerase is a reverse transcriptase,
which mediates the de novo synthesis of telomeric sequences
at the ends of chromosomes in order to maintain telomere length
(5). In adult humans, telomerase
activity is only detected in germ cells, certain stem cells and
peripheral lymphocytes (6). In
addition, telomeres have been observed to shorten with each round
of cell division (7). When
telomere length shortens to a critical length (termed the Hayflick
limit), it loses its ability to protect the genetic integrity of
chromosomes, resulting in genetic instability and ultimately
replicative senescence (8).
Telomere length shortens with age in the majority of
tissues and cells, although the rate of shortening has been
reported to vary between individuals (9). In addition, telomere shortening with
cell division is considered an inherent mechanism of cellular
senescence (10). Thus, telomere
length may represent a potential marker for aging (11–14),
in addition to being an accurate marker of the age of an
individual.
The kidney is an organ that is known to be affected
by aging (15). The incidence of
chronic kidney disease (CKD) and end-stage renal disease in the
elderly has been increasing and thus the kidney has become a focus
in aging-associated studies. Although the mechanisms of aging in
the kidney remain unclear, it is hypothesized that telomere
shortening may be involved (16).
For example, Melk et al (16) previously demonstrated that telomere
shortening progresses in an age-dependent manner in human kidneys.
In addition, Westhoff et al (17) confirmed that telomere shortening
enhances kidney damage in animal models and this can reduce the
capacity to repair the kidneys following injury.
Few studies on telomere length and kidney function
in the elderly have been conducted (17). Thus, the aim of the present study
was to investigate telomere length with respect to age.
Specifically, Southern blotting was used to measure the telomere
restriction fragment (TRF) length of peripheral blood leukocytes
obtained from healthy volunteers of various ages. These data were
also compared with various surrogate markers of kidney function in
order to investigate the relevance of telomere length to renal
function in a healthy cohort.
Materials and methods
Sample screening
In 2011, 139 healthy volunteers were selected from
673 candidate volunteers, aged 35 to 90 years, from the Han
population in Beijing, China. The volunteers were not taking any
medication and had not been hospitalized in the previous three
years. All participants gave informed consent subsequent to a clear
explanation of the potential risks of the study. The study
inclusion criteria and a flow chart for the study enrollment
process were previously described (18,19).
The current study was approved by the Ethics Committee on Human
Experimentation of the Chinese People’s Liberation Army General
Hospital (Beijing, China) and was conducted in accordance with the
Declaration of Helsinki and subsequent amendments. The volunteers
that were selected were divided into five groups according to age:
35–44, 45–54, 55–64, 65–74 and >75 years.
Detection of indices
Various patient characteristics were recorded for
each volunteer, including gender, age, height, weight, waist
circumference, hip circumference and blood pressure, including
systolic blood pressure, diastolic blood pressure, pulse pressure
and pulse pressure index. Laboratory indicators were also assayed
AU500 (Beckman Coulter, Brea, CA, USA) that included the following:
Blood urea nitrogen, creatinine, uric acid, creatinine clearance
rate, triglyceride, total cholesterol, low density lipoprotein,
high density lipoprotein, blood and urine routine analyses, serum
levels of cystatin C (CYSC) and estimated glomerular filtration
rate (eGFR) was measured using the Chronic Kidney Disease
Epidemiology Collaboration (CKD-EPI) formula (20).
Genomic DNA was isolated using a cell tissue genomic
DNA extraction kit (Tiangen Biotech (Beijing) Co., Ltd., Beijing,
China) and TRF length was measured using the Telo TTAGGG telomere
length assay (Roche Diagnostics GmBH, Mannheim, Germany). In brief,
genomic DNA (1.5 mg) was digested with the restriction enzymes,
RsaI and HinfI (Roche Diagnostics GmBH), for 2 h at
37°C. The resulting DNA fragments were separated on a 0.8% agarose
gel by electrophoresis, then were subsequently denatured,
neutralized, transferred to a nylon membrane (Roche Diagnostics
GmBH) via capillary transfer at 15–20°C using a 20X saline-sodium
citrate transfer buffer (Roche Diagnostics GmBH) and cross-linked
with UV light (UVP, Inc., Upland, CA, USA). Blotted DNA fragments
were then incubated with a telomeric probe [digoxigenin (DIG)
3′-end labeled 5′-(CCCTAA)3; Roche Diagnostics GmBH] at 42°C for 3
h, followed by incubation with a DIG-specific polyclonal sheep
antibody (Roche Diagnostics GmBH) covalently coupled to alkaline
phosphatase. Binding sites of the telomere probe were visualized
using a highly sensitive chemiluminescence substrate that
metabolizes alkaline phosphatase. TRF lengths were then compared
with molecular weight markers (Roche Diagnostics GmBH) (2) and mean TRF lengths were estimated
using Quantity One 1-D analysis software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) (2).
Statistical analysis
Statistically significant differences were
identified using a linear regression model. Data were expressed as
the mean ± standard deviation and P<0.05 was considered to
indicate a statistically significant difference. All statistical
calculations were performed using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA).
Results
General characteristics of the study
subjects
A total of 139 healthy subjects were enrolled (69
males and 70 females) and divided into five groups according to
age: 35–44, 45–54, 55–64, 65–74 and >75 years (Table I). Peripheral blood leukocytes were
collected from each subject and genomic DNA was isolated.
 | Table ICharacteristics of the cohort. |
Table I
Characteristics of the cohort.
| Age group
(years) | Male | Female | Total |
|---|
| 35–44 | 14 | 13 | 27 |
| 45–54 | 13 | 13 | 26 |
| 55–64 | 13 | 14 | 27 |
| 65–74 | 15 | 16 | 31 |
| >75 | 14 | 14 | 28 |
| Total | 69 | 70 | 139 |
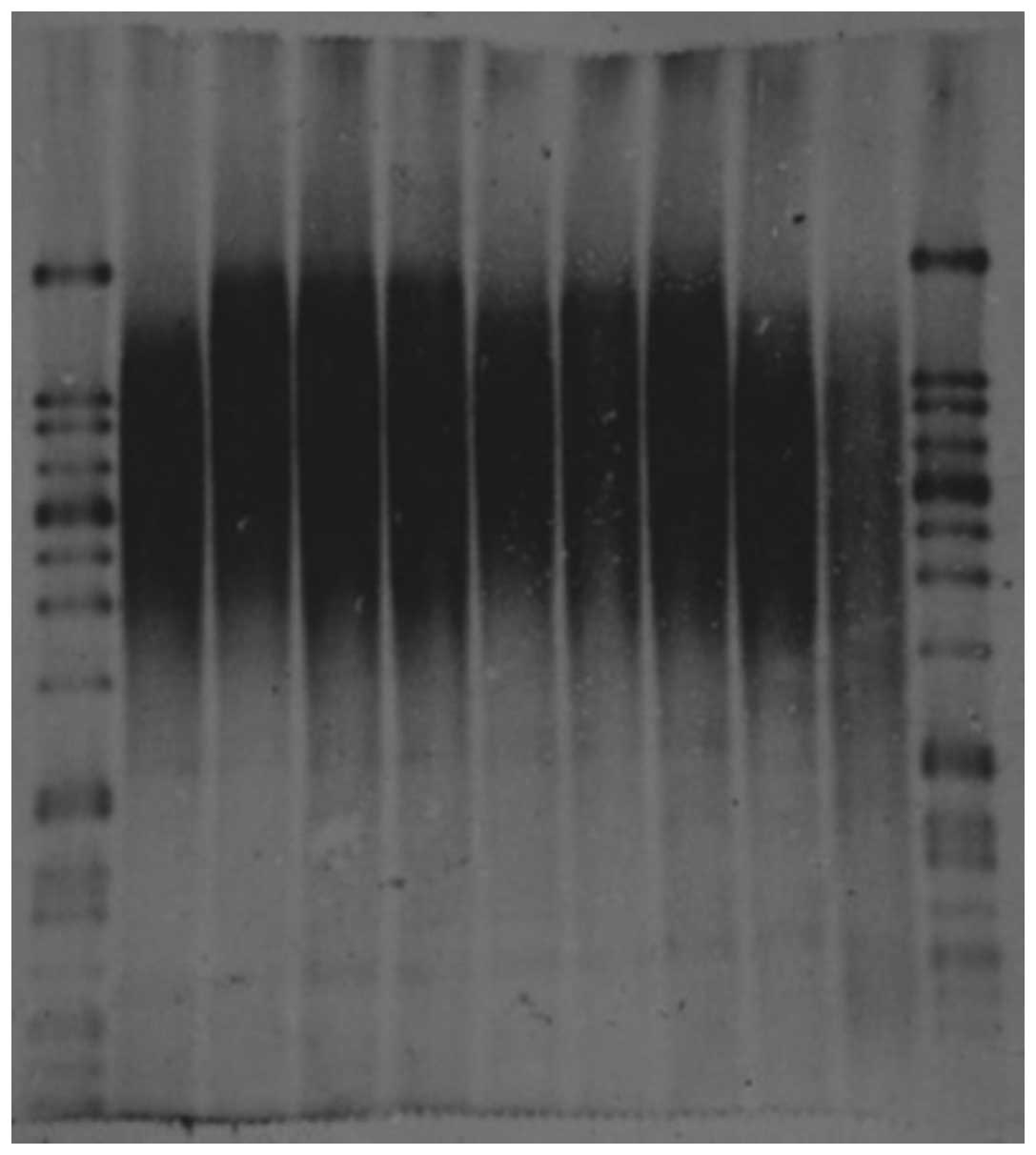
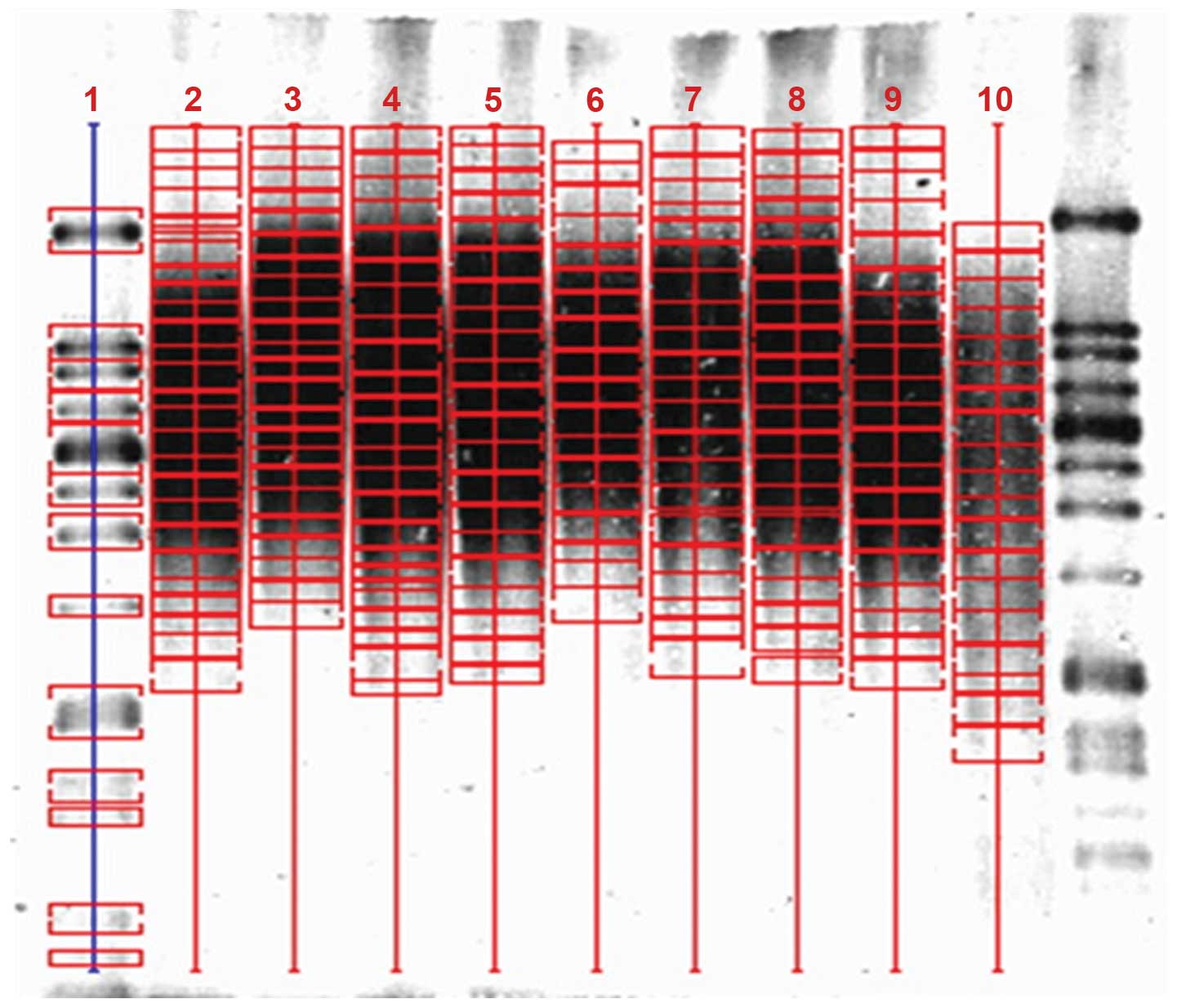
Detection of TRF lengths
Using a Telo TTAGGG telomere length assay, TRF
lengths for each sample were analyzed using Southern blotting
(Fig. 1, Table II). Mean TRF lengths were then
examined using Quantity One software (Fig. 2). TRF length for the >75 years
age group was observed to be significantly shorter than the 35–44,
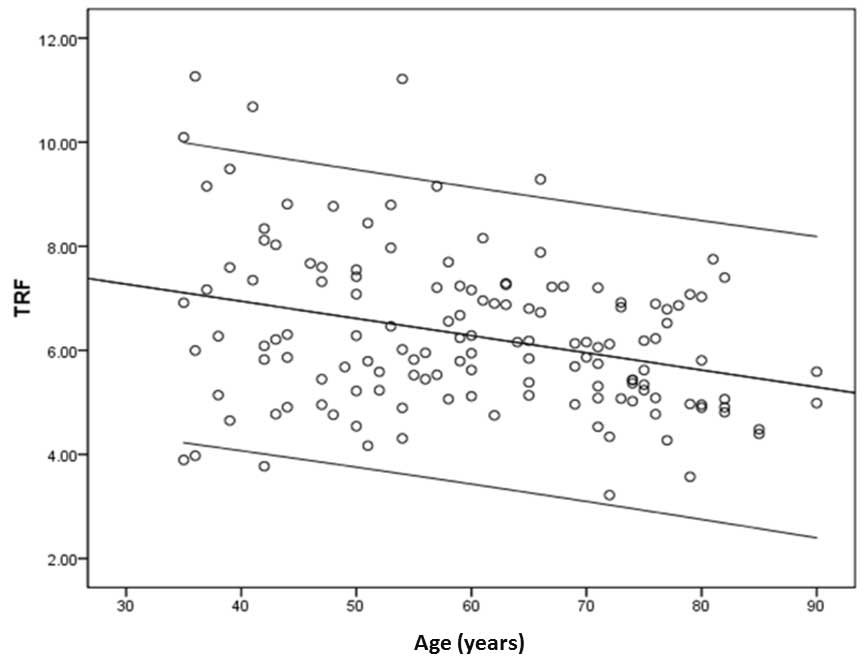
45–54 and 55–64 years age groups (P<0.05; Table III). In addition, a scatter plot
of TRF lengths and age (Fig. 3)
generated a regression equation of: Y=−0.033X+8.269 (Y, mean TRF
length; X, age) and a correlation coefficient of −0.314. Thus, the
correlation between TRF length and age was demonstrated to be
significant (P<0.001). To investigate gender-specific
alterations in telomere length in human peripheral blood
leukocytes, TRF lengths for males versus females were compared for
all five age groups. However, no significant difference in TRF
lengths with regard to gender was observed in any of the age groups
analyzed (P>0.05; Table
IV).
 | Table IICharacteristics of the telomeric DNA
samples analyzed. |
Table II
Characteristics of the telomeric DNA
samples analyzed.
| Lane | Number | Age (years) | Gender |
|---|
| 3 | H44 | 71 | Male |
| 4 | H50 | 63 | Male |
| 5 | H54 | 77 | Male |
| 6 | H58 | 57 | Female |
| 7 | H62 | 68 | Female |
| 8 | H67 | 62 | Female |
| 9 | H70 | 60 | Female |
| 10 | H74 | 79 | Female |
 | Table IIIMean TRF length determined for each
age group. |
Table III
Mean TRF length determined for each
age group.
| Age group
(years) | Number of
samples | Mean TRF
(kilobases) | P-value |
|---|
| 35–44 | 27 | 6.924a | 0.001 |
| 45–54 | 26 | 6.514a | 0.028 |
| 55–64 | 27 | 6.464a | 0.036 |
| 65–74 | 31 | 5.944 | 0.406 |
| >75 | 28 | 5.626 | 1 |
 | Table IVMean TRF lengths according to gender
(x ± SD). |
Table IV
Mean TRF lengths according to gender
(x ± SD).
| Age group
(years) | Number
| Mean ± SD TRF (kb)
| t-value | P-value |
|---|
| Males | Females | Male | Female |
|---|
| 35–44 | 14 | 13 | 6.96±2.37 | 6.87±1.84 | 0.106 | 0.916 |
| 45–54 | 13 | 13 | 6.59±1.47 | 6.43±1.97 | 0.239 | 0.813 |
| 55–64 | 13 | 14 | 6.49±1.16 | 6.42±0.91 | 0.173 | 0.864 |
| 65–74 | 15 | 16 | 5.89±0.85 | 5.99±1.43 | −0.257 | 0.799 |
| >75 | 14 | 14 | 5.79±1.08 | 5.46±1.08 | 0.818 | 0.421 |
Correlation between TRF lengths and
kidney function indices
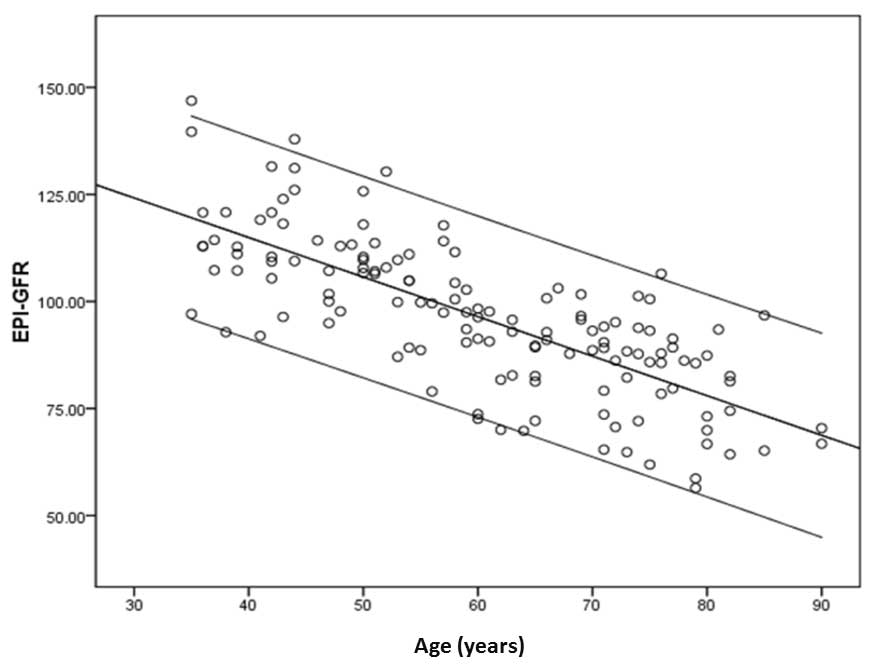
In order to investigate the relevance of telomere
length and alterations in renal function, eGFR CKD-EPI was
calculated using serum creatinine levels in order to estimate GFR
(20). It was observed that eGFR
reduced with age (Fig. 4) and was
associated with a correlation coefficient of −0.746 (P<0.001).
However, when the correlation between eGFR and TRF length of
peripheral blood leukocytes was examined, a Spearman correlation
coefficient of −0.184 (P=0.03) was observed, indicating that an
association exists between eGFR and TRF.
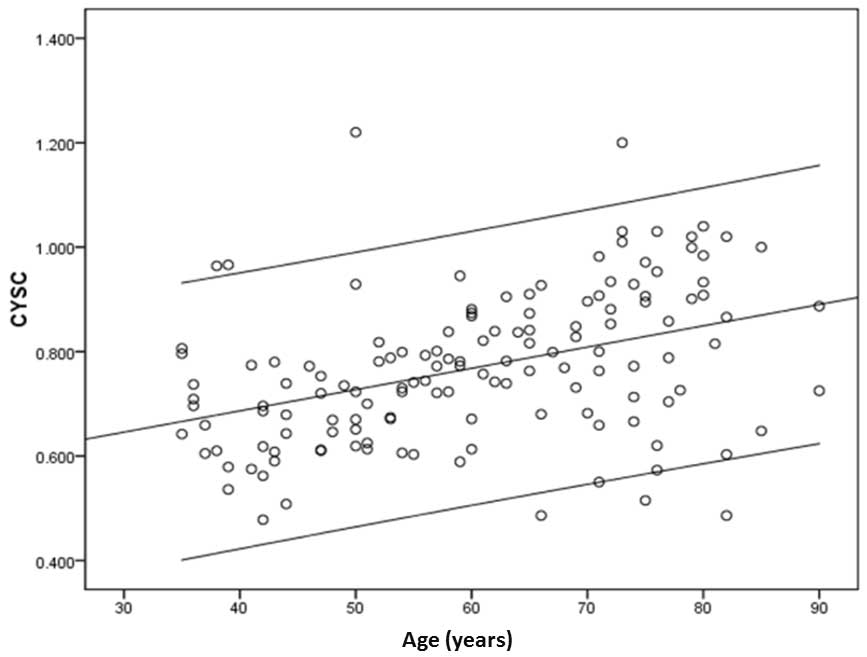
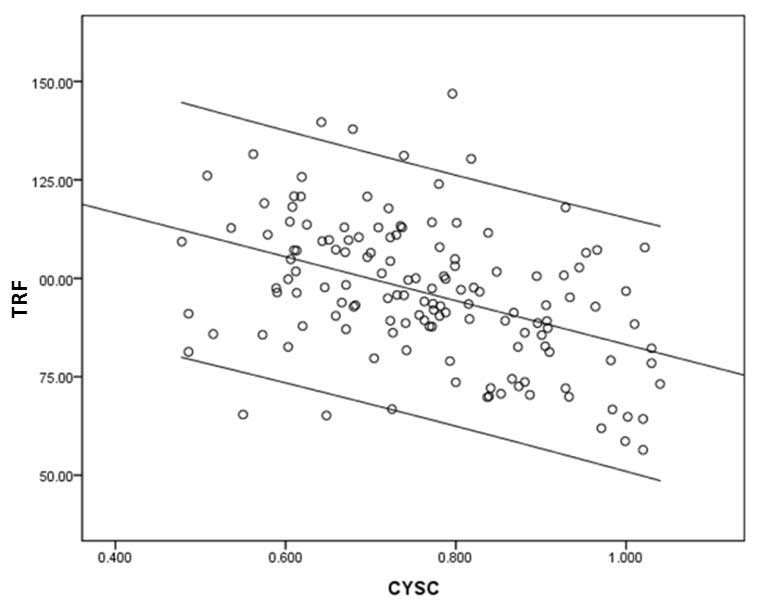
Serum levels of CYSC also increased with age
(r=0.405, P<0.001; Fig. 5) and
appeared to correlate with TRF length (r=−0.195, P=0.02) (Fig. 6).
Discussion
Aging is a degenerative process that involves the
gradual degradation of biological systems. The accumulation of
irreversible degenerative alterations can result in an increased
vulnerability to disease and eventual mortality. Telomeres are
highly conserved regions of DNA that maintain the stability and
integrity of chromosomes and therefore, the stability of a genome.
In order to achieve this, telomeres act as a buffer during the
process of cell division by preventing the shortening of
chromosomes and a loss of genetic material (4). A previous study demonstrated that
when telomeres become sufficiently shortened, this buffer function
is lost (21) and the cell
recognizes that cell division must be stopped. As a result,
telomere length has the potential to act as a biological clock,
thereby regulating the aging process (22).
In a study by Aubert et al (1), telomere length in peripheral blood
lymphocytes of 400 healthy individuals was observed to gradually
shorten with increasing age. In addition, rates of shortening were
increased in infancy and old age. Valdes et al (23) also analyzed TRF length for
peripheral blood leukocytes obtained from a general population.
These results indicated that there was a significant negative
correlation between TRF length and increasing age. In the present
study, TRF was assessed in 139 healthy individuals and telomere
length was demonstrated to shorten with increasing age. The
correlation between TRF length and age observed (r=−0.314) was
consistent with a review of 124 cross-sectional studies of telomere
length and age, which indicated that telomere length was negatively
correlated with age independent of absolute telomere length
(r=−0.338) or relative telomere length (r=−0.295) (24).
It has been reported that females have longer
telomeres and life expectancies than males (25). However, in the present study, TRF
was not observed to differ between males and females, and these
results are consistent with the analysis of an Amish population by
Cawthon et al (26).
Therefore, further studies are required to determine whether there
is a correlation between telomere length and gender.
The kidney is an organ affected by aging and the
histological alterations associated with this process include:
Progressive regression of renal mass associated with
glomerulosclerosis, tubular atrophy, interstitial fibrosis and
fibrous thickening of the arterial intima (27,28).
In addition, these functional alterations are associated with
increased renal vascular resistance, reduced renal blood flow and a
progressive decline in GFR. In a previous study, GFR was observed
to be stable until the age of 30–40 years old and subsequently
declined linearly at an average rate of ~8 ml/min/decade (29).
CYSC is an endogenous indicator of renal function,
which has recently been demonstrated to provide improved
sensitivity, specificity and accuracy compared with creatinine
(30–32). CYSC is an endogenous cysteine
protease inhibitor that is constitutively expressed and
persistently secreted in all nucleated cells. Since CYSC is
produced at a constant rate, freely filtered by the glomerulus and
is almost entirely reabsorbed and disassimilated in the proximal
tubule (33), it is only excreted
through the kidneys. Thus, it is recommended as a marker of GFR. In
healthy individuals, the concentrations of CYSC have been observed
to vary with age, thus have been suggested as a biomarker of
healthy aging (20). For example,
when Finney et al (34)
examined 401 British elderly individuals (65–101 years old), it was
observed that CYSC increased with age. In addition, an analysis of
309 healthy blood donors demonstrated that concentrations of CYSC
were higher in donors that were older than 50 years of age
(35). Consistently, an additional
study observed that in urine samples from 338 healthy subjects
(age, 0–95 years), the levels of CYSC were varied according to age
(36). In the present study, CYSC
was also observed to increase with age (r=0.405, P<0.001),
although CYSC was only observed to be associated with TRF length
(r=−0.195, P=0.02). Similarly, a cardiovascular health study of 419
individuals >65 years old from the United States demonstrated a
correlation between human TRF length and CYSC (37). The results of the present study are
consistent with these observations. Therefore, human peripheral
blood leukocyte telomere length is suggested to serve as a marker
of kidney function. In order to verify this, large-scale,
multi-center prospective studies are required. In particular, if a
correlation between CYSC and telomere length were to be confirmed,
early alterations in renal function may aid in the identification
of individuals at risk for age-associated kidney disease.
In the present study, eGFR CKD-EPI was calculated
using serum creatinine levels in order to estimate GFR. eGFR values
were observed to reduce with age and these results are consistent
with those of previous studies (38,39).
When eGFR and TRF length in peripheral blood leukocytes were
compared, a correlation was observed (r=−0.184, P<0.05). This
result is consistent with previous study in Holland of 866 patients
with heart failure, in which an association between telomere length
and GFR (r=0.123, P<0.001) was identified (40). By contrast, a study by Melk et
al (16) demonstrated that the
association between telomere length and GFR was not relevant. It
should be considered that the number of samples analyzed in the
present study was limited. Therefore, the exact association between
telomere DNA length and GFR requires further investigation.
The association between telomere length and kidney
function in aging remains to be confirmed; however, the current
study suggests that telomere length serves a role in the aging and
function of kidneys. Therefore, further investigation of the
association between telomere length, aging and renal function are
required in order to improve preserve renal function and prevent
associated morbidity and mortality. It is hypothesized that
progress in this field may result in reduced healthcare costs for
aging populations.
Acknowledgments
The authors would like to thank those who
participated in the current study. The study was supported by the
National Basic Research Program of China (2103CB530800) and the
National Key Technology R&D Program (2011BAI10B00) and the
Natural Science Foundation of China (81270819).
References
|
1
|
Aubert G and Lansdorp PM: Telomeres and
aging. Physiol Rev. 88:557–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuji A, Ishiko A, Takasaki T and Ikeda N:
Estimating age of humans based on telomere shortening. Forensic Sci
Int. 126:197–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuldt A: Telomeres: Damage response cut
short. Nat Rev Mol Cell Biol. 13:1372012.PubMed/NCBI
|
|
4
|
Wong JM and Collins K: Telomere
maintenance and disease. Lancet. 362:983–988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lingner J, Hughes TR, Shevchenko A, et al:
Reverse transcriptase motifs in the catalytic subunit of
telomerase. Science. 276:561–567. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiyama E and Hiyama K: Telomere and
telomerase in stem cells. Br J Cancer. 96:1020–1024. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allsopp RC, Chang E, Kashefi-Aazam M, et
al: Telomere shortening is associated with cell division in vitro
and in vivo. Exp Cell Res. 220:194–200. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pickett HA and Reddel RR: The role of
telomere trimming in normal telomere length dynamics. Cell Cycle.
11:1309–1315. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Djojosubroto MW, Choi YS, Lee HW and
Rudolph KL: Telomeres and telomerase in aging, regeneration and
cancer. Mol Cells. 15:164–175. 2003.PubMed/NCBI
|
|
10
|
Blackburn EH: Telomere states and cell
fates. Nature. 408:53–56. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baker GT III and Sprott RL: Biomarkers of
aging. Exp Gerontol. 23:223–239. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mooradian AD: Biomarkers of aging: do we
know what to look for? J Gerontol. 45:B183–B186. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arking R: The Biology of Aging:
Observations and Principles. 3rd. Oxford University Press; New
York: 2006
|
|
14
|
Vasto S, Scapagnini G, Bulati M, et al:
Biomarkes of aging. Front Biosci (Schol Ed). 2:392–402. 2010.
View Article : Google Scholar
|
|
15
|
Zhou XJ, Rakheja D, Yu X, et al: The aging
kidney. Kidney Int. 74:710–720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Melk A, Ramassar V, Helms LM, et al:
Telomere shortening in kidneys with age. J Am Soc Nephrol.
11:444–453. 2000.PubMed/NCBI
|
|
17
|
Westhoff JH, Schildhorn C, Jacobi C, et
al: Telomere shortening reduces regenerative capacity after acute
kidney injury. J Am Soc Nephrol. 21:327–336. 2010. View Article : Google Scholar :
|
|
18
|
Zhang WG, Bai XJ and Chen XM: SIRT1
variants are associated with aging in a healthy Han Chinese
population. Clin Chim Acta. 411:1679–1683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai X, Han L, Liu Q, et al: Evaluation of
biological aging process-a population-based study of healthy people
in China. Gerontology. 56:129–140. 2010. View Article : Google Scholar
|
|
20
|
Levey AS, Stevens LA, Schmid CH, et al: A
new equation to estimate glomerular filtration rate. Ann Intern
Med. 150:604–612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Olovnikov AM: A theory of marginotomy: the
incomplete copying of template margin in enzymic synthesis of
polynucleotides and biological significance of the phenomenon. J
Theor Biol. 41:181–190. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shawi M and Autexier C: Telomerase,
senescence and ageing. Mech Ageing Dev. 129:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Valdes A, Andrew T, Gardner J, et al:
Obesity, cigarette smoking, and telomere length in women. Lancet.
366:662–664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Müezzinler A, Zaineddin AK and Brenner H:
A systematic review of leukocyte telomere length and age in adults.
Ageing Res Rev. 12:509–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tarry-Adkins JL, Chen JH, Smith NS, Jones
RH, Cherif H and Ozanne SE: Poor maternal nutrition followed by
accelerated postnatal growth leads to telomere shortening and
increased markers of cell senescence in rat islets. FASEB J.
23:1521–1528. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cawthon RM, Smith KR, O’Brien E,
Sivatchenko A and Kerber RA: Association between telomere length in
blood and mortality in people aged 60 years or older. Lancet.
361:393–395. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou XJ, Saxena R, Liu Z, Vaziri ND and
Silva FG: Renal senescence in 2008: progress and challenges. Int
Urol Nephrol. 40:823–839. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Silva FG: The aging kidney: a review -
part I. Int Urol Nephrol. 37:185–205. 2005. View Article : Google Scholar
|
|
29
|
Morrissey PE and Yango AF: Renal
transplantation: older recipients and donors. Clin Geriatr Med.
22:687–707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ozer BA, Dursun B, Baykal A, Gultekin M
and Suleymanlar G: Can cystatin C be a better marker for the early
detection of renal damage in primary hypertensive patients? Ren
Fail. 27:247–253. 2005.PubMed/NCBI
|
|
31
|
Cepeda F, Fernández E, Pobes A and Baños
L: Utility of cystatin-C in hospitalized patients. Comparing with
different methods of assessing renal function. Nefrologia.
27:168–174. 2007.In Spanish.
|
|
32
|
Hojs R, Bevc S, Ekart R, Gorenjak M and
Puklavec L: Serum cystatin C-based equation compared to serum
creatinine-based equations for estimation of glomerular filtration
rate in patients with chronic kidney disease. Clin Nephrol.
70:10–17. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobsson B, Lignelid H and Bergerheim US:
Transthyretin and cystatin C are catabolized in proximal tubular
epithelial cells and the proteins are not useful as markers for
renal cell carcinomas. Histopathology. 26:559–564. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Finney H, Bates CJ and Price CP: Plasma
cystatin C determinations in a healthy elderly population. Arch
Gerontol Geriatr. 29:75–94. 1999. View Article : Google Scholar
|
|
35
|
Finney H, Newman DJ and Price CP: Adult
reference ranges for serum cystatin C, creatinine and predicted
creatinine clearance. Ann Clin Biochem. 37:49–59. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pennemans V, Rigo JM, Faes C, Reynders C,
Penders J and Swennen Q: Establishment of reference values for
novel urinary biomarkers for renal damage in the healthy
population: are age and gender an issue? Clin Chem Lab Med.
51:1795–1802. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sanders JL, Fitzpatrick AL, Boudreau RM,
et al: Leukocyte telomere length is associated with noninvasively
measured age-related disease: the Cardiovascular Health Study. J
Gerontol A Biol Sci Med Sci. 67:409–416. 2012. View Article : Google Scholar
|
|
38
|
Sun X, Chen Y, Chen X, et al: Change of
glomerular filtration rate in healthy adults with aging. Nephrology
(Carlton). 14:506–513. 2009. View Article : Google Scholar
|
|
39
|
Lindeman RD, Tobin J and Shock NW:
Longitudinal studies on the rate of decline in renal function with
age. J Am Geriatr Soc. 33:278–285. 1985.PubMed/NCBI
|
|
40
|
Wong LS, van der Harst P, de Boer RA, et
al: Renal dysfunction is associated with shorter telomere length in
heart failure. Clin Res Cardiol. 98:629–634. 2009. View Article : Google Scholar : PubMed/NCBI
|