Introduction
The majority of patients with ovarian cancer
(>80%) are found to have metastasis when they are first
diagnosed (1). Therefore,
cytoreductive surgery and subsequent chemotherapy is common in the
treatment of ovarian cancer. For the last 30 years, the
chemotherapy regimens for ovarian cancer have been developed using
one or a combination of cytotoxic agents, including melphalan,
cyclophosphamide, doxorubicin, cisplatin, carboplatin and
paclitaxel (2,3). Following a long-term follow-up study
by Goldberg et al, which demonstrated the significant
antiproliferative effect of paclitaxel in platinum-refractory
patients (4), the addition of
paclitaxel to platinum-based chemotherapy has been established as a
standard treatment in ovarian cancer. While the majority of
patients initially respond to the first-line chemotherapy of a
combination of paclitaxel and cisplatin/carboplatin, it has been
reported that ~30% of patients in advanced stages are unresponsive,
exhibiting intrinsic chemoresistance, and initial responders also
eventually acquire chemoresistance over time (5–8).
These intrinsic and acquired resistances to chemotherapeutic agents
have been a major obstacle in the treatment of ovarian cancer, and
the underlying molecular mechanisms remain to be fully
elucidated.
There are >90 types of protein tyrosine kinase in
the human genome, 58 of which are receptor tyrosine kinases (RTK)
containing a transmembrane domain, and they have been classified
into 20 families (9). The TAM
family, a subfamily of RTK, consists of three RTKs; Tyro3, also
termed Sky; Axl, also termed Ark and Ufo; and Mer (10–13).
These RTKs have similar extracellular regions, two
immunoglobin-like domains and two fibronectin type III repeats, and
a conserved kinase domain within the cytoplasmic region (14). The growth arrest-specific 6 (Gas 6)
and protein S vitamin K-dependent proteins have been identified as
the biological ligands, which bind to TAM RTKs and transduce a
variety of intracellular downstream signals including those
involved in cell proliferation and survival, inhibition of
apoptosis, cell adhesion, cell morphology and motility (15,16).
TAM receptors have been demonstrated as being important in normal
cellular function involved in the initiation and progression of
several types of cancer (17).
Notably, the protein levels of Axl, Mer and/or Gas 6 have been
reported to be elevated in numerous cancer cell lines and patient
samples, including leukemia (18,19),
multiple myeloma (20), breast
cancer (21,22), colon cancer (23), gastric cancer (24), liver cancer (25), melanoma (26), ovarian cancer (27), prostate cancer (28) and renal cell carcinoma (29). Therefore, these TAM RTKs and their
ligands may be reliable biomarkers for the diagnosis and prognosis
of various types of cancer and offer potential targets for the
their treatment.
In the present study, SKOV3/TR taxol-resistant
ovarian cancer cells were established to investigate the biological
effects of TAM RTKs and the mechanisms underlying the development
of resistance, which may serve as possible interventions to restore
the chemosensitivity of these resistant cells.
Materials and methods
Reagents and antibodies
Primers for Axl, Tyro3, Mer and GAPDH were
synthesized from Bioneer, Inc. (Daejoun, Korea). TRIzol reagent was
obtained from Solgent (Daejoun, Korea). AmpliTaq DNA polymerase was
obtained from Roche diagnostics (Indianapolis, IN, USA). For
western blot analysis, 10× Tris-buffered saline with Tween-20
(TBST; cat. no. sc-24953) and specific antibodies against
phosphorylated (phospho)-Akt (cat. no. sc-7985-R; anti-rabbit), Akt
(cat. no. sc-8312; anti-rabbit), Axl (cat. no. sc-1096; anti-goat),
phospho-signal transducers and activators of transcription 3
(STAT3; cat. no. sc-8059; anti-mouse), STAT3 (cat. no. sc-482;
anti-rabbit), Tyro3 (cat. no. sc-1095; anti-goat), GAPDH (cat. no.
sc-25778; anti-rabbit) and secondary antibodies (goat anti-rabbit
IgG-HRP, cat. no. sc-2004; rabbit anti-goat IgG-HRP, cat. no.
sc-2768; goat anti-mouse IgG-HRP; cat. no. sc-2005) were obtained
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Taxol
(paclitaxel; cat. no. T7402) and NAC (cat no. A7250), were
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The SKOV3 cells were purchased from American Type
Culture Collection and grown in RPMI-1640 (Gibco-BRL, Grand Island,
N Y, USA), containing 10% FBS, 2 mM L-glutamine, 10 U/ml penicillin
and 10 g/ml streptomycin, at 37°C in 5% CO2 in a
humidified atmosphere.
Establishment of taxol-resistant
cells
The SKOV3/TR taxol-resistant ovarian cancer cells,
which are a variant of SKOV3, were established by stepwise exposure
of the SKOV3 cells to increasing concentrations of taxol, ranging
between 1.5 and 24 nM over 6 months.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The cells (3×105) were seeded into a 60
mm culture dish and grown overnight at 37°C. Total RNA was
extracted using TRIzol reagent and subjected to cDNA synthesis and
RT-qPCR. Briefly, 2 μg total RNA was reverse transcribed
into cDNA using SuperScript First-Strand Synthesis System
(Invitrogen Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Using specific primers, the obtained
cDNAs were amplified using a TGradient Thermal Cycler (Biometra,
Goettingen, Germany) and AccuPower PCR PremixTM (Bioneer, Daejeon,
Korea), according to the manufacturer’s instructions. The cycling
conditions were as follows: 3 min denaturation at 94°C, 30 cycles
of denaturation at 94°C for 30 sec, annealing at 55°C for 1 min and
extension at 72°C for 30 sec, followed by a final cycle of 94°C for
30 sec and 60°C for 5 min. The specific primers were as follows:
Axl, sense 5′-AACCTTCAACTCCTGCCTTCTCG-3′ and antisense
5′-CAGCTTCTCCTTCAGCTCTTCAC-3′; Tyro3, sense
5′-GTGTGTGGCTGACTTCGGAC-3′ and antisense
5′-CACGTCCTCCATACACTCCG-3′; Mer, sense 5′-CGACTAAGCAGCAGGATGGA-3′
and anti-sense 5′-GAGGGGGCATAATCTACCCA-3′; and GAPDH, sense
5′-GGAGCCAAAAGGGTCATCAT-3′ and antisense
5′-GTGATGGCATGGACTGTGGT-3′.
Western blot analysis
The cells were treated with N-acetyl cysteine (NAC)
at 37°C for 24 h. The whole cell lysates were prepared from the
cells using lysis buffer, containing 1% Triton X-100, 50 mM Tris
(pH 8.0), 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 1 mM
Na3VO4, and protease inhibitor cocktail. The
protein concentrations were determined using a Bio-Rad protein
assay kit II (cat. no. 500-0002; Bio-rad Laboratories, Inc.,
Hercules, CA, USA). The proteins from the whole cell lysates (20
μg for Axl and Tyro3 detection or 40 μg for p-Akt,
Akt, p-STAT3 and STAT3 detection) were separated using 12%
SDS-PAGE, and electrotransferred onto nitrocellulose membranes. The
membranes were blocked for 30 min at room temperature in
Tris-buffered saline with 0.05% Tween-20 (TTBS), containing 5%
non-fat dry milk, and incubated with TTBS containing primary
antibody (1:1,000 dilution) for 4 h at room temperature. Following
three 10 min washes in TTBS, the membranes were incubated with
peroxidase-conjugated secondary antibody (1:3,000 dilution) for 1
h. Following three additional 10 min washes with TTBS, the protein
bands were visualized using an enhanced chemiluminescence detection
system (Amersham™ ECL™ Prime Western Blotting Detection reagent; GE
Healthcare, Piscataway, NJ, USA). Images of protein bands were
captured using an LAS-3000 Image Analysis system (FujiFilm, Tokyo,
Japan) and the relative quantities of each protein band were
analyzed using Image Gauge 3.01 software (Fuji Photofilm;
Fujiflm).
Colony-forming assay
The cells (1 to 2×103/dish) were seeded
into 35 mm culture dishes and allowed to grow for between 7 days
(for untreated cells) and 10 days (for experiments using siRNA or
NAC) to form colonies. Colonies containing >50 cells were
visualized by crystal violet staining (in 60% methanol; Junsei
Chemical Co, Ltd., Tokyo, Japan). Images of the cells were captured
using an LAS 3000 Image Analysis system (FujiFilm, Tokyo,
Japan).
Cell viability assay
The viability of the cells was measured using a Cell
Counting Kit-8 assay kit (Dojindo Laboratories, Kumamoto, Japan).
The cells (1×103 cells/well) were seeded into 96-well
plates and grown overnight at 37°C. The cells were subsequently
incubated at 37°C for 24, 48 and 72 h to measure cell proliferation
rate or treated with 0.001, 10, 100, and 1,000 nM taxol for 72 h to
observe the acquisition of taxol resistance. Following treatment,
10 μl CCK-8 solution was added and the cells were incubated
for 4 h. The absorbance at 450 nm was measured using a microplate
reader (Model 680 microplate reader; Bio-Rad Laboratories, Inc.).
The values are expressed as the mean ± standard deviation for
triplicate wells, and normalized to that of the control group to
determine the percentage viability.
Small interfering (si)RNA
transfection
RNA interference silencing was performed to decrease
the protein level of Tyro3. The SKOV3 or SKVO3/TR cells
(1×106) were seeded into 100 mm culture dishes and grown
overnight. The cells were then transfected with 50 nM siRNA against
Tyro3 (cat. no. sc-36438; Santa Cruz Biotechnology, Inc.), or
control siRNA (cat. no. sc-37007; Santa Cruz Biotechnology, Inc.).
For the cell proliferation and colony formation assays, the cells
were harvested 24 h after transfection and were reseeded into 35 mm
culture dishes and allowed to grow for 10 days. The cells were
harvested 48 h after transfection and western blot analysis were
performed to evaluate the protein levels of Tyro3.
ROS measurement
Fluorescence-activated cell sorting analysis was
performed to determine the intracellular levels of ROS. The cells
(1×106) were seeded into 100 mm culture dishes and grown
overnight. The cells were then stained with 5 mg/μl 2′,
7′-dichlorodihydrofluorescin diacetate for 30 min and subjected to
flow cytometry using a Becton-Dickinson FACSCalibur
(Becton-Dickinson, San Jose, CA, USA) and analyzed using CellQuest
software (Becton-Dickinson).
Statistical analysis
All data are expressed as the mean ± standard
deviation of triplicate samples or at least three independent
experiments. Student’s t-test was used to determine statistical
significance and P<0.05 was considered to indicate a
statistically significant difference.
Results
Long-term exposure of ovarian cancer
cells to taxol results in taxol resistance and a reduction in
proliferation capacity
To characterize and investigate the underlying
mechanisms of chemoresistance in ovarian cancer cells,
taxol-resistant SKOV3/TR cells were established by stepwise
long-term exposure of the SKOV3 cells to gradually increasing
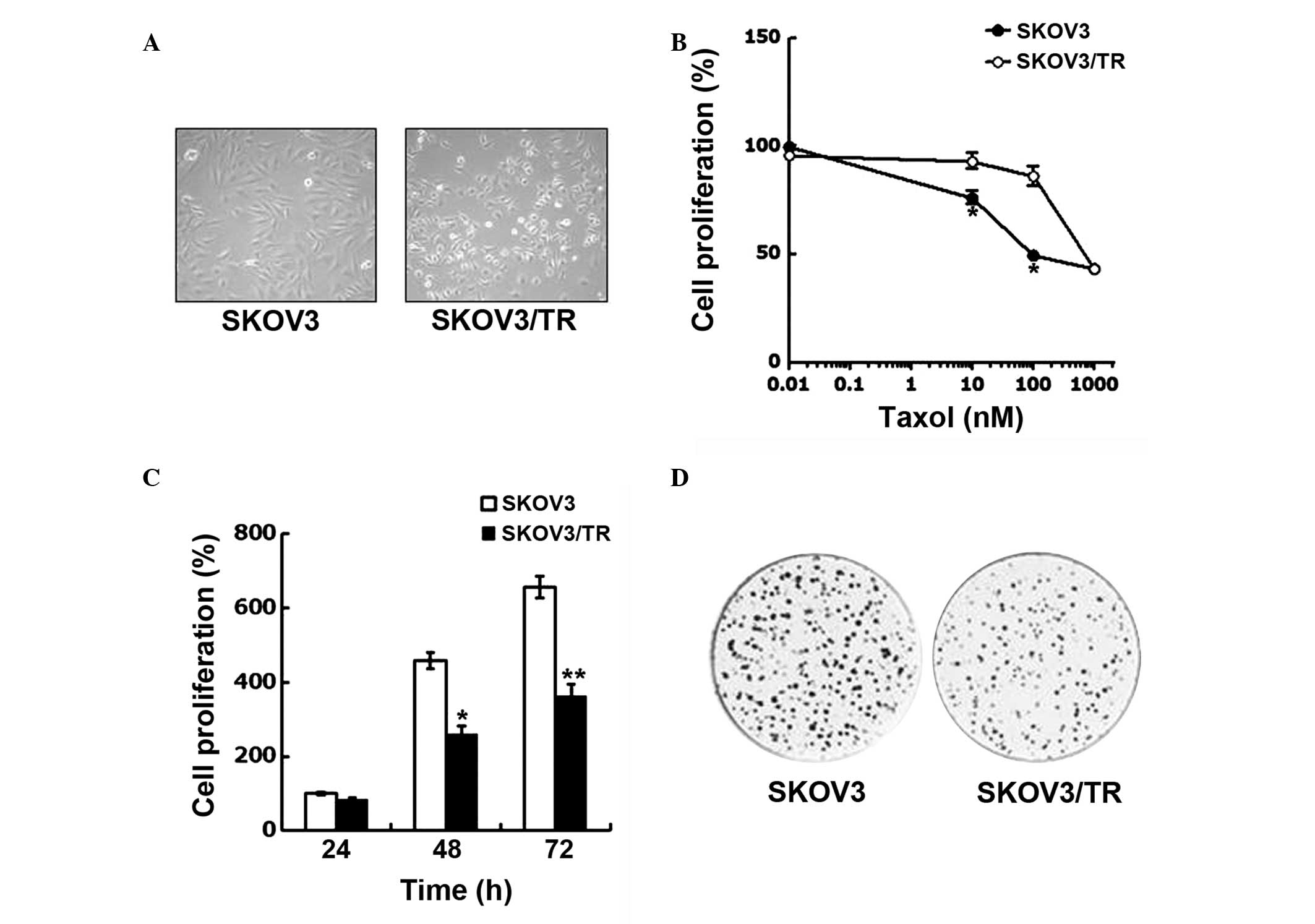
concentrations of taxol over 6 months. As shown in Fig. 1A, the SKOV3/TR cells were found to
be significantly smaller in size and more rounded in shape compared
with the parental SKOV3 cells, indicating that the SKOV3/TR cells
exhibited different morphological features.
The acquisition of taxol resistance was evaluated by
comparing the viability of the parental SKOV3 cells and SKOV3/TR
cells following treatment with taxol at concentrations ranging
between 0.01 and 1,000 μM. The cell viability was determined
using a CCK-8 assay. Taxol treatment decreased the proliferation of
the parental SKOV3 cells and the variant SKOV3/TR cells in
dose-dependent manner. However, at concentrations of taxol >10
μM, the SKOV3 cells were significantly more sensitive to
taxol treatment compared with the SKOV3/TR cells, suggesting the
acquisition of taxol resistance (Fig.
1B).
The present study also found that proliferation rate
of the SKOV3/TR cells was decreased compared with that of the
parental SKOV3 cells (Fig. 1C),
indicating the prolonged population doubling time (PDT) of the
SKOV3/TR cells. The results of the colony-forming assay further
demonstrated the prolonged PDT of the SKOV3/TR cells, since the
SKOV3/TR cells formed significantly fewer colonies, compared with
the SKOV3 cells (Fig. 1D). In
addition, the sizes of the single colonies formed by the SKOV3/TR
cells were smaller than that formed by the SKOV3 cells, indicating
a smaller number of SKOV3/TR cells compared with the SKOV3 cells.
Taken together, these results indicated that taxol resistance had
developed in the SKOV3/TR, with a concomitant reduction in
proliferative capacity.
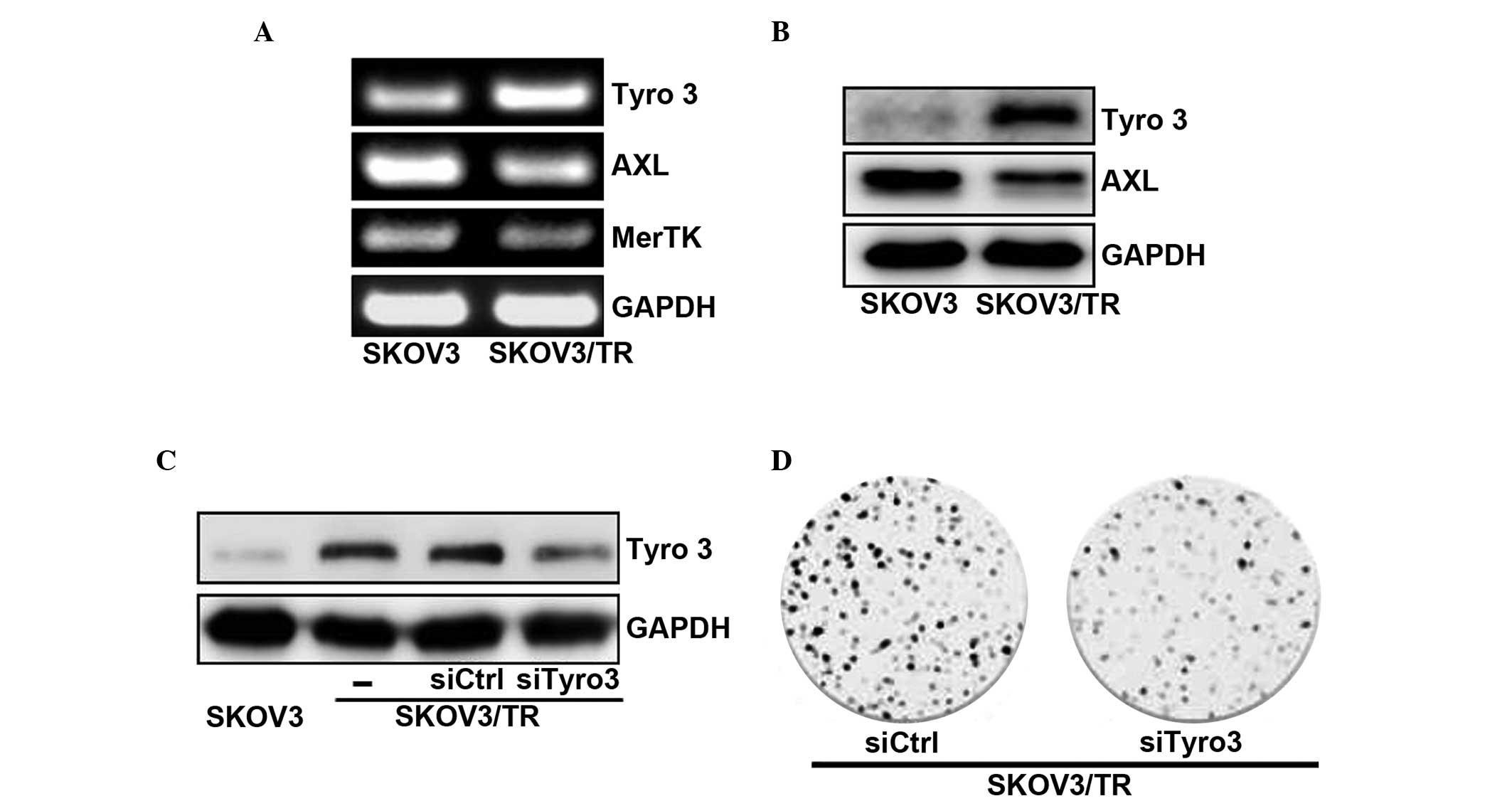
Expression of Tyro3 RTK is upregulated in
taxol-resistant cells
Since the TMA family of RTKs has been reported as
being important on cell survival and proliferation (30), the present study examined whether
the acquisition of taxol resistance affected the mRNA and protein
expression levels of TAM RTKs. Tyro3, Axl, and Mer in the SKOV3 and
SKOV3/TR cells. The RT-qPCR results revealed that the mRNA
expression of Tyro3 in the SKOV3/TR cells was increased compared to
that of the parental SKOV3 cells, while the mRNA levels of Axl and
Mer were decreased (Fig. 2A). The
upregulation of Tyro3 in the SKOV3/TR cells was further confirmed
using western blot analysis. As shown in Fig. 2B, the protein levels of Tyro3 and
Axl in the SKOV3/TR cells were higher and lower than those in the
parental SKOV3 cells, respectively, consistent with the RT-qPCR
results. Of note, these results suggested that, among the TAM RTKs,
only the expression of Tyro3 was upregulated and responsible for
the acquisition of taxol resistance in the SKOV3/TR cells.
Tyro3 specific siRNA transfection into
taxol-resistant cells suppresses cell proliferation
The present study also examined the effect of
overexpression of the Tyro3 protein on the proliferation of the
SKOV3/TR cells. The SKOV3/TR cells were transfected with siTyro3
and their viability was determined. Western blot analysis
demonstrated that transfection with siTyro3, but not control siRNA,
into the SKOV3/TR cells significantly decreased the protein
expression of Tyro3 (Fig. 2C). The
effect of siTyro3 on cell proliferation was determined using a
colony-forming assay. As shown in Fig.
2D, the SKOV3/TR cells transfected with siTyro3 formed fewer
colonies compared with the same cells transfected with siCtrl
(Fig. 2D). Taken together, these
results demonstrated that upregulation of the expression of Tyro3
was responsible for the proliferation of SKOV3/TR cells, eventually
causing the acquisition of taxol resistance.
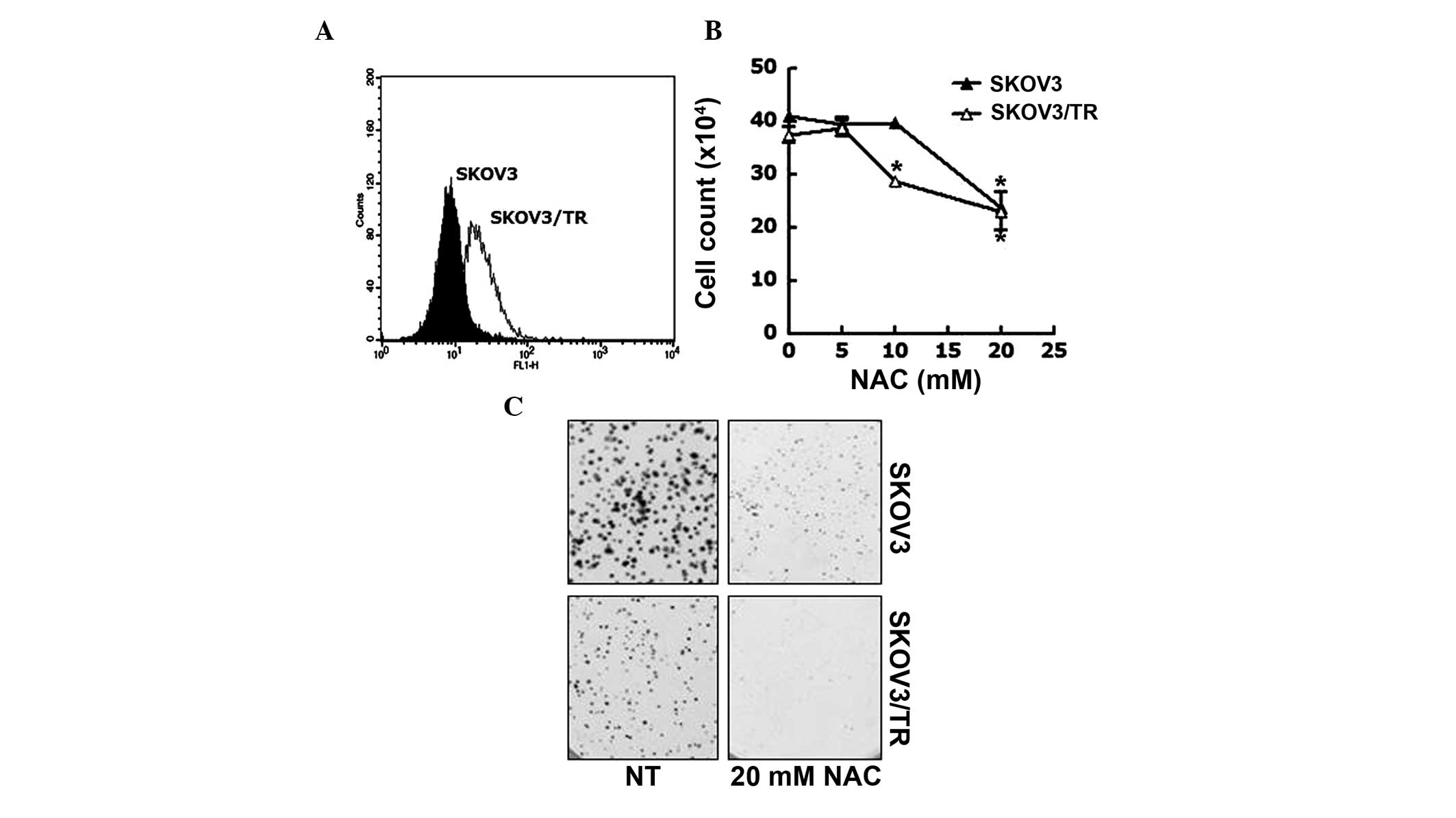
Anti-oxidant NAC reduces the
proliferation of taxol-resistant cells via downregulation of the
expression of Tyro3 and inhibiting the phosphorylation of Akt
The production of ROS banticancer drugs is a
well-known and essential mechanism to induce apoptosis, which in
turn, abrogate the proliferation of cancer cells (31,32).
Therefore, the present study assessed the involvement of ROS in the
acquired taxol resistance of SKOV3/TR cells. FACS analysis was
performed to measure ROS contents of SKOV3 and SKOV3/TR cells. As
shown in Fig. 3A, the
intracellular ROS content of SKOV3/TR cells was higher than that of
the parental cells. To investigate the biological relevance of the
increased level of intracellular ROS in the SKOV3/TR cells the
effect of the NAC antioxidant reagent on the proliferation of SKOV3
and SKOV3/TR cells was examined.. The cells were treated with 5, 10
or 20 mM NAC for 24, following which the viable cell numbers were
counted. The results demonstrated that the viability of the
SKOV3/TR cells treated with 10 mM NAC was slightly reduced (21%
reduction), while that of the SKOV3 cells was unaffected by this
dose of NAC. However, at 20 mM NAC, the viability of each cell was
reduced to 69% (SKOV3 cell) and 50% (SKOV3/TR cells), indicating
that SKOV3/TR cells were more sensitive to NAC treatment compared
with the parental cells (Fig. 3B).
This profound antiproliferative effect of NAC on the SKOV3/TR cells
was further verified using a colony-forming assay. As shown in
Fig. 3C, the SKOV3/TR cells were
found to form significantly smaller colony sizes and fewer colonies
in the presence of 20 mM NAC compared with the parental cells.
These results indicated that the elevated ROS in taxol-resistant
SKOV3/TR cells was responsible for the proliferation of the
cells.
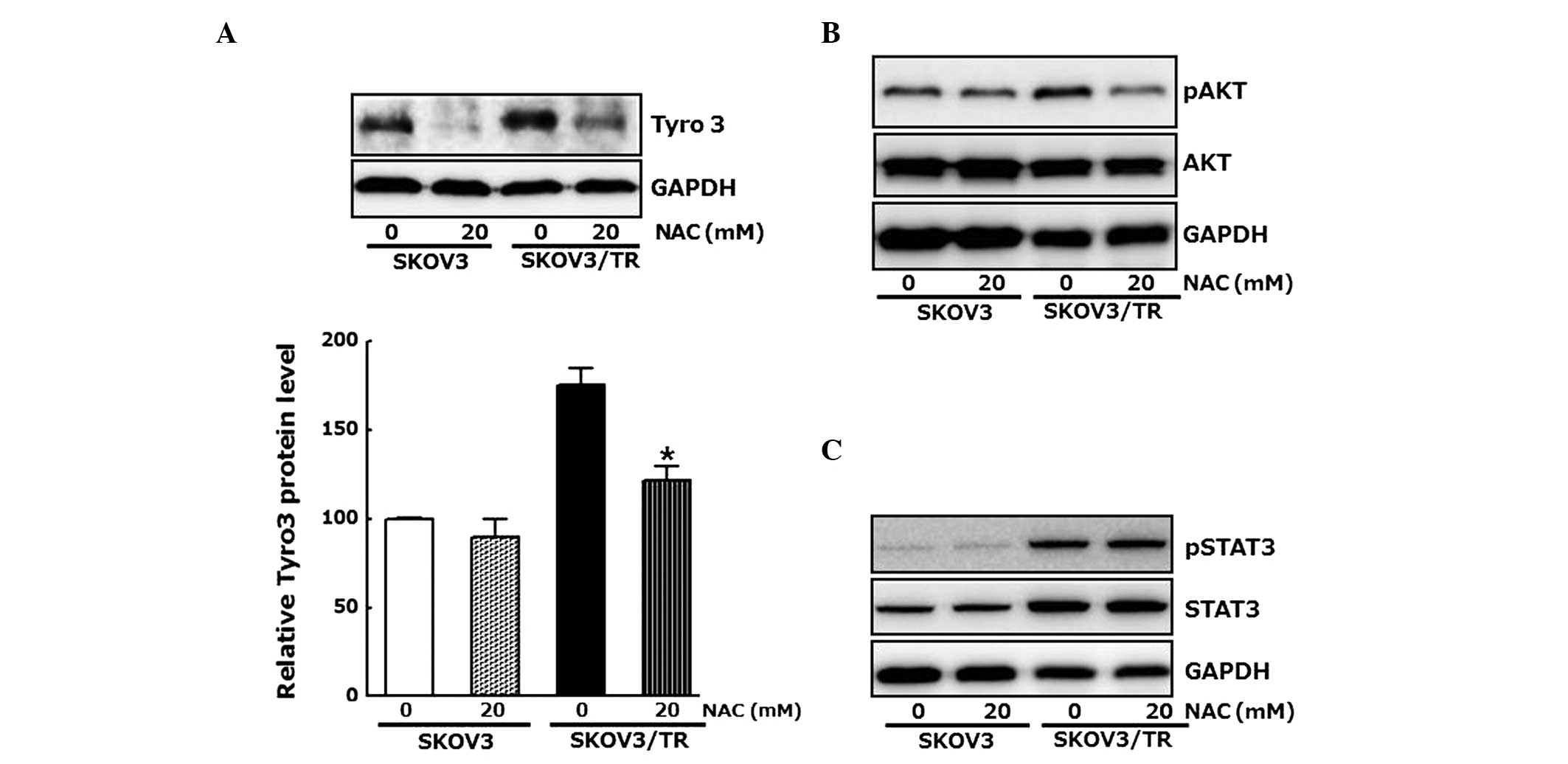
As a reduction in the protein level of Tyro3 by
Tyro-specific siRNA had antiproliferative effects in the SKOV3/TR
cells, whether the antiproliferative effect of NAC also results
from modulation of the expression of Tyro3 was examined. The cells
were treated with 20 mM NAC and the protein levels of Tyro3 were
determined using western blot analysis. As shown in Fig. 4A, the expression of Tyro3 was
inhibited by NAC, confirming that the elevated level of
intracellular ROSinduced the expression of Tyro3, which
subsequently resulted in proliferation of the SKOV3/TR cells
Western blot analysis further revealed the inhibitory effect of NAC
on Akt phosphorylation, which transduces proliferative signal and
was increased in the SKOV3/TR cells (Fig. 4B). However, NAC treatment had no
effect on the phosphorylation or the induction of STAT3, a
well-known positive regulator of cell proliferation (Fig. 4C). Taken together, these results
indicate that upregulation of the expression of Tyro3 ant
phosphorylation of Akt, but not the phosphorylation or induction of
STAT3, were associated with the increased intracellular ROS
contents in the SKOV3/TR cells, resulting in proliferation of the
cells.
Discussion
Ovarian cancer is the first leading cause of death
among gynecological cancers (33).
The majority of patients with ovarian cancer are confronted with
refractoriness against standard chemotherapy, a combination of
platinum, containing cisplatin, carboplatin and paclitaxel, and
develop further resistance to subsequent treatments (1). Therefore, the characterization and
understanding of the molecular mechanisms associated with
chemoresistance is valuable in overcoming cases of ovarian cancer
relapse.
The present study developed SKOV3/TR taxol-resistant
ovarian cancer cells, which were found to exhibit a reduction in
size and slower growth rates compared with the parental SKOV cells.
While the prolonged PDT of the taxol-resistant SKOV3/TR cells was
consistent with a phenotype of therapy-induced senescence (TIS),
their morphological characteristics of a reduced size and increased
roundedness, compared with the parental cells, is opposite to those
observed in TIS (34), since the
morphology of senescent cells is enlarged and fattened. It may be
beneficial to examine wether SKOV3/TR cells have other
characteristics similar to TIS, including the activation of
senescence-associated β-galactosidase, the development of
senescence-associated heterochromatin foci or the secretion of
excessive tumor-promoting cytokines, as TIS has inhibitory effects
on proliferation and apoptosis, and senescent cancer cells require
elimination for the successful treatment of cancer.
Resistance to tyrosine kinase inhibitors, including
imatinib and nilotinib, has been correlated with the activation and
overexpression of TAM RTKs in chronic myeloid leukemia cells
(35,36). Consistent with the these reports,
the present study demonstrated that the expression of Tyro3 RTK was
elevated in the taxol-resistant SKOV3/TR cells, and knockdown of
Tyro3 using specific siRNA sensitized the SKOV3/TR cells to taxol.
However, the expression levels of the other TAM RTKs, Axl and Mer,
were inversely downregulated in these cells, indicating that the
increased expression of Tyro3 plays is important in the survival
and proliferation of SKOV3/TR cells, while decreased levels of Axl
and Mer may be responsible for their prolonged PDT.
One of the antiproliferative mechanisms of
chemotherapeutic drugs is the induction of ROS, which leads to
apoptosis of cancer cells (31).
The role of ROS in developing chemoresistance is complex, as
elevation and reduction are observed in the levels of ROS during
the acquisition of drug resistance (37). The present study found that, in the
SKOV3/TR cells, the intracellular ROS contents were increased and
their proliferation was suppressed by NAC treatment. The
correlation between the production of ROS and the development of
taxol resistance in the SKOV3/TR cells was further confirmed by
western blot analysis, which demonstrated the inhibitory effect of
NAC on the expresion of Tyro3. Taken together, these results
suggested that the levels of ROS were increased during the
acquisition of taxol resistance in the SKOV3/TR cells, which
upregulated the expression of Tyro3, and concomitantly led to the
survival and proliferation of these cells via overexpression of the
Tyro 3 protein.
The present study also demonstrated that NAC
treatment reduced the level of phospho-Akt, which was increased in
the SKOV3/TR cells, suggesting that Tyro3 signaled through the Akt
pathway to promote cell proliferation. This result was consistent
with that of a previous study, which reported that
phosphoinositide-3-kinase/Akt pathway is linked to the activation
of Tyro3 (38). However, NAC did
not affect the phosphorylation or protein levels of STAT3,
indicating that the increased phosphorylation and expression of
STAT3 in the SKOV3/TR cells may not be associated with the elevated
level of intracellular ROS in the cells.
In conclusion, the present study demonstrated that
long-term exposure of human ovarian cancer SKOV3 cells resulted in
the formation of taxol-resistant SKOV3/TR cells via the induction
of intracellular ROS, subsequent upregulation in the expression of
Tyro3 and the activation of Akt, which promoted the survival and
proliferation of the resistant cells. Thus, modulation of the
expression of Tyro3 and/or its role in the downstream signaling
pathway may offer a potential target in overcoming taxol
resistance.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology (grant.
no. 2006-2005303).
References
|
1
|
Agarwal R and Kaye SB: Ovarian cancer:
strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reed E, Kohn EC, Sarosy G, et al: M
Paclitaxel, cisplatin, and cyclophosphamide in human ovarian
cancer: molecular rationale and early clinical results. Semin
Oncol. 22(3 Suppl 6): 90–96. 1995.PubMed/NCBI
|
|
3
|
Raja FA, Counsell N, Colombo N, et al:
Platinum versus platinum-combination chemotherapy in
platinum-sensitive recurrent ovarian cancer: a meta-analysis using
individual patient data. Ann Oncol. 24(12): 3028–3034. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Einzig AI, Wiernik PH, Sasloff J, Runowicz
CD and Goldberg GL: Phase II study and long-term follow-up of
patients treated with taxol for advanced ovarian adenocarcinoma. J
Clin Oncol. 10:1748–1753. 1992.PubMed/NCBI
|
|
5
|
Tian C, Ambrosone CB, Darcy KM, et al:
Common variants in ABCB1, ABCC2 and ABCG2 genes and clinical
outcomes among women with advanced stage ovarian cancer treated
with platinum and taxane-based chemotherapy: a Gynecologic Oncology
Group study. Gynecol Oncol. 124:575–581. 2012. View Article : Google Scholar :
|
|
6
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McGuire WP 3rd: Current status of taxane
and platinum-based chemotherapy in ovarian cancer. J Clin Oncol.
21(Suppl 10): 133–135. 2003. View Article : Google Scholar
|
|
8
|
Ozols RF: Systemic therapy for ovarian
cancer: current status and new treatments. Semin Oncol. 33:3–11.
2006. View Article : Google Scholar
|
|
9
|
Robinson DR, Wu YM and Lin SF: The protein
tyrosine kinase family of the human genome. Oncogene. 19:5548–5557.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohashi K, Mizuno K, Kuma K, Miyata T and
Nakamura T: Cloning of the cDNA for a novel receptor tyrosine
kinase, Sky, predominantly expressed in brain. Oncogene. 9:699–705.
1994.PubMed/NCBI
|
|
11
|
O’Bryan JP, Frye RA, Cogswell PC, et al:
axl, a transforming gene isolated from primary human myeloid
leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell
Biol. 11:5016–5031. 1991.
|
|
12
|
Janssen JW, Schulz AS, Steenvoorden AC, et
al: A novel putative tyrosine kinase receptor with oncogenic
potential. Oncogene. 6:2113–2120. 1991.PubMed/NCBI
|
|
13
|
Rescigno J, Mansukhani A and Basilico C: A
putative receptor tyrosine kinase with unique structural topology.
Oncogene. 6:1909–1913. 1991.PubMed/NCBI
|
|
14
|
Sasaki T, Knyazev PG, Clout NJ, et al:
Structural basis for Gas6-Axl signalling. EMBO J. 25:80–87. 2006.
View Article : Google Scholar
|
|
15
|
Stitt TN, Conn G, Gore M, et al: The
anticoagulation factor protein S and its relative, Gas6, are
ligands for the Tyro 3/Axl family of receptor tyrosine kinases.
Cell. 80:661–670. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hafizi S and Dahlbäck B: Gas6 and protein
S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase
subfamily. FEBS J. 273:5231–5244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zwick E, Bange J and Ullrich A: Receptor
tyrosine kinase signalling as a target for cancer intervention
strategies. Endocr Relat Cancer. 8:161–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Challier C, Uphoff CC, Janssen JW and
Drexler HG: Differential expression of the ufo/axl oncogene in
human leukemia-lymphoma cell lines. Leukemia. 10:781–787.
1996.PubMed/NCBI
|
|
19
|
Graham DK, Salzberg DB, Kurtzberg J, et
al: Ectopic expression of the proto-oncogene Mer in pediatric
T-cell acute lymphoblastic leukemia. Clin Cancer Res. 12:2662–2669.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Vos J, Couderc G, Tarte K, et al:
Identifying intercellular signaling genes expressed in malignant
plasma cells by using complementary DNA arrays. Blood. 98:771–780.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meric F, Lee WP, Sahin A, Zhang H, Kung HJ
and Hung MC: Expression profile of tyrosine kinases in breast
cancer. Clin Cancer Res. 8:361–367. 2002.PubMed/NCBI
|
|
22
|
Tavazoie SF, Alarcón C, Oskarsson T, et
al: Endogenous human microRNAs that suppress breast cancer
metastasis. Nature. 451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Craven RJ, Xu LH, Weiner TM, et al:
Receptor tyrosine kinases expressed in metastatic colon cancer. Int
J Cancer. 60:791–797. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu CW, Li AF, Chi CW, et al: Clinical
significance of AXL kinase family in gastric cancer. Anticancer
Res. 22:1071–1078. 2002.PubMed/NCBI
|
|
25
|
Tsou AP, Wu KM, Tsen TY, et al: Parallel
hybridization analysis of multiple protein kinase genes:
identification of gene expression patterns characteristic of human
hepatocellular carcinoma. Genomics. 50:331–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Györffy B and Lage H: A Web-based data
warehouse on gene expression in human malignant melanoma. J Invest
Dermatol. 127:394–399. 2007. View Article : Google Scholar
|
|
27
|
Macleod K, Mullen P, Sewell J, et al:
Altered ErbB receptor signaling and gene expression in
cisplatin-resistant ovarian cancer. Cancer Res. 65:6789–6800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sainaghi PP, Castello L, Bergamasco L,
Galletti M, Bellosta P and Avanzi GC: Gas6 induces proliferation in
prostate carcinoma cell lines expressing the Axl receptor. J Cell
Physiol. 204:36–44. 2005. View Article : Google Scholar
|
|
29
|
Chung BI, Malkowicz SB, Nguyen TB,
Libertino JA and McGarvey TW: Expression of the proto-oncogene Axl
in renal cell carcinoma. DNA Cell Biol. 22:533–540. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
van Ginkel PR, Gee RL, Shearer RL, et al:
Expression of the receptor tyrosine kinase Axl promotes ocular
melanoma cell survival. Cancer Res. 64:128–134. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ozben T: Oxidative stress and apoptosis:
impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lau AT, Wang Y and Chiu JF: Reactive
oxygen species: current knowledge and applications in cancer
research and therapeutic. J Cell Biochem. 104:657–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Permuth-Wey J and Sellers TA: Epidemiology
of ovarian cancer. Methods Mol Biol. 472:413–437. 2009.
|
|
34
|
Collado M and Serrano M: Senescence in
tumours: evidence from mice and humans. Nat Rev Cancer. 10:51–57.
2010. View
Article : Google Scholar
|
|
35
|
Gioia R, Leroy C, Drullion C, et al:
Quantitative phosphoproteomics revealed interplay between Syk and
Lyn in the resistance to nilotinib in chronic myeloid leukemia
cells. Blood. 118:2211–2221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dufies M, Jacquel A, Belhacene N, et al:
Mechanisms of AXL overexpression and function in Imatinib-resistant
chronic myeloid leukemia cells. Oncotarget. 2:874–885.
2011.PubMed/NCBI
|
|
37
|
Achuthan S, Santhoshkumar TR, Prabhakar J,
Nair SA and Pillai MR: Drug-induced senescence generates
chemoresistant stemlike cells with low reactive oxygen species. J
Biol Chem. 286:37813–37829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhong Z, Wang Y, Guo H, et al: Protein S
protects neurons from excitotoxic injury by activating the TAM
receptor Tyro3-phosphatidylinositol 3-kinase-Akt pathway through
its sex hormone-binding globulin-like region. J Neurosci.
30:15521–15534. 2010. View Article : Google Scholar : PubMed/NCBI
|