Introduction
Cardiovascular disease (CVD) is an important
prognostic factor in patients with chronic kidney disease (CKD).
Mortality due to CVD in patients with CKD accounted for 44–51% of
the total mortality, which is the leading cause of mortality in
these patients. Levels of mortality in dialysis patients with CVD
was 30 times higher than that of the general population at the same
age (1) and atherosclerosis was
the most common cardiovascular complication. Endothelial cells are
responsible for lining the inner surface of the entire vascular
system; they are involved in the maintenance of normal blood flow
and are the basis of the closed conduits, which form the
cardiovascular system. Dysfunction of the endothelium has an
important role in the incidence of atherosclerosis. A large number
of uremic toxins accumulating in uremic patients lead to vascular
endothelial cell dysfunction (2)
and accelerate the development of CVD. The concentration of
parathyroid hormone (PTH) in uremia patients with secondary
hyperparathyroidism (SHPT) is several times higher than that in
non-SHPT uremic patients. High PTH levels may increase blood
pressure, exacerbating the development of hyperlipidemia and are
also an important factor for the development of atherosclerosis in
patients with end-stage renal disease (3). A meta-analysis recently indicated
that high concentrations of PTH were closely associated with an
increased risk of suffering a cardiovascular event (4).
In 1997, Kuro-o et al (4) identified a novel gene associated with
aging, termed Klotho. Klotho gene knockout mice (kl−/−mice) present
with a variety of similar phenotypes to that observed in aging
humans, including shortened life expectancy, hearing loss,
infertility, atherosclerosis, soft tissue calcification, skin
atrophy, osteoporosis and emphysema (5–8).
Klotho is highly expressed in the normal kidney. With a decline in
the glomerular filtration rate, Klotho gene and protein expression
levels significantly decrease in CKD patients (9). Previous studies have demonstrated
that Klotho protein has a protective effect on endothelial cell
damage and dysfunction induced by angiotensin-II and tumor necrosis
factor α (10,11), as well as the uremic toxin indoxyl
(12). It is hypothesized that
reduced Klotho in the peripheral organs is closely associated with
the complications of uremia and increased Klotho levels may improve
the complications of uremia. In the present study, by simulating
the state of SHPT in vivo, the proliferation, apoptosis and
nitric oxide (NO) synthesis of human umbilical vein endothelial
cells (HUVECs) in the serum from patients with SHPT was
investigated and the effect of recombinant Klotho protein on HUVECs
and the possible mechanism of this effect was examined.
Materials and methods
Materials
HUVECs were kindly provided by the Central
Laboratory of the First Affiliated Hospital of Nanjing Medical
University (Nanjing, China). HUVECs were purchased from ATCC
(Manassa, VA, USA; cat. no. PCS-100–010) RPMI-1640 medium, fetal
bovine serum, penicillin and streptomycin were purchased from
Gibco-BRL (Grand Island, NY, USA). The cell counting kit-8 (CCK-8)
detection kit (Beyotime, Shanghai, China) was used to assess cell
viability. PD98059 (ERK1/2 inhibitor), total ERK1/2 (t-ERK1/2)
mouse antibody (cat. no. 9107S) and phosphorated ERK1/2 (p-ERK1/2)
antibody (cat. no. 9106S) were purchased from Cell Signaling
Technology, Inc, (Danvers, MA, USA). A rabbit anti-GAPDH antibody
(cat. no. BA2913; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) was used for western blotting analysis. SDS PAGE gels were
purchased from Beyotime Institute of Biotechnology (Shanghai,
China). Recombinant Klotho protein was purchased from PeproTech
(cat. no. 100–53; Rocky Hill, NJ, USA). The annexin V-fluorescein
isothiocyanate apoptosis detection kit (KeyGEN, Nanjing, China) was
used to assess the levels of cell apoptosis using flow cytometry. A
human Klotho ELISA kit was purchased from USCN Life Science Inc.
(Wuhan, China). An NO detection kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China) was used to assess the
production of NO in the supernatants.
A total of three types of mixed serum were
collected, inactivated and preserved at -80°C. The first type was
from 15 patients with SHPT scheduled for parathyroidectomy, 8 males
and 7 females, aged 28–59 (45.3±9.7). This sera was collected
between July 1st and October 30th 2012 from
patients without infection. Every patient iPTH was >800 pg/ml.
The second was from 10 CKD-stage 5 without SHPT patients: 6 males
and 4 females, aged 36–79 (52.7±16.7). This sera was collected from
patients between July 1st and October 30th
2012 from patients whose eGFR was <15 ml/min, without infection.
Every patient iPTH was <300 pg/ml. The third was from 15 healthy
volunteers, 12 males and 3 females, aged 24–71 (41.4±19.5). The
present study was approved by the ethics committee of Nanjing
Medical University, Nanjing, China.
Cell culture
Cells were routinely cultured in RPMI-1640 medium
supplemented with 10% fetal bovine serum, 100 U/ml penicillin and
100 U/ml streptomycin at 37°C in a humidified atmosphere with 5%
CO2. All experiments were conducted using cells at
passage 5–10.
Assessment of the main constituents of
the three types of mixed serum
The three groups of sera were analyzed using an
automatic chemistry analyzer analyzer (AU5400; Beckman Coulter,
Fullerton, CA, USA) to detect blood urea nitrogen (BUN), creatinine
(Cr), uric acid (UA), calcium (Ca) and phosphate (P) in the
clinical testing center of The First Affiliated Hospital of Nanjing
Medical University. Intact parathyroid hormone (iPTH) and
C-reactive protein (CRP) were evaluated by chemiluminescence
apparatus (DXI800; Beckman Coulter) and an immunoturbidi-metric
automatic protein analyzer (BNII; Simens, Inc., Munich, Germany),
respectively. The levels of Klotho in the three types of mixed
serum were quantified using the human Klotho ELISA kit, according
to the manufacturer’s instructions.
Cell viability assay
The viability of cells was measured using the CCK-8
assay. HUVECs were cultured at a density of 104
cells/well in a 96-well plate for 24 h and then incubated in
vitro with 10% SHPT serum or 10% serum of stage 5 CKD patients
without SHPT or serum from healthy individuals (10%) as the
control. HUVECs were incubated with various concentrations of SHPT
sera (5, 10 or 20%) for 24 h and incubated with 10% SHPT sera for
6, 12 and 24 h. To observe different concentrations of Klotho with
or without PD98059 on the proliferation of HUVECs, cells were
cultured with serum from healthy individuals (10%) as the control
(C), 10% SHPT serum (S), 10% SHPT serum with different
concentrations of Klotho (25, 50 or 100 ng/ml; K) and 10% SHPT
serum with different concentrations of Klotho and PD98059 (P) for
24 h. All experiments were repeated at least three times.
Apoptosis assay
To determine whether SHPT serum induced cell
apoptosis of HUVECs and the protective role of Klotho, HUVECs were
cultured with 10% SHPT serum, 10% SHPT serum + Klotho (50 or 100
ng/ml), 10% SHPT serum + Klotho (50 or 100 ng/ml) + PD98059 (10
µmol/l) and serum from healthy individuals (10%) as the
control. Following incubation for 24 h, cells were harvested using
0.25% trypsin (without ethylene diamine tetraacetic acid) and
washed twice with cold phosphate-buffered saline (PBS). Following
staining with Annexin V/propidium iodide, the quantitative analysis
of cell apoptosis was determined using flow cytometry (FACSCalibur;
BD Biosciences, San Jose, CA, USA). All experiments were repeated
at least three times.
Immunoblotting
HUVECs were grouped as in the apoptosis assay
experiment section. Following incubation for 24 h, the cells were
washed with cold PBS. Protein from the cells was homogenized in
lysis buffer and was quantified. The protein (50 µg) for each
sample was separated using SDS-PAGE and the gel (percentage of
spacer gel and separation gel was 8% and 10%, respectively) was
transferred onto nitrocellulose membranes. The membranes were
blocked with 5% nonfat dry milk for 2 h and then incubated
overnight at 4°C with the corresponding primary antibodies,
p-ERK1/2 (1:1,000) or t-ERK1/2 (1:1,000). Subsequently, secondary
antibodies were applied (1:5,000) and the signals developed with an
ECL plus western blotting detection system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Densitometric analysis was performed with
Image J software version 1.41 (National Institutes of Health,
Bethesda, MD, USA). All experiments were repeated at least three
times.
Measurement of NO production
HUVECs were incubated in vitro with serum
from healthy individuals (10%) as a control, 10% SHPT serum or 10%
SHPT serum with Klotho (50 or 100 ng/ml) for 24 h. The supernatants
were collected and the synthesis of NO was measured using the
nitrate reduction method, according to the manufacturer’s
instructions. All experiments were repeated at least three
times.
Statistical analysis
Statistical analyses were performed using SPSS 13.0
(SPSS, Inc., Chicago, IL, USA). Values are expressed as the mean ±
standard deviation. Multiple comparisons were evaluated using
one-way analysis of variance and significant differences between
two groups were analyzed using the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Main constituents of the three types of
mixed serum
The main constituents of the mixed serum from
patients with SHPT, CKD at stage 5 without SHPT and healthy
volunteers are shown in Table I.
The levels of BUN, Cr, UA, Ca and P of the mixed serum from
patients with SHPT were significantly higher than those from
healthy volunteers and the iPTH from patients with SHPT was higher
than that from patients with CKD at stage 5 without SHPT. The
levels of Klotho in the mixed serum from healthy volunteers,
patients with SHPT and CKD at stage 5 without SHPT were
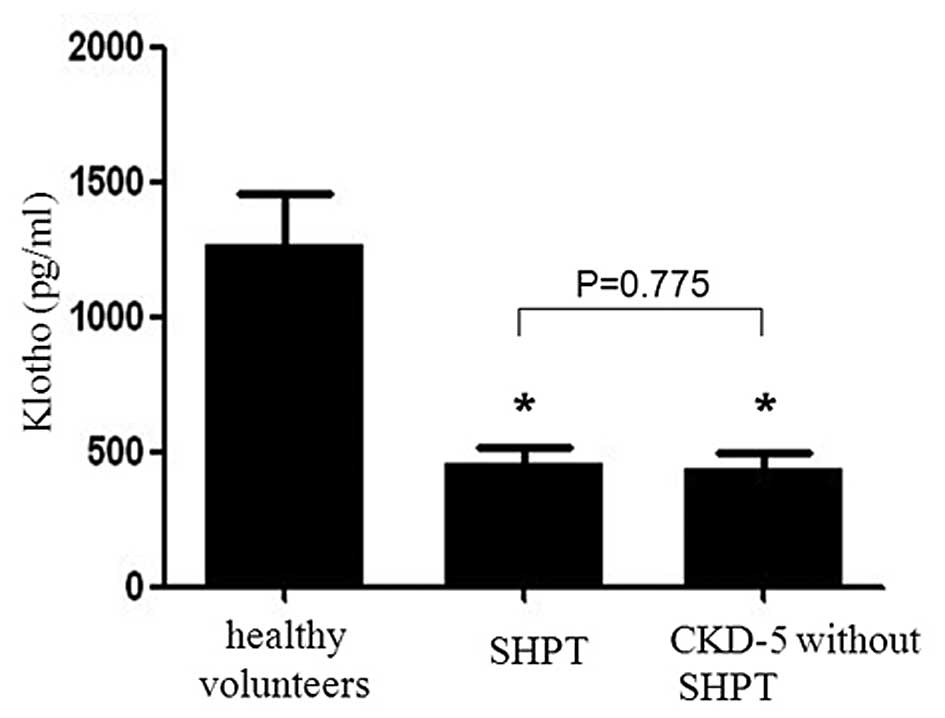
1,264.1±192.3 pg/ml, 458.5±57.3 pg/ml and 438.4±55.2 pg/ml,
respectively (Fig. 1). A
statistically significant difference was observed between the
healthy group and the latter two groups (P<0.001). However, no
statistically significant difference was observed between the SHPT
group and the CKD group (P=0.775).
 | Table IMain constituents of the mixed serum
from patients with secondary hyperparathyroidism, chronic kidney
disease at stage 5 without hyperparathyroidism and healthy
volunteers. |
Table I
Main constituents of the mixed serum
from patients with secondary hyperparathyroidism, chronic kidney
disease at stage 5 without hyperparathyroidism and healthy
volunteers.
| Patients | BUN (mmol/l) | Cr (µmol/l) | UA (µmol/l) | Ca (mmol/l) | P (mmol/l) | iPTH (pg/ml) | CRP (ng/l) |
|---|
| Healthy
volunteers | 3.18 | 43.8 | 305.9 | 2.08 | 1.24 | 13.5 | 3.19 |
| SHPT | 17.57 | 800.0 | 372.9 | 2.49 | 1.81 | 1435.0 | 9.77 |
| CKD-5 without
SHPT | 20.55 | 904.0 | 421.8 | 2.38 | 1.42 | 277.0 | 9.21 |
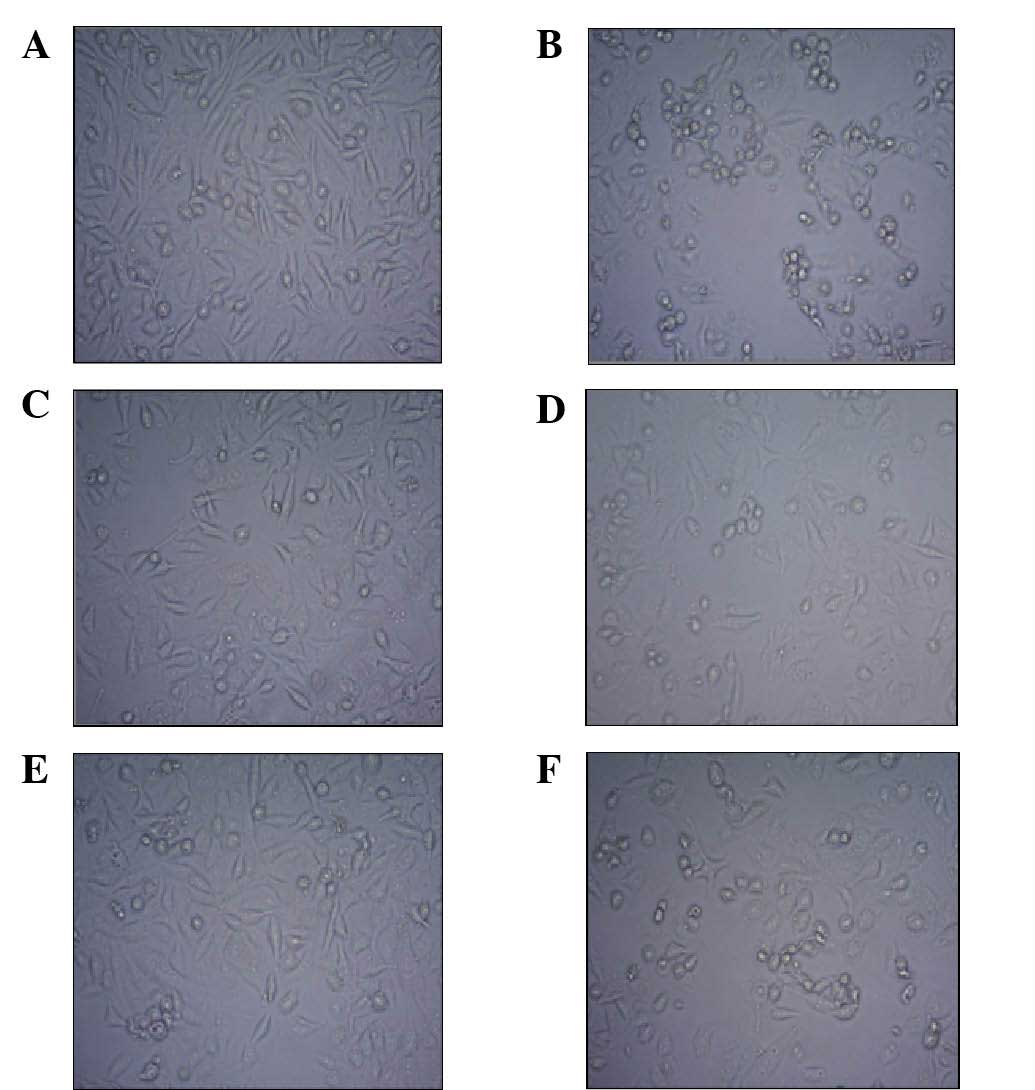
Morphology of HUVECs
The HUVEC monolayer exhibited a characteristic
cobblestone-like appearance in the control group (Fig. 2A). In the SHPT serum group, the
number of HUVECs decreased with cell shrinkage and nuclear and
cytoplasmic condensation (Fig.
2B). Compared with the SHPT group, the number of the HUVECs in
the SHPT+Klotho (50 or 100 ng/ml) group increased with the
morphology of the cells similar to that of the control (Fig. 2C and E). However, when PD98059 was
added to the SHPT + Klotho group, the number of HUVECs decreased
and the cells shrank (Fig. 2D and
F).
Cell viability and proliferation of
HUVECs incubated with the serum from SHPT or CKD patients at stage
5 is decreased
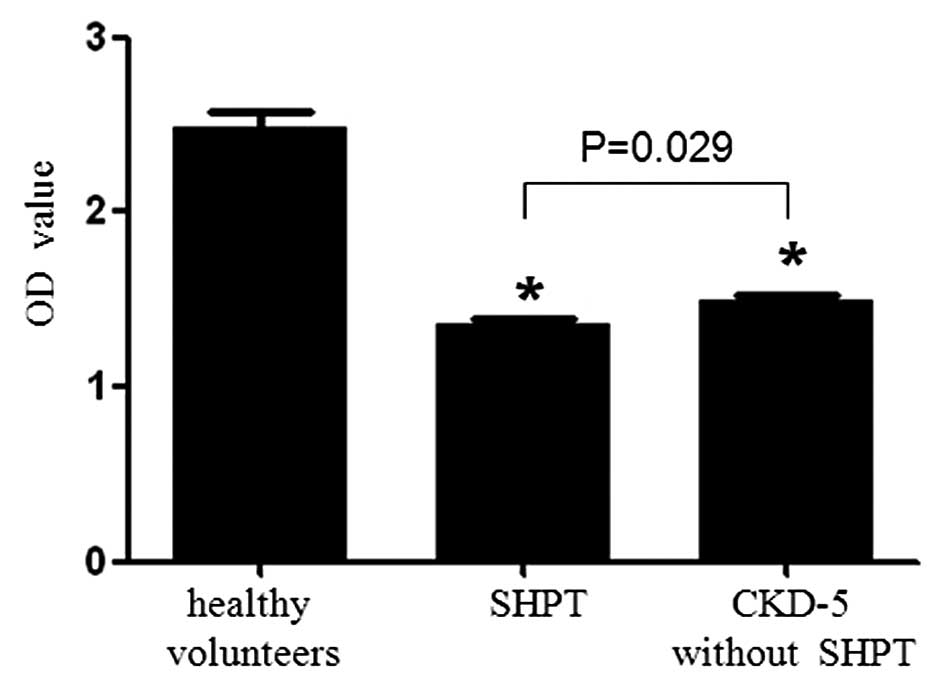
Compared with the healthy control group [optical
density (OD)=2.478±0.094], the proliferation of HUVECs in the CKD
at stage 5 without SHPT group (OD=1.498±0.027) and SHPT group
(OD=1.363±0.023) decreased significantly compared with that of the
control (P<0.001; Fig. 3).
Furthermore, the inhibition of SHPT serum on the proliferation of
HUVECs was greater than that of the CKD at stage 5 without SHPT
serum (P=0.029).
SHPT serum inhibits the proliferation of
HUVECs in a concentration-dependent manner
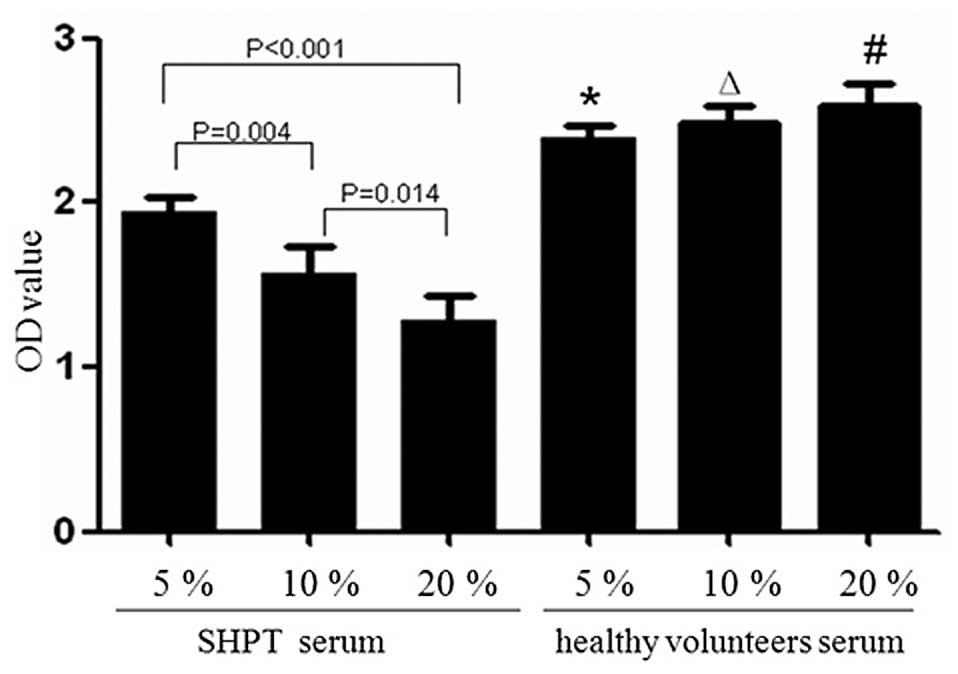
Compared with the healthy control group (5, 10 and
20%), with OD values of 2.392±0.074, 2.487±0.100 and 2.592±0.139,
respectively, the proliferation of HUVECs in the SHPT group
decreased significantly (P≤0.001; Fig.
4). At 5–20%, SHPT serum inhibited the proliferation of HUVECs
in a concentration-dependent manner (P<0.05). The OD values for
the SHPT group (5, 10 and 20%) were 1.934±0.088, 1.570±0.160 and
1.282±0.150, respectively (5 vs. 10% SHPT group, P=0.004; 5 vs. 20%
SHPT group, P<0.001 and 10 vs. 20% SHPT group, P=0.014).
SHPT serum inhibits the proliferation of
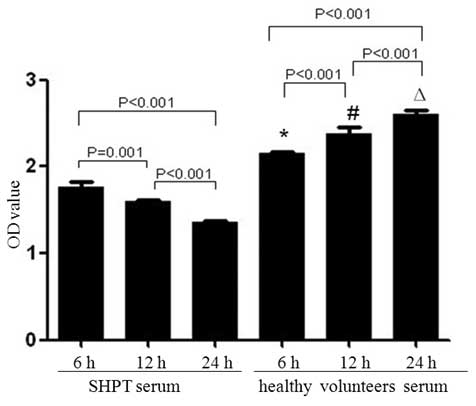
HUVECs in a time-dependent manner
Compared with the healthy control group, with OD
values at 6, 12 and 24 h of 2.146±0.027, 2.373±0.081 and
2.608±0.047, respectively, the OD values in the SHPT group
decreased significantly (1.767±0.062, 1.599±0.018 and 1.353±0.026
at 6, 12 and 24 h, respectively; 6 h group vs. 12 h group, P=0.001;
6 h group vs. 24 h group, P<0.001; 12 h group vs. 24 h group,
P<0.001; Fig. 5).
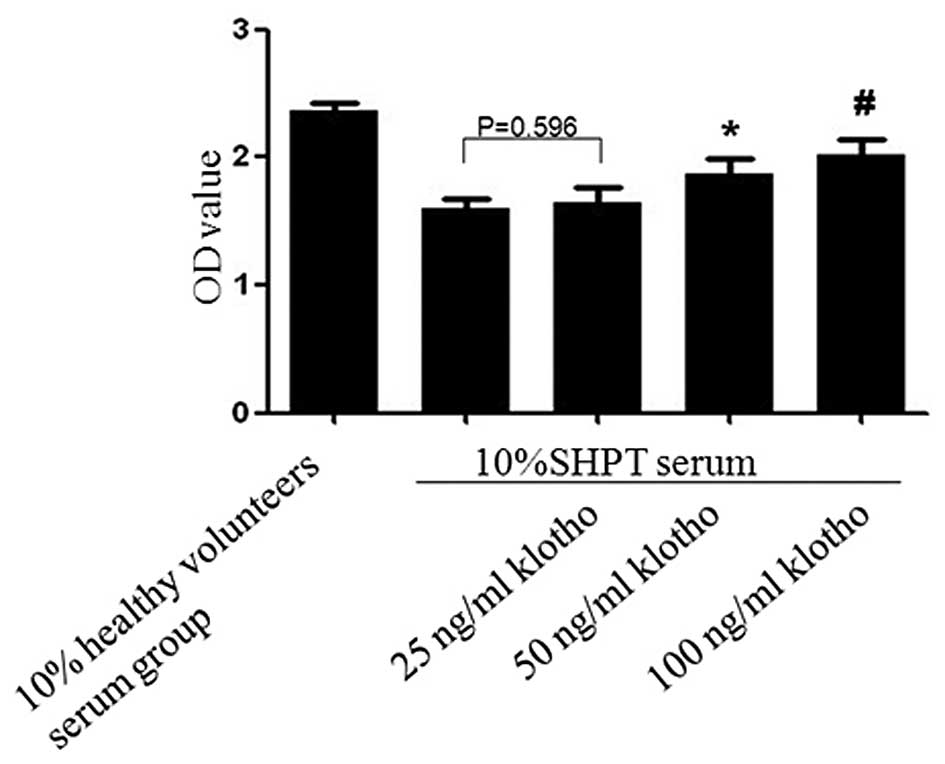
Klotho alleviates the inhibitory effect
of SHPT serum on the proliferation of HUVECs in a
concentration-dependent manner
Compared with the SHPT group (OD=1.598±0.078), the
proliferation of HUVECs was increased in the SHPT + Klotho (50 or
100 ng/ml) group (OD=1.869±0.118 and 2.021±0.123, respectively;
SHPT group vs. 50 ng/ml Klotho + SHPT group, P=0.01 and SHPT group
vs. 100 ng/ml Klotho + SHPT group, P=0.001; Fig. 6). However, there was no
statistically significant difference between the 25 ng/ml Klotho +
SHPT group (OD=1.645±0.114) and the SHPT group (P=0.596).
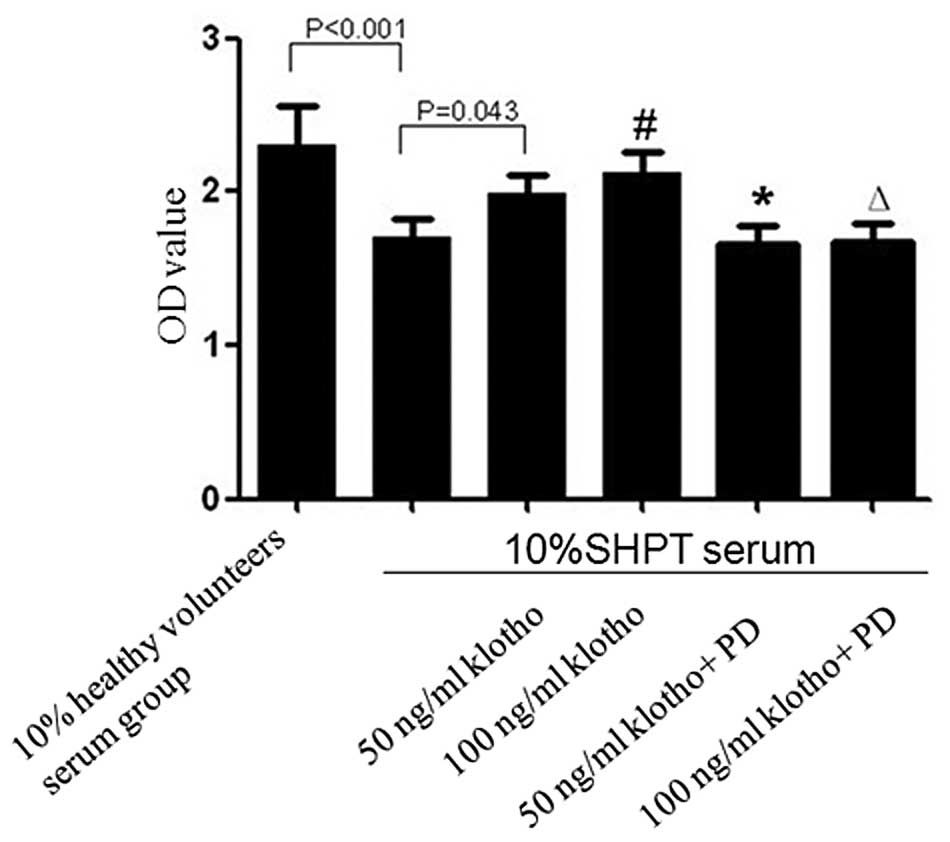
The ERK1/2 inhibitor PD98059 abolishes
the alleviating effect of Klotho on the inhibitory effect of SHPT
serum on the proliferation of HUVECs
Compared with the healthy control group
(OD=2.305±0.255), the proliferation of HUVECs in the SHPT group
decreased significantly (OD=1.707±0.113; P<0.001; Fig. 7). The proliferation was partly
restored when 50 or 100 ng/ml Klotho was added into the 10% SHPT
serum, with OD values of 1.991±0.121 (50 ng/ml Klotho; P=0.043) and
2.117±0.136 (100 ng/ml Klotho; P=0.007). However, this effect was
blocked by PD98059. When PD98059 was added to the 50 or 100 ng/ml
Klotho group, the OD value decreased to 1.656±0.129 or 1.681±0.117
(50 ng/ml Klotho group vs. 50 ng/ml Klotho + PD98059, P=0.021; 100
ng/ml Klotho vs. 100 ng/ml Klotho + PD98059, P=0.005; Fig. 7).
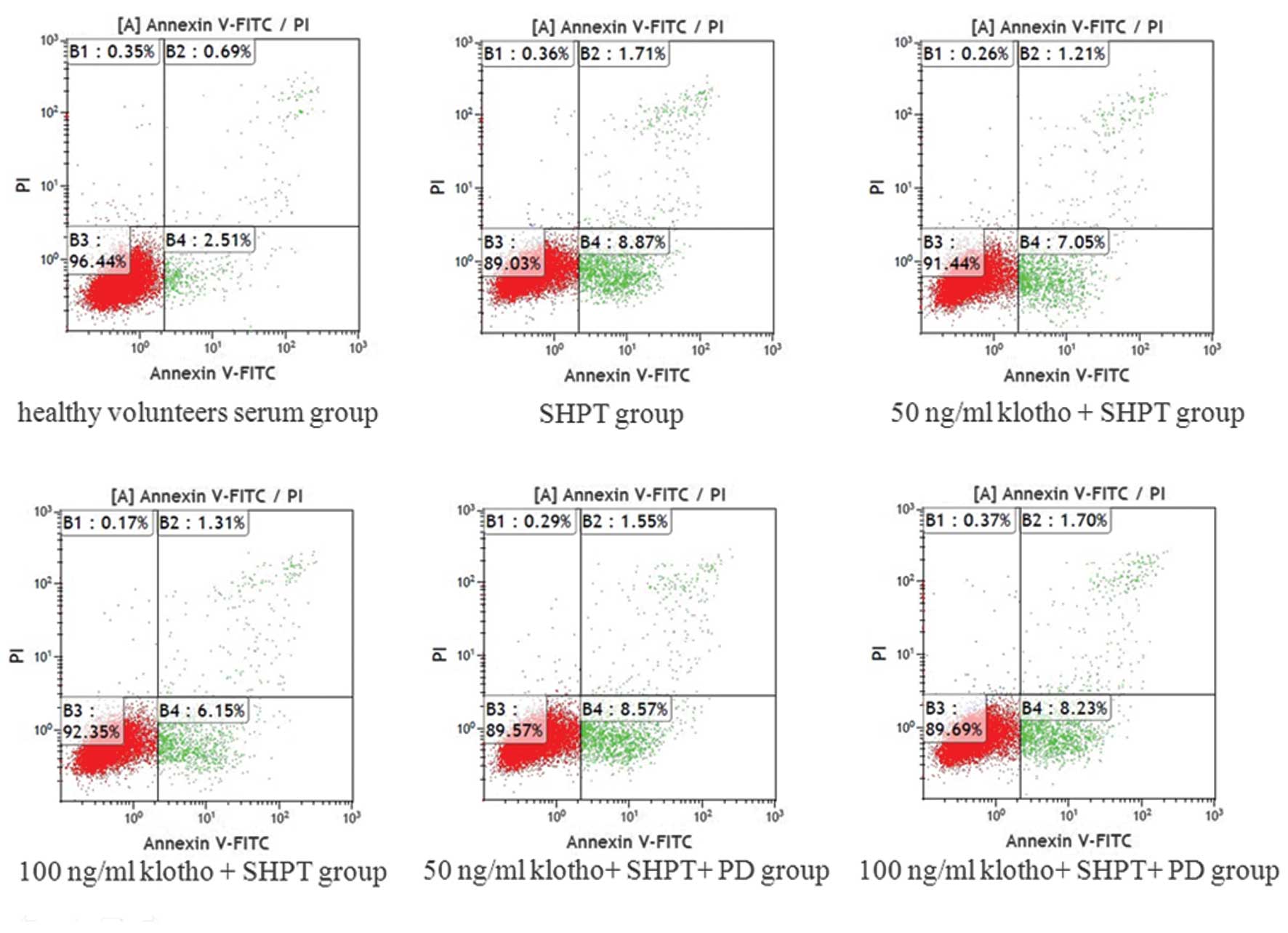
Klotho inhibits apoptosis of HUVECs
induced by SHPT serum
Compared with the healthy control group, SHPT serum
induced apoptosis of HUVECs. The apoptotic rates of the SHPT group
and healthy control group were 8.53±0.81 and 3.21±0.59%,
respectively, and this difference was statistically significant
(P<0.001). Of note, the apoptotic rate was reduced when 50 or
100 ng/ml Klotho was added to the 10% SHPT serum, resulting in
apoptotic rates of 6.14±0.86 and 5.86±0.98%, respectively (50 ng/ml
Klotho + SHPT group vs. SHPT group, P=0.001; 100 ng/ml Klotho +
SHPT group vs. SHPT group, P=0.001). In addition, the
anti-apoptotic effect was blocked by the ERK1/2 inhibitor PD98059.
In the 50 ng/ml Klotho + SHPT + PD98059 group, the apoptotic rate
was 8.12±1.03%, which was significantly different compared with
that in the 50 ng/ml Klotho + SHPT group (P=0.006). In the 100
ng/ml Klotho + SHPT + PD98059 group, the apoptotic rate was
7.87±0.65%, which was significantly different compared with that in
the 100 ng/ml Klotho + SHPT group (P=0.005; Fig. 8).
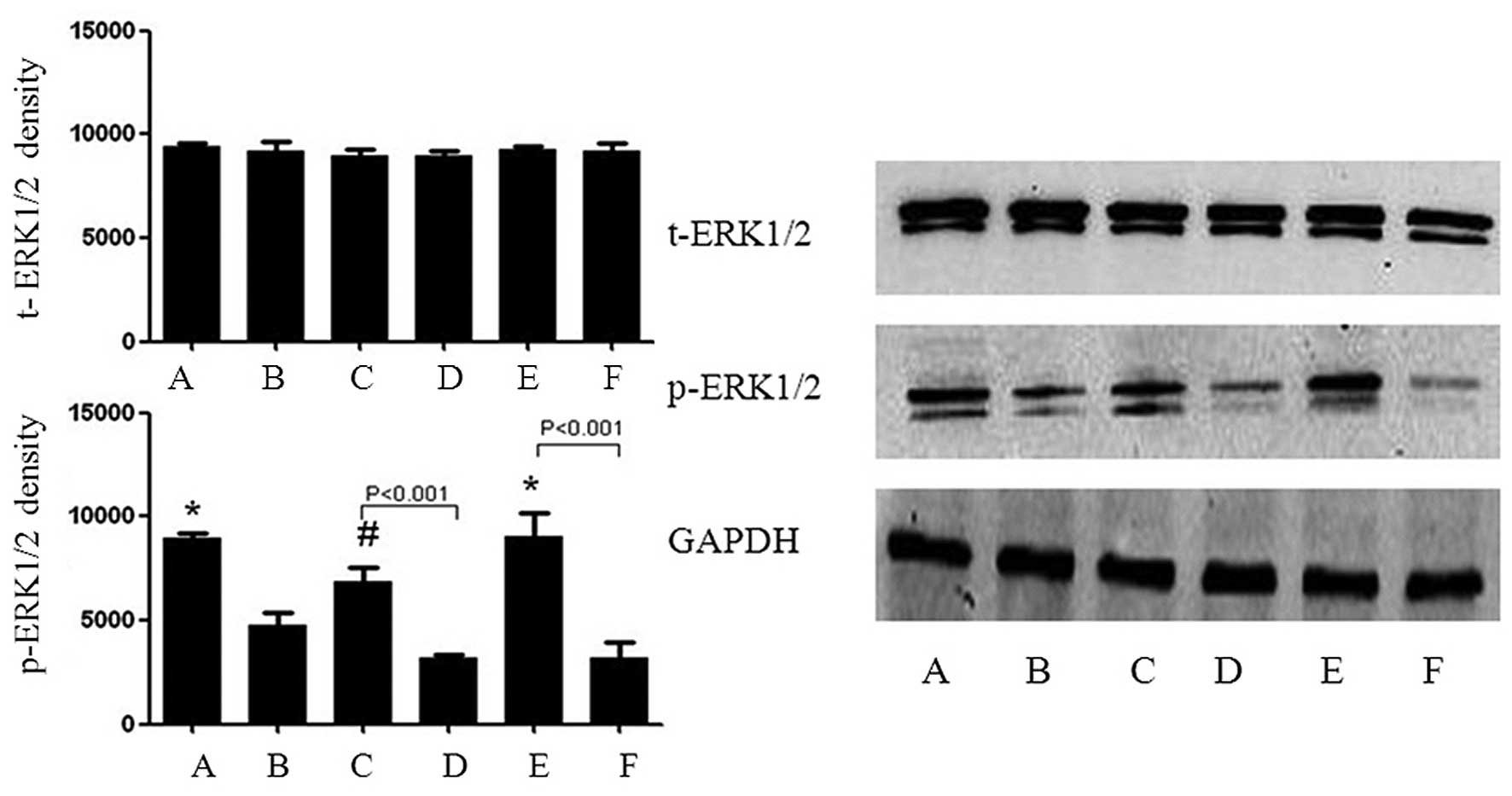
Klotho inhibits the SHPT serum-stimulated
repression of p-ERK1/2 in HUVECs
Compared with the SHPT group, the expression of
t-ERK1/2 was not affected by Klotho; however, p-ERK1/2 was markedly
upregulated. This effect of Klotho was blocked by PD98059 (Fig. 9).
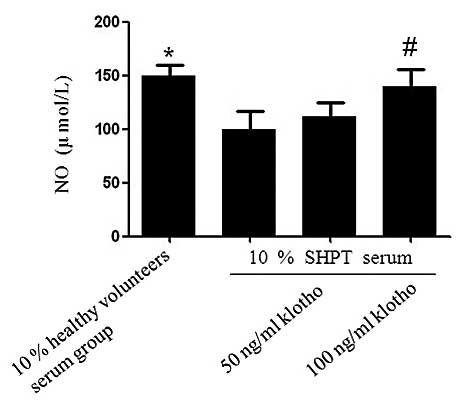
Klotho abrogates the SHPT serum-mediated
inhibition of NO production in HUVECs
Compared with the healthy control group, the
production of NO in the SHPT group decreased significantly for the
incubation period of 24 h (99.94±16.28 µmol/l vs. 149.50±10.16
µmol/l; P=0.03; Fig. 10).
However, NO production increased in the SHPT + Klotho group (50
ng/ml Klotho, 111.35±13.33 µmol/l and 100 ng/ml Klotho,
139.78±15.89 µmol/l). There was a statistically significant
difference between the 100 ng/ml Klotho + SHPT group and the SHPT
group (P=0.039; Fig. 10).
Discussion
Linder et al (13) proposed the concept of ‘accelerated
atherosclerosis’ in 1974 and hypothesized that accelerated
atherosclerosis is a major risk factor affecting the long-term
survival of hemodialysis patients. The particular
pathophysiological state in uremia patients may result in
endothelial cell dysfunction (2,14).
Compared with non-SHPT uremia, in uremic patients with SHPT,
despite of urea, creatinine, indole, phenolic compounds,
guanidines, amines and other toxins (15), their PTH concentrations are several
times higher than normal levels. Serum PTH levels >70 pg/ml may
become independent risk factors for CVD in CKD stage 3–4 patients
(14,16). Excessive PTH is another risk factor
for increasing vascular endothelial damage and for these patients,
their symptoms of CVD are more serious. Slowing the development of
CVD in patients with SHPT is a priority in treating SHPT. The
prevention and treatment of accelerated atherosclerosis, which may
reduce the morbidity and mortality of cardiovascular events may
improve the outcome of patients with SHPT. Atherosclerosis is a
state in which a series of molecular and cellular changes are
involved, resulting from endothelial injury. Vascular endothelial
injury and dysfunction is a critical link to atherosclerosis
formation. Regarding the risk factors for atherosclerosis in
patients with SHPT, traditional risk factors, including high
cholesterol, smoking status, diabetes and hypertension are
important. In addition, other non-traditional factors, including a
variety of uremic toxins, immune dysfunction, calcium and
phosphorus metabolism, high PTH, hyperlipidemia, oxidative stress,
micro-inflammation, hyperhomocysteinemia, advanced glycation end
products and advanced oxidation protein products may all induce
endothelial cell dysfunction and are involved in promoting the
formation of atherosclerosis (17,18).
Endothelial cells are the basic structural and functional unit of
the vascular endothelium, which has an important role in barrier
function and regulating the exchange of substances inside and
outside the vessel. The repair of injured endothelial cells
predominantly relies on the movement and proliferation of adjacent
normal cells (19). The growth and
proliferation of endothelial cells and endothelial repair are
closely associated with the incidence of atherosclerosis (20). The present study demonstrated that,
compared with healthy human serum, the proliferation of HUVECs was
inhibited by the serum of SHPT patients and CKD-5 patients without
SHPT. Of note, the inhibition by SHPT serum was greater than that
by serum from patients with CDK-5 withouth SHPT, suggesting that
the SHPT state can accelerate the occurrence of atherosclerosis
more markedly.
In recent years, the Klotho gene and protein, which
have a key role in aging and aging-associated diseases aroused
growing interest. Human Klotho exists in two forms (21–23),
as membrane-bound proteins and a secreted form. Klotho protein may
act as a hormonal factor to protect against endothelial cell injury
induced by oxidative stress (24,25).
It has been demon strated that the expression of Klotho was
negatively correlated with the development of atherosclerosis in
the uremia model of apolipoprotein E knockout mice induced by
unilateral renal cortical electrical cautery plus contralateral
kidney resection (26). Decreased
levels of Klotho may be involved in the process of atherosclerosis
in uremia (26). Of note,
researchers have proposed in recent years that the uremia itself is
an uncontrolled process of aging. Anti-aging therapy may be a novel
approach to prevent uremia (27).
The present study found that Klotho protein can partially restore
proliferation and inhibit apoptosis of HUVECs treated with SHPT
serum, suggesting that atherosclerosis induced by SHPT serum may be
partly antagonized by Klotho protein.
As has been established, NO is one of the most
important vascular relaxing factors derived from endothelial cells.
As a cellular messenger molecule, in addition to vasodilation, NO
also has a variety of effects on the blood vessels, including the
maintenance of vascular elasticity, inhibition of platelet
aggregation induced by adenosine diphosphate, effective platelet
disaggregation, and inhibition of lymphocyte, granulocyte and
monocyte adhesion to the vascular endothelium (28–30).
These effects of NO make it an important protective factor against
the formation of atherosclerosis. The alterations in the production
of NO from endothelial cells are likely to affect the normal
protective mechanisms of blood vessels. The present study found
that NO production was inhibited by SHPT serum and enhanced by
Klotho, suggesting that Klotho protein may protect endothelial
cells by inducing the synthesis of NO.
The mitogen-activated protein kinase (MAPK) signal
transduction pathway is an important signal transduction system
in vivo (31). The ERK1/2
signal transduction pathway belongs to the MAPK family, which has
an important role in cell growth, the cell cycle, cell stress,
apoptosis and other physiological and pathological processes
(32,33). It can regulate cell proliferation,
differentiation and cytoskeletal rearrangement as well as a number
of other biological activities. p-ERK1/2 is an important activated
form in the ERK1/2 signaling pathway, which is involved in the
expression of genes, migration, differentiation and proliferation
in cells (34–37). The present study identified that
Klotho protein may partially restore the proliferation and vitality
of HUVECs and inhibit their apoptosis when treated with SHPT serum,
accompanied by p-ERK1/2 upregulation. In addition, the effects of
Klotho may be inhibited by PD98059, a specific ERK1/2 inhibitor,
suggesting that the ERK1/2 signaling pathway is involved in the
vascular protective effect of the Klotho protein.
These experimental results revealed that Klotho
protein had a protective effect on endothelial cells and is a
potential therapeutic factor for the prevention of atherosclerosis
with SHPT. Based on these findings, further study of the Klotho
protein is warranted.
Acknowledgments
The present study was supported in part by research
grants from the National Science and Technology Pillar Program
during the Twelfth Five-year Plan Period (grant no. 2011BAI 10B00),
the Medical Scientific Research Foundation of Jiangsu Province
(grant no. Z201002) and the Priority Academic Program
Development(PAPD) of Jiangsu Higher Education Institutions.
References
|
1
|
Foley RN, Parfrey PS and Sarnak MJ:
Clinical epidemiology of cardiovascular disease in chronic renal
disease. Am J Kidney Dis. 32(5 Suppl 3): S122–S129. 1998.
View Article : Google Scholar
|
|
2
|
Jourde-chiche N, Dou L, Cerini C, et al:
Vascular incompetence in dialysis patients-protein-bound uremic
toxins and endothelial dysfunction. Semin Dial. 24:327–337. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Van Ballegooijen AJ, Reinders I, Visser M,
et al: Parathyroid hormone and cardiovascular disease events: A
systematic review and meta-analysis of prospective studies. Am
Heart J. 165:655–664. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuro-o M, Matsumura Y, Aizawa H, et al:
Mutation of the mouse klotho gene leads to a syndrome resembling
aging. Nature. 3909:45–51. 1997. View
Article : Google Scholar
|
|
5
|
Kamemori M, Ohyama Y, Kurabayashi M, et
al: Expression of klotho protein in the inner ear. Hear Res.
171:103–110. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagai T, Yamada K, Kim HC, et al:
Cognition impairment in the genetic model of aging klotho gene
mutant mice: a role of oxidative stress. FASEB J. 17:50–52.
2003.
|
|
7
|
Anamizu Y, kawaguchi H, Seichi A, et al:
Klotho insufficiency causes decrease of ribosomal RNA gene
transcription activity, cytoplasmic RNA and rough ER in the spinal
anterior horn cells. Acta Neuropathol. 109:457–466. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu MC, Shi M, Zhang J, et al: Klotho
deficiency causes vascular calcification in chronic kidney disease.
J Am Soc Nephrol. 22:124–136. 2011. View Article : Google Scholar :
|
|
9
|
Rakugi H, Matsukawa N, Ishikawa K, et al:
Anti-oxidative effect of klotho on endothelial cells through cAMP
activation. Endocrine. 31:82–87. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carracedo J, Buendia P, Merino A, et al:
klotho modulates the stress response in human senescent endothelial
cells. Mech Ageing Dev. 133:647–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang K, Nie L, Huang Y, et al: Ameliration
of uremic toxin indoxyl sulfate-induced endothelial cell
dysfunction by klotho protein. Toxicol Lett. 215:77–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komaba H and Fukagawa M: FGF23-parathyroid
interaction: implications in chronic kidney disease. Kidney Int.
77:292–298. 2010. View Article : Google Scholar
|
|
13
|
Linder A, Charra B and Sherrard DJ:
Accelerated atherosclerosis in prolonged maintenance hemodialysis.
N Eng J Med. 290:697–701. 1974. View Article : Google Scholar
|
|
14
|
Eberhardt RT, Forgione MA, Cap A, et al:
Endothelial dysfunc dysfunction in a murine model of mild
hyperhomocyst(e)inemia. J Clin Invest. 106:483–491. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raff AC, Meyer TW and Hostetter TH: New
insights into uremic toxicity. Curr Opin Nephrol Hypertens.
17:560–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhuriva R, Li S, Chen SC, et al: Plasma
parathyroid hormone level and prevalent cardiovascular disease in
CKD stages 3 and 4: an analysis from the Kidney Early Evaluation
Program. Am J Kidney Dis. 5(Suppl 4): 3–10. 2009. View Article : Google Scholar
|
|
17
|
de Groot K, Bahlmann FH, Sowa J, et al:
Uremia causes endo-thelial progenitor cell deficiency. Kidney Int.
66:641–646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi JH, Kim KL, Huh W, et al: Decreased
number and impaired angiogenic function of endothelial progenitor
cells in patients with chronic renal failure. Arterioscler Thromb
Vasc Biol. 24:1246–1252. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carmeliet P, Moons L, Stassen JM, et al:
Vascular wound healing and neointima formation induced by
perivascular electric injury in mice. Am J Patho. 150:761–776.
1997.
|
|
20
|
Goligorsky MS, Yasuda K and Ratliff B:
Dysfunctional endothelial progenitor cells in chronic kidney
disease. J Am Soc Nephrol. 21:911–919. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsumura Y, Aizawa H, Nakamura T, et al:
Identification of the human klotho gene and its two transcripts
encoding membrance and secreted klotho protein. Biochem Biophys Res
Commun. 242:626–630. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiraki-lida T, Aizawa H, Matsumura Y, et
al: Structure of the mouse klotho gene and its two transcripts
encoding membrane and secreted protein. FEBS Lett. 424:6–10.
1998.
|
|
23
|
Tohyma O, Imura A, Iwano A, et al: Klotho
is novel beta-glucuronidase capable of hydrolyzing steroid
beta-glucuronidase. J Biol Chem. 279:9777–9784. 2004. View Article : Google Scholar
|
|
24
|
Kachiwala SJ, Harris SE, Wright AF, et al:
Genetic influences on oxidative stress and their association with
normal cognitive aging. Neurosci Lett. 386:116–120. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamamoto M, Clark JD, Pastor JV, et al:
Regulation of oxidative stress by the anti-aging hormone klotho. J
Biol Chem. 280:38029–38034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jie YU, Deng M, Zhao J, et al: Decreased
expression of klotho gene in uremic atherosclerosis in
apolipoprotein E-deficient mice. Biochem Biophys Res Commun.
391:261–266. 2010. View Article : Google Scholar
|
|
27
|
Kooman JP, Broers NJ, Uswat L, et al: Out
of control: accelerated aging in uremia. Nephrol Dial Transplant.
28:48–54. 2013. View Article : Google Scholar
|
|
28
|
Zou MH, Shi C and Cohen RA: Oxidation of
the zinc-thiolate complex and uncoupling of endothelial nitric
oxide synthase by peroxynitrite. J Clin Invest. 109:817–826. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moncada S and Higgs EA: Nitric oxide and
the vascular endothelium. Handb Exp Pharmacol. 176(Pt 1): 213–254.
2006.PubMed/NCBI
|
|
30
|
Lubos E, Handy DE and Loscalzo J: Role of
oxidative stress and nitric oxide in atherothrombosis. Front
Biosci. 13:5323–5344. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lawrence MC, Jivan A, Shao C, et al: The
roles of MAPKs in disease. Cell Res. 18:436–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cross TG, Scheel-Toellner D, Henriquez NV,
et al: Serine/threonine protein kinases and apoptosis. Exp Cell
Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogenactivated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
34
|
Cobb MH: MAP kinase pathways. Prog Biophys
Mol Biol. 71:479–500. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Z, Dickens M, Raingeaud J, et al:
Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis.
Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steelman LS, Bertrand FE and McCubrey JA:
The complexity of PTEN: mutation, marker and potential target for
therapeutic intervention. Expert Opin Ther Targets. 8:537–550.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Steelman LS, Pohnert SC, Shelton JG, et
al: JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle
progression and leukemogenesis. Leukemia. 18:189–218. 2004.
View Article : Google Scholar : PubMed/NCBI
|