Introduction
Metastasis is the leading cause of morbidity and
mortality associated with cancer. The development of metastasis
consists of numerous processes, in which cancer cells initially
detach from the primary tumor, invade the surrounding tissues and
intravasate into blood and/or lymphatic systems. This is followed
by extravasation from the vasculature and subsequent colonization
of target organs (1). The
extracellular matrix (ECM) provides both structural support and
extracellular cues that regulate invasive tumor growth.
Tumor-associated changes in the ECM contribute to cancer
progression (2). Cell migration
involves the assembly and disassembly of focal adhesions. The
migration of cells is stimulated extracellularly and is initiated
by integrins and intracellular signaling proteins, which are
located within focal adhesions (2,3).
Integrins are α and β heterodimeric cell-surface receptors that
mediate cell-ECM interactions, and have important roles in the
regulation of normal and tumor cell migration and survival
(2,3). The binding of a ligand to the
extracellular integrin domain induces conformational changes and
integrin clustering, that results in the activation of signaling
cascades and recruitment of multi-protein complexes to focal
adhesions (4). Subsequently,
integrins lacking kinase activity transmit signals through a
variety of intracellular protein kinases and adaptor molecules,
including focal adhesion kinase (FAK), integrin-linked kinase (ILK)
and paxillin (5–7).
ILK has a central role in mediating signal
transduction, initiated by cell-ECM interactions. This leads to the
regulation of numerous biochemical processes, including
proliferation, survival, differentiation, migration, invasion and
angiogenesis (8). ILK was
initially described as an integrin β1 subunit-binding protein, with
involvement in kinase signaling pathways (5). However, previous studies have
demonstrated that ILK is a pseudokinase that acts as a distinct
adaptor protein linking integrin and α-parvin (9,10).
Structurally, ILK is comprised of an N-terminal ankyrin repeat
domain and a C-terminal kinase-like domain (10,11).
Through the ankyrin repeat domain, ILK binds to PINCH1 (12). The kinase-like domain of ILK
interacts directly with other components of integrin-based adhesion
plaques, including α-parvin (9,10,13).
ILK, PINCH1, and α-parvin form the ternary complex IPP, which has
emerged as an essential constituent of integrin-containing adhesion
sites. IPP functions both as a structural complex, that connects
integrins to the actin cytoskeleton, and as a signaling platform,
that modulates numerous cellular processes (6,8). The
IPP complex is a central constituent of β1 and β3
integrin-containing adhesion sites, where it regulates numerous
signaling pathways, including Akt, extracelullar signal-regulated
kinase (ERK)1/2, and Rac1 (6,8,13).
Chelidonine is a major benzophenanthridine alkaloid
derived from the plant extract of Chelidonium majus, which
is also known as the greater celandine (family Papaveraceae), and
is widely distributed in Europe and Asia. The plant extract
exhibits notable antitumor and antiviral activities (14). Chelidonine is the major component
of the anticancer drug Ukrain, which is a semisynthetic derivative
of C. majus alkaloids and has antitumor activity (15). This alkaloid has been shown to
induce apoptosis of primary human uveal melanoma cells (16); arrest mitosis through the
inhibition of tubulin polymerization and activation of the
stress-activated protein kinase/c-Jun N-terminal protein kinase
(17); and down-regulate the
expression of telomerase in HepG2 cells (18). The effects of chelidonine on cell
migration and invasion, however, have not yet been determined. The
aim of the present study was to explore whether chelidonine
inhibited the migration and invasion of MDA-MB-231 human breast
cancer cells, and to further elucidate the underlying
mechanisms.
Materials and methods
Cell culture and reagents
MDA-MB-231 human breast cancer cells were purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were maintained in RPMI-1640 medium, supplemented with 100
U/ml penicillin and 100 µg/ml streptomycin (Invitrogen Life
Technologies, Carsbad, CA, USA) and 10% heat-inactivated fetal
bovine serum (HyClone Laboratories, Inc., Logan, UT, USA), in a
humidified 5% CO2 atmosphere at 37°C. The chelidonine
used for the present study was isolated from C. majus as
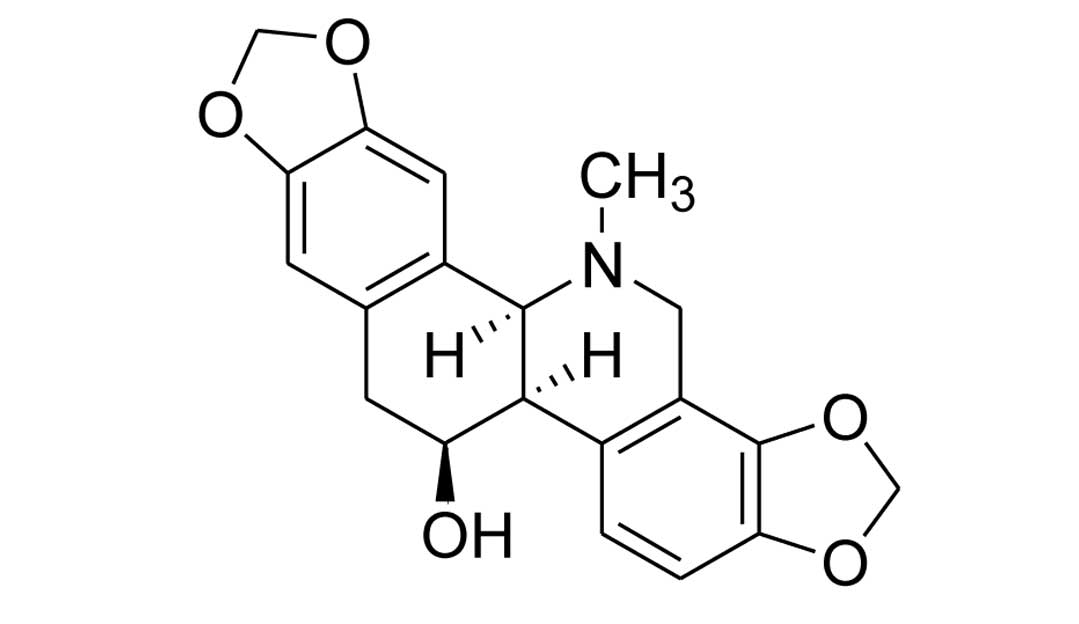
described by previous methods (19). The chemical structure of
chelidonine is shown in Fig. 1.
The purity of the compound was determined to be >98%, by high
performance liquid chromatography analysis. HPLC was performed
using a Gilson UV/VIS 156 system (Gilson Inc., Middleton, WI, USA),
in reverse phase mode on an Agilent Eclipse XD8-C18 (5 µm;
4.6 × 250 cm) column (Agilent Technologies, Santa Clara, CA, USA)
at an elution rate of 1 ml/min, in the gradient 15–80% of
acetonitrile in 10 mM 1-hexane sulphonic acid sodium. The
chelidonine was solubilized in 100% dimethyl sulfoxide (DMSO) and
used at a final concentration of <0.05% DMSO. Antibodies against
phospho-Akt (Ser473), Akt, ERK1/2, phospho-ERK1/2 (Thr202/Tyr204),
phospho-FAK (Tyr397), FAK, and ILK were obtained from Santa Cruz
Biotechnology, (Santa-Cruz, CA, USA). Antibodies against α-tubulin
and PINCH1 were from Sigma-Aldrich (St. Louis, MO, USA). Protein
A/G agarose beads and antibodies against GAPDH, ErbB2, and α-parvin
were obtained from Santa Cruz Biotechnology Inc. (Dallas, TX, USA).
Fibronectin and type 1 collagen (COL-I) were purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Fluorescein isothiocyanate
(FITC)-conjugated phalloidin was from Enzo Life Sciences (San
Diego, CA, USA).
Cell viability assay
The cytotoxic activity of chelidonine was determined
using an MTT-based colorimetric assay. Briefly, the cells
(1×104 cells/well) were seeded in 96-well plates and
allowed to grow for 24 h. Chelidonine was added to the wells at the
following concentrations: 0, 1, 3, 10 µm. Following an
additional 24 h incubation, the MTT solution (5 mg/ml) was added
and the cells were incubated for 4 h. The experiment was performed
in triplicate and the cell viability was presented as a percentage
of the control.
Immunoprecipitation and western
blotting
Immunoprecipitation and western blotting were
performed as previoulsy described (20,21).
Briefly, the cells were lysed in lysis buffer, containing 50 mM
Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 5 mM sodium
orthovanadate, 1% NP40 and a protease inhibitor cocktail (BD
Biosciences), and centrifuged at 22,000 × g for 10 min at 4°C. A
total of 25 µg protein per lane was separated by sodium
dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene difluoride membrane (Millipore,
Bedford, MA, USA.). The membrane was blocked with 5% non-fat milk
for 2 h and subsequently incubated with the corresponding primary
antibody (1:1,000 dilution) overnight at 4°C. Following washing and
binding of an appropriate secondary antibody (1:5,000 dilution)
coupled to horseradish peroxidase for 2 h at room temperature, the
signals were visualized by enhanced chemiluminescence according to
the manufacturer’s instructions (Animal Genetics Inc, Kyonggi-do,
Korea). For the immunoprecipitation, equal quantities of cell
lysate were incubated with the appropriate antibodies, followed by
an incubation with protein A/G agarose beads. The
immunoprecipitates were extensively washed and the eluted
precipitates were resolved, transferred and probed with the
appropriate antibodies.
Cell fractionation
The cells were washed with phosphate-buffered saline
(PBS), incubated for 20 min in hypotonic lysis buffer (50 mM
Tris-HCl, pH 7.0, 1 mM EDTA, 0.1% β-mercaptoethanol, 5 mM sodium
orthovanadate, protease inhibitors cocktail) and 1 ml of the
protein extract was lysed using a pre-chilled Dounce homogenizer
(Thomas Scientific, Swedesboro, NJ, USA), with a tight-fitting
pestle (15 strokes). Unbroken cells and nuclei were pelleted at
1,000 × g at 4°C for 10 min, the supernatants were further
centrifuged at 21,000 × g at 4°C for 45 min. The pellets,
containing the cellular membranes, were washed three times in
hypotonic lysis buffer and resuspended in lysis buffer prior to
western blot analysis.
Cell migration and invasion assays
The cell migration and invasion assays were
performed using a modified Boyden chamber (8 mm pore size; Corning
Costar, Cambridge, MA, USA) as described by previous methods
(20,21). Briefly, the lower surface of the
filters were coated with COL-I or fibronectin as a chemoattractant.
The upper surface of the filter was coated with Matrigel™ (BD
Biosciences) for the invasion assay, or left uncoated for the
migration assay. MDA-MB-231 cells were seeded at a density of
5×104 cells in 100 µl RPMI, containing 0.5%
bovine serum albumin (migration) or RPMI, containing 10% FBS
(invasion) in the upper compartment of transwell. The lower
compartment contained 800 µl RPMI, containing 10% FBS.
Following incubation for 8 h (migration) or 24 h (invasion) at 37°C
in 5% CO2, the cells which had not penetrated the filter
were completely wiped away using a cotton swab and the cells which
had migrated to the lower surface of the filter were fixed with
methanol. The cells were subsequently stained and counted in ≥5
randomly selected microscopic fields (magnification, ×100) per
filter using an Olympus CKX41 inverted microscope (Olympus, Tokyo,
Japan).
Adhesion and spreading assays
The cell adhesion assays were carried out using
96-well tissue culture plates. The plates were coated with 10
µg/ml of COL-I overnight at 4°C. Each well was rinsed with
1X PBS and blocked with PBS supplemented with 0.1% bovine serum
albumin for 1 h. The cells were plated at a density of
2×104 cells/well. Following a 1 h incubation, the
unbound cells were removed from the wells by gentle aspiration and
washed three times with PBS. The attached cells were quantified by
measuring the acid phosphatase activity. Briefly, the attached
cells were treated with lysis buffer (0.1 M sodium acetate, pH 5.0,
0.1% triton X-100) containing 5 mM p-nitrophenyl phosphate and
incubated for 1 h at 37°C, followed by the addition of 1 M NaOH.
The absorbance was measured at 405 nm using a microplate
reader.
For the spreading assay, the cells were seeded into
a 24-well plate coated with 10 mg/ml of COL-I (1×105
cells/well) and incubated for 1 h. Following the incubation, the
cells were fixed in 3.7% formaldehyde. The proportion of spread
cells was determined using a light microscope. Non-spread cells
were observed as small, round cells with little or no membrane
protrusions, whereas spread cells were observed as large cells with
extensive visible lamellipodia. The results represent the
percentage of spread cells in five randomly selected microscopic
fields (magnification, ×200).
F-actin staining and confocal
microscopy
The cells were grown on glass coverslips coated with
COL-I, fixed with 4% paraformaldehyde and permeabilized in 0.2%
Triton X-100. The F-actin staining was performed using fluorescein
isothiocyanate (FITC)-conjugated phalloidin. Confocal images were
acquired using a Zeiss LSM510 META NLO inverted laser scanning
confocal microscope (Carl Zeiss AG, Oberchocken, Germany; Korea
Basic Science Institute Chuncheon Center) equipped with an external
argon HeNe laser and HeNe laser II. The images were captured at the
colony midsection using a C-Apochromat 63X NA1.2 water immersion
objective (Carl Zeiss AG).
Statistical analyses
The data represent the means ± standard deviation of
three independent experiments, each repeated in triplicate. The
data were analyzed by Student’s t-test. A P<0.05 was considered
to indicate a statistically significant difference, as compared
with the controls.
Results
Chelidonine inhibits COL-I-induced
migration of MDA-MB-231 cells without affecting cell viability
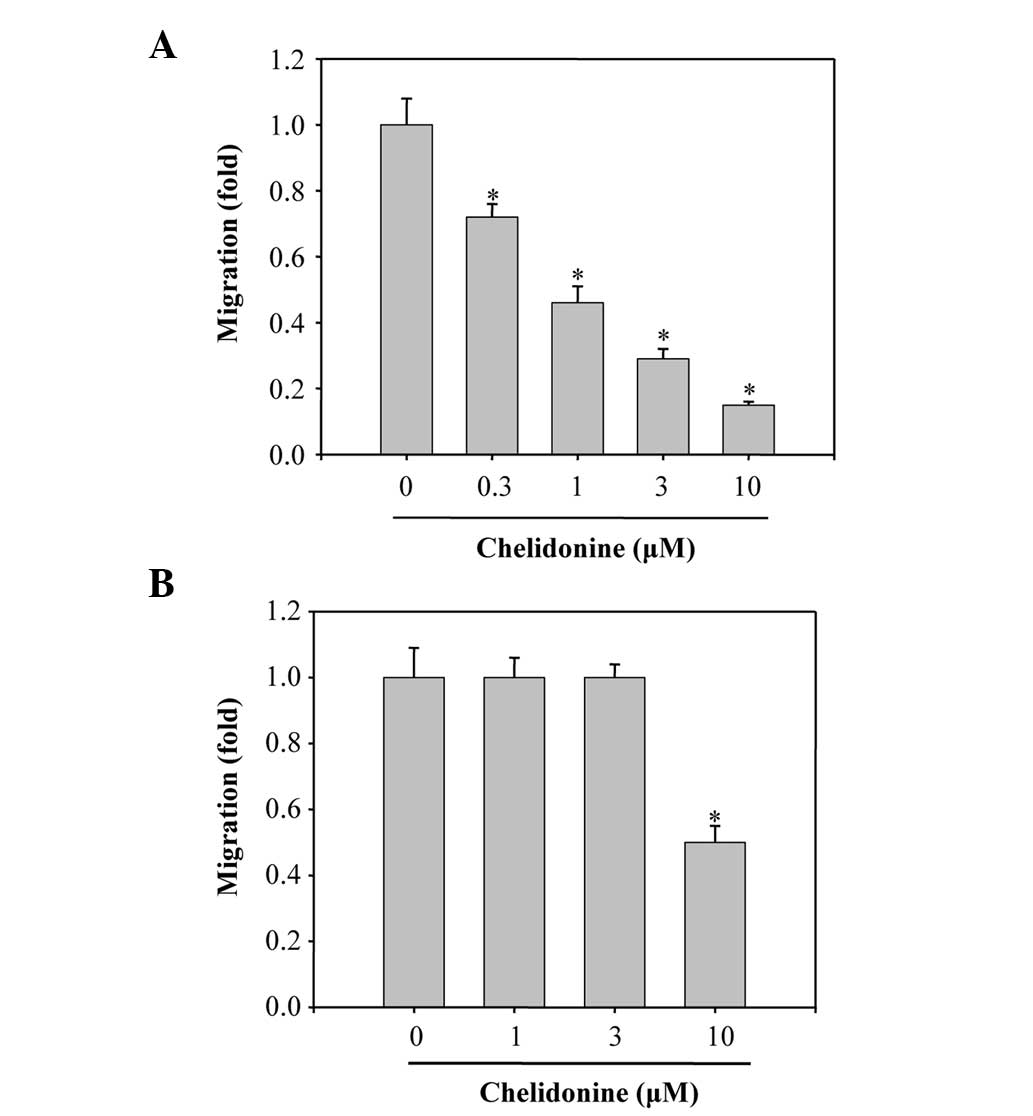
The Boyden chamber migration assay was performed to
determine the inhibitory effects of chelidonine on the migratory
ability of MDA-MB-231 cells. The lower surface of the filters was
coated with COL-I and the migrated cells were analyzed following a
12 h incubation with chelidonine. Chelidonine significantly
suppressed the migration of the cells towards COL-I, in a
concentration-dependent manner, with a half maximal inhibitory
concentration (IC50) value of 1.0±0.1 µM (Fig. 2A). The effects of chelidonine on
the migration of MDA-MB-231 cells induced by fibronectin, were also
investigated. Chelidonine treatment weakly inhibited the migration
of the cells towards fibronectin, with an IC50 value of
>10 µM (Fig. 2B). These
results suggest that chelidonine was more effective at suppressing
COL-I-induced cell migration, as compared with fibronectin-induced
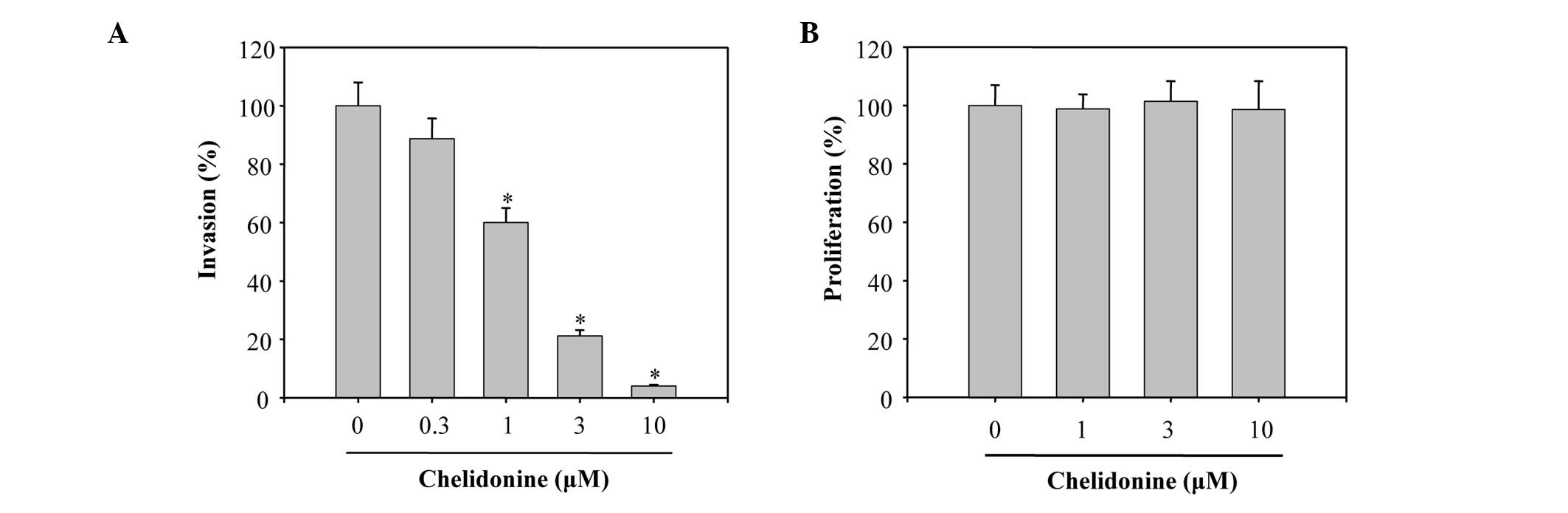
cell migration. The present study also examined whether chelidonine
was capable of suppressing the invasive abilities of MDA-MB-231
cells, using a Boyden chamber coated with Matrigel™. The number of
cells invading the lower chamber were significantly decreased when
the cells were treated with chelidonine, in a
concentration-dependent manner, with an IC50 value of
1.4±0.2 µM (Fig. 3A). To
verify that the concentrations of chelidonine used in the
experiments did not affect the cell viability, MDA-MB-231 cells
were incubated with various concentrations of chelidonine for 24 h
and cell viability was evaluated using an MTT assay. Chelidonine
did not cause any significant toxic effects on the cells <10
µM (Fig. 3B), suggesting
that chelidonine is effective in inhibiting MDA-MB-231 cell
migration and invasion.
Chelidonine inhibits cell spreading and
actin cytoskeleton reorganization, during adhesion to COL-I
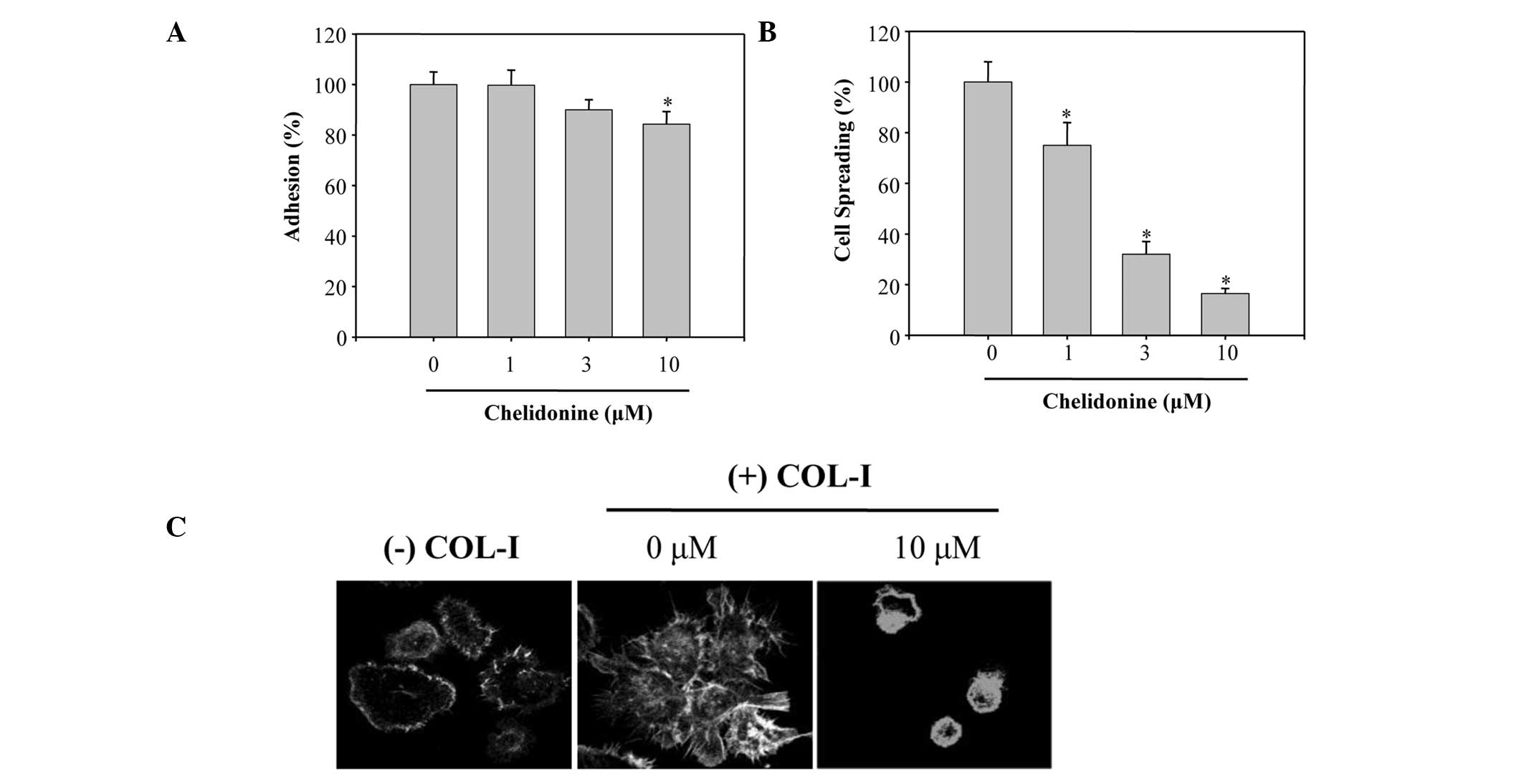
Integrin-mediated cancer cell adhesion and spreading
is an important step in cell migration and invasion (22). Therefore, the present study
determined whether chelidonine prevented the adhesion of MDA-MB-231
cells to COL-I. Treatment of MDA-MB-231 cells with chelidonine
slightly impaired the adhesion of cells to COL-I (Fig. 4A). The percentage of adhesion
inhibition, in the cells treated with 10 µM chelidonine, was
15±2.5%, which was markedly less than that of the migratory
inhibition towards COL-I. The effects of chelidonine on the
spreading of MDA-MB-231 cells on COL-I were also determined.
Chelidonine inhibited the spreading of cells on COL-I, in a
concentration-dependent manner, with an IC50 value of
2.5±0.3 µM (Fig. 4B). COL-I
stimulation also induced a dramatic reorganization of the actin
cytoskeleton in MDA-MB-231 cells; however, treatment of the cells
with 10 µM chelidonine completely blocked the COL-I-induced
reorganization of the actin cytoskeleton (Fig. 4C). These results suggest that
chelidonine may inhibit migration of MDA-MB-231 cells by
suppressing the reorganization of the actin cytoskeleton and cell
spreading.
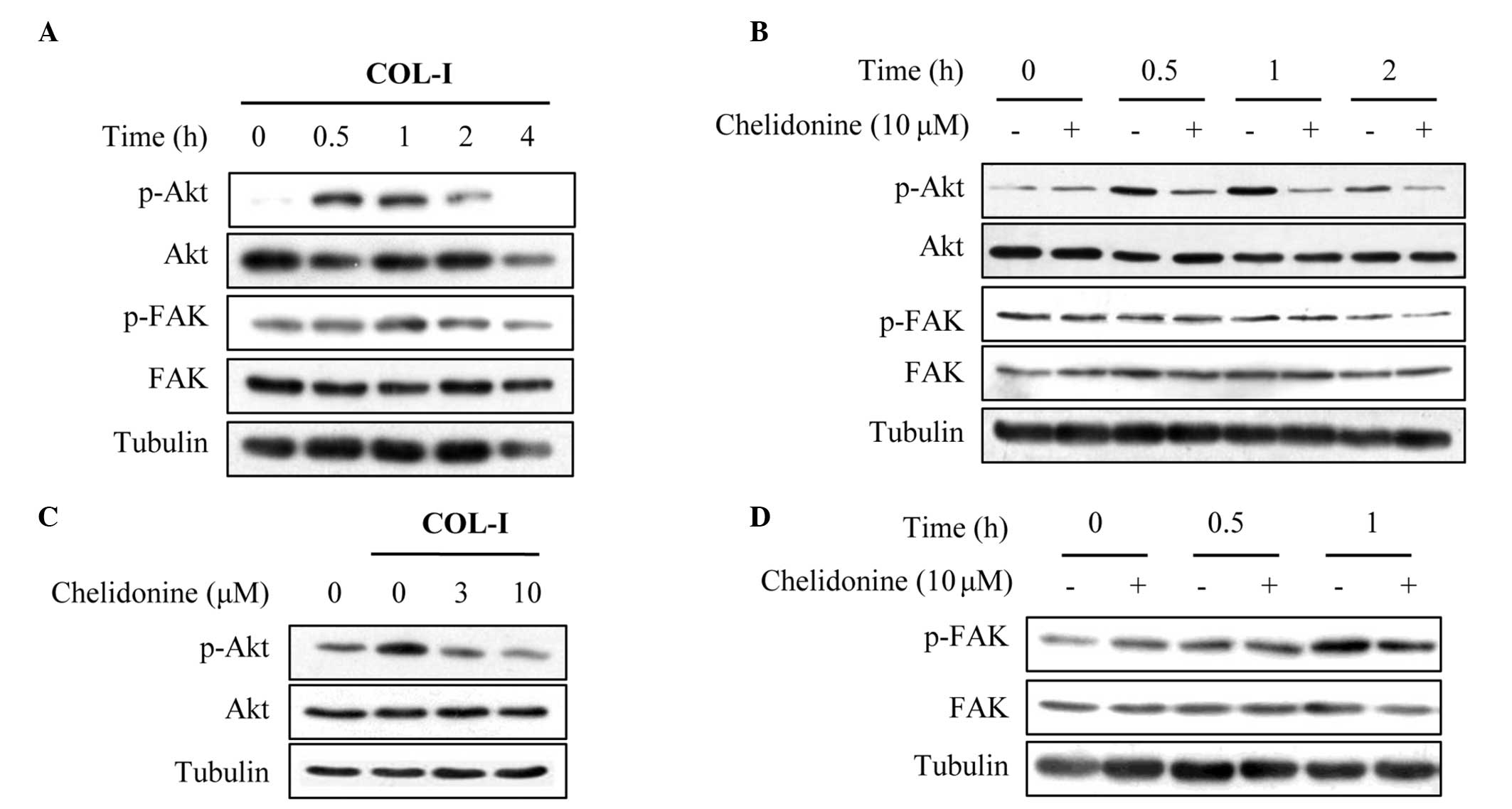
Chelidonine inhibits COL-I-induced
activation of Akt
Integrins transmit signals through a variety of
intracellular protein kinases and adaptor molecules, including FAK
and ILK (9,23). Activation of FAK results in
numerous cellular processes, including cell attachment, migration
and invasion (24). Therefore, the
effects of chelidonine on COL-I-induced FAK activation were
determined. COL-I stimulation did not significantly increase the
protein expression levels of phospho-FAK (Tyr397) in MDA-MB-231
cells (Fig. 5A). However, COL-I
stimulation did significantly increase the protein expression
levels of phospho-Akt (Ser473) within 30 min, after which it
gradually returned to the basal level (Fig. 5A). These results suggest that the
activation of Akt may be induced at an early stage during adhesion
to COL-I, in this cancer cell line. Notably, treatment of
MDA-MB-231 cells with chelidonine significantly decreased
phosphor-Akt (Ser473) protein expression levels (Fig. 5B), in a concentration dependent
manner (Fig. 5C). Conversely,
fibronectin increased the protein expression levels of phospho-FAK
(Tyr397), and chelidonine treatment did not significantly alter the
fibronectin-induced phosphorylation of FAK (Fig. 5D).
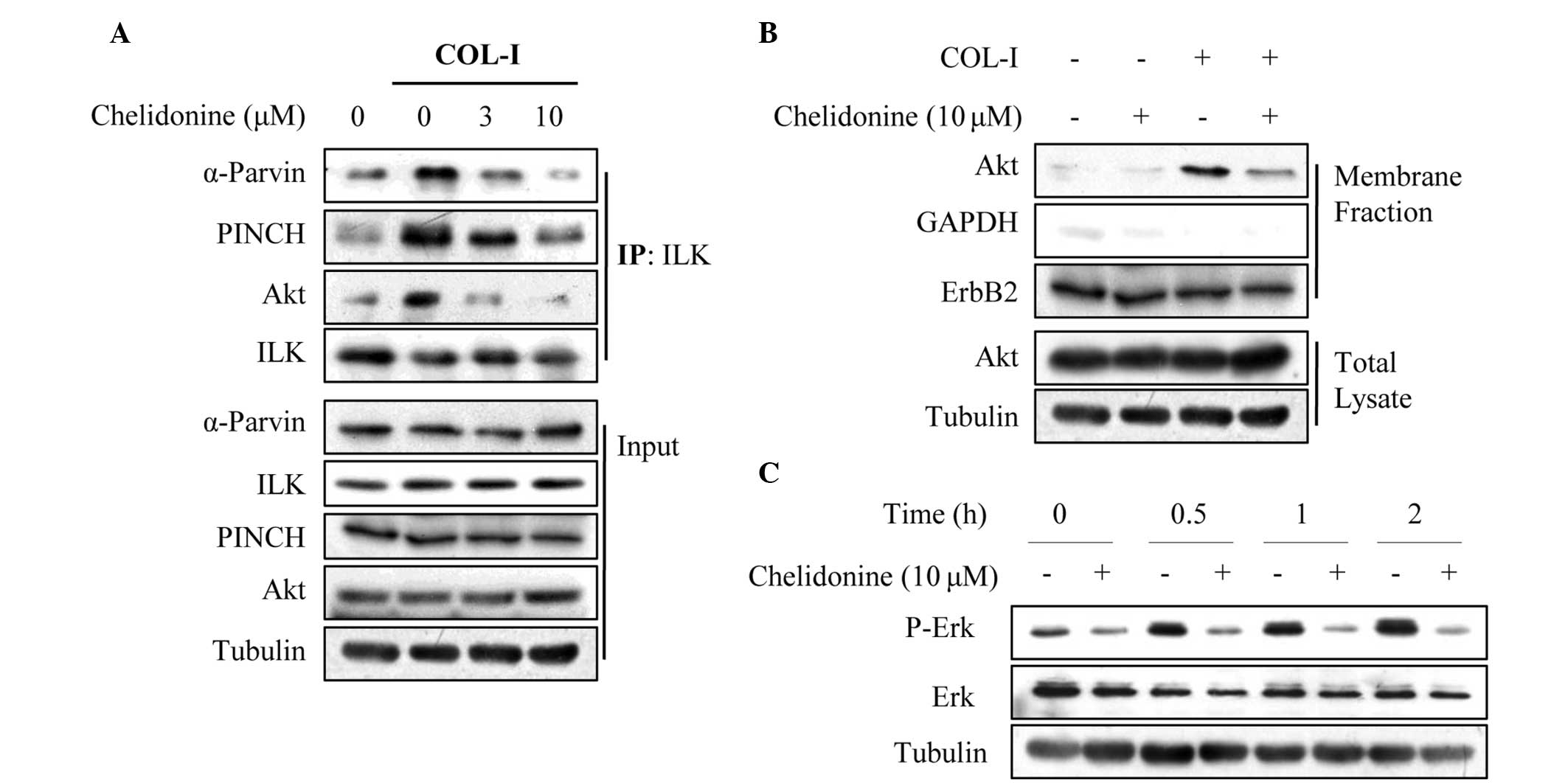
Chelidonine inhibits the COL-I-induced
formation of the IPP complex
The IPP complex regulates multiple integrin
signaling pathways, including Akt, ERK1/2 and Rac1 small GTPase
(6,23). Therefore, the effects of
chelidonine on the COL-I-induced formation of the IPP complex were
determined using a co-immunoprecipitation assay. COL-I stimulation
increased ILK association with PINCH1 and α-parvin, as well as Akt;
however, chelidonine suppressed this association in a
concentration-dependent manner (Fig.
6A). Membrane fractionation revealed that chelidonine
significantly suppressed the COL-I-induced plasma membrane
translocation of Akt (Fig. 6B),
which is an essential process for Akt activation (25,26).
To further confirm that chelidonine inhibited the COL-I-induced
formation of the IPP complex, the effects of chelidonine on IPP
downstream signaling molecules, such as ERK1/2, were evaluated.
COL-I significantly increased the phosphorylation of ERK1/2 in
MDA-MB-231 cells, in a time-dependent manner; however, chelidonine
treatment decreased the protein expression levels of COL-I-induced
phospho-ERK1/2 (Fig. 6C). These
results suggest that chelidonine may exert its anti-migratory
effects through interfering with the formation of the IPP
complex.
Discussion
Chelidonium majus has a history in
phytomedicine for the treatment of numerous diseases and health
disturbances. It contains isoquinoline alkaloids, particularly
protoberberine and benzophenanthridine alkaloids (27). The plant extract exhibits both
antitumor and antiviral activities (14), in addition to hepatoprotective and
anti-genotoxic effects in mice (28). Ukrain, a semi-synthetic derivative
of C. majus alkaloids, has been used in therapy to treat
various types of solid tumor. Previous pre-clinical and clinical
investigations have established Ukrain as an anticancer drug
(29). Chelidonine, the major
component of Ukrain, induces apoptosis (16), mitotic arrest (17) and reduction of telomerase activity
(18) in cancer cells. However,
the underlying mechanisms behind the anticancer effects of
chelidonine remain to be elucidated.
In the present study, the anti-migratory and
anti-invasive effects of chelidonine on MDA-MB-231 human breast
cancer cells were investigated. The results demonstrated that
chelidonine exhibited a potent anti-migratory effect on MDA-MB-231
cells induced by COL-I, without affecting cell viability. Based on
these results, the mechanism of action of chelidonine, for
inhibiting COL-I-induced migration, was further explored.
Chelidonine was shown to be capable of suppressing COL-I-induced
reorganization of the actin cytoskeleton and cell spreading.
Notably, chelidonine treatment suppressed the formation of the IPP
complex and subsequent activation of IPP downstream signaling
molecules, including Akt and ERK1/2 induced by COL-I. These results
suggest that the anti-migratory mechanisms of chelidonine may be
associated with inhibition of integrin signaling, by suppressing
the COL-I-induced formation of the IPP complex.
Integrin-mediated signaling regulates a variety of
biological processes, including cell migration, survival and
proliferation. The ternary IPP complex is also a central
constituent of adhesion sites, where it regulates multiple
signaling pathways, including Akt, ERK1/2, and Rac1 (6,8,10).
The present study demonstrated that chelidonine suppressed the
COL-I-induced association of ILK with PINCH1, α-parvin and Akt.
Furthermore, chelidonine suppressed COL-I-induced reorganization of
the actin cytoskeleton, cell spreading and migration, as well as
activation of the downstream IPP signaling molecules, including Akt
and ERK1/2. Therefore, chelidonine may inhibit cell migration by
suppressing COL-I-induced integrin signaling, through inhibiting
the formation of the IPP complex in MDA-MB-231 cells. However, the
detailed mechanisms by which chelidonine interferes with IPP
complex formation induced by COL-I, remain to be elucidated.
Chelidonine was shown to be more effective at
suppressing COL-I-induced cell migration, as compared with
fibronectin-mediated cell migration. The binding of integrins to
their ligands induces conformational changes and integrin
clustering, resulting in the activation of signaling cascades and
recruitment of multi-protein complexes to focal adhesions (30). FAK is predominantly activated in
focal adhesions and is important in cell-ECM interactions that
affect cell migration, proliferation and survival (4,7).
Autophosphorylation of FAK at Tyr397, occurs in response to
numerous stimuli, including integrin engagement. FAK
phosphorylation promotes the Src homology domain 2-dependent
binding of the Src family tyrosine kinases and the formation of an
activated FAK-Src complex (7). FAK
activation at focal adhesion sites enhances cytoskeletal
reorganization, cellular adhesion and cell survival (7). Previous studies have shown that
stimulation of MDA-MB-231 cells with fibronectin predominantly
induces the activation of FAK (20,21).
Conversely, COL-I stimulation does not significantly increase FAK
phosphorylation at Tyr397, but induces the activation of Akt in
MDA-MB-231 cells, suggesting that the formation of the IPP complex
could be induced at an early time during adhesion to COL-I, in this
cancer cell line. Chelidonine did not significantly decrease the
level of FAK phosphorylation induced by fibronectin, but it did
effectively suppress the COL-I-induced formation of the IPP
complex, and activation of IPP downstream signaling molecules,
including Akt. These results indicate that chelidonine may
preferentially inhibit the IPP complex, rather than suppress FAK
activation. This may explain why chelidonine was more effective at
suppressing COL-I-induced cell migration, as compared with
fibronectin-mediated cell migration.
The anticancer effects of chelidonine have
previously been reported, however this is the first report, to the
best of our knowledge, demonstrating that chelidonine inhibits
migration and invasion of MDA-MB-231 cells. These effects may be
achieved through the inhibition of the IPP complex and subsequent
activation of IPP downstream signaling molecules, such as Akt and
ERK1/2. The results of the present study also support potential,
additional biological activities of chelidonine, and may provide a
basis for the development of more specific cancer chemotherapeutic
agents derived from natural products.
Acknowledgments
The present study was supported by grants from the
Leaders in Industry-University Cooperation Project (no.
C1011325-01-01), supported by the Ministry of Education, and the
National Research Foundation of Korea (no.
2012R1A2A2A06046921).
References
|
1
|
Valastyan S and Weinberg RA: Tumor
metastasis: molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watt FM: Role of integrins in regulating
epidermal adhesion, growth and differentiation. EMBO J.
21:3919–3926. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC and Dedhar
S: Regulation of cell adhesion and anchorage-dependent growth by a
new beta 1-integrin-linked protein kinase. Nature. 379:91–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Legate KR, Montañez E, Kudlacek O and
Fässler R: ILK, PINCH and parvin: the tIPP of integrin signalling.
Nat Rev Mol Cell Biol. 7:20–31. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaller MD and Parsons JT: Focal adhesion
kinase and associated proteins. Curr Opin Cell Biol. 6:705–710.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
McDonald PC, Fielding AB and Dedhar S:
Integrin-linked kinase - essential roles in physiology and cancer
biology. J Cell Sci. 121:3121–3132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda K, Gupta S, Chen K, Wu C and Qin J:
The pseudoactive site of ILK is essential for its binding to
alpha-Parvin and localization to focal adhesions. Mol Cell.
36:819–830. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wickström SA, Lange A, Montanez E and
Fässler R: The ILK/PINCH/parvin complex: the kinase is dead, long
live the pseudokinase! EMBO J. 29:281–291. 2010. View Article : Google Scholar :
|
|
11
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC and Dedhar
S: Regulation of cell adhesion and anchorage-dependent growth by a
new beta 1-integrin-linked protein kinase. Nature. 379:91–96. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiswell BP, Zhang R, Murphy JW, Boggon TJ
and Calderwood DA: The structural basis of integrin-linked
kinase-PINCH interactions. Proc Natl Acad Sci USA. 105:20677–20682.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: a cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colombo ML and Bosisio E: Pharmacological
activities of Chelidonium majus L. (Papaveraceae). Pharmacol Res.
33:127–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hohenwarter O, Strutzenberger K, Katinger
H, Liepins A and Nowicky JW: Selective inhibition of in vitro cell
growth by the anti-tumour drug Ukrain. Drugs Exp Clin Res. 18:1–4.
1992.PubMed/NCBI
|
|
16
|
Kemény-Beke A, Aradi J, Damjanovich J,
Beck Z, Facskó A, Berta A and Bodnár A: Apoptotic response of uveal
melanoma cells upon treatment with chelidonine, sanguinarine and
chel-erythrine. Cancer Lett. 237:67–75. 2006. View Article : Google Scholar
|
|
17
|
Panzer A, Joubert AM, Bianchi PC, Hamel E
and Seegers JC: The effects of chelidonine on tubulin
polymerisation, cell cycle progression and selected signal
transmission pathways. Eur J Cell Biol. 80:111–118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Noureini SK and Wink M: Transcriptional
down regulation of hTERT and senescence induction in HepG2 cells by
chelidonine. World J Gastroenterol. 15:3603–3610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JE, Cuong TD, Hung TM, Lee I, Na M,
Kim JC, Ryoo S, Lee JH, Choi JS, Woo MH and Min BS: Alkaloids from
Chelidonium majus and their inhibitory effects on LPS-induced NO
production in RAW264.7 cells. Bioorg Med Chem Lett. 21:6960–6963.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hwangbo C, Park J and Lee JH:
mda-9/Syntenin protein positively regulates the activation of Akt
protein by facilitating integrin-linked kinase adaptor function
during adhesion to type I collagen. J Biol Chem. 286:33601–33612.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwangbo C, Kim J, Lee JJ and Lee JH:
Activation of the integrin effector kinase focal adhesion kinase in
cancer cells is regulated by crosstalk between protein kinase
Calpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res.
70:1645–1655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hehlgans S, Haase M and Cordes N:
Signalling via integrins: implications for cell survival and
anticancer strategies. Biochim Biophys Acta. 1775:163–180.
2007.
|
|
24
|
van Nimwegen MJ and van de Water B: Focal
adhesion kinase: a potential target in cancer therapy. Biochem
Pharmacol. 73:597–609. 2007. View Article : Google Scholar
|
|
25
|
Restuccia DF and Hemmings BA: Cell
signaling. Blocking Akt-ivity. Science. 325:1083–1084. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filippa N, Sable CL, Hemmings BA and Van
Obberghen E: Effect of phosphoinositide-dependent kinase 1 on
protein kinase B translocation and its subsequent activation. Mol
Cell Biol. 20:5712–5721. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niu CQ and He LY: Determination of
isoquinoline alkaloids in Chelidonium majus L. by ion-pair
high-performance liquid chromatography. J Chromatogr. 542:193–199.
1991. View Article : Google Scholar
|
|
28
|
Biswas SJ, Bhattacharjee N and
Khuda-Bukhsh AR: Efficacy of a plant extract (Chelidonium majus L.)
in combating induced hepatocarcinogenesis in mice. Food Chem
Toxicol. 46:1474–1487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ernst E and Schmidt K: Ukrain-a new cancer
cure? A systematic review of randomised clinical trials. BMC
Cancer. 5:692005. View Article : Google Scholar
|
|
30
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|