Introduction
Transient global cerebral ischemia leads to
selective neuronal damage via triggering a complex series of
biochemical events in certain regions of the brain, including the
hippocampus and neocortex (1,2). The
hippocampal CA1 region, in particular, is well known as the most
vulnerable region (3–5). Neuronal death in the hippocampal CA1
region occurs a few days following transient ischemic insult and is
referred to as ‘delayed neuronal death’ (2). It has been demonstrated that cerebral
ischemia leads to the production of excessive reactive oxygen
species (ROS), although the underlying mechanisms, which are
associated with the delayed neuronal death and selective neuronal
damage, remain to be fully elucidated (6–8). The
cerebral ischemia-induced overproduction of ROS can cause
morphological and functional alterations of cells, including the
alteration of intracellular Ca2+ homeostasis, which has
been considered the basis of excitotoxicity injury mechanisms
(9–11).
The thioredoxin (Trx) and peroxiredoxin (Prx) redox
system is important in cellular function by reducing oxidative
stress via the regulation of intracellular ROS levels (12–15).
Among the subtypes of Trx and Prx, Trx2 and Prx3 are exclusively
expressed in the mitochondrial compartment (16,17),
and are involved in the control of the antioxidant defence system,
cell survival and apoptosis (18–20).
In addition, Trx2 and Prx3 are associated with neuronal damage and
neuroprotective effects in the brain in response to
neurodegenerative disorders and various insults, including brain
ischemia (9,21–24).
In our previous studies, time-dependent changes were
reported in the expression levels of Trx2 and Prx3 and the
neuroprotective effects of Trx2 and Prx3 in a gerbil model of
transient cerebral ischemia (22,25).
However, transient cerebral ischemia-induced neuronal damage in the
hippocampal CA1 region is also affected by various factors,
including the duration of ischemia/reperfusion and the age of the
experimental animals (26–28). In addition, the time-dependent
changes in the expression levels of Trx2 and Prx3 following
cerebral ischemia remain to be fully elucidated in aged animals. In
the present study, therefore, ischemia-induced changes in the
protein expression levels of Trx2 and Prx3 in the hippocampal CA1
region were compared between adult and aged gerbils following 5 min
of transient global cerebral ischemia.
Materials and methods
Experimental animals
Male Mongolian gerbils (Meriones
unguiculatus) were obtained from the Experimental Animal
Center, Kangwon National University (Chuncheon, South Korea). The
Mongolian gerbils were aged 6 months (body weight, 65–75 g) in the
adult group, and 24 months (body weight, 75–85 g) in the aged
group. The animals (n=196) were housed in a conventional state
under stable temperature (23°C) and humidity (60%) with a 12-h
light/12-h dark cycle, and were provided with free access to food
and water. The procedures for animal handling and care adhered to
guidelines in compliance with the current international laws and
policies (Guide for the Care and Use of Laboratory Animals, The
National Academies Press, 8th Ed., 2011) (29), and were approved by the
Institutional Animal Care and Use Committee at Kangwon National
University (Chuncheon, South Korea; approval no. KW-130424-1). All
of the experiments were performed in a manner to minimize the
number of animals used and the suffering caused by the
procedures.
Induction of transient cerebral
ischemia
The animals were anesthetized with a mixture of 2.5%
isoflurane (Ilsung Pharmaceuticals, Seoul, Korea) in 33% oxygen and
67% nitrous oxide. The bilateral common carotid arteries were
isolated and occluded using non-traumatic aneurysm clips (Yasargil
FE 723K; Aesculap, Tuttlingen, Germany). The complete interruption
of blood flow was confirmed by observing the central artery in
retinae under an opthalmoscope (HEINE K180®; Heine
Optotechnik, Herrsching, Germany). Following 5 min occlusion, the
aneurysm clips were removed from the common carotid arteries. The
body (rectal) temperature under free-regulating or normothermic
(37±0.5°C) conditions was monitored using a rectal temperature
probe (TR-100; Fine Science Tools, Foster City, CA, USA) and
maintained using a thermometric blanket prior to, during and
following surgery until the animals were completely recovered from
anesthesia. Thereafter, the animals were maintained on the thermal
incubator (Mirae Medical Industry, Seoul, South Korea) to maintain
the body temperature of the animals until the animals were
sacrificed. The sham-operated animals were subjected to the same
surgical procedures, with the exception that the common carotid
arteries were not occluded.
Tissue processing for histology
For histological analysis, section were prepared
from the sham- and ischemia-operated adult and aged gerbils (n=7 at
each time point) at designated time-points (1, 2, 4, 5 and 7 days)
following reperfusion. The animals were anesthetized with sodium
pentobarbital (JW Pharm. Co., Ltd., Korea, 40 mg/kg, i.p) and
perfused transcardially with 0.1 M phosphate-buffered saline (PBS;
pH 7.4; Sigma-Aldrich, St. Louis, MO, USA) followed by 4%
paraformaldehyde (Sigma-Aldrich) in 0.1 M phosphate-buffer (PB; pH
7.4; Sigma-Aldrich). The brains were removed and postfixed in the
same fixative for 6 h. The brain tissues were then cryoprotected by
infiltration with 30% sucrose (Sigma-Aldrich) overnight.
Thereafter, frozen tissues were serially sectioned on a cryostat
(Leica Microsystems, GmbH, Wetzlar, Germany) into 30 μm
coronal sections, which were then collected into six-well plates
containing PBS.
Staining for neuronal damage
To confirm the delayed neuronal death in the
hippocampal CA1 region between the adult and aged gerbils following
transient cerebral ischemia, NeuN immunohistochemistry was
performed, according to the methods of the previous studies
(27,28). In brief, for NeuN
immunohistochemistry, the sections were sequentially treated with
0.3% hydrogen peroxide (H2O2; Sigma-Aldrich)
in PBS for 30 min and 10% normal goat serum (Vector Laboratories,
Inc., Burlingame, CA, USA) in 0.05 M PBS for 30 min. The sections
were then incubated with diluted mouse anti-NeuN, a neuron-specific
soluble nuclear antigen (1:1,000; cat. no. MAB377; Millipore,
Temecula, CA, USA) overnight at 4°C. Thereafter the tissues were
exposed to biotinylated goat anti-mouse immunoglobulin (Ig) G
(1:200; Vector Laboratories Inc., Burlingame, CA, USA) and
streptavidin peroxidase complex (Vector Laboratories, Inc.,
Burlingame, CA, USA) for 2 h at room temperature. The sections (6
sections/animal) were visualized by staining with
3,3′-diaminobenzidine (Sigma-Aldrich) in 0.1 M Tris-HCl buffer and
mounting on gelatin-coated slides. Following dehydration, the
sections were mounted using Canada balsam (Kanto, Tokyo,
Japan).
In order to quantitatively analyze NeuN
immunoreactivity, digital images of the hippocampal tissues were
captured using an AxioM1 light microscope (Carl Zeiss AG,
Oberkochen, Germany) equipped with a digital camera (Axiocam; Carl
Zeiss AG) connected to a PC monitor. The number of
NeuN-immunoreactive neurons were counted in a 250×250 μm
square applied approximately at the center of the CA1 region using
an image analyzing system (Optimas 6.5; CyberMetrics, Inc.,
Scottsdale, AZ, USA). The tissue sections were selected at 120
μm intervals, and cell counts were obtained by averaging the
counts from each animal.
Immunohistochemistry for Trx2 and
Prx3
To compare the changes of Trx2 and Prx3 in the
hippocampal CA1 region between adult and aged gerbils,
immunohistochemistry for rabbit anti-Trx2 (1:500; cat. no.
LF-PA0024, Ab Frontier, Seoul, Korea) and mouse anti-Prx3 (1:500;
cat. no. LF-MA0045; Ab Frontier) was performed, according to the
above-mentioned method. In order to establish the specificity of
the immunostaining, a negative control was used, with only the
secondary antibody and without primary antibody. This negative
control resulted in the absence of immunoreactivity in any
structures.
A total of six sections with a 120 μm
interval per animal were selected to quantitatively analyze the
Trx2 and Prx3 immunoreactivity. Digital images of the hippocampal
CA1 region were captured using an AxioM1 light microscope (Carl
ZeissAG), equipped with a digital camera (Axiocam; Carl Zeiss AG)
connected to a PC monitor. According to the methods of our previous
study (7), semi-quantification of
the immunostaining intensities were evaluated using digital image
analysis software (MetaMorph 4.01; Universal Imaging Corporation,
Downingtown, PA, USA). The level of immunoreactivity was scaled as
−, ±, +, ++ or +++ representing no staining (gray scale value
≥200), weakly positive (gray scale value=150–199), moderate (gray
scale value=100–149), marked (gray scale value=50–99), or very
marked (gray scale value ≤49), respectively.
Western blot analysis for Trx2 and
Prx3
To examine changes in the protein levels of Trx2 and
Prx3 in the hippocampal CA1 region following transient cerebral
ischemia, the sham- and ischemia-operated adult and aged animals
(n=5 at each time point) were analyzed using western blot analysis
in the sham, group and 2 and 5 days following reperfusion.
Following sacrifice of the animals and removal of their brains, the
brains were serially and transversely cut to a thickness of 400
μm on a vibratome (Leica Microsystems GmbH), and the
hippocampal CA1 regions were then dissected using a surgical blade.
The tissues were homogenized in 50 mM PBS (pH 7.4) containing 0.1
mM ethylene glycol bis (2-aminoethyl Ether)-N,N,N’,N’ tetraacetic
acid (pH 8.0; Sigma-Aldrich), 0.2% Nonidet P-40 (Sigma-Aldrich), 10
mM ethylendiamine tetraacetic acid (pH 8.0; Sigma-Aldrich), 15 mM
sodium pyrophosphate (Sigma-Aldrich), 100 mM β-glycerophosphate
(Sigma-Aldrich), 50 mM NaF (Sigma-Aldrich), 150 mM NaCl
(Sigma-Aldrich), 2 mM sodium orthovanadate (Sigma-Aldrich), 1 mM
phenylmethylsulfonyl fluoride and 1 mM dithiothreitol (DTT;
Sigma-Aldrich). Following centrifugation at 16,000 × g for 20 min
at 4°C, the protein levels were determined in the supernatants
using a Micro BCA protein assay kit, with bovine serum albumin as
the standard (Pierce Biotechnology, Inc., Rockford, IL, USA).
Aliquots containing 20 μg total protein were boiled for 5
min in loading buffer containing 150 mM Tris (pH 6.8), 3 mM DTT, 6%
SDS, 0.3% bromophenol blue (Sigma-Aldrich) and 30% glycerol. The
aliquots were then loaded onto a 10% polyacrylamide gel. Following
electrophoresis, the gels were transferred onto nitrocellulose
transfer membranes (Pall Life Sciences, East Hills, NY, USA). To
reduce background staining, the membranes were incubated with 5%
non-fat dry milk in PBS containing 0.1% Tween 20 (Sigma-Aldrich)
for 45 min at room temperature, followed by incubation with rabbit
anti-Trx2 (1:1,000; Chemicon International, Temecula, CA, USA) or
mouse anti-Prx3 (1:1000; cat. no. LF-PA0024; Ab Frontier), and
peroxidase-conjugated donkey anti-rabbit IgG or goat anti-mouse IgG
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h at room
temperature, and an ECL kit (Pierce Biotechnology, Inc.).
The result of the western blot analyses were
scanned, and densitometric analysis for the quantification of the
bands was performed using Image 1.46 (National Institutes of
Health, Bethesda, MD, USA), which was used to count the relative
optical density (ROD). The ratio of the ROD was calibrated as the
percentage, with the adult sham-operated group designated as
100%.
Statistical analysis
The data are expressed as the mean ± standard error
of the mean. Differences in the means among the groups were
statistically analyzed using one-way analysis of variance with
Bonferroni’s multiple comparison post-hoc test in order to
elucidate ischemia-associated differences among the experimental
groups using SPSS 17.0 software (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Delayed neuronal death in the
hippocampus
Neuronal damage in the hippocampal CA1 of the adult
and aged gerbils following transient cerebral ischemia was examined
using NeuN immunohistochemistry. In the adult and aged
sham-operated gerbils, NeuN-immunoreactive neurons in the stratum
pyramidale (SP) of the CA1 region were well observed. At 4 days
after ischemia-reperfusion, fewer NeuN-immunoreactive neurons were
detected in the SP of the CA1 region in the adult gerbil, due to
delayed neuronal death. However, in the aged gerbil, numerous
NeuN+ neurons were found in the SP of the CA1 region 4
days after ischemia-reperfusion, with delayed neuronal death in the
aged group observed 5 days after ischemia-reperfusion (data not
shown). This finding was consistent with that of our previous study
(27,28).
Changes in Trx2 immunoreactivity
Moderate Trx2 immunoreactivity was detected in the
SP of the CA1 region in the adult sham-group, and was marginally
higher, compared with that in the aged sham-group (Table I; Fig.
1A and B). No change in Trx2 immunoreactivity was observed in
the SP 1 day after ischemia-reperfusion (Table I; Fig.
1C). However, as shown in Table
I and Fig. 1E, G and I, from 2
days after ischemia-reperfusion, Trx2 immunoreactivity in the SP
was markedly decreased in the ischemic CA1 region the adult group,
and was almost undetectable, whereas in the aged group, Trx2
immunoreactivity in the SP was significantly increased 1 and 2 days
after ischemia-reperfusion, marginally decreased after 4 days after
ischemia-reperfusion, was weak 5 days after ischemia-reperfusion
(Table I; Fig. 1D, F, H and J) and almost
undetectable 7 days after ischemia-reperfusion (Table I).
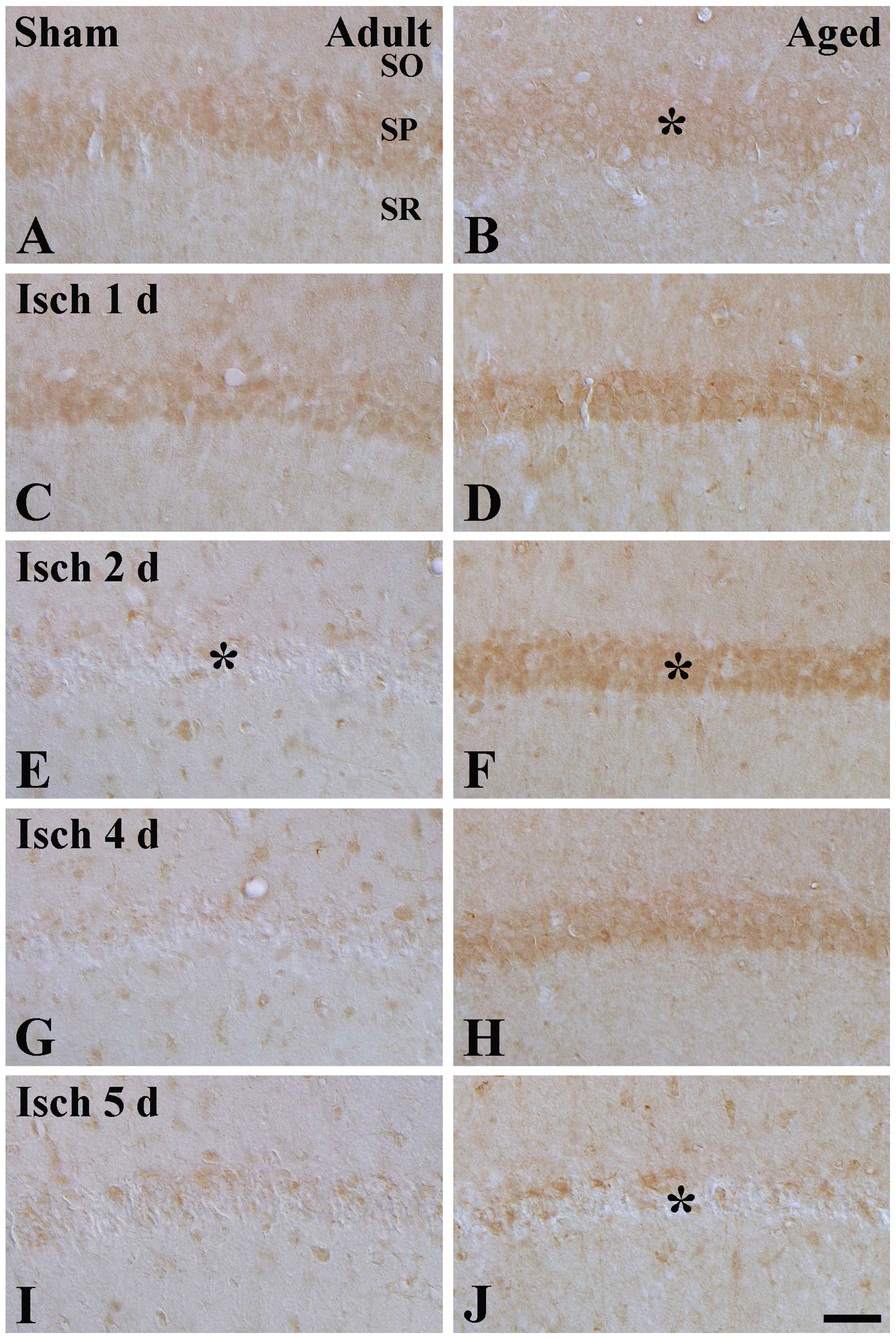 | Figure 1Trx2 immunohistochemistry in the CA1
region of (A and B) sham-operated and (C-J) ischemia-operated (A,
C, E, G and I) adult and (B, D, F, H and J) aged gerbils. In the
adult ischemia-group, Trx2 immunoreactivity in the SP was decreased
2 days after ischemia-reperfusion (* in E). In the aged sham-group,
Trx2 immunoreactivity (* in B) was marginally lower than that in
the adult sham-group; however, Trx2 immunoreactivity in the aged
ischemia-group was markedly increased (* in F) 1–4 days after
ischemia-reperfusion, and significantly decreased (* in J) 5 days
after ischemia-reperfusion. Scale Bar=100 μm. SO, stratum
oriens; SR, stratum radiatum; SP, stratum pyramidale; Trx,
thioredoxin; Prx, peroxiredoxin; sham, no ischemia-reperfusion. |
 | Table ITime-dependent levels of Trx2 and Prx3
immunoreactivity in the stratum pyramidale of the hippocampal CA1
region between adult and aged gerbils following transient cerebral
ischemia. |
Table I
Time-dependent levels of Trx2 and Prx3
immunoreactivity in the stratum pyramidale of the hippocampal CA1
region between adult and aged gerbils following transient cerebral
ischemia.
| Protein | Time following
ischemia-reperfusion (days)
| |
|---|
| Sham | 1 | 2 | 4 | 5 | 7 |
|---|
| Trx2 | | | | | | |
| Adult | + | + | − | − | − | − |
| Aged | + | ++ | ++ | ++ | ± | − |
| Prx3 | | | | | | |
| Adult | + | + | + | ± | ± | − |
| Aged | + | ++ | ++ | + | ± | − |
Changes in Prx3 immunoreactivity
In the adult sham-group, moderate Prx3
immunoreactivity was detected in the SP of the CA1 region (Table I; Fig.
2A), which was higher than that in the aged sham-group
(Fig. 2B). Prx3 immunoreactivity
in the SPs of the adult and aged groups was increased 1 and 2 days
after ischemia-reperfusion (Table
I; Fig. 2C–F). However, as
shown in Table I and Fig. 2 G–J, Prx3 immunoreactivity in the
SP of the adult and aged groups was weak 4 and 5 days after
ischemia-reperfusion, particularly in the adult group, and almost
undetectable 7 days after ischemia-reperfusion (Table I).
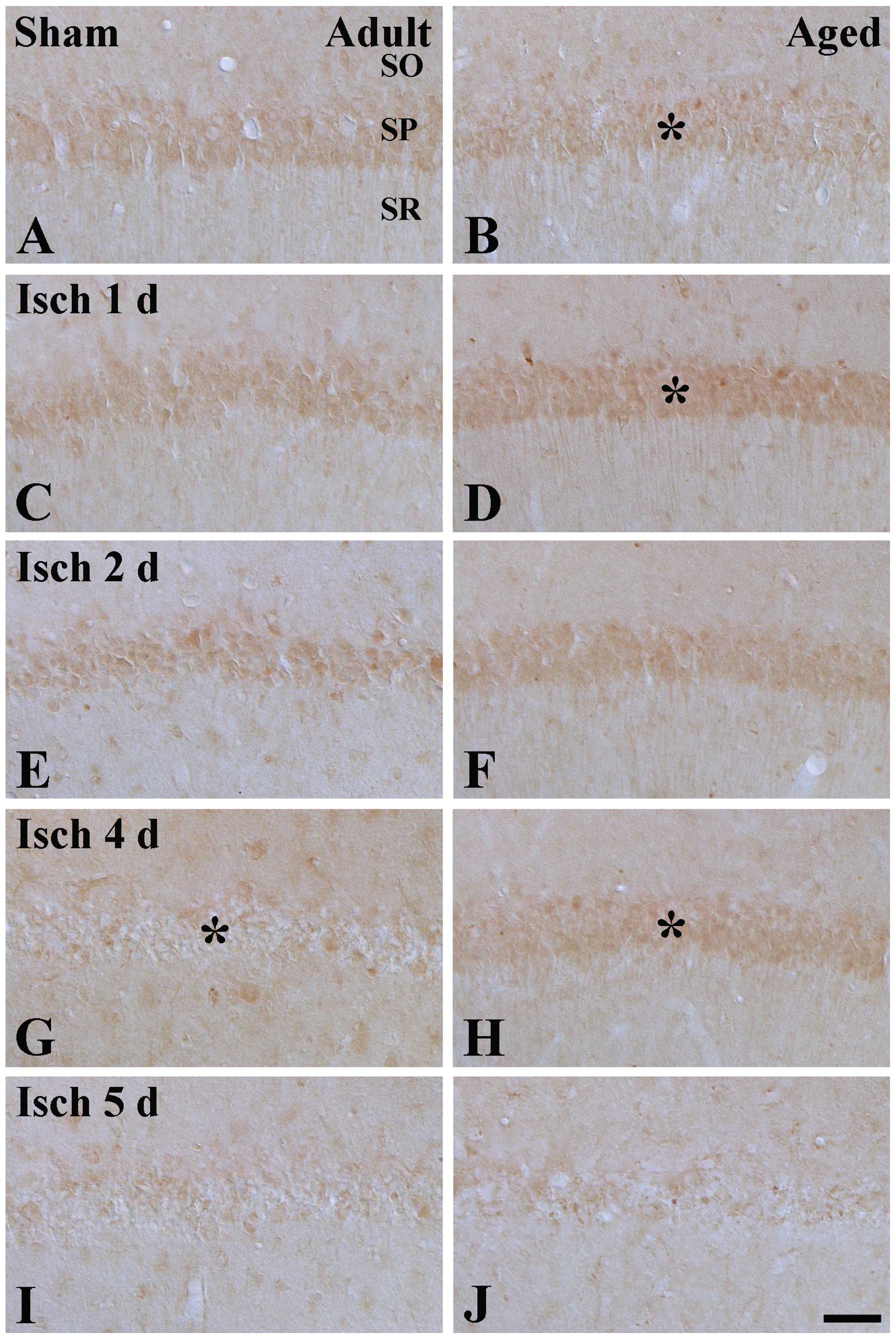 | Figure 2Prx3 immunohistochemistry in the CA1
region of the (A and B) sham-operated and (C-J) ischemia-operated
(A, C, E, G and I) adult and (B, D, F, H and J) aged gerbils. In
the adult ischemia-group, Prx3 immunoreactivity in the SP (* in G)
was decreased from 4 days post-ischemia. In the SP of the aged
sham-group, Prx3 immunoreactivity (* in B) was marginally lower,
compared with the adult sham-group. However, Prx3 immunoreactivity
in the aged ischemia-group was significantly higher (* in D and H),
compared with that in the adult ischemia-group. Scale Bar=100
μm. SO, stratum oriens; SR, stratum radiatum; SP, stratum
pyramidale; Trx, thioredoxin; Prx, peroxiredoxin; sham, no
ischemia-reperfusion. |
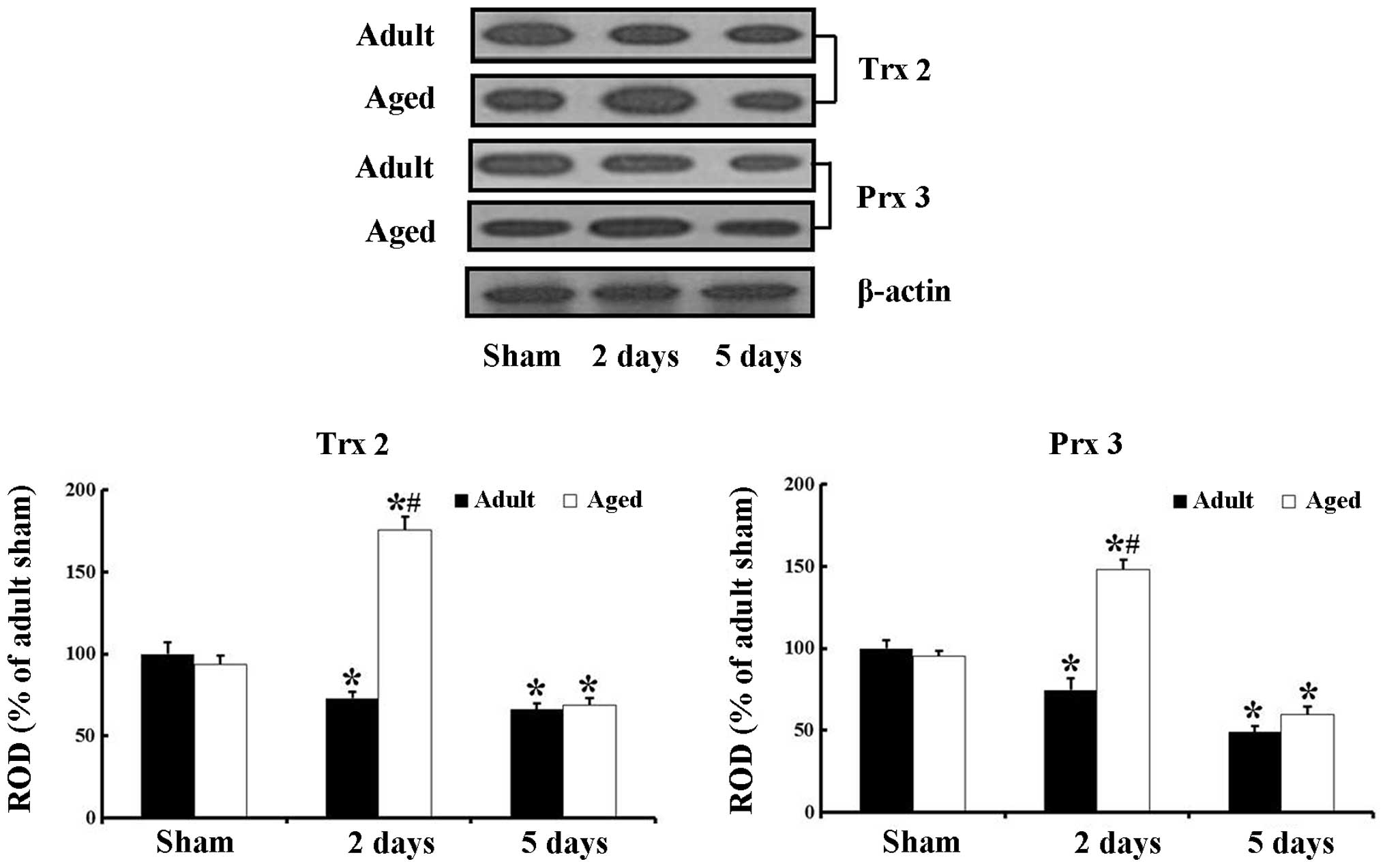
Changes in the protein levels of Trx2 and
Prx3
The results of the western blot analysis revealed a
similar pattern of changes in the protein levels of Trx2 and Prx3
in the adult and aged hippocampal CA1 region following ischemic
damage to those observed in the immunohistochemical data (Fig. 3).
In the adult animals, the protein level of Trx2 was
decreased (P=0.0155) from 2 days after ischemia-reperfusion. In the
aged sham-group, the protein level of Trx2 was marginally lower,
compared with that in the adult sham-group. In the aged
ischemia-group, the protein level of Trx2 was significantly
increased (P<0.0001) 2 days after ischemia-reperfusion, and a
significantly decreased (P=0.0147) 5 days post-ischemia.
The pattern of change in the protein level of Prx3
was similar to that of Trx2. The protein level of Prx3 was also
slightly lower, compared with that in the adult sham-group. In the
aged ischemia-group, the protein level of Prx3 was also
significantly increased (P=0.0001) 2 days post-ischemia, and
significantly decreased (P=0.0038) 5 days post-ischemia.
Discussion
Aging is one of major risk factors affecting
neuronal damage in cerebral ischemia (30). In the present study, the transient
cerebral ischemia-induced delay of neuronal death was significantly
slower in aged gerbils, compared with that in the adult gerbils.
This result is in line with other studies and our previous studies,
which reported that cerebral ischemia-induced neuronal damage in
aged animals is delayed more than in adult animals due to the
effects of aging on changes of lysosomes and the
caspase-3-dependent apoptotic pathway in the hippocampal CA1 region
(27,28,31,32).
In the present study, the protein expression levels
of Trx2 and Prx3 in the aged sham-group were marginally lower than
those in the adult sham-group. This is the first study, to the best
of our knowledge, to demonstrate decreased expression levels of
Trx2 and Prx3 in the aged hippocampus. A previous study reported
that the protein levels of Prx3 and glutathione-S-transferase ω1 in
the human cerebellum is inversely correlated with age (21), and it was suggested that the
negative correlation between age and antioxidant proteins was
associated with normal aging and oxidative stress. Therefore, it
was hypothesized that the decreases of Trx2 and Prx3 in the aged
gerbil hippocampus may be associated with increased oxidative
stress in aging.
In the present study, the Trx2 immunoreactivity in
the SP of the adult ischemia-group was minimal 2 days after
ischemia-reperfusion. In the aged animals, Trx2 immunoreactivity in
the sham-group was marginally lower than that in the adult
sham-group. In the aged ischemia-group, Trx2 immunoreactivity in
the SP was significantly higher at 1, 2 and 4 days post-ischemia,
compared with that in the adult ischemia-group. At 5 days
post-ischemia, Trx2 immunoreactivity was significantly decreased in
the SP. Prx3 immunoreactivity in the SP of the adult ischemia-group
was significantly decreased from 4 days after ischemia-reperfusion.
In the aged animals, Prx3 immunoreactivity in the sham-group was
also marginally lower than that in the adult sham-group. Prx3
immunoreactivity in the aged ischemia-group also significantly
higher at 1, 2 and 4 days post-ischemia, compared with the adult
ischemia-group; however, Prx3 immunoreactivity at 5 days
post-ischemia was significantly decreased. The results of the
western blot analysis demonstrated similar patterns of change in
the protein levels of Trx2 and Prx3 in the adult and aged
hippocampal CA1 region following ischemic damage to those observed
in the immunohistochemical data. These findings indicated that
cerebral ischemia led to different protein expression levels of
Trx2 and Prx3 in the hippocampal CA1 region between adult and aged
gerbils, and these differences may be associated with increased
delay of neuronal death in the aged gerbil hippocampus following
transient global cerebral ischemia.
In the present study, ischemia-induced changes in
the protein expression levels of Trx2 and Prx3 in the hippocampal
CA1 region were examined between adult and aged gerbils. The
protein expression levels of Trx2 and Prx3 were markedly decreased
in the hippocampal CA1 region of the adult ischemia-group from 2
days after ischemia-reperfusion, and minimal Trx2 and Prx3
immunoreactivity was detected in the adult SP 4 days after
ischemia-reperfusion. This result was consistent with those of a
previous study, which observed that the immunoreactivities of Trx
and Trx mRNA were decreased in the ischemic core region of the rat
brain following focal cerebral ischemia (33). By contrast, the present study
demonstrated that the immunoreactivities and protein levels of Trx2
and Prx3 in the aged ischemia-group were significantly increased
between 1 and 4 days following ischemia-reperfusion, compared with
those in the adult ischemia-group. In addition, their levels of
expression were almost undetectable in the SP of the aged
ischemia-group at 5 days post-ischemia, when delayed neuronal death
occurred in the aged ischemia-group. This finding indicated marked
expression levels of Trx2 and Prx3 in the neurons of the SP of the
aged hippocampal CA1 region, which were maintained significantly
longer than those in the adult CA1 region, following transient
cerebral ischemia. The present study is also the first, to the best
of our knowledge, to demonstrate the changes in the protein
expression levels of Trx2 and Prx3 in the aged hippocampus
following ischemic insult, therefore, the cause of the difference
in the protein expression levels of Trx2 and Prx3 protein
expressions between the adult and aged ischemic hippocampal CA1
region is difficult to determine. However, it has is known that
age-related changes in mitochondria can lead to the reduction in
the production of adenosine triphosphate and excessive oxidative
stress, and reductions in antioxidant detoxification mechanisms are
associated with increased susceptibility to ischemic damage
(34). Therefore, the marked
increases in the protein expression levels of Trx2 and Prx3 in the
aged ischemia-group may be associated with a compensatory mechanism
for increased susceptibility against ischemic damage, although the
basal levels of Trx2 and Prx3 in the aged sham-group were
marginally lower than those in the adult sham-group.
By contrast, it is widely accepted that the Trx/Prx
redox system is closely associated with protective effects against
neuronal damage following various insults, including cerebral
ischemia (23,24,35).
In our previous study, the administration of Prx3 and Prx3/Trx2
into ischemic brains resulted in a substantial neuroprotective
effect against ischemic damage by reducing oxidative stress induced
by transient ischemia (22).
In conclusion, the results of the present study
demonstrated that transient cerebral ischemia led to more marked
increase and longer maintenance in the protein expression levels of
Trx2 and Prx3 in the hippocampal CA1 region of aged gerbils,
compared with adult gerbils. The results indicated that differences
in the protein expression levels of Trx2 and Prx3 in the aged
gerbil may be associated with the difference in delayed neuronal
death in the CA1 region observed between aged and adult gerbils
following transient global cerebral ischemia.
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee for
their technical assistance. This study was supported by the Basic
Science Research Program through the National Research Foundation
of Korea, funded by the Ministry of Science, ICT and Future
Planning (grant. no. NRF-2012R1A1A1007298).
References
|
1
|
Kirino T and Sano K: Selective
vulnerability in the gerbil hippocampus following transient
ischemia. Acta Neuropathol. 62:201–208. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kirino T: Delayed neuronal death in the
gerbil hippocampus following ischemia. Brain Res. 239:57–69. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang ZH, Wu LN, Song JG and Li WQ:
Correlations between cognitive impairment and brain-derived
neurotrophic factor expression in the hippocampus of post-stroke
depression rats. Mol Med Rep. 6:889–893. 2012.PubMed/NCBI
|
|
4
|
Ding DX, Tian FF, Guo JL, et al: Dynamic
expression patterns of ATF3 and p53 in the hippocampus of a
pentylenetetrazole-induced kindling model. Mol Med Rep. 10:645–651.
2014.PubMed/NCBI
|
|
5
|
Chen L, Lv Y, Cui Z, et al: Tetrandrine
ameliorates cognitive impairment via inhibiting astrocyte-derived
S100B activation in a rat model of chronic cerebral hypoperfusion.
Neurol Res. 35:614–621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rastogi L, Godbole MM, Ray M, et al:
Reduction in oxidative stress and cell death explains
hypothyroidism induced neuroprotection subsequent to
ischemia/reperfusion insult. Exp Neurol. 200:290–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee CH, Park JH, Yoo KY, et al: Pre- and
post-treatments with escitalopram protect against experimental
ischemic neuronal damage via regulation of BDNF expression and
oxidative stress. Exp Neurol. 229:450–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan PH: Mitochondria and neuronal
death/survival signaling pathways in cerebral ischemia. Neurochem
Res. 29:1943–1949. 2004. View Article : Google Scholar
|
|
9
|
Yamagata K, Tagami M, Ikeda K, Yamori Y
and Nara Y: Altered gene expressions during hypoxia and
reoxygenation in cortical neurons isolated from stroke-prone
spontaneously hypertensive rats. Neurosci Lett. 284:131–134. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White BC, Sullivan JM, DeGracia DJ, et al:
Brain ischemia and reperfusion: molecular mechanisms of neuronal
injury. J Neurol Sci. 179:1–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Starkov AA, Chinopoulos C and Fiskum G:
Mitochondrial calcium and oxidative stress as mediators of ischemic
brain injury. Cell Calcium. 36:257–264. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Go YM and Jones DP: Mitochondrial
thioredoxin-2/peroxiredoxin-3 system functions in parallel with
mitochondrial GSH system in protection against oxidative stress.
Arch Biochem Biophys. 465:119–126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nordberg J and Arner ES: Reactive oxygen
species, antioxidants and the mammalian thioredoxin system. Free
Radic Biol Med. 31:1287–1312. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Drechsel DA and Patel M:
Respiration-dependent H2O2 removal in brain
mitochondria via the thioredoxin/peroxiredoxin system. J Biol Chem.
285:27850–27858. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Das KC: Thioredoxin and its role in
premature newborn biology. Antioxid Redox Signal. 7:1740–1743.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Watabe S, Hiroi T, Yamamoto Y, et al:
SP-22 is a thioredoxin-dependent peroxide reductase in
mitochondria. Eur J Biochem. 249:52–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powis G and Montfort WR: Properties and
biological activities of thioredoxins. Annu Rev Biophys Biomol
Struct. 30:421–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka T, Hosoi F, Yamaguchi-Iwai Y, et
al: Thioredoxin-2 (TRX-2) is an essential gene regulating
mitochondria-dependent apoptosis. EMBO J. 21:1695–1703. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rhee SG, Kang SW, Chang TS, Jeong W and
Kim K: Peroxiredoxin, a novel family of peroxidases. IUBMB Life.
52:35–41. 2001. View Article : Google Scholar
|
|
20
|
Damdimopoulos AE, Miranda-Vizuete A,
Pelto-Huikko M, Gustafsson JA and Spyrou G: Human mitochondrial
thioredoxin. Involvement in mitochondrial membrane potential and
cell death. J Biol Chem. 277:33249–33257. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krapfenbauer K, Engidawork E, Cairns N,
Fountoulakis M and Lubec G: Aberrant expression of peroxiredoxin
subtypes in neurodegenerative disorders. Brain Res. 967:152–160.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang IK, Yoo KY, Kim DW, et al: Changes
in the expression of mitochondrial peroxiredoxin and thioredoxin in
neurons and glia and their protective effects in experimental
cerebral ischemic damage. Free Radic Biol Med. 48:1242–1251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hattori I, Takagi Y, Nakamura H, et al:
Intravenous administration of thioredoxin decreases brain damage
following transient focal cerebral ischemia in mice. Antioxid Redox
Signal. 6:81–87. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hattori F, Murayama N, Noshita T and
Oikawa S: Mitochondrial peroxiredoxin-3 protects hippocampal
neurons from excitotoxic injury in vivo. J Neurochem. 86:860–868.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan BC, Park JH, Ahn JH, et al: Comparison
of the immunoreactivity of Trx2/Prx3 redox system in the
hippocampal CA1 region between the young and adult gerbil induced
by transient cerebral ischemia. Neurochem Res. 37:1019–1030. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu DK, Yoo KY, Shin BN, et al: Neuronal
damage in hippocampal subregions induced by various durations of
transient cerebral ischemia in gerbils using Fluoro-Jade B
histofluorescence. Brain Res. 1437:50–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CH, Yoo KY, Choi JH, et al: Neuronal
damage is much delayed and microgliosis is more severe in the aged
hippocampus induced by transient cerebral ischemia compared to the
adult hippocampus. J Neurol Sci. 294:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee CH, Yoo KY, Choi JH, et al: Comparison
of phosphorylated extracellular signal-regulated kinase 1/2
immunoreactivity in the hippocampal Ca1 region induced by transient
cerebral ischemia between adult and aged gerbils. Cell Mol
Neurobiol. 31:449–457. 2011. View Article : Google Scholar
|
|
29
|
Institute of Laboratory Animal Research,
Committee for the Update of the Guide for the Care and Use of
Laboratory Animals, National Research Council: Guide for the care
and use of laboratory animals. 8th. Washington, (DC): National
Academies Press; pp. 2202011
|
|
30
|
Xu K, Puchowicz MA, Sun X and LaManna JC:
Mitochondrial dysfunction in aging rat brain following transient
global ischemia. Adv Exp Med Biol. 614:379–386. 2008.PubMed/NCBI
|
|
31
|
Tamagaki C, Murata A, Asai S, et al:
Age-related changes of cornu ammonis 1 pyramidal neurons in gerbil
transient ischemia. Neuropathology. 20:221–227. 2000. View Article : Google Scholar
|
|
32
|
He Z, Meschia JF, Brott TG, Dickson DW and
McKinney M: Aging is neuroprotective during global ischemia but
leads to increased caspase-3 and apoptotic activity in hippocampal
neurons. Curr Neurovasc Res. 3:181–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takagi Y, Horikawa F, Nozaki K, Sugino T,
Hashimoto N and Yodoi J: Expression and distribution of redox
regulatory protein, thioredoxin during transient focal brain
ischemia in the rat. Neurosci Lett. 251:25–28. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baltan S: Ischemic injury to white matter:
an age-dependent process. Neuroscientist. 15:126–133. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L and Jiang DM: Neuroprotective
effect of Buyang Huanwu Decoction on spinal ischemia/reperfusion
injury in rats. J Ethnopharmacol. 124:219–223. 2009. View Article : Google Scholar : PubMed/NCBI
|