Introduction
Parkinson's disease (PD) is the second-most common
neurodegenerative disease worldwide (1). The typical pathological feature is
the abnormal accumulation of α-synuclein (SYN) in substantia nigra
(SN) dopaminergic neurons, causing the formation of Lewy bodies
(LBs) and the degeneration and apoptosis of neurons. To date, the
etiology and pathogenesis of abnormal SYN accumulation have
remained elusive (2,3). Studies have shown that inflammatory
and abnormal immune responses have a crucial role in the occurrence
of PD (4–7). These immune-associated types of
inflammation exist not only in the brain (7,8), but
the peripheral immune system is also thought to contribute to the
onset and progression of the neurodegenerative process in PD
(9–12). Inflammation in the peripheral
immune system is hypothesized to contribute to the onset and
progression of the neurodegenerative process observed in PD, due to
serum αSYN-specific antibodies (10), and lymphocyte infiltration into the
brains of patients with PD (7). In
light of these studies, it is indicated that the function of immune
cells is essential during inflammation-induced PD.
CD4+ T cells are important immune cells with
functions including the identification of viruses, bacteria and
other pathogens, and the coordination of other immune cells,
directing them to attack these pathogens (13). CD4+ T cells were found to
infiltrate into the SN in PD patients as well as mouse models of PD
(14–17). Naive CD4+ T cells can differentiate
into T-helper (Th1, Th2 and Th17) and regulatory T (Treg) cells in
order to execute their functional activities in the immune system.
These sub-sets are not differentiated stereotypical cells, but have
a certain plasticity (17,18). They affect and restrain each other
through the secretion of cytokines, forming a network to maintain
homeostasis. An imbalance in the sub-set ratios can cause immune
dysfunction and lead to immune damage of organs (19). Studies have shown that circulating
CD4+ T-cell sub-sets and sub-set balance may have an active role in
the progression of PD (20,21).
Only a small number of studies have demonstrated that circulating
CD4+ T cell sub-sets are altered in PD patients, however, with
conflicting results (22,23).
In the present study, the fractions of Th1, Th2,
Th17 and Treg cells which differentiated from CD4+ T cells were
assessed in patients with PD. Furthermore, these sub-sets and their
rations were correlated with Unified Parkinson's Disease Rating
Scale III (UPDRS-III) scores of PD patients. The present study
indicated that imbalances in CD4+ sub-sets may be utilized as novel
biomarkers for PD.
Materials and methods
Subjects
Blood samples were obtained aseptically by
venipuncture from PD patients (n=60) and age-, gender- and
environment-matched controls (n=40). Sixty PD patients (32 males
and 28 females) received treatment at the First Affiliated Hospital
of Bengbu Medical College (Bengbu, China) were enrolled between
January 2012 and October 2013. Their age range was 40–74 years with
an average age of 61.4 years. In addition, 40 healthy volunteers
(average age, 60.8 years; 20 males and 20 females) from the
patients' families (spouses) and the First Affiliated Hospital of
Bengbu Medical Center, whose age and gender matched those of the PD
group, were recruited as the control group. All patients were asked
to provide details on their medical history, including time of PD
onset, duration, onset process, past history, family history and
medication. The relevant scores, as determined by the Unified
Parkinson's Disease Rating Scale-III (UPDS-III), were established
by specifically trained neurologists. Patients with a history of
autoimmune or inflammatory disorders and those receiving chronic
immunosuppressive therapy were excluded from the present study.
Written informed consent was obtained from all
participants and the study was conducted in accordance with the
declaration of Helsinki with approval from the Ethics Committee of
Bengbu Medical College (Bengbu, China).
Sample collection
Sterile quantitative blood collection tubes
containing EDTA-2 K (BD Vacutainer; BD Biosciences, Franklin Lakes,
NJ, USA) were used to draw 8 ml peripheral venous blood. Samples
were coded and stored at room temperature until processing, which
occurred within 2 h of collection. A complete blood cell count with
differential analysis was performed using an XT-4000i automated
hematology analyzer (Sysmex, Kobe, Japan) based on the
semiconductor laser fluorescence streaming technology.
Flow cytometric phenotype analysis
Reagents
Multicolor flow cytometric analysis was performed
using a FACSCalibur flow cytometer (BD Biosciences) with BD
FACSDiva hardware (BD Biosciences) and CellQuest Pro software (BD
Biosciences). Cells were labeled with fluorochrome-conjugated
monoclonal antibodies against the following antigens: Anti-human
CD8 fluorescein isothiocyanate (FITC) (cat. no. 11-0087), mouse
immunoglobulin (Ig)G1 phycoerythrin (PE) (cat. no. 12-4714),
anti-human interleukin (IL)-4 PE (cat. no. 12-7049), anti-human
interferon (IFN)-γ PE (cat. no. 12-7319), IL-17A PE (cat. no.
12-7178), CD3 FITC (cat. no. 1538-03), CD4 FITC (cat. no. 347324),
CD25 PE (cat. no. 347643), CD127 peridinin chlorophyll (PerCP)-Cy
5.5 (cat. no. 557938) (all from BD Biosciences). Heparin RPMI 1640
medium, ionomycin mixture, monensin mixture were from MultiScience
Lianke Biotech Co., Ltd. (Hangzhou, China). Isotype-matched mouse
or rat monoclonal antibodies were used as negative controls.
Th1 and Th2 cell detection
The specific cytokines IFN-γ and IL-4 secreted by
Th1 and Th2 cells were used to distinguish these two cell types.
Anti-CD3 and CD8 labeling with reverse mapping of CD4+ cells was
used to prevent propylene glycol methyl ether acetate (PMA)-induced
CD4 cell-surface endocytosis. Fresh sterile blood samples (200
µl), heparin anti-coagulant and 200 µl RPMI-1640
[MultiScience (Lianke) Biotech Co., Ltd., Hangzhou, China] were
added to a 10-µl PMA (11 µg/ml), 8-µl
ionomycin (50 µg/ml) and 8-µl monensin (0.1 mg/ml)
working solution [MultiScience (Lianke) Biotech Co., Ltd.]. The
mixture was incubated in a 5% CO2 incubator at 37°C for
4–6 h. CD3-FITC and CD8-FITC (BD Biosciences) were added, followed
by incubation. The mixture was divided into four aliquots, and to
each of them, 100 µl stained whole blood was added. Fixative
(100 µl; BD Biosciences) was added and incubated. Following
centrifugation, the supernatant was discarded, and 100 µl
solubilizing agent (BD Biosciences) was added, followed by mouse
IgG1-PE, IFN-γ-PE, rat IgG1-PE and IL-4-PE and the mixture was
incubated in the dark at room temperature for 15 min. Cells were
then washed, centrifuged at a speed of 206 × g and the supernatant
was discarded. Cells (1×106) were resuspended in 0.5 ml
phosphate-buffered saline (PBS), then detection of the proportions
of CD3 + CD8-IFN-γ+cells (Th1 cells) and CD3 + CD8-IL-4+cells (Th1
cells) with a FACS Calibur flow cytometer (BD Biosciences) was
performed.
CD4+-cell and Th17-cell detection
Fresh sterile blood samples (2 ml) were mixed with
100 µl heparin RPMI-1640 medium [MultiScience (Lianke)
Biotech Co., Ltd.]. 5 µl 2 µg/ml phorbol ester, 5
µl 50 µg/ml ionomycin and 4 µl 5 mg/ml
brefeldin were added, and the cells were incubated at 37°C in a 5%
CO2 incubator for 5 h. Fixative (100 µl; BD
Biosciences) was added and the mixture was incubated in the dark at
room temperature for 15 min. Cells were then washed, centrifuged at
a speed of 206 x g, and the supernatant was discarded. Solubilizing
agent (100 µl; BD Biosciences) was added to disrupt the cell
membrane. Cells were then stained with allophycocyanine
(APC)-conjugated mouse anti-human CD3 (BD Biosciences),
PerCP-labeled mouse anti-human CD4 BD Biosciences) and PE-labeled
IL-17A-directed monoclonal antibodies (Abcam) or PE-labeled isotype
control at 35°C in the dark for 15 min. After rinsing twice and
centrifugation at a speed of 206 × g, the supernatant was
discarded. PBS was added and the samples were examined using the
FACSCalibur flow cytometer to determine the proportion of CD3+,
CD4+, and IL-17A+ cells.
Treg detection
Tregs were defined as CD3+ CD4+ CD25high
CD127diminished (dim), as it has been shown that these cells
represent the large majority of forkhead box (Fox) P3+ Tregs
(24,25). In the present study, this
definition was used instead of the commonly accepted definition
(CD3+ CD4+ CD25high FoxP3+) to avoid excessive cell loss from blood
samples during the fixation and washing steps required for FoxP3
staining. Fresh sterile blood (100 µl) containing heparin
was incubated with APC-conjugated mouse anti-human CD3,
FITC-labeled mouse anti-human CD25, PerCP-labeled mouse anti-human
CD4 and PE-conjugated mouse anti-human CD127 monoclonal antibodies
(all BD Biosciences) and the appropriate isotype controls at 35°C
in the dark for 15 min. FACS lysis solution (BD Biosciences) was
added and the two samples, which were sample treated with all
antibodies and IgG1-PE isotype control were incubated for 5 min in
the dark at 35°C for 15 min. Cells were then washed and centrifuged
at a speed of 206 × g, and the supernatant was discarded. The cells
were fixed with 0.5 ml of 1% paraformaldehyde. Multicolor flow
cytometric analysis was performed using the FACSCalibur flow
cytometer.
Statistical analysis
Data were analyzed using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA), and graphs were drawn using Graphpad Prism 4
(Graphpad Inc., La Jolla, CA, USA). Comparison between the
experimental and control groups was performed using a two-sample
t-test. Each index comparison between different groups was
performed using analysis of variance and a pairwise
Student-Newman-Keuls comparison test. P<0.01 was considered to
indicate a statistically significant difference.
Results
Lymphocyte and CD3+ CD4+ T-cell
frequencies in patients with PD
Increasing evidence suggested that CD4+ T cells have
an important role in the pathogenesis of PD (14–16).
Therefore, the numbers of CD4+ T cells in PD patients were
determined by flow cytometry. CD4+ T cells are a type of
lymphocytes, the latter accounting for 20–40% of white blood cells
(WBC). Thus, the present study first determined the WBC count and
the main components of peripheral WBC, neutrophils and lymphocytes.
The results in Table I show that
neutrophils were enhanced in the PD group, while the absolute
lymphocyte value, the lymphocyte proportion of WBCs, the CD4+
T-cell proportion and the absolute CD4+ T-cell number in
lymphocytes were decreased in the PD group compared with those in
the control group. The CD4+ T-cell proportion and the absolute CD4+
T-cell number in lymphocytes were also higher in the PD group than
those in the control group. These results suggested that peripheral
blood CD4+ T cells may be involved in the pathogenesis of PD.
 | Table IComplete blood counts and
differential counts of PD patients and controls. |
Table I
Complete blood counts and
differential counts of PD patients and controls.
| Blood cell
count | Controls
| PD patients
| P-value | t-value |
|---|
| n | Mean | SD | SEM | n | Mean | SD | SEM |
|---|
| Absolute WBC
×109/l | 40 | 5.89 | 1.13 | 0.18 | 45 | 6.06 | 1.33 | 0.20 | 0.483 | 0.615 |
| Absolute
neutrophils ×109/µl | 40 | 3.33 | 0.75 | 0.12 | 45 | 3.82 | 1.14 | 0.17 | 0.030 | 2.306 |
| Neutrophils
(%) | 40 | 56.42 | 5.57 | 0.88 | 45 | 62.58 | 8.98 | 1.33 | 0.003 | 3.841 |
| Absolute
lymphocytes ×109/l | 40 | 1.90 | 0.45 | 0.07 | 45 | 1.72 | 0.58 | 0.07 | 0.141 | −1.538 |
| Lymphocyte (%) | 40 | 31.88 | 4.00 | 0.63 | 45 | 28.93 | 8.19 | 1.22 | 0.001 | −2.143 |
| CD3+ CD4+ (%) | 40 | 38.00 | 3.18 | 0.50 | 45 | 34.96 | 5.19 | 0.77 | <0.001 | −3.288 |
| Absolute CD3+ CD4+
count/µla | 40 | 719.68 | 175.82 | 27.80 | 45 | 603.51 | 221.91 | 33.08 | 0.071 | −2.652 |
PD patients show an enhanced Th1 and
Th17-type immune response
Naive CD4+ T cells can differentiate into T-helper
cells (Th1, Th2 and Th17) and Treg cells, which affect each other.
An imbalance can cause immune dysfunction and lead to immune damage
of organs (19). Studies have
shown that circulating CD4+ T-cell sub-sets and the sub-set balance
may have an active role in the progression of PD (20,21).
The fact that peripheral blood CD4+ T cells may be involved in the
pathogenesis of PD suggested that the occurrence of PD may be
correlated with CD4+-cell sub-sets (Th1, Th2, Th17 and Tregs) and
sub-set balances (Th1/Th2 and Th17/Treg). To test this hypothesis,
the present study examined the numbers of Thl, Th2, Thl7 and Treg
cells in the peripheral blood of patients with PD. The ratios of
Th1/Th2 and Th17/Treg were then calculated.
To quantify Thl and Th2 cells, the specific
cytokines IFN-γ and IL-4, which are secreted by Th1 and Th2,
respectively, were used to identify those two types of cell.
anti-CD3 and CD8 labeling with reverse mapping of CD4+ cells was
used to prevent PMA-induced CD4 cell surface endocytosis. To test
for Thl7, the specific cytokine IL-17, which is secreted by Th17,
was used to distinguish Th17 cells. Tregs were defined as CD3+ CD4+
CD25high and CD127dim cells. In the present study, this definition
was used instead of the 'gold standard' (CD3+ CD4+ CD25high FoxP3+)
to avoid excessive cell loss from blood samples during the fixation
and washing steps required for FoxP3 staining; studies have shown
that CD3+ CD4+ CD25high CD127dim cells represent the large majority
of FoxP3+ Tregs (24,25).
The results showed that Thl and Thl7 cells were
obviously enhanced in PD patients group, while the proportion of
Th2 and Treg cells significantly decreased (Table II). The Th1/Th2 and Th17/Treg
ratios in the PD group were significantly higher than those in the
control group, and were shifted towards Th1 and Th17. The
enhancement of Th1-type response (the Th1/Th2 balance shifting
towards Th1) indicated that the cellular immunity was in the active
state and the host cell defended intracellular pathogen infection.
This is consistent with the pathological features comprising the
abnormal accumulation of SYN in SN dopamine neurons in PD patients.
This intracellular abnormal SYN is similar to intracellular
pathogens and activates the immune system, leading to the observed
Th1-type response. Th17 cells promote inflammation, mainly by
producing cytokines, while Tregs inhibit the immune response.
Therefore, Th17 and Tregs regulate each other. In the present
study, similarly to the Th1-type response, the enhancement of the
Th17-type response (Th17/Treg balance shifting towards Th17) also
suggested that the inflammation was initiated.
 | Table IICD4+ T-cell sub-sets and sub-set
balance in PD patients and controls. |
Table II
CD4+ T-cell sub-sets and sub-set
balance in PD patients and controls.
| T-cell sub-set | Controls
| PD patients
| P-value | t-value |
|---|
| n | Mean | SD | n | Mean | SD |
|---|
| Th1 | 40 | 11.23 | 1.34 | 60 | 17.88 | 6.21 | <0.001 | −8.026 |
| Th2 | 40 | 1.82 | 0.19 | 60 | 1.67 | 0.26 | 0.001 | −3.282 |
| Th17 | 40 | 0.52 | 0.16 | 60 | 0.86 | 0.18 | <0.001 | −9.798 |
| Treg | 40 | 5.91 | 0.42 | 60 | 3.89 | 0.42 | <0.001 | 15.125 |
| Th1/Th2 | 40 | 6.22 | 0.88 | 60 | 10.92 | 4.02 | <0.001 | −8.767 |
| Th17/Treg | 40 | 0.09 | 0.03 | 60 | 0.23 | 0.06 | <0.001 | −14.645 |
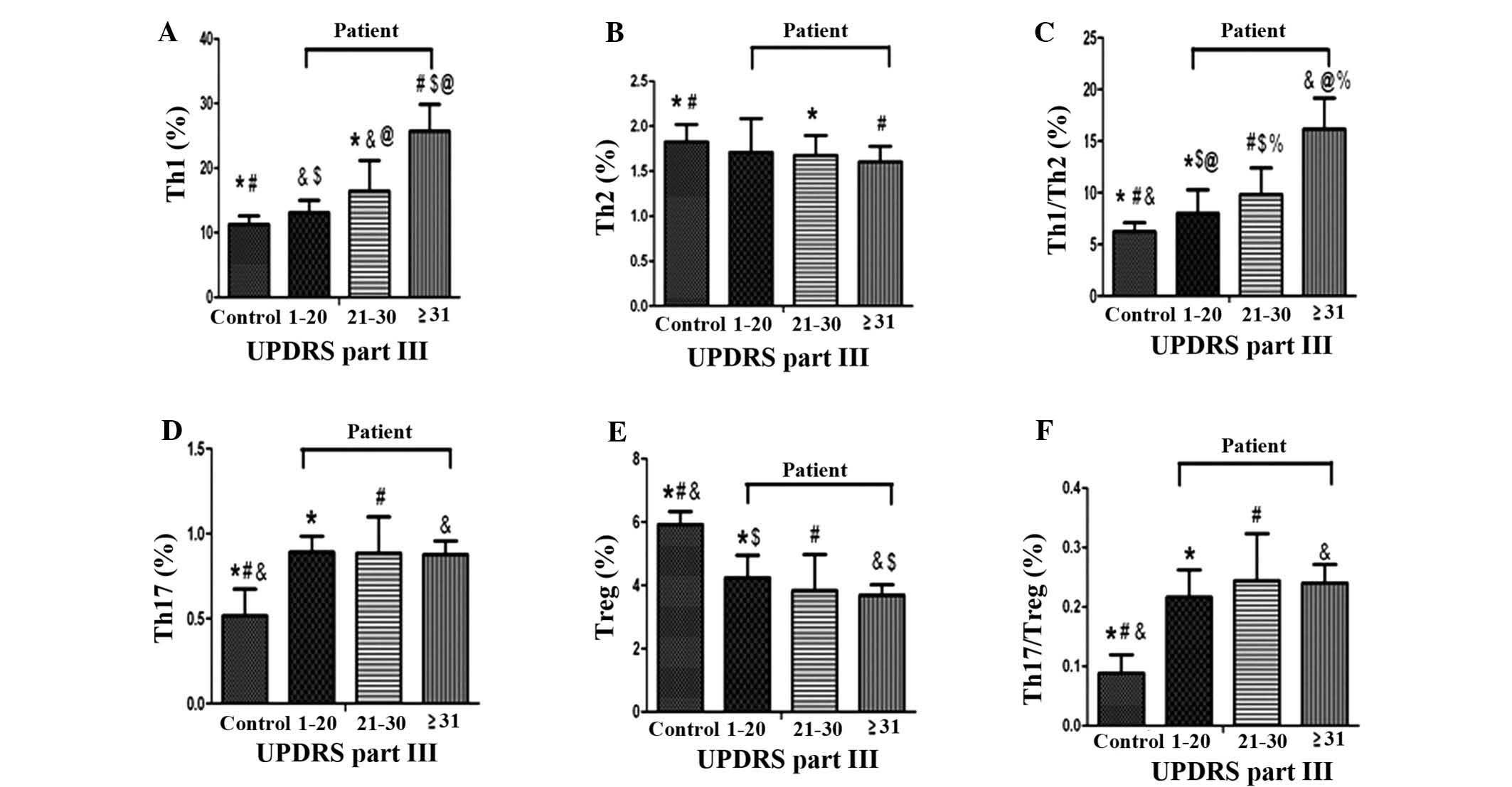
Severity of PD is correlated with CD4+
T-cell sub-sets and sub-set balance
To investigate the association between motor
dysfunction severity and CD4+ T-cells sub-sets and sub-set balance,
the flow cytometric data of the PD group were correlated with the
UPDRS-III scores. Three groups were segregated according to
UPDRS-III scores of 1–20 (n=15), 21–30 (n=29) and ≥31 (n=16).
Correlation analysis showed that the Th1-type response was
associated with motor function scores determined by UPDRS-III.
However, no correlation between changes in the Th17/Treg-cell
balance and UPDRS-III was identified (Fig. 2, P>0.05).
Discussion
Inflammation is a defensive reaction against harmful
stimuli that can induce a defensive response in the body. In the
present study, peripheral blood parameters of PD patients (the
general parameters of WBCs, as shown in Table I) were within the normal range
(4–10×109/l), however, the proportion of neutrophils in
PD patients was high. In PD patients, the proportion of CD4+ cells
in lymphocytes was low, but the difference from the healthy control
group was not statistically significant. Further study confirmed
that the distribution of CD4+-cell sub-sets in PD patients was
obviously abnormal, which indicated alterations in the immune
response in PD patients. This result was consistent with the
findings of previous studies, which suggested that peripheral
immunological challenges and chronic inflammatory diseases
influence the pathogenesis and progression of PD (26–29).
These studies indicated that CD4+ cells have an important role in
the development of PD.
In the immune system, CD4+ T lymphocytes are
important immune cells, and all CD4+ T cells can be classified into
T helper (Th1, Th2, Th17) and regulatory T (Treg) cell sub-sets.
Through the secretion of cytokines, they affect and restrain each
other, and form a network to maintain homeostasis for maintaining
the normal immune function. Mu et al (30) first presented the concept that
functional imbalance of Th1-, Th2-, Th17- and Treg-cell sub-sets
leads to multiple sclerosis. The present study confirmed the
changes in the distribution of CD4+-cell sub-sets in PD patients.
However, if the above-mentioned network balance is broken, it will
lead to a disease (30).
The present study reported that the Th1-cell
population was increased and the Th1/Th2 balance was shifted
towards Th1 in PD patients. The normal proportion of Th1 cells
among CD4+ cells is 10–15% (the proportion of the subsets refers to
the total CD4+-cell population and these reference values are
derived from the application of flow cytometry, which was performed
for Th1/Th2 detection). The normal range of Th1 was 11.8–25.8% and
the normal range of Th2 was 0.8–2.4%. In the present study,
Th1-cell populations in the PD group and control group were 17.88
and 11.23%, respectively; therefore, the Th1-cell population in the
PD group was higher than the measured value of the control group.
The normal value for the Th2-cell population is 0.8–2.4%. In the
present study, Th2-cell populations in the PD group and control
group were 1.82 and 1.67%, respectively. Although the Th2
population in the PD group was decreased, the difference from the
control group was no statistically significant and values were
within the normal range. The Th1/Th2 balance shifted towards the
Th1 cells, indicating an enhanced Th1-type response in PD patients.
Previous studies indicated that Th1 cells mediate cell immunity
through secretion of cytokines and have an important role in
removing intracellular microbial infections (13). Th2 cells mediate the humoral immune
response through the secretion of cytokines. Under normal
conditions, the immune function of Th1 and Th2 cells is in a state
of dynamic equilibrium. Once the equilibrium is disturbed, normal
immune function cannot be maintained with the emergence of various
associated diseases (18,19). It is known that the Th1/Th2-cell
imbalance is closely associated with infectious diseases,
autoimmune diseases and cancer. The Th1-type response is associated
with the enhanced immune defense of a host against viral and
intracellular pathogen infections (19,31,32).
The results of the present study indicated that the Th1-type
response was enhanced in PD patients, and PD was based mainly on
cell-mediated immunity. This is consistent with the pathological
features of PD, comprising the abnormal accumulation of SYN in SN
dopamine neurons (3). This
accumulated SYN in the affected neurons has a similar effect to
that of intracellular pathogens, thereby activating the immune
system and leading to a Th1-type response and a shift in the
Th1/Th2 balance towards Th1. As long as SYN is accumulated in the
SN dopamine neurons, it continues to activate the immune system,
and the resulting excessive or prolonged immune response leads to
PD progression and loss of dopamine neurons. The more the cells are
affected, the more severe the disease, and the more marked the
Th1-type response and the shifting of the Th1/Th2 balance.
Therefore, the present study assessed the correlation between Th1,
Th2, the Th1/Th2 balance and UPDRS-III scores in patients with PD.
UPDRS-III is the most commonly used assessment of disease severity,
based on PD motor symptoms. The correlation analysis showed that in
PD patients, Th1 and Th1/Th2 were associated with the motor
function scores determined by UPDRS-III. PD patients with UPDRS-III
scores of 30 or higher had increased Th1 and Th1/Th2, indicating
more severe motor symptoms of PD and the presence of a correlation
between the Th1 response and the severity of PD.
The present study also found that Th17 cells were
increased and Tregs were decreased in PD patients. The normal
proportion of Tregs in human peripheral blood is 5–10% (33); in the present study, Tregs were
reduced to 4.12% in PD patients and the Th17/Treg balance was
shifted towards Th17. Previous studies have shown that Th17 cells
promote inflammation mainly through production of cytokines, and
are primarily associated with mucosal inflammation (34,35).
Normally, Th17 cells and Tregs restrain each other, and there is a
Th17/Treg balance, similar to the Th1/Th2 balance. The disturbance
of the Th17/Treg balance is a key factor in numerous inflammatory
and autoimmune diseases (34,36).
In the present study, the increased Th17-cell population in PD
patients indicated enhanced PD-associated inflammation, while
reduced Tregs in PD patients suggested weakened inhibition of
inflammation. This further confirmed not only the existence of an
excessive inflammatory response, but also weakened immune
inhibition in PD patients. The latter caused the relative
enhancement of inflammation, which is responsible for the
aggravation of the inflammatory damage to tissues and organs in PD.
However, the present study found no significant correlation between
the proportion of Th17 cells and the UPDRS-III schore in patients
with PD. This imay be because UPDRS-III may not fully reflect the
severity of PD, as it only considers motor function. The clinical
diagnosis is primarily based on motor symptoms of PD, and
therefore, the most commonly used assessment of disease severity is
the UPDRS-III score, which is based on motor symptoms. However, in
recent years, with the deeper understanding of PD, non-motor
symptoms in PD patients also received increased attention (37,38).
Numerous studies have shown that PD is a multicentric
neurodegenerative process that also affects neuronal structures
outside the central nervous system in the SN (13,39).
Studies have identified abnormal accumulation of SYN in olfactory
mucosa and the colon, and in the clinic, PD patients generally
present with symptoms including the loss of smell and constipation.
These symptoms can appear prior to or after the motor symptoms, and
therefore, it is unreasonable to exclusively consider motor
symptoms to assess the degree of PD. A preliminary study by our
group found that non-specific inflammation in the colonic mucosa,
which is associated with long-term inflammation-induced colonic
transit constipation, is present in PD patients. The proportion of
Th17 cells and Treg cells as well as the Th17/Treg ratio were found
to be correlated with constipation of Parkinson's disease patients
(results not shown) (40). Th17
cells are closely associated with mucosal inflammation, and Treg
cells can be transformed into Th17 cells in the mucous membrane
(35), which may explain the
present findings that Th17 cells, Treg cells and Th17/Treg were not
correlated with the severity of motor symptoms in PD patients.
Experiments addressing the phenotype of pathogenic
CD4+T-cells have shown that Th1 and Th17-cells autoreactive cells
are important for the promotion of neuronal loss in PD (41). Conversely, other T-cell subsets,
such as Tregs-cells and Th2-cells, could contribute to microglial
acquisition of an anti-inflammatory phenotype and to promote
neuronal protection (41,42).
In conclusion, the results of the present study
indicated that chronic immune stimulation, specifically the
imbalance of the Th1/Th2 CD4+-cell sub-set, is linked to PD
pathobiology and disease severity. The Th1/Th2 equilibrium and its
imbalance may therefore represent a novel biomarker or therapeutic
target for PD.
Acknowledgments
The present study was supported by the key projects
of Anhui Provinal Department of Education (no. KJ2014A163), the
China Postdoctoral Science Foundation (no. 20090461139) and the
National Natural Science Foundation of China (no. 81001457).
References
|
1
|
Dorsey ER, Constantinescu R, Thompson JP,
et al: Projected number of people with Parkinson's disease in the
most populous nations, 2005 through 2030. Neurology. 68:384–386.
2007. View Article : Google Scholar
|
|
2
|
Mori F, Nishie M, Kakita A, Yoshimoto M,
Takahashi H and Wakabayashi K: Relationship among alpha-synuclein
accumulation, dopamine synthesis, and neurodegeneration in
Parkinson disease substantia nigra. J Neuropathol Exp Neurol.
65:808–815. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Croisier E, Moran LB, Dexter DT, Pearce RK
and Graeber MB: Microglial inflammation in the parkinsonian
substantia nigra: Relationship to alphaa-synuclein deposition. J
Neuroinflammation. 2:142005. View Article : Google Scholar
|
|
4
|
Stevens CH, Rowe D, Morel-Kopp MC, Orr C,
Russell T, Ranola M, Ward C and Halliday GM: Reduced T helper and B
lymphocytes in Parkinson's disease. J Neuroimmunol. 252:95–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Umemura A, Oeda T, Tomita S, Hayashi R,
Kohsaka M, Park K, Sugiyama H and Sawada H: Delirium and high Fever
are associated with subacute motor deterioration in Parkinson
disease: A nested case-control study. PLOS One. 9:e949442014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noelker C, Morel L, Osterloh A,
Alvarez-Fischer D, Lescot T, Breloer M, Gold M, Oertel WH, Henze C,
Michel PP, et al: Heat shock protein 60: An endogenous inducer of
dopaminergic cell death in Parkinson disease. J Neuroinflammation.
11:862014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
More SV, Kumar H, Kim IS, Song SY and Choi
DK: Cellular and molecular mediators of neuroinflammation in the
pathogenesis of Parkinson's disease. Mediators Inflamm.
2013:9523752013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bakay RA, Boyer KL, Freed CR and Ansari
AA: Immunological responses to injury and grafting in the central
nervous system of nonhuman primates. Cell Transplant. 7:109–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luk KC, Kehm VM, Zhang B, O'Brien P,
Trojanowski JQ and Lee VM: Intracerebral inoculation of
pathological α-synuclein initiates a rapidly progressive
neurodegenerative α-synucleinopathy in mice. J Exp Med.
209:975–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maetzler W, Apel A, Langkamp M, Deuschle
C, Dilger SS, Stirnkorb JG, Schulte C, Schleicher E, Gasser T and
Berg D: Comparable autoantibody serum levels against amyloid- and
inflammation-associated proteins in Parkinson's disease patients
and controls. PLOS One. 9:e886042014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamza TH, Zabetian CP, Tenesa A, Laederach
A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E,
et al: Common genetic variation in the HLA region is associated
with late-onset sporadic Parkinson's disease. Nat Genet.
42:781–785. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Melief J, Koning N, Schuurman KG, Van De
Garde MD, Smolders J, Hoek RM, Van Eijk M, Hamann J and Huitinga I:
Phenotyping primary human microglia: Tight regulation of LPS
responsiveness. Glia. 60:1506–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shannon KM, Keshavarzian A, Dodiya HB,
Jakate S and Kordower JH: Is alpha-synuclein in the colon a
biomarker for premotor Parkinson's disease? Evidence from 3 cases.
Mov Disord. 27:716–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirsch EC and Hunot S: Neuroinflammation
in Parkinson's disease: A target for neuroprotection? Lancet
Neurol. 8:382–397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brochard V, Combadière B, Prigent A,
Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer
D, Callebert J, Launay JM, et al: Infiltration of CD4+lymphocytes
into the brain contributes to neurodegeneration in a mouse model of
Parkinson disease. J Clin Invest. 119:182–192. 2009.
|
|
16
|
González H, Contreras F, Prado C, Elgueta
D, Franz D, Bernales S and Pacheco R: Dopamine receptor D3
expressed on CD4+ T cells favors neurodegeneration of dopaminergic
neurons during Parkinson's disease. J Immunol. 190:5048–5056. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation. Immunity.
30:646–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wan YY: Multi-tasking of helper T cells.
Immunology. 130:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu L, Sun B, Kong Q, Wang J, Wang G, Zhang
S, Wang D, Liu Y, Liu Y, An H, et al: Disequilibrium of T helper
type 1, 2 and 17 cells and regulatory T cells during the
development of experimental autoimmune myasthenia gravis.
Immunology. 128:e826–e836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nie K, Zhang Y, Gan R and Wang L, Zhao J,
Huang Z, Tang H and Wang L: Polymorphisms in immune/inflammatory
cytokine genes are related to Parkinson's disease with cognitive
impairment in the han chinese population. Neurosci Lett.
541:111–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sanchez-Guajardo V, Annibali A, Jensen PH
and Romero-Ramos M: α-Synuclein vaccination prevents the
accumulation of parkinson disease-like pathologic inclusions in
striatum in association with regulatory T cell recruitment in a rat
model. J Neuropathol Exp Neurol. 72:624–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saunders JA, Estes KA, Kosloski LM, Allen
HE, Dempsey KM, Torres-Russotto DR, Meza JL, Santamaria PM, Bertoni
JM, Murman DL, et al: CD4+ regulatory and effector/memory T cell
subsets profile motor dysfunction in Parkinson's disease. J
Neuroimmune Pharmacol. 7:927–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niwa F, Kuriyama N, Nakagawa M and
Imanishi J: Effects of peripheral lymphocyte subpopulations and the
clinical correlation with Parkinson's disease. Geriatr Gerontol
Int. 12:102–107. 2012. View Article : Google Scholar
|
|
24
|
Fazekas de St Groth B, Zhu E, Asad S and
Lee L: Flow cytometric detection of human regulatory T cells.
Methods Mol Biol. 707:263–279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McClymont SA, Putnam AL, Lee MR, Esensten
JH, Liu W, Hulme MA, Hoffmüller U, Baron U, Olek S, Bluestone JA,
et al: Plasticity of human regulatory T cells in healthy subjects
and patients with type 1 diabetes. J Immunol. 186:3918–3926. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pott Godoy MC, Ferrari CC and Pitossi FJ:
Nigral neurode-generation triggered by striatal AdIL-1
administration can be exacerbated by systemic IL-1 expression. J
Neuroimmunol. 222:29–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hasegawa Y, Inagaki T, Sawada M and
Suzumura A: Impaired cytokine production by peripheral blood
mononuclear cells and monocytes/macrophages in Parkinson's disease.
Acta Neurol Scand. 101:159–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brodacki B, Staszewski J, Toczyłowska B,
Kozłowska E, Drela N, Chalimoniuk M and Stepien A: Serum
interleukin (IL-2, IL-10, IL-6, IL-4), TNF alpha and INF gamma
concentrations are elevated in patients with atypical and
idiopathic parkinsonism. Neurosci Lett. 441:158–162. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reale M, Iarlori C, Thomas A, Gambi D,
Perfetti B, Di Nicola M and Onofrj M: Peripheral cytokines profile
in Parkinson's disease. Brain Behav Immun. 23:55–63. 2009.
View Article : Google Scholar
|
|
30
|
Mu L, Sun B, Kong Q, Wang J, Wang G, Zhang
S, Wang D, Liu Y, Liu Y, An H, et al: Disequilibrium of T helper
type 1, 2 and 17 cells and regulatory T cells during the
development of experimental autoimmune myasthenia gravis.
Immunology. 128(1 Suppl): e826–e836. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hawkins RD, Larjo A, Tripathi SK, Wagner
U, Luu Y, Lönnberg T, Raghav SK, Lee LK, Lund R, Ren B, Lähdesmäki
H and Lahesmaa R: Global chromatin state analysis reveals
lineage-specific enhancers during the initiation of human T helper
1 and T helper 2 cell polarization. Immunity. 38:1271–1284. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Olson NC, Sallam R, Doyle MF, Tracy RP and
Huber SA: T helper cell polarization in healthy people:
Implications for cardiovascular disease. J Cardiovasc Transl Res.
6:772–786. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wan YY and Flavell RA: How diverse - CD4
effector T cells and their functions. J Mol Cell Biol. 1:20–36.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Homey B: After TH1/TH2 now comes
Treg/TH17: Significance of T helper cells in immune response
organization. Hautarzt. 57:730–732. 2006.In German. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sakaguchi S, Ono M, Setoguchi R, Yagi H,
Hori S, Fehervari Z, Shimizu J, Takahashi T and Nomura T: Foxp3+
CD25+ CD4+ natural regulatory T cells in dominant self-tolerance
and autoimmune disease. Immunol Rev. 212:8–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing
Y, Deng Z, Yao Z, Tsun A and Li B: FOXP3 and RORγt: Transcriptional
regulation of Treg and Th17. Int Immunopharmacol. 11:536–542. 2011.
View Article : Google Scholar
|
|
37
|
Charles PD, Esper GJ and Davis TL: A
definition of “on”: Patient diaries compared to the UPDRS.
Parkinsonism Relat Disord. 5:99–101. 1999. View Article : Google Scholar
|
|
38
|
Longhi M, Ricciardi G, Tommasi G, Nicolato
A, Foroni R, Betrolasi L, Beltramello A, Moretto G, Tinazzi M and
Gerosa M: The role of 3T magnetic resonance imaging for targeting
the human subthalamic nucleus in deep brain stimulation for
Parkinson Disease. J Neurol Surg A Cent Eur Neurosurg. 76:181–189.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Braak H, Sastre M, Bohl JR, de Vos RA and
Del Tredici K: Parkinson's disease: Lesions in dorsal horn layer I,
involvement of parasympathetic and sympathetic pre- and
postganglionic neurons. Acta Neuropathol. 113:421–429. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Yu M, Liu X, Qu H, Chen Q, Qian W,
Wei D, Xu W, Ma B and Wu W: Clinical characteristics and peripheral
T cell subsets in Parkinson's disease patients with constipation.
Int J Clin Exp Pathol. 8:2495–2504. 2015.PubMed/NCBI
|
|
41
|
Reynolds AD, Stone DK, Hutter JA, Benner
EJ, Mosley RL and Gendelman HE: Regulatory T cells attenuate Th17
cell-mediated nigrostriatal dopaminergic neurodegeneration in a
model of Parkinson's disease. J Immunol. 184:2261–2271. PubMed/NCBI
|
|
42
|
Appel SH: CD4+ T cells mediate
cytotoxicity in neurodegenerative diseases. J Clin Invest.
119:13–15. 2009.
|