Introduction
Hepatoma-derived growth factors (HDGFs) are novel
multifunctional growth factors that were first purified from the
HuH-7 hepatoma cell line (1) and
rat metanephrotic mesenchymal cells (2). Subsequently, five HDGF-associated
proteins (HRP1-4) and lens epithelium-derived growth factor (LEDGF)
were identified. HRP1-4 and LEDGF constitute the HDGF family.
Members of the HDGF family are expressed in a broad range of
organs, including the brain, testes, lungs, skeletal muscles and
the spleen (3–6).
HDGF has mitogenic activity in numerous cell types,
including hepatocellular carcinoma (HCC) cells, fibroblasts,
endothelial cells, vascular smooth muscle cells, neuronal cells and
fetal hepatocytes (1,2,7–9).
HDGF is also involved in the development of the kidney, liver and
lungs, in addition to cardiovascular differentiation (2,4,5,10–12).
HDGF has been suggested to be associated with tumorigenesis, due to
the fact that upregulation of HDGF expression has been observed in
human gastric cancer (13),
non-small-cell lung cancer (14,15),
HCC (16), esophageal carcinoma
(17) and pancreatic cancer
(18) cells. HDGF has additionally
been reported to stimulate DNA synthesis and cell proliferation
upon entering the nuclei of tumor cells and serve important roles
in angiogenesis, tumor relapse, distant metastasis and malignancy
(19).
HDGF is a novel trophic factor for motor neurons,
promoting neurite extension and the survival of spinal motor
neurons in primary cultures, to an extent equivalent to the effects
of the well-known ciliary neurotrophic factor and brain-derived
neurotrophic factor (20,21). HDGF expression was reported to be
increased in spinal motor neurons in a mouse model of motor neuron
degeneration and in polyglutamine-tract-binding protein-1
transgenic mice prior to the onset of degeneration (22). In addition, an in vivo study
in newborn rats demonstrated that HDGF represses cell death of
motor neurons following facial nerve sectioning (22). Although the expression of HDGF in
numerous organs has been reported previously, the expression of
HDGF-2 in the development and injury of the spinal cord has
remained to be fully elucidated. It was hypothesized in the present
study that HDGF-2 is involved in spinal cord development and spinal
cord recovery following spinal cord injury (SCI). The purpose of
the present study was to investigate alterations in the expression
of HDGF-2 in normal fetal and adult rat spinal cords and in injured
spinal cords following spinal cord contusion.
Materials and methods
The current study was approved by the institutional
and licensing committee of Shantou University Medical College
(STUMC; Shantou, China).
Animals
A total of 120 Adult Sprague-Dawley rats
weighing an average of 250 g (220–270 g; two months old) were used
in the current study (provided by the Experimental Animal Center of
STUMC). Adult rats were assigned randomly to the SCI groups
(including the day 1, day 21 and day 45 subsequent to SCI groups;
n=30 for each group) and the normal adult rat group (n=30). Rats at
embryonic day 19 (E19; n=40), post-natal day 7 (P7; n=30) and P14
(n=30) were used. Rats were housed in cages (7–8 rats per cage for
each group) and maintained under a 12-h light/dark cycle at 21±1°C
and 50±5% humidity. All animals were acclimated to their
environment and had ad libitum access to tap water and a
standard rodent diet. All experimental procedures were performed
according to the guidelines of STUMC and were approved by the
Medical Animal Care and Welfare committee of STUMC at the Second
Affiliated Hospital Shantou University Medical College for the care
of animals.
Adult rat model of SCI
Rats were anesthetized by intraperitoneal injection
of pentobarbital (40 mg/kg; Beijing Chemical Reagent Company,
Beijing, China). Anesthesia was considered complete when animals
failed to exhibit hind limb withdrawal in response to a noxious
foot pinch. The spinal cords of adult rats were contused
extradurally with a 10-g Modified Allen's weight-drop impactor
comprising a 10 g weight dropping 25.0 mm onto the T9-T10 region of
the spinal cord exposed by laminectomy as described previously
(23). All animal care and
surgical procedures were approved by the institutional animal care
and use committee of Shantou University.
Western blot analysis
The spinal cords from the T9-T10 region were
dissected from the E19, P7 and adult rats. Cord tissues were
suspended in lysis buffer (Cell Signaling Technology, Danvers, MA,
USA) and homogenized in a dounce homogenizer (Ningbo SCIENTZ
Biotechnology Co., Ltd., Ningbo, China) on ice. Tissue homogenates
were centrifuged at 3,948 x g for 10 min at 4°C and then stored at
−30°C. Protein concentrations were determined by a Bio-Rad protein
assay kit II (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
according to the manufacturer's instructions. For the western blot
analysis, 20 µl of each sample was separated by 12% SDS-PAGE
and proteins were transferred to polyvinylidene difluoride
membranes. Blots were blocked with 5% non-fat dry milk in
Tris-buffered saline for 1 h at room temperature and then incubated
with 1:200 diluted monoclonal rabbit antibodies against HDGF-2
(cat. no. sc-292373; Bioss Biotech Co., Ltd, Beijing, China)
overnight at 4°C Membranes were then processed with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:500;
Sigma-Aldrich, St. Louis, MO, USA). Immunoreactive bands were
quantified using image analysis software.
Immunohistochemistry
Rats were deeply anesthetized and perfused via
cardiac puncture with 0.1 M phosphate-buffered saline (PBS; pH 7.4)
and subsequently with 4% paraformaldehyde in 0.1 M PBS.
The spinal cord was carefully dissected from the
T9-T10 region (an 8-mm segment containing the injured epicenter was
dissected out in the injury group). Isolated spinal cords were
post-fixed by immersion in 4% paraformaldehyde for 3 h, then
cryoprotected by immersion in PBS containing 20% sucrose (Beijing
Chemical Reagent Company) overnight. The tissue segments were then
embedded in optimal cutting temperature compound for frozen
sections and frozen transverse spinal sections (10 µm) were
cut with a Leica CM1800 cryostat (Leica Microsystems GmbH, Wetzlar,
Germany). Immunohistochemical staining was then performed using an
avidin-biotin complex method as described previously (23,24).
The rabbit anti-HDGF-2 primary antibody and biotinylated
anti-rabbit antibody were diluted to 1:200.
Evaluation of staining
Immunohistochemically-stained tissue sections were
evaluated for clinical parameters by three pathologists blinded to
the treatment groups. HDGF-2 expression in the nuclei was
independently evaluated. Five fields of vision were randomly
selected at 400× to count positive cells, and the average number of
positive cells was calculated. The criteria for positive staining
were as follows: Positive staining in vascular endothelial cell
nuclei, 1; staining intensity of HDGF-2 in the nuclei was rated as
weak (1), mild (2) and strong (3). Positive HDGF-2 expression was
additionally evaluated in the cytoplasm, in which the percentage of
brown-yellow cells was scored as negative <5%, (−); or positive
5–25%, (+); 25.1–50%, (++); >50%, (+++). The same principle was
applied in the assessment of spinal cord anterior horn motor
neurons.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical significance was assessed by one-way
analysis of variance. Significant differences between the two pairs
of groups were assessed by Pearson's χ2 test.
Statistical analysis was performed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
HDGF-2 is highly expressed in spinal
cords of neonatal rats
To examine the expression of HDGF-2 during
development of the spinal cord, the expression of HDGF-2 was
detected in the spinal cord homogenates prepared from rats at
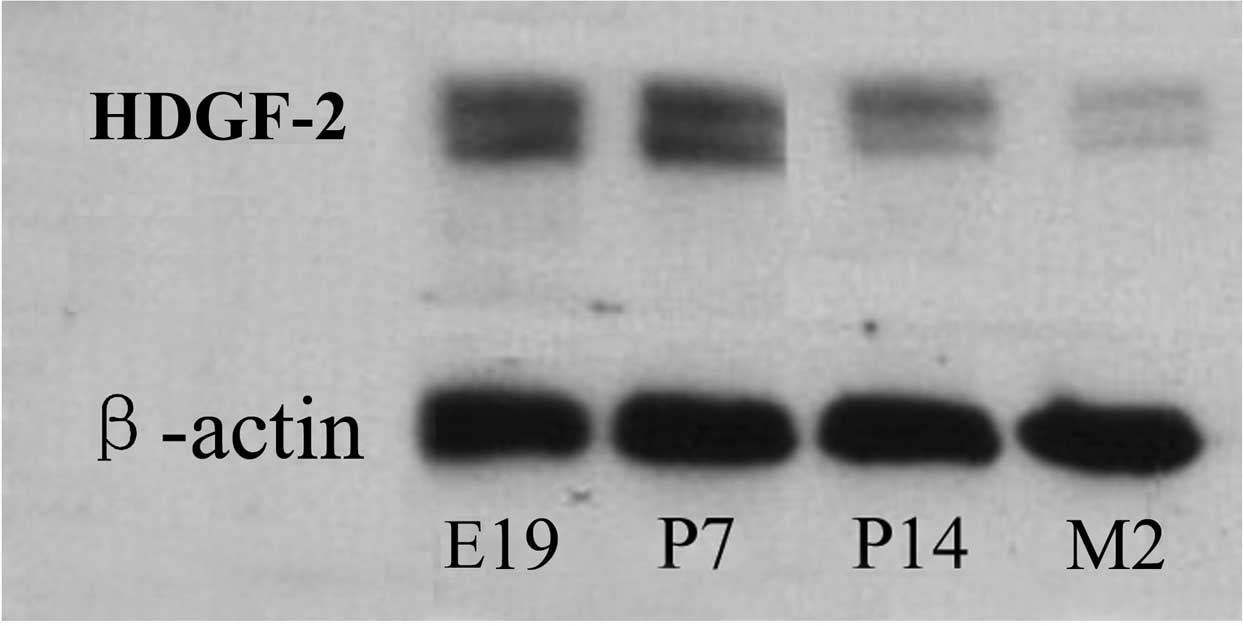
different developmental stages using western blot analysis. HDGF-2
protein expression was identified to be highest in the spines of
E19 rats and remained high at P7; however, it became weak at P14
and was the lowest at two months (Fig.
1).
In order to identify the distribution pattern of
HDGF-2 protein and the cell populations that express HDGF-2,
immunohistochemical staining was performed. In the P7 rats, HDGF-2
protein expression was primarily restricted to neurons, astrocytes
and oligodendrocytes and it was particularly expressed in motor
neurons of the anterior horn. HDGF-2 immunoreactivity was observed
at low levels within the white matter. At the sub-cellular level,
HDGF-2 was primarily localized within cell nuclei but was
additionally detected in the cytoplasm (Fig. 2). HDGF-2 was highly expressed in
the E19 rat spinal cords, whereas it was weakly expressed in the
spinal cords of the two-month-old rats.
In order to deduce the possible role of HDGF-2 in
spinal cord development and recovery from SCI, the expression of
HDGF-2 was assessed using immunohistochemical s-p staining in
embryonic and adult spinal cords and injured spinal cords following
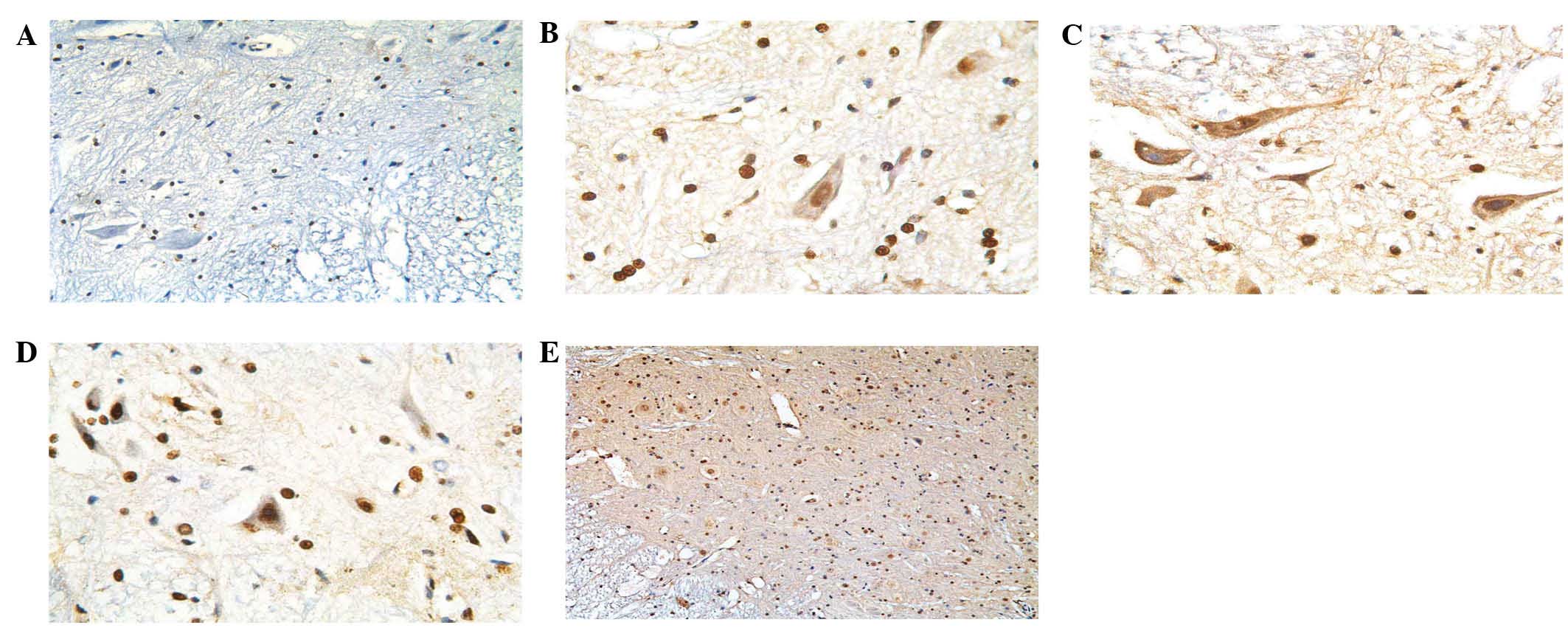
SCI. HDGF-2 immunoreactivity was minimally observed in normal adult
rat spinal cords (Fig. 2A),
however, it was highly expressed in E19 embryonic rat spinal cords
(Fig. 2B). As early as one day
subsequent to injury, HDGF-2 expression was identified in neurons
within the gray matter in the lesion area (Fig. 2C). HDGF-2 was highly expressed
until day 21 subsequent to injury (Fig. 2D) and almost declined to basal
levels 45 days following injury (Fig.
2E).
HDGF-2 protein was highly expressed in the spinal
cords of rat embryos and adult rats at the early stages of SCI. The
expression of HDGF-2 at days 1, 21 and 45 subsequent to SCI was
significantly higher than that in the normal rats (141±62, 107±32
and 92±18 vs. 50±9, respectively). Significant differences between
the different time-points following SCI were identified
(P<0.01). Details of the positive HDGF-2 expression observed in
the nuclei are presented in Table
I and that observed in the cytoplasm are presented in Table II. The percentage of cells stained
positive for HDGF-2 was 16.67% in the spinal cords of normal adult
rats, whereas the percentage was significantly increased in fetal
rats and SCI rats, with rates of 67.50 and 77.78%, respectively
(P<0.05). The expression of HDGF-2 in the spinal cord following
SCI and in fetal rats was significantly higher than that in the
normal adult rats (P<0.05). Pearson's χ2 tests
demonstrated that the expression levels of HDGF-2 in the anterior
horn motor neurons of SCI rats were different among various
post-operative stages (P<0.01) and the expression levels of
HDGF-2 correlated with different time-points following SCI.
 | Table ICorrelation between time period
subsequent to SCI and expression of hepatoma-derived growth
factor-2 (staining intensity) (n=30 per group). |
Table I
Correlation between time period
subsequent to SCI and expression of hepatoma-derived growth
factor-2 (staining intensity) (n=30 per group).
| Parameter | Normal | SCI
|
|---|
| 1 day | 21 days | 45 days |
|---|
| Positive score | 50.23±8.76 | 141±61.76 | 107±33.28 | 92±17.92 |
| t-value | | 57.07 | 46.29 | 20.31 |
 | Table IICorrelation between group and
cytoplasmic expression of hepatoma-derived growth factor-2. |
Table II
Correlation between group and
cytoplasmic expression of hepatoma-derived growth factor-2.
| Group | n | Negative (n)
| Positive (n)
| Positive rate
(%) | P-value |
|---|
| − | + | ++ | +++ |
|---|
| Normal | 30 | 25 | 2 | 2 | 1 | 16.67 | |
| Fetal | 40 | 13 | 5 | 10 | 12 | 67.50 | <0.05 |
| SCI | 90 | 20 | 8 | 20 | 42 | 77.78 | <0.05 |
Discussion
HDGF is a novel trophic factor for motor neurons,
promoting neurite extension and the survival of spinal motor
neurons in primary cultures (20,21).
An in vivo study in newborn rats demonstrated that HDGF
represses the cell death of motor neurons following facial nerve
sectioning (22). Although the
expression of HDGF in numerous organs had been previously reported
(3–6), the expression of HDGF-2 in the
development and injury of the spinal cord has remained elusive. It
was hypothesized that HDGF-2 is involved in spinal cord development
and spinal cord recovery following SCI. The purpose of the present
study was to investigate alterations in the expression levels of
HDGF-2 in the spinal cord of fetal and normal adult rats as well as
in rats following SCI.
HDGF-2 has previously been demonstrated to have
neurotrophic activity (8,22). Abouzied et al (25) reported that the expression levels
of HDGF and HDGF-2 are regulated during brain development, with the
highest levels around the time-point of birth followed by a decline
by post-natal day 9. In order to gain an improved understanding of
the normal organization and development of the human spinal cord,
numerous studies have been conducted regarding spinal cord
development in lower vertebrates and mammals. For example, Clowry
et al (26) used a
restricted set of immunohistochemical markers to follow the
development of sensorimotor components of the spinal cord and
identified that human fetal spinal cord development during
gestational weeks 7.5–17 is similar to the late embryonic/early
post-natal period of rodent development (E16.5-P5). During this
period, motor neurons segregate into motor columns. Therefore, E19
embryonic rat spinal cords were selected for the present study and
it was observed that HDGF-2 was highly expressed in the fetal
spinal cord and anterior horn motor neurons in the spinal cord, and
that HDGF-2 was predominantly localized in the nuclei. Statistical
analysis additionally suggested that the expression of HDGF-2 in
the fetal spinal cord was greater than that in the normal spinal
cord from protein expression levels, suggesting that HDGF-2 may be
involved in the development of the nervous system.
HDGF-2 expression is induced following SCI,
particularly during the early phase of recovery from SCI. It has
been previously reported that HDGF promotes the proliferation and
survival of neurons (27).
El-Tahir et al (3)
hypothesized that the function of HDGF during neuronal development
changes from promoting proliferation to promoting cellular
survival. In the present study, it was demonstrated that the HDGF-2
protein expression levels were the highest in the E19 spinal cord
and it was also highly expressed in P7 rat spinal cords; however,
expression became weak at P14 and was not detectable at two months.
HDGF-2 expression in the fetal rat spinal cord and injured spinal
cord was higher than that in uninjured adult spinal cord tissues.
The expression of HDGF-2 was the highest at the early phases
following SCI, suggesting that HDGF-2 may be involved in the early
recovery from SCI. Therefore, it was hypothesized that HDGF-2 may
be involved in fetal spinal cord development and the repair of
damage from SCI.
In conclusion, the present study showed assessed the
expression of HDGF-2 in the rat spinal cord at different
developmental stages and following SCI. Expression of HDGF-2
following SCI and in fetal rat spinal cord tissues was greater than
that in normal adult spinal cord tissues. Expression of HDGF-2 was
additionally significantly elevated in the early period following
SCI as compared with that in the later stages following recovery
from SCI. Thus, the results of the present study suggested that
HDGF-2 may be a novel therapeutic substance to aid in the recovery
from SCI.
Acknowledgments
The current study was supported by grants from the
Natural Science Foundation of Guangdong Province, China (grant no.
S2011010005018) and the Foundation of Guangdong Technology Bureau,
China (grant no. 2009B080701040). The authors would like to thank
Dr Stanley Lin for his assistance with the revision of the
manuscript (Shantou University Medical College, Shantou,
China).
References
|
1
|
Nakamura H, Izumoto Y, Kambe H, Kuroda T,
Mori T, Kawamura K, Yamamoto H and Kishimoto T: Molecular cloning
of complementary DNA for a novel human hepatoma-derived growth
factor. Its homology with high mobility group-1 protein. J Biol
Chem. 269:25143–25149. 1994.PubMed/NCBI
|
|
2
|
Oliver JA and Al-Awqati Q: An endothelial
growth factor involved in rat renal development. J Clin Invest.
102:1208–1219. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Tahir HM, Dietz F, Dringen R, Schwabe
K, Strenge K, Kelm S, Abouzied MM, Gieselmann V and Franken S:
Expression of hepatoma-derived growth factor family members in the
adult central nervous system. BMC Neurosci. 7:62006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Everett AD, Narron JV, Stoops T, Nakamura
H and Tucker A: Hepatoma-derived growth factor is a pulmonary
endothelial cell-expressed angiogenic factor. Am J Physiol Lung
Cell Mol Physiol. 286:L1194–L1201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori M, Morishita H, Nakamura H, Matsuoka
H, Yoshida K, Kishima Y, Zhou Z, Kida H, Funakoshi T, Goya S, et
al: Hepatoma-derived growth factor is involved in lung remodeling
by stimulating epithelial growth. Am J Respir Cell Mol Biol.
30:459–469. 2004. View Article : Google Scholar
|
|
6
|
Li SZ, Zhao YB, Cao WD, Qu Y, Luo P, Zhen
HN, Chen XY, Yan ZF and Fei Z: The expression of hepatoma-derived
growth factor in primary central nervous system lymphoma and its
correlation with angiogenesis, proliferation and clinical outcome.
Med Oncol. 30:6222013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou Y, Zhou N, Fang W and Huo J:
Overexpressed HDGF as an independent prognostic factor is involved
in poor prognosis in Chinese patients with liver cancer. Diagn
Pathol. 5:582010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Yamamoto Y, Sugai F, Yoshida K,
Kishima Y, Sumi H, Nakamura H and Sakoda S: Hepatoma-derived growth
factor is a neurotrophic factor harbored in the nucleus. J Biol
Chem. 279:27320–27326. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Enomoto H, Yoshida K, Kishima Y, Okuda Y
and Nakamura H: Participation of hepatoma-derived growth factor in
the regulation of fetal hepatocyte proliferation. J Gastroenterol.
37(Suppl 14): 158–161. 2002. View Article : Google Scholar
|
|
10
|
Everett AD: Identification, cloning and
developmental expression of hepatoma-derived growth factor in the
developing rat heart. Dev Dyn. 222:450–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Enomoto H, Yoshida K, Kishima Y, Kinoshita
T, Yamamoto M, Everett AD, Miyajima A and Nakamura H:
Hepatoma-derived growth factor is highly expressed in developing
liver and promotes fetal hepatocyte proliferation. Hepatology.
36:1519–1527. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enomoto H, Nakamura H, Liu W, Yoshida K,
Okuda Y, Imanishi H, Saito M, Shimomura S, Hada T and Nishiguchi S:
Hepatoma-derived growth factor is induced in liver regeneration.
Hepatol Res. 39:988–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang KC, Tai MH, Lin JW, Wang CC, Huang
CC, Hung CH, Chen CH, Lu SN, Lee CM, Changchien CS, et al:
Hepatoma-derived growth factor is a novel prognostic factor for
gastrointestinal stromal tumors. Int J Cancer. 121:1059–1065. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iwasaki T, Nakagawa K, Nakamura H, Takada
Y, Matsui K and Kawahara K: Hepatoma-derived growth factor as a
prognostic marker in completely resected non-small-cell lung
cancer. Oncol Rep. 13:1075–1080. 2005.PubMed/NCBI
|
|
15
|
Ren H, Tang X, Lee JJ, Feng L, Everett AD,
Hong WK, Khuri FR and Mao L: Expression of hepatoma-derived growth
factor is a strong prognostic predictor for patients with
early-stage non-small-cell lung cancer. J Clin Oncol. 22:3230–3237.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida K, Tomita Y, Okuda Y, Yamamoto S,
Enomoto H, Uyama H, Ito H, Hoshida Y, Aozasa K, Nagano H, et al:
Hepatoma-derived growth factor is a novel prognostic factor for
hepatocellular carcinoma. Ann Surg Oncol. 13:159–167. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto S, Tomita Y, Hoshida Y, Morii E,
Yasuda T, Doki Y, Aozasa K, Uyama H, Nakamura H and Monden M:
Expression level of hepatoma-derived growth factor correlates with
tumor recurrence of esophageal carcinoma. Ann Surg Oncol.
14:2141–2149. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uyama H, Tomita Y, Nakamura H, Nakamori S,
Zhang B, Hoshida Y, Enomoto H, Okuda Y, Sakon M, Aozasa K, et al:
Hepatoma-derived growth factor is a novel prognostic factor for
patients with pancreatic cancer. Clin Cancer Res. 12:6043–6048.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Ren H, Yuan P, Lang W, Zhang L
and Mao L: Down-regulation of hepatoma-derived growth factor
inhibits anchorage-independent growth and invasion of non-small
cell lung cancer cells. Cancer Res. 66:18–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arakawa Y, Sendtner M and Thoenen H:
Survival effect of ciliary neurotrophic factor (CNTF) on chick
embryonic motoneurons in culture: Comparison with other
neurotrophic factors and cytokines. J Neurosci. 10:3507–3515.
1990.PubMed/NCBI
|
|
21
|
Sendtner M, Holtmann B, Kolbeck R, Thoenen
H and Barde YA: Brain-derived neurotrophic factor prevents the
death of motoneurons in newborn rats after nerve section. Nature.
360:757–759. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marubuchi S, Okuda T, Tagawa K, Enokido Y,
Horiuchi D, Shimokawa R, Tamura T, Qi ML, Eishi Y, Watabe K, et al:
Hepatoma-derived growth factor, a new trophic factor for motor
neurons, is up-regulated in the spinal cord of PQBP-1 transgenic
mice before onset of degeneration. J Neurochem. 99:70–83. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang XJ, Kong KM and Qi WL: Interleukin-1
beta induction of neuron apoptosis depends on p38 mitogen-activated
protein kinase activity after spinal cord injury. Acta Pharmacol
Sin. 26:934–942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomlinson A, Appleton I, Moore AR, Gilroy
DW, Willis D, Mitchell JA and Willoughby DA: Cyclo-oxygenase and
nitric oxide synthase isoforms in rat carrageenin-induced pleurisy.
Br J Pharmacol. 113:693–698. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abouzied MM, Baader SL, Dietz F, Kappler
J, Gieselmann V and Franken S: Expression patterns and different
subcellular localization of the growth factors HDGF
(hepatoma-derived growth factor) and HRP-3 (HDGF-related protein-3)
suggest functions in addition to their mitogenic activity. Biochem
J. 378:169–176. 2004. View Article : Google Scholar
|
|
26
|
Clowry GJ, Moss JA and Clough RL: An
immunohistochemical study of the development of sensorimotor
components of the early fetal human spinal cord. J Anat.
207:313–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bremer S, Klein K, Sedlmaier A, Abouzied
M, Gieselmann V and Franken S: Hepatoma-derived growth factor and
nucleolin exist in the same ribonucleoprotein complex. BMC Biochem.
14:22013. View Article : Google Scholar : PubMed/NCBI
|