Introduction
Following the confirmation by Lo et al
(1) of the presence of cell-free
fetal DNA (cffDNA) in maternal plasma and serum in 1997,
investigations have focussed on the utilization of cffDNA in
non-invasive prenatal testing (NIPT). To date, cffDNA analysis is
widely used in numerous NIPT, including for fetal gender
determination (2), Rhesus blood
group D (RhD) antigen status determination (3), and for the assessment of monogenic
diseases and chromosomal aneuploidies prenatally (4).
CffDNA is widely accepted to originate predominantly
from the product of placenta trophoblast apoptosis (5), and exhibits a distinctive molecular
characteristic. CffDNA molecules are generally <300 bps in
length, while the maternally-derived cell-free plasma DNA are
>300 bps in length (6,7). Based on these observations, it is
possible to separate cffDNA molecules from the overwhelming
quantity of maternal-derived DNA.
Several techniques are used for analyzing cffDNA,
including methylated DNA immunoprecipitation, digital polymerase
chain reaction (PCR) and massively parallel sequencing (8–10).
Quantitative PCR (qPCR) is the most fundamental, cost efficient and
common method used for cffDNA analysis, however, its accuracy is
affected by a number of external and internal factors, including
the quantity of the initial samples, the quality of templates and
the PCR efficiency (11).
Therefore, it is necessary to normalize the gene level. At present,
the use of control genes as a standard normalizer (12) is the most common method. Control
genes are commonly defined as genes, which ubiquitously exist at
stable levels in different biological contexts and are used to
confirm the presence and quality of DNA in each sample, as well as
measure the quantity of total (maternal and fetal) DNA in each
sample (13). However, no single
universal and entirely constant control gene has been reported.
Accumulating evidence has indicated that the content levels of
widely used control genes vary significantly in different
independent studies (14–16). Therefore, it is essential to
compare and evaluate the content stability of each control gene
prior to use for normalization in cffDNA analysis. To the best of
our current knowledge, the commonly used control genes for cffDNA
analysis are selected, almost without any preliminary evaluation of
their content suitability.
The present study aimed to examine the content
stability of six commonly used control genes, which exist as
differently sized maternal plasma DNA molecules, including those
>300 bps, considered maternally-derived DNA, and <300 bps,
considered fetally-derived DNA. These control genes are β-globin
(HBB), telomerase (TERT), glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), albumin (ALB), β-actin (ACTB) and T cell receptor γ (TRG),
and they were selected based on previous reports on cffDNA using
the qPCR method. In the present study, three common statistical
algorithm programs, geNorm (17),
NormFinder (18) and BestKeeper
(19), were used to evaluate the
content stabilities of the six genes. The results of the present
study aimed to reveal optimal control gene selections for further
investigations on cffDNA.
Materials and methods
Plasma sample collection and DNA
extraction
The present study was approved by the Ethical
Committee of Second Hospital, Jilin University (Jilin, China). For
the investigation, 2 ml of peripheral blood was collected from the
cubital vein of 20 pregnant females (gestational age, 18.67±0.58
weeks) and written informed consent was obtained from each
individual prior to commencement of the investigation. The blood
samples were anticoagulated using EDTA (1.5%). DNA was extracted
from the plasma of each sample using a QIAamp DNA mini kit (Qiagen,
Hilden, Germany), according to the manufacturer's instructions,
within 4 h of blood collection.
Separation of maternal-and fetal-derived
DNA
The extracted DNA was subjected to 1% agarose gel
electrophoresis (Invitrogen Life Technologies, Carlsbad, CA, USA)
(7,20), and was visualized under ultraviolet
light (GIS-2008, Peiqing Science & Technology, Shanghai,
China). Each lane was cut at a position of 300 bps into two
discrete sections, according to the DL500 DNA marker (Takara Bio,
Inc., Otsu, Japan) and extracted from the agarose using a AxyPrep
Gel Extraction kit (Axygen Biosciences, Union City, CA, USA),
according to the manufacturer's instructions. DNA with a length
<300 bps was defined as fetal-derived DNA (fetal group) and DNA
with lengths >300 bps was defined as maternal-derived DNA
(maternal group).
qPCR analysis
The subsequent qPCR analysis was performed using an
ABI PRISM 7500 Sequence Detection system (Applied Biosystems Life
Technologies, Foster City, CA, USA). The primers of the control
genes were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China) and the sequences are presented in Table I.
 | Table IPrimer sequences, product sizes and
PCR efficiency. |
Table I
Primer sequences, product sizes and
PCR efficiency.
| Symbol | Primer
sequence | Product size
(bp) | PCR efficiency |
R2-value |
|---|
| HBB |
F-GTGCACCTGACTCCTGAGGAGA | 101 | 2.58 | 0.97 |
|
R-CCTTGATACCAACCTGCCCAG | | | |
| TERT |
F-GGTGAACCTCGTAAGTTTATGCAA | 97 | 2.00 | 0.97 |
|
R-GGCACACGTGGCTTTTCG | | | |
| GAPDH |
F-GGACTGAGGCTCCCACCTTT | 157 | 1.72 | 0.99 |
|
R-GCATGGACTGTGGTCTGCAA | | | |
| ALB |
F-TGAAACATACGTTAACCCAAAGAGTTT | 80 | 1.79 | 0.99 |
|
R-CTCTCCTTCTCAGAAAGTGTGCATAT | | | |
| ACTB |
F-CCTGTACGCCAACACAGTGC | 211 | 2.08 | 0.98 |
|
R-ATACTCCTGCTTGCTGATCC | | | |
| TRG |
F-AGGGTTGTGTTGGAATCAGG | 160 | 1.82 | 0.97 |
|
R-CGTCGACAACAAGTGTTGTTCCAC | | | |
The qPCR reactions were performed in a 20 µl
volume containing DNA (8 ng) using a SYBR Premix Ex Taq kit (Takara
Bio, Inc.), including 10 µl SYBR Premix Ex Taq (2X), 0.4
µl ROX Reference Dye (50X) and 1 µl forward/reverse
primer (10 µM each), made up to 20 µl with deionized
water, according to the manufacturer's instructions. The
amplification was performed using an ABI PRISM 7500 Sequence
Detection system (Applied Biosystems Life Technologies) and
subjected to the following cycling steps: Initial step of 95°C for
10 min, followed by 50 cycles of 95°C for 15 sec, 58°C for 15 sec
and 72°C for 30 sec. Each assay was performed four times. The
results of the qPCR results were subjected to 1% agarose gel
electrophoresis. To estimate the efficiencies of amplification, a
standard curve was generated by Microsoft Excel (Microsoft
Corporation, Redmond, WA, USA) for each primer pair based on four
points of serial 2-fold dilutions of the DNA template and performed
qPCR reactions as described above.
Statistical analysis
Microsoft Excel was used to calculate the mean and
standard deviation (SD) values. The amplification efficiencies were
calculated using the slope of the calibration curve with the
equation, E = 2−1/slope, following which the correlation
coefficients (R2 values) were determined.
The content stabilities of the six candidate control
genes were assessed using three commonly used programs: geNorm
(http://medgen.ugent.be/~jvdesomp/genorm/), NormFinder
(http://moma.dk/normfinder-software)
and BestKeeper (http://www.gene-quantification.de/bestkeeper.html),
according to the manufacturer's instructions. In geNorm and
NormFinder, the threshold cycle (Ct) values were converted into
relative quantities using the 2−ΔCt formula (ΔCt = Ct −
lowest Ct). For BestKeeper, the raw Ct values were used directly.
These three programs were all based on Microsoft Excel, using
different algorithms to determinate the stability of the control
genes.
Results
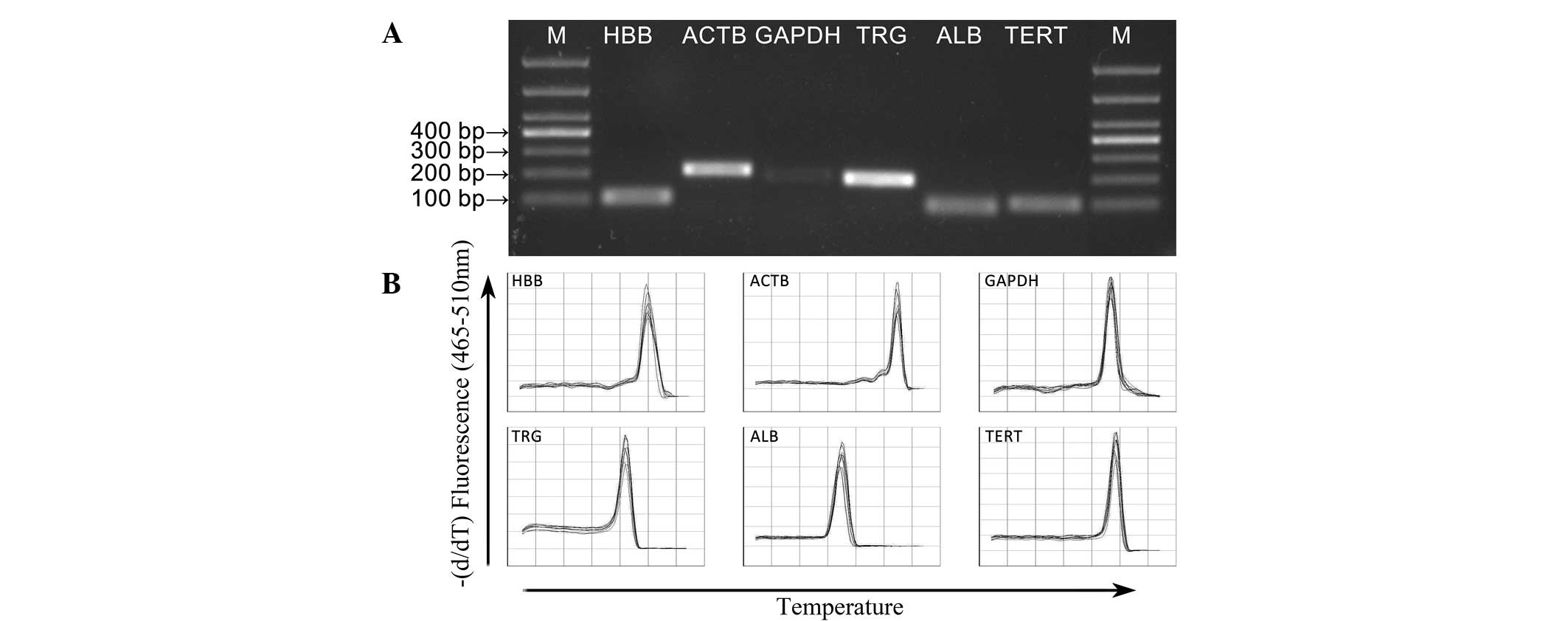
Amplification performance of primers
The qPCR amplification product was detected using 1%
agarose gel electrophoresis and was of the expected size with no
primer dimers (Fig. 1A). A single
peak was obtained in each amplification reaction during the
analysis of the dissociation curves, which confirmed the specific
amplification of the primers (Fig.
1B). The sequences, corresponding amplicon sizes, PCR
efficiencies of the primers and R2 values are listed in
Table I.
Amplification profile of the candidate
control genes
The amplification profiles of the candidate control
genes were estimated according to the Ct values of the six
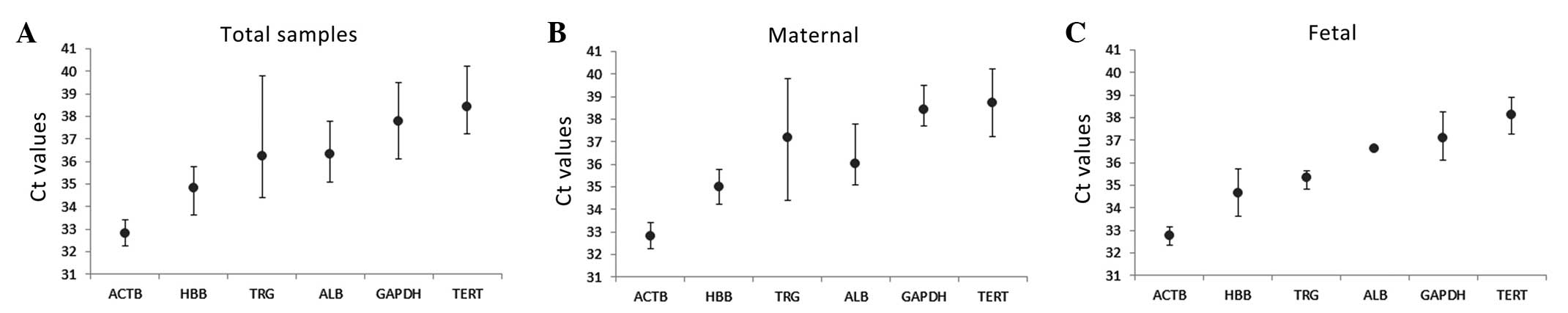
biological samples. As shown in Fig.
2, the mean Ct values of each gene in the maternal group and
fetal group were determined.
The control genes exhibited Ct values varying
between 32.78, for ACTB, and 38.74, for TERT, in the total samples
(Fig. 2A). Among these genes, ACTB
exhibited the lowest Ct value (32.25–33.43) and TERT exhibited the
highest Ct value (37.23–40.22), followed by GAPDH (36.13–39.48), as
shown in Fig. 2A. Among these six
candidate control genes, TRG was the most variable in terms of
content, with a high SD value (2.63) in the maternal group
(Fig. 2B). ACTB was the candidate
control gene with the lowest SD values (0.34 and 0.49 in the
maternal and fetal group, respectively; Fig. 2B and C). No significant difference
in Ct values were observed between the maternal and fetal group for
any of the genes.
Content stability of the candidate
control genes
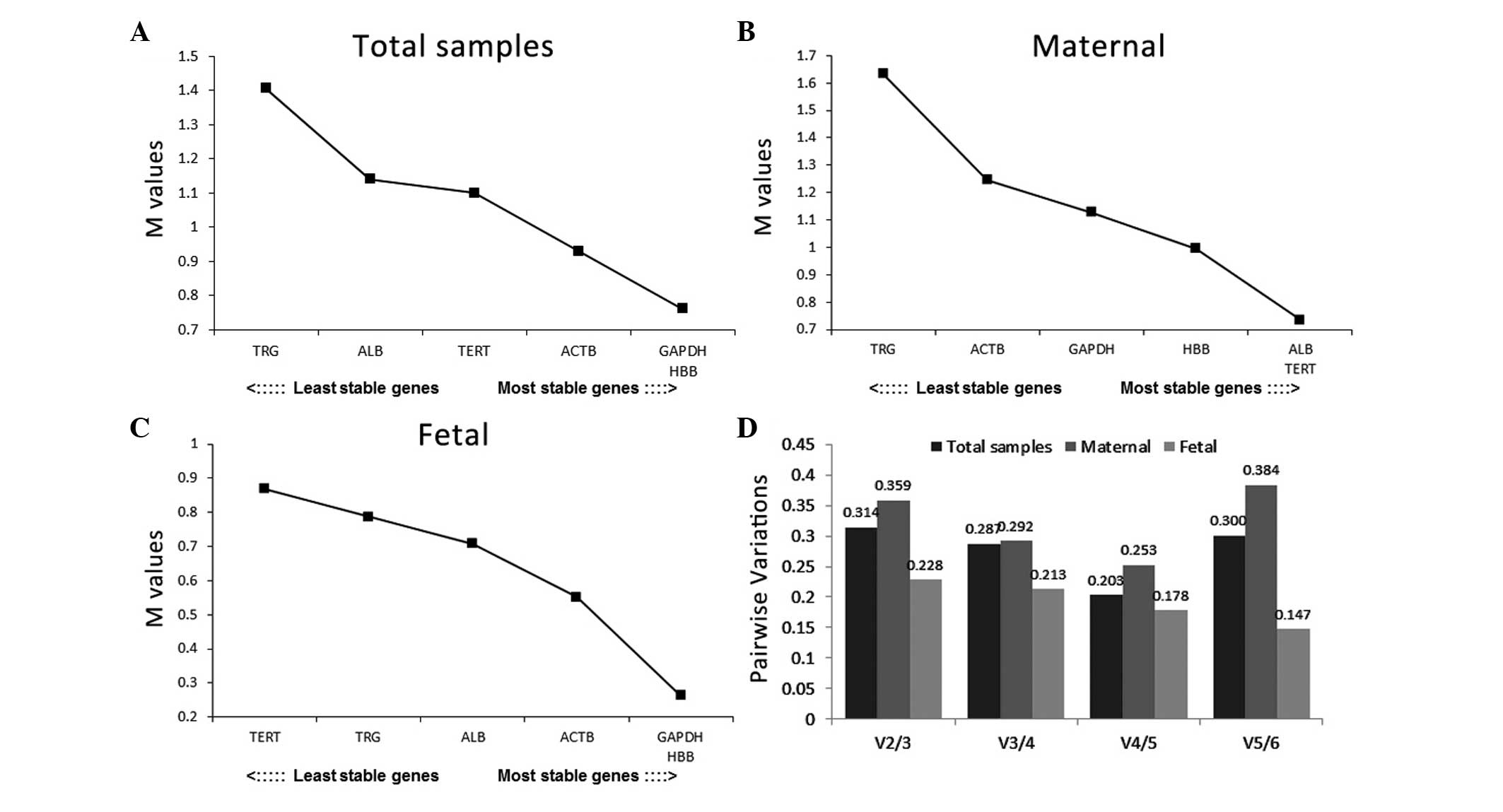
According to the geNorm database, HBB and GAPDH were
the most stable genes, with the lowest M values, which were
followed by ACTB, in the total sample and fetal group, whereas ALB
and TERT were ranked as the most stable genes in the maternal group
(Fig. 3A–C). TRG was considered to
be an unstable gene in all three groups. Notably, almost none of
the pairwise variation values were below the cutoff value (V=0.15),
with the exception of V5/6 in the fetal group (Fig. 3D). This result indicated that
combining five control genes together in the fetal group increased
the stability for normalization. No optimal combination number of
control genes were identified for normalization in the other
groups.
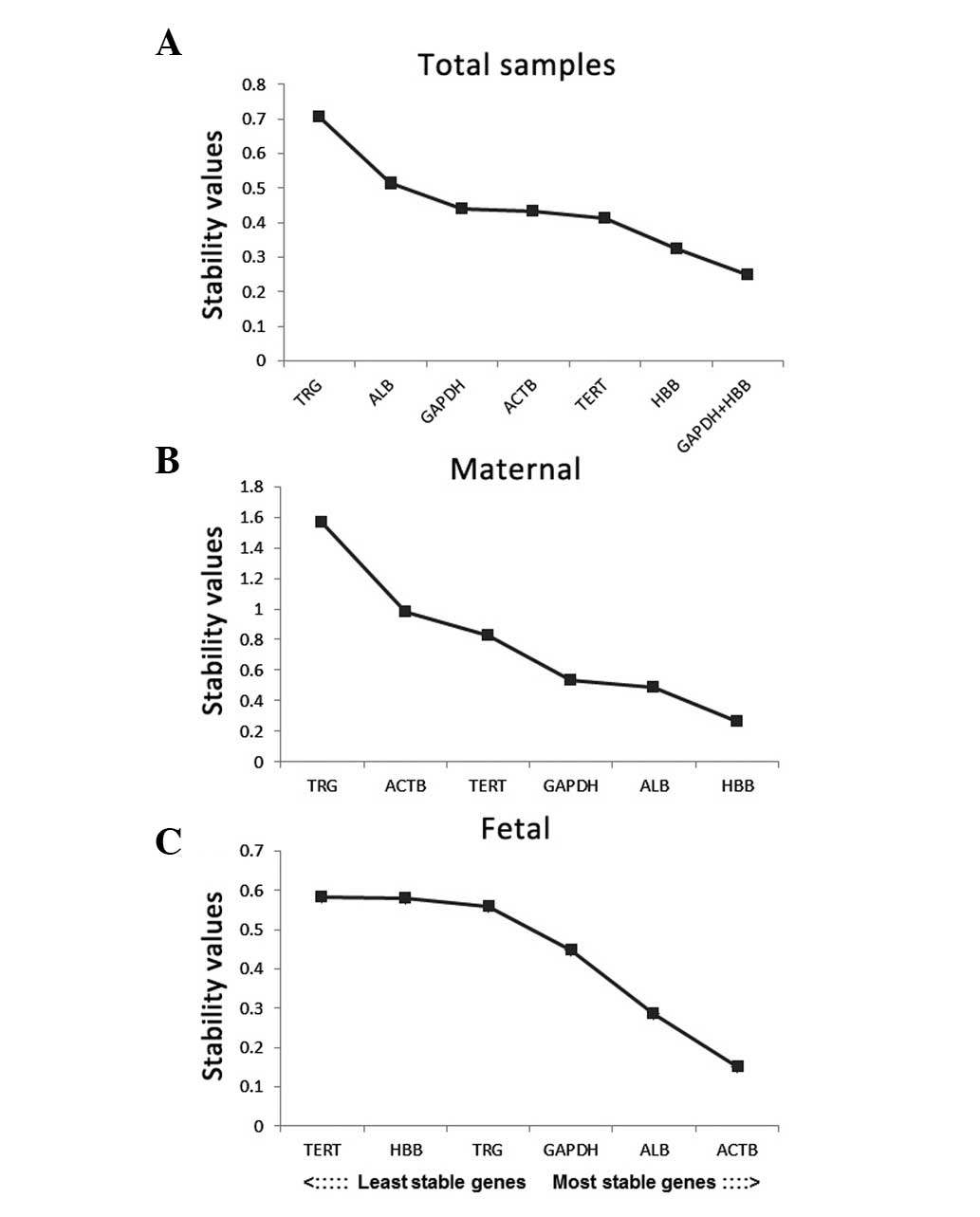
The results of the NormFinder analysis indicated
that HBB was the most content stable gene, with a stability value
of 0.325, in the total samples (Fig.
4A). The optimal combination of two genes was determined to be
HBB and GAPDH. The stability value of the HBB/GADPH combination
(0.249) was lower than that of HBB alone (0.325), which suggested
that the combination of these two genes provided higher stability,
compared with HBB alone (Fig. 4A).
HBB and ACTB were identified as the most content stable genes in
the maternal and fetal group, respectively (Fig. 4B and C).
The results of the BestKeeper analysis revealed that
HBB demonstrated the highest stability in the total samples and in
the maternal group, whereas GAPDH was determined as the optimal
performer n the fetal group (Fig.
5A–C). On examination of the variance (Fig. 5D–F), the SD value of TRG was
>1.00 in the total samples and maternal group, therefore, it was
considered unacceptable and eliminated from the stability
analysis.
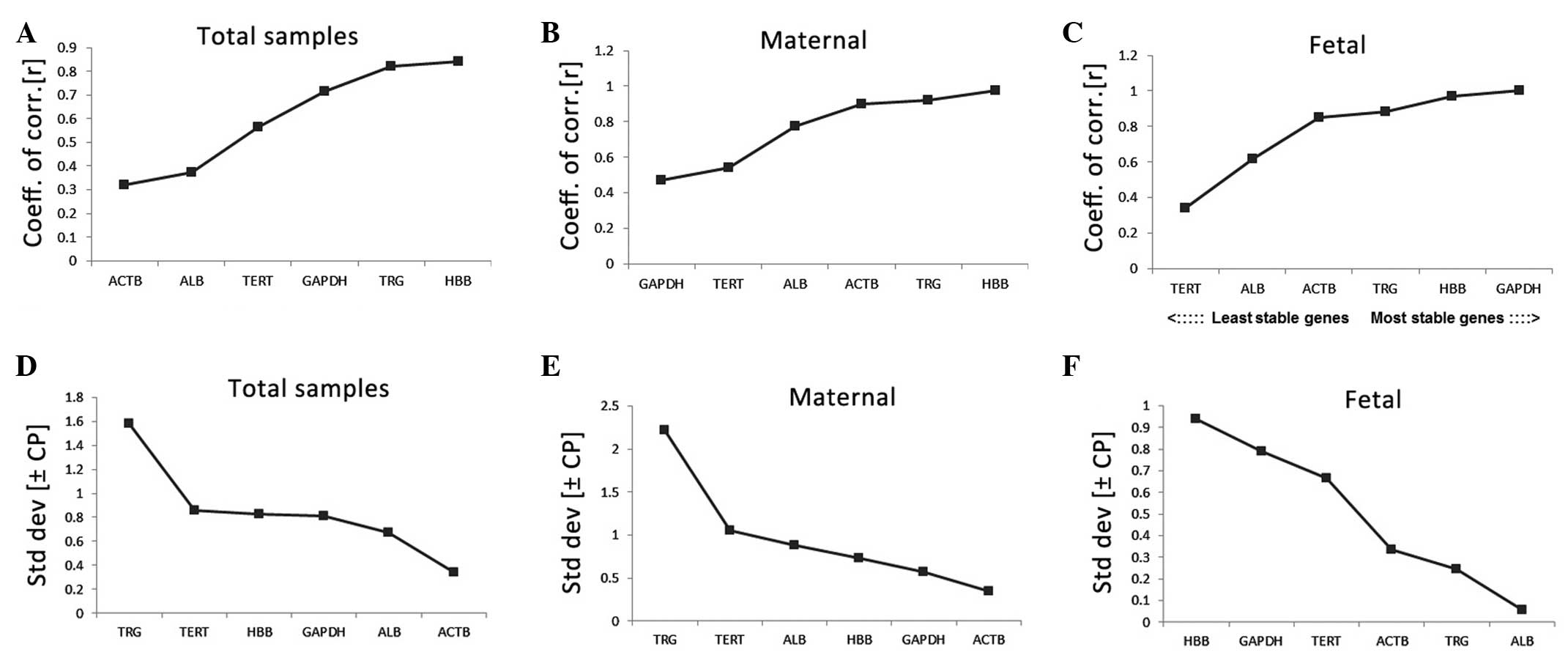 | Figure 5Stability values of the candidate
control genes evaluated using BestKeeper. The coeff. of corr.
values of the candidate control genes were plotted in the (A) total
samples (B) maternal groups and (C) fetal groups. The higher the
stability value, the higher the stability. The std dev values of
the candidate control genes were plotted in the (D) total samples,
(E) maternal groups and (F) fetal groups. HBB, β-globin; TERT,
telomerase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ALB,
albumin; ACTB, β-actin; TRG, T cell receptor γ; coeff. of corr.,
coefficient of correlation; std dev, standard deviation. |
Discussion
The identification of cffDNA in maternal plasma has
become a primary target for NIPT (1). In healthy gravidae, cffDNA can be
detected in maternal plasma as early as the seventh week following
conception (21), which then
increases as pregnancy progresses (22) and reaches a plateau in the ensuing
three months, being cleared from the maternal plasma to become
absent within 2 h of delivery (23). Furthermore, cffDNA molecules are
generally shorter than 300 bps in length, whereas maternal-derived
molecules are longer than 300 bps in length (6,7),
which enables cffDNA molecules to be readily separated from the
original maternal DNA using electrophoresis. These properties have
rendered cffDNA as an optimal material for NIPT. At present, qPCR
is the most fundamental, cost efficient and commonly used method in
investigations of cffDNA. Due to its low cost and ease of
operation, qPCR is constantly being applied to attempt to diagnose
numerous types of hereditary disease. To date, gender determination
(24,25) and several diseases, including
β-thalassemia (26,27), RhD fetal blood group genotyping
(28–30), trisomy 21 (31) and X chromosome aneuploidies
(32) have been successfully
diagnosed using qPCR. In the process of quantitative
investigations, control genes are important. A suitable control
genes is required to be stably expressed in both maternal- and
fetal-derived DNA. An ideal control gene in maternal plasma is that
which is not affected or regulated by pregnancy conditions, stress
response, stimulation or any other physiological or pathological
state throughout the pregnancy process (33). However, there is accumulating
evidence suggesting that the content levels of widely used control
genes vary significantly in different independent investigations,
for example the single-copy DNA control gene, HBB, which is used to
represent the cell number has been suggested to be not the most
reliable control gene (13). Our
previous study also revealed that the content stability of widely
used control genes for DNA demonstrated significant variation in
the plasma DNA of pregnant and non-pregnant individuals (14). It is essential to normalize the
control gene content levels and determine reliable control genes
prior to any qPCR analysis. To the best of our knowledge, the
present study is the first to evaluate the content stability of
control genes commonly used in maternal- and fetal-derived DNA,
respectively. The present study collected blood samples in the
second trimester of gestation, at which stage the content of cffDNA
is stable. Subsequently, six candidate control genes were assessed,
including HBB, TERT, GAPDH, ALB, ACTB and TRG, which were estimated
using the geNorm, NormFinder and BestKeeper statistical
algorithms.
Onn analysis of the raw Ct values, ACTB exhibited
the lowest mean Ct values, followed by HBB and TRG. By contrast,
ACTB exhibited the lowest variation in content levels, indicated by
the SD values, whereas TERT exhibited the highest mean Ct values.
TRG exhibited the highest SD values, which indicated that its
content varied markedly.
On the basis of the results obtained from the three
statistical software programmes, HBB was confirmed as the most
content stable gene when analyzing maternal- and fetal-derived DNA
together and maternal-derived DNA alone; GAPDH was considered the
most content stable gene in fetal-derived DNA. The ranking order of
the candidate genes in terms of stability differed marginally.
These differences may have been caused by the different calculation
algorithms used in the three software programmes (34) and indicated different features of
the correlations between these control genes.
The optimal number of control genes for
normalization was suggested by genes with a V-value below the
cutoff value of 0.15 in geNorm (17). No optimal combination of the
selected control genes had a V-value below the cutoff value, with
the exception of the use of five genes in the fetal group. Thalita
(35) reported that the
combination of genes cannot increase the accuracy definitely and it
is suggested, if conditions permit, that three of the most stable
control genes are used, rather than a single gene (36). The number of control genes also
depends on the experimental conditions.
Of note, the concentration of cffDNA in plasma is
low (22) and the majority
originates from the apoptosis of placental trophoblasts resulting
in fragments shorter than 300 bps in length. These characteristics
affect the PCR amplification of cffDNA, as the length of the cffDNA
template is limited at 300 bps, whereas a longer template of the
target gene increases the number of opportunities to be digested in
the process of apoptosis (2).
Therefore, amplicon sizes are required to be sufficiently short to
ensure adequate effective templates for PCR amplification.
Increasingly, studies are focusing on the clinical
application of cffDNA, which is relevant to NIPT. However, to the
best of our knowledge, the control genes used in analysis of cffDNA
are selected without confirmation of the content stability of these
control genes in maternal plasma DNA. The present study validated
the most content stable control genes in maternal- and
fetal-derived DNA at the second trimester of gestational age, which
can be used as a criterion in subsequent investigations.
In conclusion, the present study indicated that the
content stability of control genes used for analyzing plasma DNA
exhibited significant variation between maternal- and fetal-derived
DNA, therefore, all qPCR performed to analyze cffDNA requires the
initial selection of an appropriate control gene individually. The
results of the present study also indicated that HBB in maternal-
and fetal-derived DNA, and in maternal-derived DNA alone, and GAPDH
in fetal-derived DNA enable efficient normalization for qPCR
investigations in maternal plasma DNA. These results also present
an appropriate strategy for the evaluation of candidate control
genes for genomic DNA qPCR analysis.
Acknowledgments
This study was supported by the Project supported by
the Key Foundation of Jilin Provincial Science & Technology
Department, China (grant. no. 20130727038YY), the Jilin Provincial
Science & Technology Department, China (grant. nos. 20100942
and 20110740) and the Jilin Provincial Development and Reform
Commission, China (grant. no. 20101928).
References
|
1
|
Lo YM, Corbetta N, Chamberlain PF, Rai V,
Sargent IL, Redman CW and Wainscoat JS: Presence of fetal DNA in
maternal plasma and serum. Lancet. 350:485–487. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khorram Khorshid HR, Zargari M, Sadeghi
MR, Edallatkhah H, Shahhosseiny MH and Kamali K: Early fetal gender
determination using real-time PCR analysis of cell-free fetal DNA
during 6th–10th weeks of gestation. Acta Med Iran.
51:209–214. 2013.PubMed/NCBI
|
|
3
|
Teitelbaum L, Metcalfe A, Clarke G,
Parboosingh JS, Wilson RD and Johnson JM: Costs and benefits of
non-invasive fetal RhD determination. Ultrasound Obstet Gynecol.
45:84–88. 2015. View Article : Google Scholar
|
|
4
|
Xiong L, Barrett AN, Hua R, Tan TZ, Ho SS,
Chan JK, Zhong M and Choolani M: Non-invasive prenatal diagnostic
testing for β-thalassaemia using cell-free fetal DNA and next
generation sequencing. Prenat Diagn. 35:258–265. 2015. View Article : Google Scholar
|
|
5
|
Alberry M, Maddocks D, Jones M, Abdel Hadi
M, Abdel-Fattah S, Avent N and Soothill PW: Free fetal DNA in
maternal plasma in anembryonic pregnancies: Confirmation that the
origin is the trophoblast. Prenat Diagn. 27:415–418. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan KC, Zhang J, Hui AB, Wong N, Lau TK,
Leung TN, Lo KW, Huang DW and Lo YM: Size distributions of maternal
and fetal DNA in maternal plasma. Clin Chem. 50:88–92. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Zimmermann B, Rusterholz C, Kang A,
Holzgreve W and Hahn S: Size separation of circulatory DNA in
maternal plasma permits ready detection of fetal DNA polymorphisms.
Clin Chem. 50:1002–1011. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papageorgiou EA, Fiegler H, Rakyan V, Beck
S, Hulten M, Lamnissou K, Carter NP and Patsalis PC: Sites of
differential DNA methylation between placenta and peripheral blood:
molecular markers for noninvasive prenatal diagnosis of
aneuploidies. Am J Pathol. 174:1609–1618. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lun FM, Tsui NB, Chan KC, Leung TY, Lau
TK, Charoenkwan P, Chow KC, Lo WY, Wanapirak C, Sanguansermsri T,
Cantor CR, Chiu RW and Lo YM: Noninvasive prenatal diagnosis of
monogenic diseases by digital size selection and relative mutation
dosage on DNA in maternal plasma. Proc Natl Acad Sci USA.
105:19920–19925. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan HC, Blumenfeld YJ, Chitkara U, Hudgins
L and Quake SR: Noninvasive diagnosis of fetal aneuploidy by
shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci USA.
105:16266–16271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhong Q, Zhang Q, Wang Z, Qi J, Chen Y, Li
S, Sun Y, Li C and Lan X: Expression profiling and validation of
potential reference genes during Paralichthys olivaceus
embryogenesis. Mar Biotechnol (NY). 10:310–318. 2008. View Article : Google Scholar
|
|
12
|
Dheda K, Huggett JF, Bustin SA, Johnson
MA, Rook G and Zumla A: Validation of housekeeping genes for
normalizing RNA expression in real-time PCR. Biotechniques.
37:112–114. 2004.PubMed/NCBI
|
|
13
|
Steinau M, Rajeevan MS and Unger ER: DNA
and RNA references for qRT-PCR assays in exfoliated cervical cells.
J Mol Diagn. 8:113–118. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Q, Ali HA, Yu S, Zhang L, Li X, Du Z
and Zhang G: Evaluation and validation of the suitable control
genes for quantitative PCR studies in plasma DNA for noninvasive
prenatal diagnosis. Int J Mol Med. 34:1681–1687. 2014.PubMed/NCBI
|
|
15
|
Li X, Yang Q, Bai J, Xuan Y and Wang Y:
Identification of appropriate reference genes for human mesenchymal
stem cell analysis by quantitative real-time PCR. Biotechnol Lett.
37:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Yang Q, Bai J, Yang Y, Zhong L and
Wang Y: Identification of optimal reference genes for quantitative
PCR studies on human mesenchymal stem cells. Mol Med Rep.
11:1304–1311. 2014.PubMed/NCBI
|
|
17
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:RESEARCH00342002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfaffl MW, Tichopad A, Prgomet C and
Neuvians TP: Determination of stable housekeeping genes,
differentially regulated target genes and sample integrity:
BestKeeper-Excel-based tool using pair-wise correlations.
Biotechnol Lett. 26:509–515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jorgez CJ and Bischoff FZ: Improving
enrichment of circulating fetal DNA for genetic testing: Size
fractionation followed by whole gene amplification. Fetal Diagn
Ther. 25:314–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galbiati S, Smid M, Gambini D, Ferrari A,
Restagno G, Viora E, Campogrande M, Bastonero S, Pagliano M, Calza
S, et al: Fetal DNA detection in maternal plasma throughout
gestation. Hum Genet. 117:243–248. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo YM, Tein MS, Lau TK, Haines CJ, Leung
TN, Poon PM, Wainscoat JS, Johnson PJ, Chang AM and Hjelm NM:
Quantitative analysis of fetal DNA in maternal plasma and serum:
Implications for noninvasive prenatal diagnosis. Am J Hum Genet.
62:768–775. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo YM, Zhang J, Leung TN, Lau TK, Chang AM
and Hjelm NM: Rapid clearance of fetal DNA from maternal plasma. Am
J Hum Genet. 64:218–224. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aghanoori MR, Vafaei H, Kavoshi H,
Mohamadi S and Goodarzi HR: Sex determination using free fetal DNA
at early gestational ages: A comparison between a modified mini-STR
genotyping method and real-time PCR. Am J Obstet Gynecol.
207:202.e1–e8. 2012. View Article : Google Scholar
|
|
25
|
Lim JH, Park SY, Kim SY, Kim do J, Choi
JE, Kim MH, Choi JS, Kim MY, Yang JH and Ryu HM: Effective
detection of fetal sex using circulating fetal DNA in
first-trimester maternal plasma. FASEB J. 26:250–258. 2012.
View Article : Google Scholar
|
|
26
|
Yenilmez ED, Tuli A and Evrüke IC:
Noninvasive prenatal diagnosis experience in the Çukurova Region of
Southern Turkey: Detecting paternal mutations of sickle cell anemia
and β-thalassemia in cell-free fetal DNA using high-resolution
melting analysis. Prenat Diagn. 33:1054–1062. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao T, Nie Y, Hu H and Liang Z:
Hypermethylation of IGSF4 gene for noninvasive prenatal diagnosis
of thalassemia. Med Sci Monit. 18:BR33–BR40. 2012. View Article : Google Scholar
|
|
28
|
Scheffer PG, van der Schoot CE,
Page-Christiaens GC and de Haas M: Noninvasive fetal blood group
genotyping of rhesus D, c, E and of K in alloimmunised pregnant
women: Evaluation of a 7-year clinical experience. BJOG.
118:1340–1348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gutensohn K, Müller SP, Thomann K, Stein
W, Suren A, Körtge-Jung S, Schlüter G and Legler TJ: Diagnostic
accuracy of noninvasive polymerase chain reaction testing for the
determination of fetal rhesus C, c and E status in early pregnancy.
BJOG. 117:722–729. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Orzinska A, Guz K, Brojer E and Zupanska
B: Preliminary results of fetal Rhc examination in plasma of
pregnant women with anti-c. Prenat Diagn. 28:335–337. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsaliki E, Papageorgiou EA, Spyrou C,
Koumbaris G, Kypri E, Kyriakou S, Sotiriou C, Touvana E, Keravnou
A, Karagrigoriou A, et al: MeDIP real-time qPCR of maternal
peripheral blood reliably identifies trisomy 21. Prenat Diagn.
32:996–1001. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Della Ragione F, Mastrovito P, Campanile
C, Conti A, Papageorgiou EA, Hultén MA, Patsalis PC, Carter NP and
D'Esposito M: Differential DNA methylation as a tool for
noninvasive prenatal diagnosis (NIPD) of X chromosome aneuploidies.
J Mol Diagn. 12:797–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peters IR, Peeters D, Helps CR and Day MJ:
Development and application of multiple internal reference
(housekeeper) gene assays for accurate normalisation of canine gene
expression studies. Vet Immunol Immunopathol. 117:55–66. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang E, Shi S, Liu J, Cheng T, Xue L,
Yang X, Yang W, Lan Q and Jiang Z: Selection of reference genes for
quantitative gene expression studies in Platycladus orientalis
(Cupressaceae) Using real-time PCR. PLoS One. 7:e332782012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marques TE, de Mendonca LR, Pereira MG, de
Andrade TG, Garcia-Cairasco N, Paçó-Larson ML and Gitaí DL:
Validation of suitable reference genes for expression studies in
different pilocarpine-induced models of mesial temporal lobe
epilepsy. PLoS One. 8:e718922013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liman M, Wenji W, Conghui L, Haiyang Y,
Zhigang W, Xubo W, Jie Q and Quanqi Z: Selection of reference genes
for reverse transcription quantitative real-time PCR normalization
in black rockfish (Sebastes schlegeli). Mar Genomics. 11:67–73.
2013. View Article : Google Scholar : PubMed/NCBI
|