Introduction
The small G-protein, Rho, and its downstream
effector, Rho-associated coiled-coil kinase (ROCK), mediate a
variety of cell functions, including smooth muscle contraction,
stress fiber formation, cell contraction, adhesion, proliferation,
differentiation and inflammatory responses (1–3). The
Rho/ROCK signaling pathway is involved in cardiovascular diseases,
including hypertension and heart failure, and in chronic kidney
disease (4–11).
Fasudil and Y27632, non-selective ROCK1/2
inhibitors, have been used to evaluate the role of ROCK in several
animal disease models. For example, in a rat heart
ischemiareper-fusion (IR) model, fasudil has been observed to
reduce infarct size by attenuating endoplasmic reticulum stress and
modulating the activity of sarco/endoplasmic reticulum
Ca2+-ATPase (12).
Similarly, in a rat kidney IR model, fasudil has been found to
suppress renal injury and improve kidney function (13). Furthermore, fasudil exhibits
renoprotective effects, including improving albuminuria and
mesangial matrix expansion (14).
It also suppresses renal injury via the downregulation of
hypoxia-inducible factor 1α in a diabetic nephropathy model
(14). Certain in vitro
studies, using mesangial, tubular epithelial or kidney fibroblast
cells (15–17), have reported that Y27632 inhibits
cell hypertrophy, the expression of α-smooth muscle actin (α-SMA)
and collagen, which is induced by aldosterone or transforming
growth factor-β1 (TGF-β1). These reports suggest that ROCK
inhibitors use a variety of functional mechanisms and are effective
in a wide range of diseases.
Renal tubulointerstitial fibrosis is a common
pathway in progressive renal diseases. The unilateral ureteral
obstruction (UUO) model is widely used in investigations of
progressive interstitial fibrosis that is independent of
hypertension or systemic immune disease. Following UUO, the
obstructed kidney exhibits a substantial macrophage influx into the
interstitium and develops tubulointerstitial fibrosis. Fasudil and
Y27632 have been reported to prevent tubulointerstitial fibrosis in
the UUO model by inhibiting the mRNA expression of collagen, TGF-β1
and α-SMA (18–20). However, the details of the
mechanism remain to be fully elucidated. In the present study, the
effect of a ROCK inhibitor on renal interstitial fibrosis, renal
intrinsic cells and cells infiltrating the kidney were
investigated.
Materials and methods
Animals and ethics
The present study was approved by the Experimental
Animal Care and Use Committee of Mitsubishi Tanabe Pharma
Corporation (Saitama, Japan), which was regulated by the Management
and Ethics of Animal law (Ministry of the Environment; http://www.env.go.jp/nature/dobutsu/aigo/1_law/). Male
C57BL/6 J mice (23–28 g) were purchased from Charles River
Laboratories International (Kanagawa, Japan); and were maintained
at room temperature on a 12-h light/dark cycle and provided with
access to standard laboratory chow (CRF-1, Oriental Yeast Co.,
Ltd., Tokyo, Japan) and tap water ad libitum. The animals
were housed at the departmental animal care facility of Mitsubishi
Tanabe Pharma Corporation in accordance with the relevant
protocols.
UUO model
The mice were anesthetized with sevofrane (Maruishi
Pharmaceutical Co., Ltd., Osaka, Japan) and subjected to a left
flank incision. UUO was performed by complete ligation of the left
ureter at the ureteropelvic junction using a 4-0 silk suture (cat,
no. RS5357; Niccho Kogyo Co., Ltd., Tokyo, Japan). The
sham-operated mice had their ureter exposed without ligation. All
the mice used in the experiments were 9 weeks of age, following a 1
week acclimation period. The UUO-operated mice were randomly
divided into the following groups (n=5–7; housed in groups): Sham,
UUO-control and UUO-fasudil (1 g/l, Mitsubishi Tanabe Pharma
Corporation). Fasudil was administered in the drinking water 2 days
prior to the UUO surgery until the day of sacrifice. The mice were
sacrificed under anesthesia with sevoflurane on days 3, 7 and 14
following surgery. The kidneys were then removed and divided into
several parts for RNA, hydroxyproline and immunohistochemistry
assays, and protein analysis.
Cell culture
The NRK-52E rat kidney tubular epithelial cell line,
NRK-49F fibroblast cell line and RAW264.7 mouse macrophage cell
line, obtained from American Type Culture Collection (Manassas, VA,
USA), were cultured in low glucose (5 mmol/l) Dulbecco's modified
Eagle's medium (cat. no. D6046; Sigma-Aldrich, St. Louis, MO, USA)
supplemented with 10% fetal bovine serum (cat. no. S1820; Biowest,
Nuaille, France) at 37°C in a 5% CO2 atmosphere. For
experimental treatments, the cells were seeded into 24-well plates
in 0.5 ml medium (NRK-52E, 3×104 cells/well; NRK-49F,
4×104 cells/well; RAW264.7, 2×105 cells/well)
for the determination of mRNA expression levels. The cells were
seeded into 12-well plates in 1.5 ml medium (NRK-52E,
5×104 cell/well; NRK-49F, 6×104 cells/well)
to determine the protein expression levels. After 24 h, serum was
reduced to 1% for 20 h at 37°C, and recombinant human TGF-β1 (10
ng/ml; cat. no. 100-21C; Peprotech, Rocky Hill, NJ, USA) or
lipopolysaccharide (LPS; 500 ng/ml; cat. no. L4391; Sigma-Aldrich)
were added for 24 h at 37°C. To investigate the role of ROCK under
these conditions, 10 or 30 µmol/l hydroxyfasudil (Mitsubishi Tanabe
Pharma Corporation), a bioactive metabolite of fasudil, was added
to the cells 1 h prior to TGF-β1 or LPS stimulation at 37°C.
Histology and immunohistochemistry
The kidneys were removed and immediately fixed in
10% formaldehyde neutral buffer solution (Nacalai Tesque, Inc.,
Kyoto, Japan). The formalin-fixed kidneys were embedded in
paraffin, and the paraffin sections were stained with hematoxylin
(cat. no. 115938; Merck Millipore, Darmstadt, Germany) and eosin
(cat. no. 115935; Merck Millipore) and Sirius Red (cat. no. 365548;
Sigma-Aldrich)/Fast Green (cat. no. 069-00032; Wako Pure Chemical
Industries, Ltd., Osaka, Japan). Immunohistochemistry was
performed, as described previously (21). Briefly, the kidney paraffin
sections (4µm thick) were deparaffinized, and the sections were
pretreated with 3% hydrogen peroxide (Junsei Chemicals, Tokyo,
Japan). For F4/80, the sections were processed for antigen
retrieval using protease (cat. no. 415231; Nichirei Histofine;
Nichirei Biosciences, Inc., Tokyo, Japan) treatment at 37°C for 10
min. The sections were incubated overnight at 4°C with anti-α-SMA
mouse monoclonal antibody (1:500; cat. no. A2547; Sigma-Aldrich) or
anti-F4/80 rat monoclonal antibody (1:100; cat. no. MCA497G; AbD
Serotec, Kidlington, UK), followed by incubation with horseradish
peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (cat.
no. 414321; Histofine Mouse Stain kit; Nichirei Biosciences, Inc.)
and HRP-conjugated anti-rat IgG secondary antibody (cat. no.
414311; Histofine Simple Stain Mouse MAX PO Rat reagent; Nichirei
Biosciences, Inc.), respectively. The sections were then stained
with 3,3′-diaminobenzidine (Nacalai Tesque, Inc.) and
counterstained with hematoxylin (cat. no. 30002; Muto Pure
Chemicals Co., Ltd., Tokyo, Japan). Images of the sections were
captured using a DP73 digital camera system (Olympus Corporation,
Tokyo, Japan) equipped with CellSens software (ver. 1.6; Olympus
Corporation) and a BX51 microscope (Olympus Corporation). For image
analysis, whole-slide digital images of the sections were obtained
using an Aperio Scan Scope XT (Leica Microsystems, Wetzlar,
Germany). The Sirius Red-, α-SMA-, and F4/80-positive areas were
determined using Image-Pro Plus software (ver. 6.1.0.372; Media
Cybernetics, Bethesda, MD, USA), as described previously (21).
Determination of kidney hydroxyproline
content
The collagen content in the kidney was determined
using the hydroxyproline assay, as previously described (22,23)
with modifications. In brief, the kidneys were homogenized in
phosphate-buffered saline (Gibco Life Technologies, Carlsbad, CA,
USA) at 700 µl/100 mg kidney weight, completely hydrolyzed in 6
mol/l HCl (Wako Pure Chemical Industries, Ltd.) at 120°C for 6 h,
and filtered through a 0.45 µm Millex-HV filter (Merck Millipore).
The samples were dried by vacuum centrifugation using EZ-2 Plus
(Genevac, Suffolk, UK) for 16 h. The dried samples were then
solubilized in distilled water. The samples were oxidized using
chloramine T solution, containing 1.4% sodium
p-toluenesulfonchloramide trihydrate (chloramine T; Wako
Pure Chemical Industries, Ltd.) and 10% n-propanol (Wako
Pure Chemical Industries, Ltd.) in citric acid buffer, which
consisted of 0.26 mol/l citric acid (Sigma-Aldrich), 0.88 mol/l
sodium acetate trihydrate (Wako Pure Chemical Industries, Ltd.),
0.85 mol/l sodium hydroxide (Wako Pure Chemical Industries, Ltd.)
and 1.2% acetic acid (Kanto Chemical Co., Inc., Tokyo, Japan).
Following incubation at room temperature for 20 min, Ehrlich's
solution, containing 1 mol/l 4-dimethylaminobenzaldehyde
(Sigma-Aldrich), 18% perchloric acid (Sigma-Aldrich) and 60%
n-propanol was added, and the samples were incubated at 65°C
for 40 min. Absorbance was measured at 560 nm (SpectraMax M5e;
SoftMax Pro ver. 5.4.1; Molecular Devices, Sunnyvale, CA, USA). The
concentration of hydroxyproline was estimated from a standard
curve, which was prepared using a pure solution of
l-hydroxyproline (Wako Pure Chemical Industries, Ltd.), with
the final results expressed as hydroxyproline per mg protein. The
kidney protein concentration was determined using a Bicinchoninic
Acid (BCA) protein assay (Thermo Fisher Scientific, Waltham, MA,
USA), with bovine serum albumin as a standard.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses
Total RNA was extracted from the kidney using TRIzol
reagent (Invitrogen Life Technologies) and purified using an RNeasy
Mini kit (cat. no. 74106; Qiagen, Venlo, Netherlands), according to
the manufacturer's instructions. The total RNA concentration was
determined using a NanoDrop 1000 Spectrophotometer (Thermo Fisher
Scientific). cDNA was synthesized from 1 µg of total RNA and the
reagent of the Master Mix, which included enzymes, random primers
and other reaction reagents (cat. no. 11755250; SuperScript VILO
MasterMix; Invitrogen, Life Technologies), using an iCycler Themal
Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) under the
following conditions: 25°C for 10 min, 42°C for 60 min and 85°C for
5 min. qPCR was performed using TaqMan technology. The TaqMan Gene
Expression Assay reagents, containing the mixture of primers and
TaqMan probe, were obtained from Applied Biosystems Life
Technologies (Foster City, CA, USA), as follows: ROCK1 (cat. no.
Mm00485745_m1), ROCK2 (cat. no. Mm00485761_m1), TGF-β1 (cat. no.
Mm00441724_ m1), α-SM A (cat. nos. Mm01546133_ m1 a nd
Rn01759928_g1), collagen 1a2 (cat. nos. Mm00483888_m1 and
Rn01526721_m1), collagen 3a1 (cat. no. Mm01254477_ m1), fibronectin
(cat. no. Mm01256744_m1), MCP-1 (cat. nos. Mm00441242_m1 and
Rn00580555_m1), F4/80 (cat. no. Mm00802529_m1), IL-1β (cat. no.
Mm00434228_m1), TNFα (cat. no. Mm00443258_m), IL-6 (cat. no.
Mm00446190_ m1) and 18SrRNA (cat. no. 4308329). The reaction was
performed using 1 µl of cDNA in 20 µl of final volume under the
following conditions: 50°C for 2 min, 95°C for 10 min and 40 cycles
of 95°C for 15 sec and 60°C for 1 min. This was performed using a
7500 Fast Real-Time PCR system (Applied Biosystems Life
Technologies). Data were analyzed using the standard curve method,
and the results for each gene were normalized to 18SrRNA (an
internal control).
Western blot analyses
The kidney tissues and cultured cells were
homogenized in a lysis buffer containing 50 mmol/l Tris-HCl (pH
8.0; Nacalai Tesque Co., Ltd.), 150 mmol/l NaCl (Wako Pure Chemical
Industries, Ltd.), 0.5% sodium deoxycholate (Wako Pure Chemical
Industries, Ltd.), 0.1% sodium dodecyl sulfate (Bio-Rad
Laboratories, Inc.), 1% Triton X-100 (Sigma-Aldrich), 1× protease
inhibitors (complete; cat. no. 11697498001; Roche Diagnostics,
Basel, Switzerland) and 1× phosphatase inhibitors (cat. no. P2850;
Sigma-Aldrich). Following centrifugation at 15,000 × g for 20 min
at 4°C (CF-15R; Hitachi, Tokyo, Japan), the supernatant was
collected as the lysate. The lysates were denatured for 5 min by
boiling. Protein concentration was determined using the BCA protein
assay (Thermo Fisher Scientific) with bovine serum albumin as a
standard. Equal quantities of the lysates were separated on a 4–15%
TGX Precast gel (Bio-Rad Laboratories, Inc.) and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.)
using a Trans-Blot Turbo Transfer system (Bio-Rad Laboratories,
Inc.). The blots were blocked in Starting Block T20 (PBS) Blocking
buffer (Thermo Fisher Scientific) at room temperature for 1 h. They
were subsequently incubated overnight at 4°C with the following
primary antibodies: Anti-chicken phosphorylated myosin phosphatase
target subunit-1 (p-MYPT-1; Thr850) rabbit polyclonal antibody
(1:1,000; cat. no. 36-003; Merck Millipore), anti-rabbit GAPDH
mouse monoclonal antibody (1:500; cat. no. MAB374; Merck
Millipore), anti-rat collagen type I rabbit polyclonal antibody
(1:2,000; cat. no. ABT123; Merck Millipore), anti-human p-Smad3
(Ser423+Ser425) rabbit polyclonal antibody (1:2,000; cat. no.
ab51451; Abcam, Cambridge, UK), anti-human α-SMA rabbit polyclonal
antibody (1:500; cat. no. 14395-1-AP; Proteintech, Chicago, IL,
USA) in Can Get Signal solution (Toyobo, Osaka, Japan). The blots
were then incubated with the following secondary polyclonal
antibodies: ECL donkey anti-rabbit IgG, HRP-conjugated
species-specific whole antibody (1:5,000, cat. no. NA934; GE
Healthcare Life Sciences, Chalfont, UK) and ECL sheep anti-mouse
IgG, HRP-conjugated species-specific whole antibody (1:5,000; cat.
no. NA931; GE Healthcare Life Sciences) at room temperature for 1
h. The blots were developed using enhanced chemiluminescence (cat.
no. 54-71-00; LumiGLO Reserve Chemiluminescent Substrate kit, KPL,
Inc., Gaithersburg, MD, USA). The signal intensities of the
specific bands were detected using an LAS-3000 Luminescent Image
Analyzer (Fujifilm, Tokyo, Japan) and analyzed using the Multi
Gauge program (ver. 3.0; Fujifilm). For quantification, the signal
intensities were normalized to GAPDH, which was loaded in each
well.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. The comparisons between the sham- and UUO-operated
control mice, the control and fasudil-treated mice, the
non-stimulated and stimulated cells, and the stimulated cells and
hydroxyfasudil-treated cells were performed using Student's t-test.
Statistical analyses were performed using the SAS system (ver.
8.0.0; SAS Institute Inc., Cary, NC, USA) in the biometrics
section, and P<0.05 was considered to indicate a statistically
significant difference.
Results
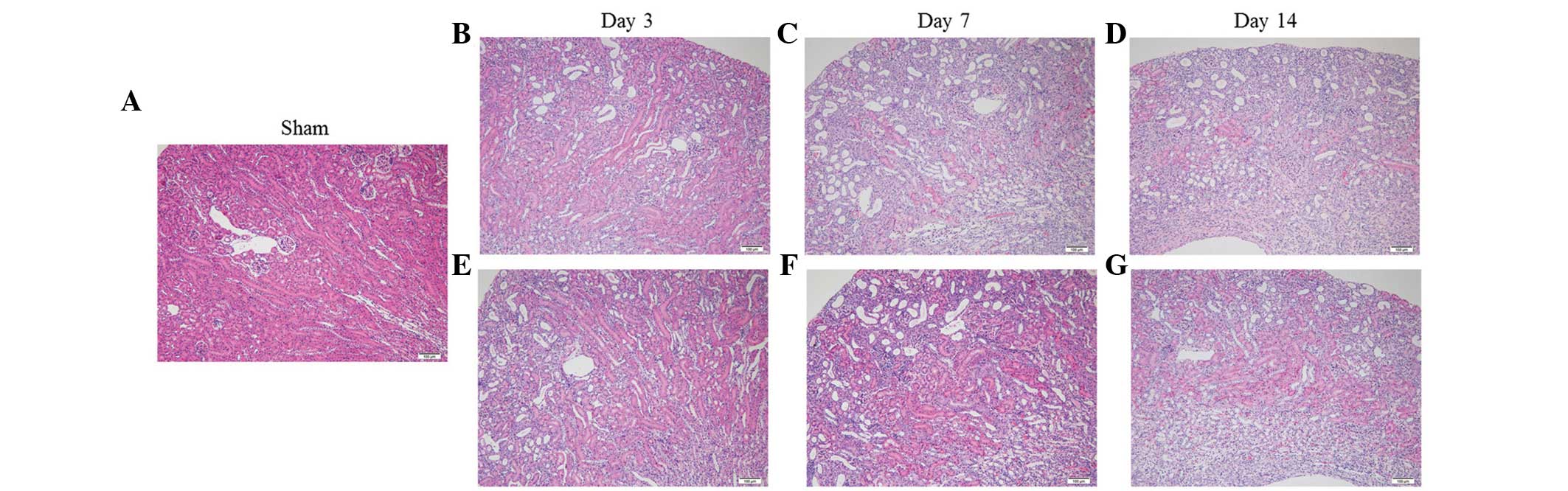
Effect of fasudil on renal histological
changes
The kidneys of the UUO mice progressively developed
renal parenchymal thinning, accompanied by severe
tubulointerstitial damage, which included tubular atrophy/dilation,
tubular regeneration and interstitial infiltration (Fig. 1), as reported previously (24,25).
Fasudil administration had minimal effect on these changes.
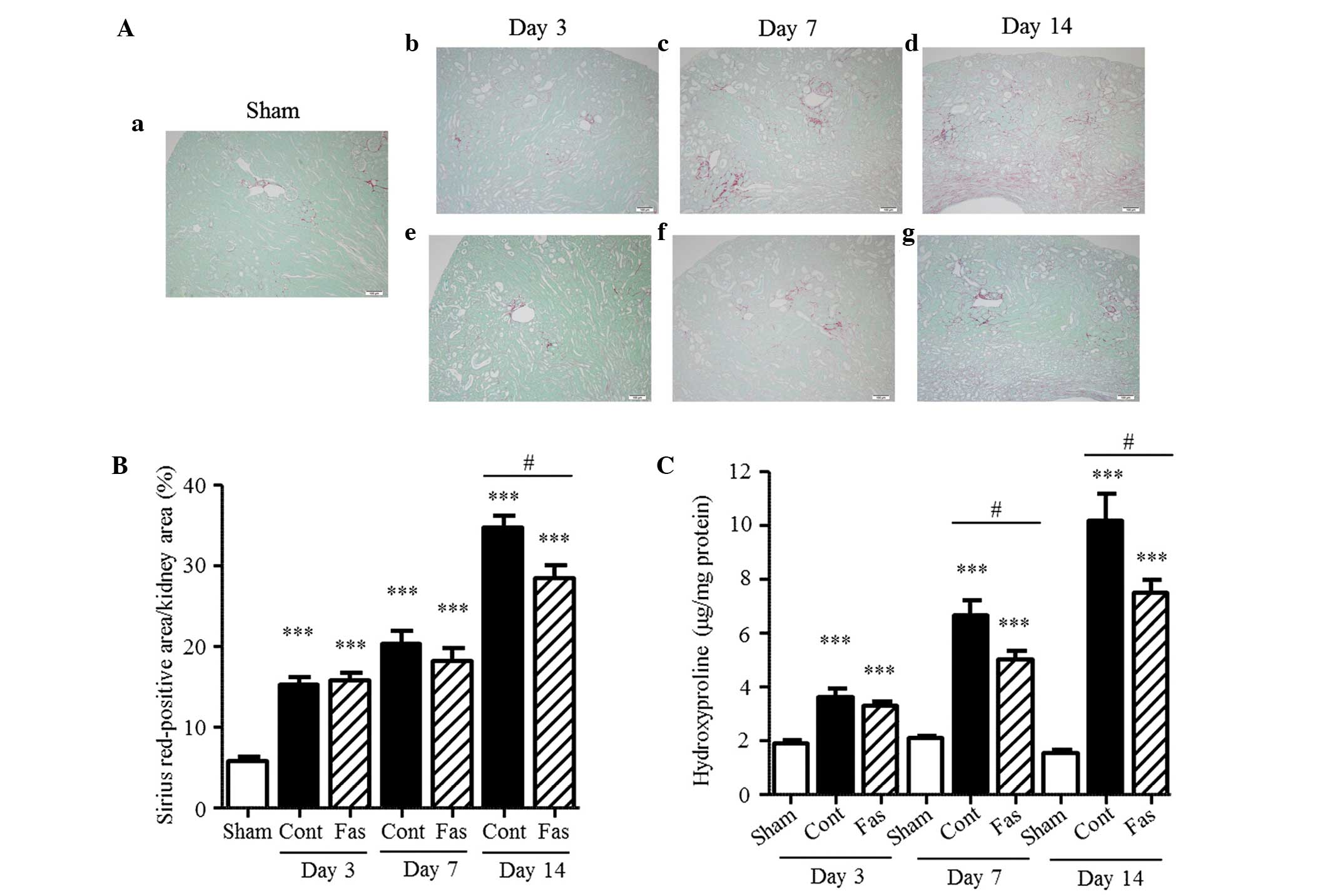
Fasudil attenuates interstitial
fibrosis
To determine the effect of fasudil on interstitial
fibrosis in the kidneys of the UUO mice, the collagen in the
interstitium was stained with Sirius Red and the hydroxyproline
content and extracellular matrix mRNA expression levels were
measured. In the obstructed kidney, the Sirius Red-positive areas
in the renal interstitium increased progressively, compared with
those in the sham-operated kidney during the 14 day period
(Fig. 2A and B), with 2.7-, 3.5-
and 6.0-fold increases on days 3, 7 and 14 post-UUO, respectively.
The content of hydroxyproline was also augmented, with 1.9-, 3.2-
and 6.6-fold increases at day 3, 7 and 14 following UUO,
respectively (Fig. 2C).
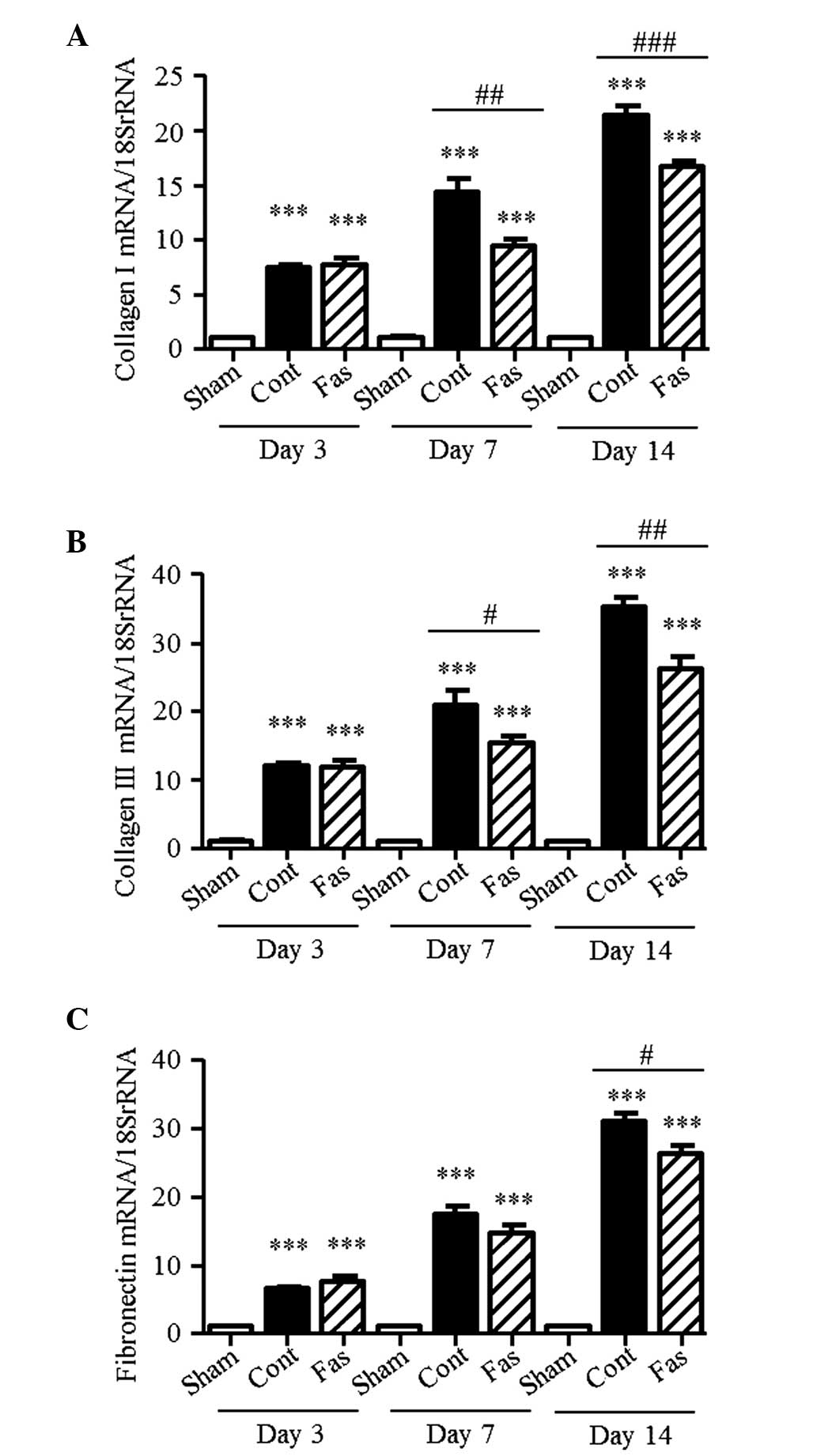
Furthermore, the mRNA expression levels of collagen I, III and
fibronectin were higher in the kidneys of the UUO mice, compared
with those of the sham group, with collagen I levels 7.4-, 14.3-
and 21.3-fold higher; collagen III levels 11.9-, 20.9- and
35.2-fold higher; and fibronectin levels 6.6-, 17.4- and 31.0-fold
higher on days 3, 7 and 14 post-UUO, respectively (Fig. 3A–C). On day 14 following UUO,
administration of fasudil significantly suppressed these augmented
levels. On day 7 following UUO, the Sirius Red-positive areas and
mRNA expression of fibronectin did not decrease significantly.
However, the mRNA expression levels of collagen I and III, and the
hydroxyproline content in the injured kidneys were markedly
suppressed by fasudil administration. These results suggested that
fasudil ameliorated the interstitial fibrosis.
Fasudil inhibits the expression of
α-SMA
The present study subsequently examined the effect
of fasudil on interstitial myofibroblasts, characterized by the
expression of α-SMA. Immunohistochemical staining revealed
increased numbers of α-SMA-positive cells in the interstitium of
the UUO mice, compared with the sham-operated mice, with 12.3-,
16.4- and 7.2-fold increases on days 3, 7 and 14 post-UUO,
respectively (Fig. 4A and B).
Similarly, the mRNA expression of α-SMA increased in the UUO mice
by 5.9-, 5.6- and 10.5-fold on days 3, 7, and 14 post-UUO,
respectively (Fig. 4C). The
immunohistochemical staining and mRNA expression analysis, which
reduced 65 and 28%, respectively, on day 7 post-UUO, demonstrated
that fasudil administration markedly inhibited the increased
expression of α-SMA in the interstitium. Furthermore, the mRNA
expression levels were significantly suppressed on day 14 following
UUO (17% reduction), compared with the control. These results
indicated that fasudil administration inhibited the transformation
of renal cells or extra-renal cells to myofibroblasts or the
proliferation of myofibroblasts.
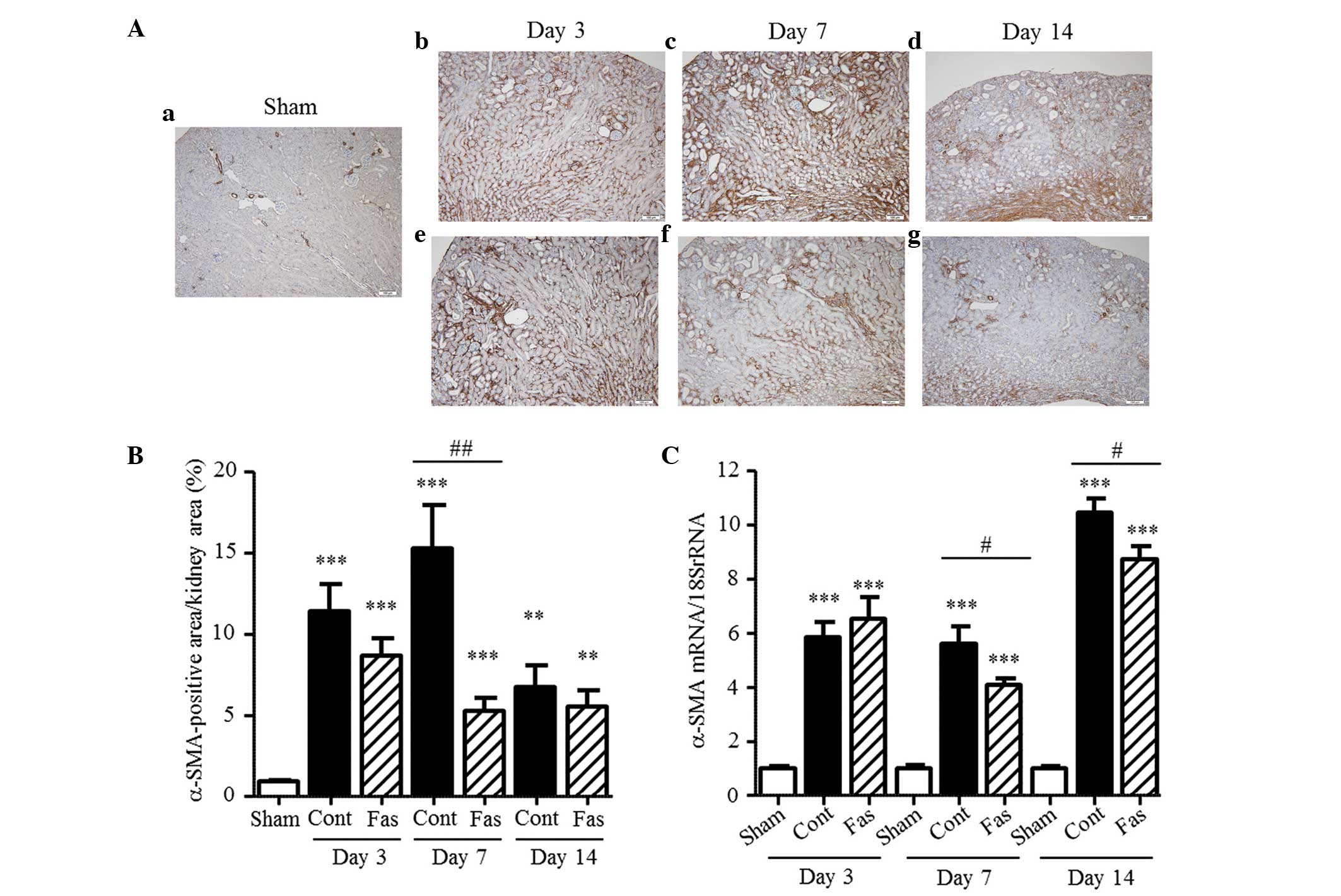 | Figure 4Expression of α-SMA in the kidney.
(A) α-SMA immunohistochemistry of the (a) Sham, (b–d) UUO-Cont and
(e–g) UUO-Fas groups on days 3, 7, and 14 following UUO
(magnification, ×10; scale bar=100 µm). α-SMA-positive fibroblasts
were stained brown. (B) α-SMA-positive area as a percentage of the
total kidney area. (C) mRNA expression of α-SMA on days 3, 7 and 14
following UUO. The results are expressed as the mean ± standard
error of the mean (n=5–7). **P<0.01 and
***P<0.001, vs. sham group; #P<0.05 and
##P<0.01, vs. UUO-Cont mice at the same time-points.
SMA, smooth muscle actin; UUO, unilateral ureteral obstruction;
Cont, control; Fas, fasudil. |
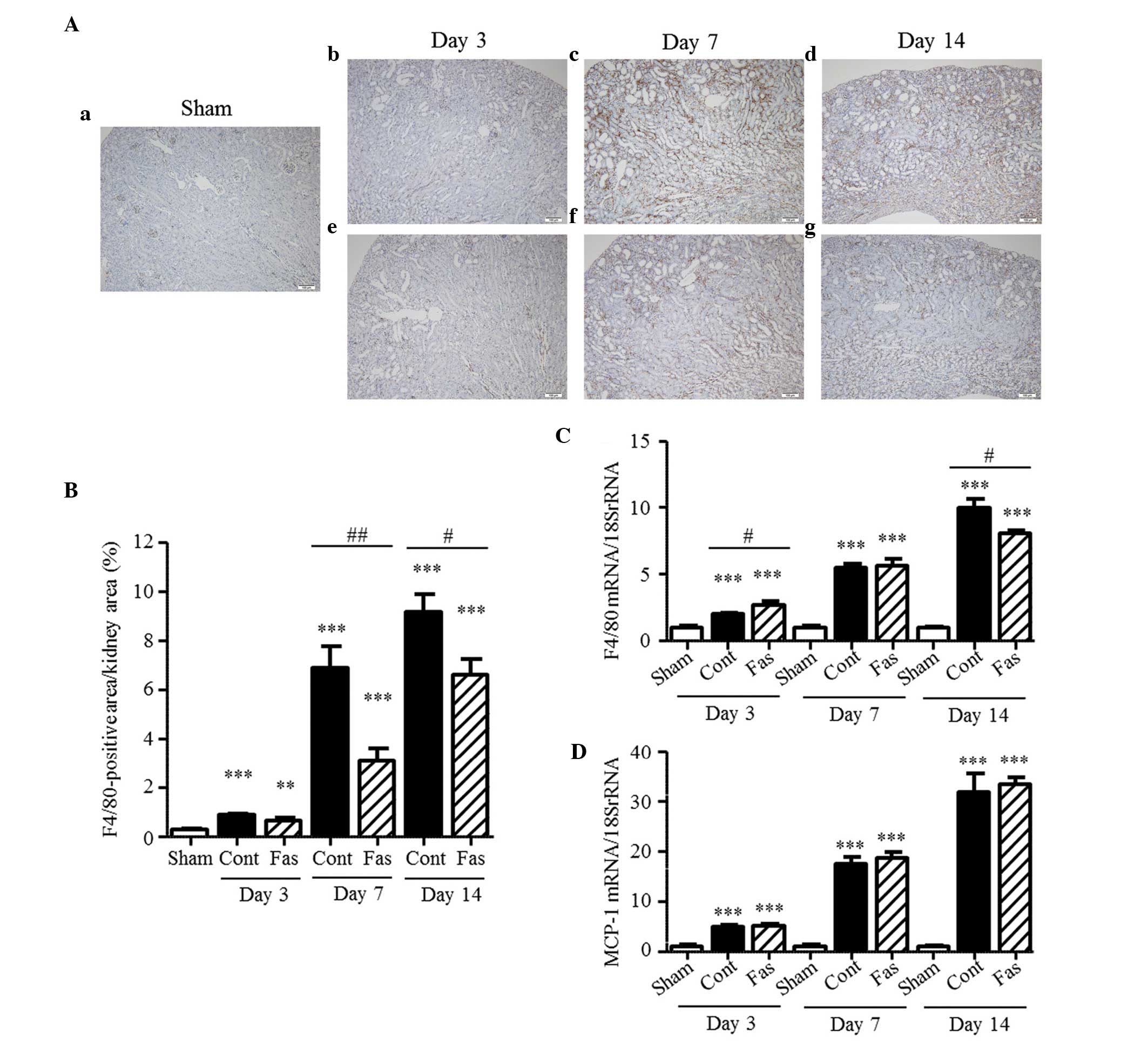
Effect of fasudil on macrophage
infiltration
ROCK inhibitors have the ability to suppress cell
migration (18–20). The present study examined the
distribution of F4/80-positive cells and mRNA expression of F4/80
to assess the effect of fasudil on the macrophage infiltration to
the kidney. The immunohistochemical staining detected
F4/80-positive cells in the interstitium of the injured kidney; and
their number in the UUO mice increased progressively, compared with
the sham group, increasing 3.0-, 22.7-, and 30.2-fold on days 3, 7
and 14 post-UUO, respectively (Fig.
5A and B). Fasudil administration clearly inhibited macrophage
infiltration into the injured kidney, with reductions of 55 and 28%
on days 7 and 14 post-UUO, respectively. Similarly, the mRNA
expression of F4/80 was increased 2.0-, 5.5- and 10.0-fold on days
3, 7, and 14 post UUO, respectively (Fig. 5C); and this increase was
significantly inhibited by the administration of fasudil on day 14
following UUO. However, the augmented mRNA expression of MCP-1 was
not affected by fasudil (Fig. 5D).
These results suggested that fasudil inhibited the migration of
macrophages to the injured kidney, but did not affect the
expression of MCP-1.
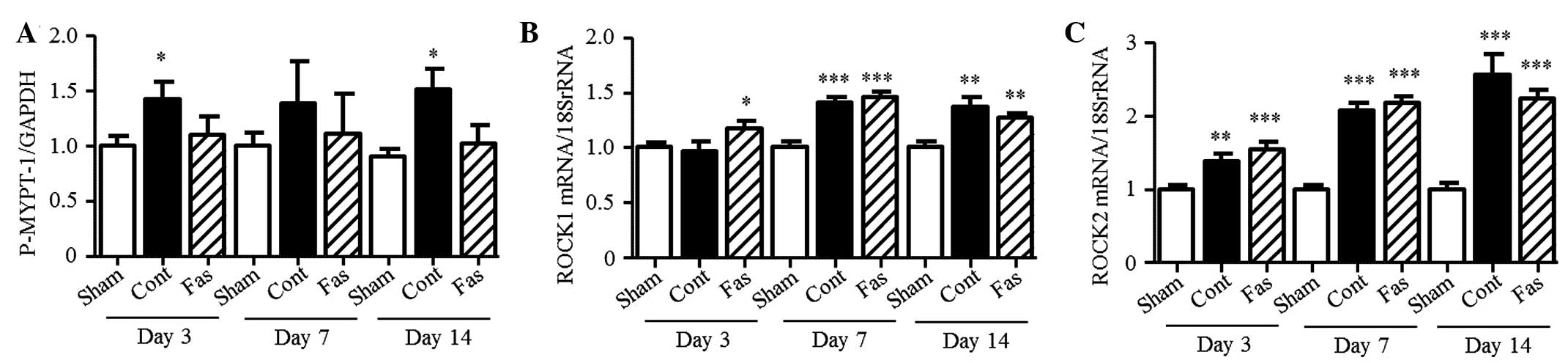
Effect of fasudil on ROCK activity
To confirm the effect of fasudil on ROCK activity,
the present study measured the phosphorylation of MYPT-1 in the
kidney. The level of phosphorylation increased in the obstructed
kidney, compared with the sham group (1.4-, 1.4- and 1.7-fold
increase on days 3, 7 and 14 post-UUO, respectively; Fig. 6A). Fasudil treatment had no
statistically significant effect on the phosphorylation levels;
however, ROCK activity was suppressed to the basal level. The mRNA
expression levels of ROCK1 and ROCK2 increased in the UUO mice, but
fasudil did not affect their levels of expression (Fig. 6B and C).
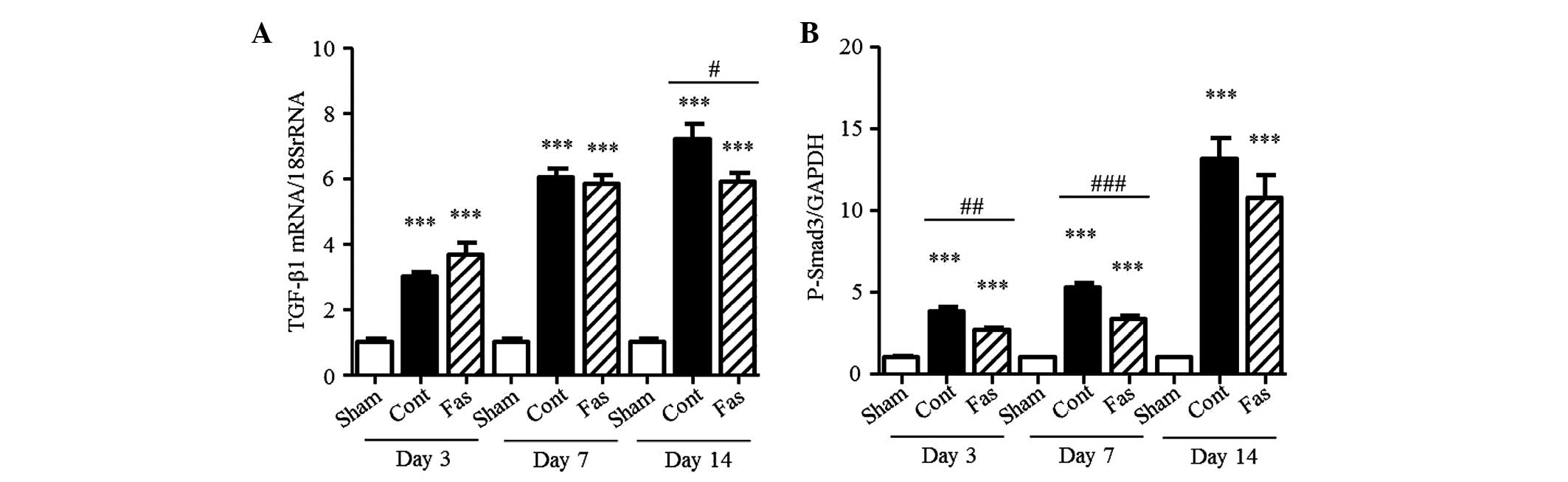
Fasudil suppresses the TGF-β-Smad
signaling pathway
It is known that the TGF-β-Smad signaling pathway is
activated in the kidneys of UUO mice (26). To examine the effect of fasudil on
this pathway, the present study measured the mRNA expression of
TGF-β1 and the level of p-Smad3. The mRNA expression of TGF-β1
increased in the UUO kidneys (3.0-, 6.0- and 7.2-fold on days 3, 7,
and 14 post-UUO, respectively; Fig.
7A), compared with that in the sham-group. Fasudil
administration significantly suppressed this increase in the
obstructed kidney, by 18%, on day 14. In the kidneys of the UUO
mice, the level of p-Smad3 progressively increased, compared with
that in the sham-operated kidneys (3.8-, 5.3- and 13.1-fold on days
3, 7 and 14 post-UUO, respectively; Fig. 7B). This increase was markedly
reduced by fasudil, by 29% on day 3 and 37% on day 7 following UUO.
These results demonstrated that fasudil inhibited the TGF-β-Smad
signaling pathway involved in interstitial fibrosis.
Effect of hydroxyfasudil on NRK-52E and
NRK-49F cells
The results from the animal experiments indicated
that fasudil attenuated renal interstitial fibrosis and the
migration of macrophages to the kidney interstitium. Using NRK-52E
and NRK-49F cells, the present study also investigated the effect
of fasudil on the intrinsic renal cells. In the kidneys of UUO
mice, TGF-β1 was recognized as a vital mediator in renal fibrosis.
In the NRK52-E and NRK-49F cells, TGF-β1 induced the mRNA and
protein expression levels of α-SMA and collagen I (Fig. 8A and B). Hydroxyfasudil, a
bioactive metabolite of fasudil, decreased the mRNA expression of
α-SMA mRNA induced by TGF-β1 in the two cell types. Furthermore, in
the NRK-49F cells, the protein expression of α-SMA was markedly
reduced following treatment with 30 µM hydroxyfasudil. However, in
the NRK-52E cells, no changes in the protein expression of α-SMA
were observed in this condition. In the NRK-49F cells, the
increased mRNA expression of collagen I was significantly
attenuated by 30 µM hydroxyfasudil treatment; however, the protein
expression was unaffected. By contrast, hydroxyfasudil did not
affect the mRNA or protein expression levels of collagen I in the
NRK-52E cells. In the kidneys of the UUO mice, the mRNA expression
of MCP-1 increased, compared with the sham-operated group. These
increased levels were unaffected by fasudil (Fig. 5D). The mRNA expression of MCP-1 was
stimulated by TGF-β1 in the NRK-52E and NRK-49F cells. Pretreatment
with hydroxyfasudil clearly suppressed the induced mRNA expression
of MCP-1 in the NRK-52E cells, but had no effect in the NRK-49F
cells (Fig. 8C).
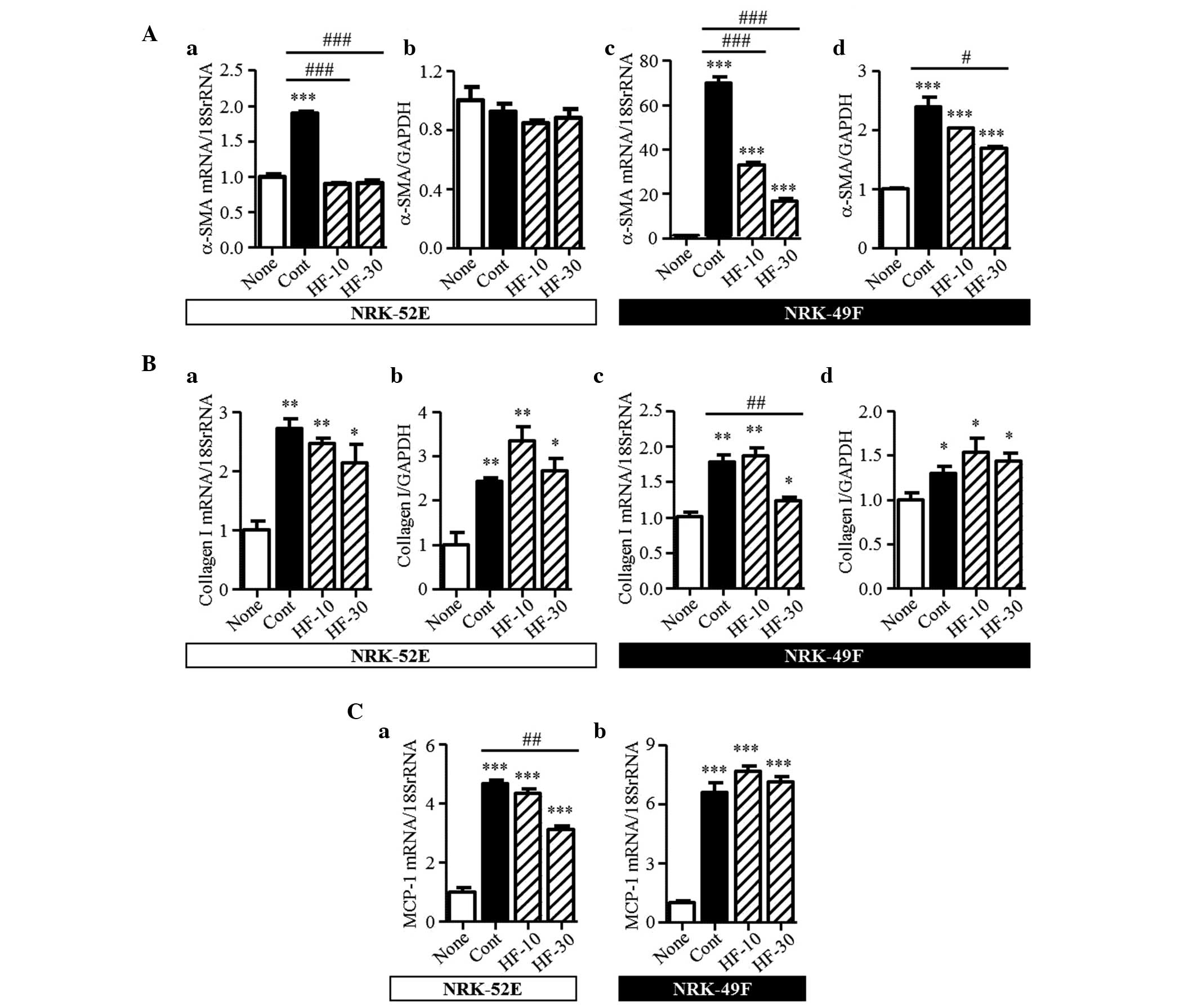 | Figure 8Expression levels of α-SMA, collagen
I and MCP-1 in the NRK-52E and NRK-49F cells. (Aa) mRNA and (Ab)
protein expression levels of α-SMA in the NRK-52E and the (Ac) mRNA
and (Ad) protein expression levels of α-SMA in the NRK-49F cells.
mRNA and protein expression levels of collagen I in the (Ba and b)
NRK-52E cells and the (Bc and d) NRK-49F cells. mRNA expression
levels of MCP-1 in the (Ca) NRK-52E and (Cb) NRK-49F cells. Results
are expressed as the mean ± standard error of the mean (n=3).
*P<0.05, **P<0.01 and
***P<0.001, vs. none; #P<0.05,
##P<0.01 and ###P<0.001, vs. Cont. SMA,
smooth muscle actin; MCP-1, monocyte chemoattractant protein-1;
None, no-stimulation; Cont, TGF-β1 stimulation; HF-10 or HF-30,
pretreatment with 10 or 30 µM hydroxyfasudil prior to TGF-β1
stimulation, respectively. |
Effect of hydroxyfasudil on RAW264.7
cells
The migration of macrophages to the kidney
interstitium was observed in the UUO model. At an early stage of
UUO, M1 macrophages, which are the classically activated
macrophages, infiltrate the kidney and are involved in inflammation
and phagocytosis (27). In the
present study, RAW264.7 cells were stimulated by LPS, as M1 subtype
cells. In these activated RAW264.7 cells, the mRNA expression
levels of MCP-1, IL-1β, IL-6 and TNFα were markedly increased
(Fig. 9A–D). Hydroxyfasudil did
not affect the expression levels of these mRNAs.
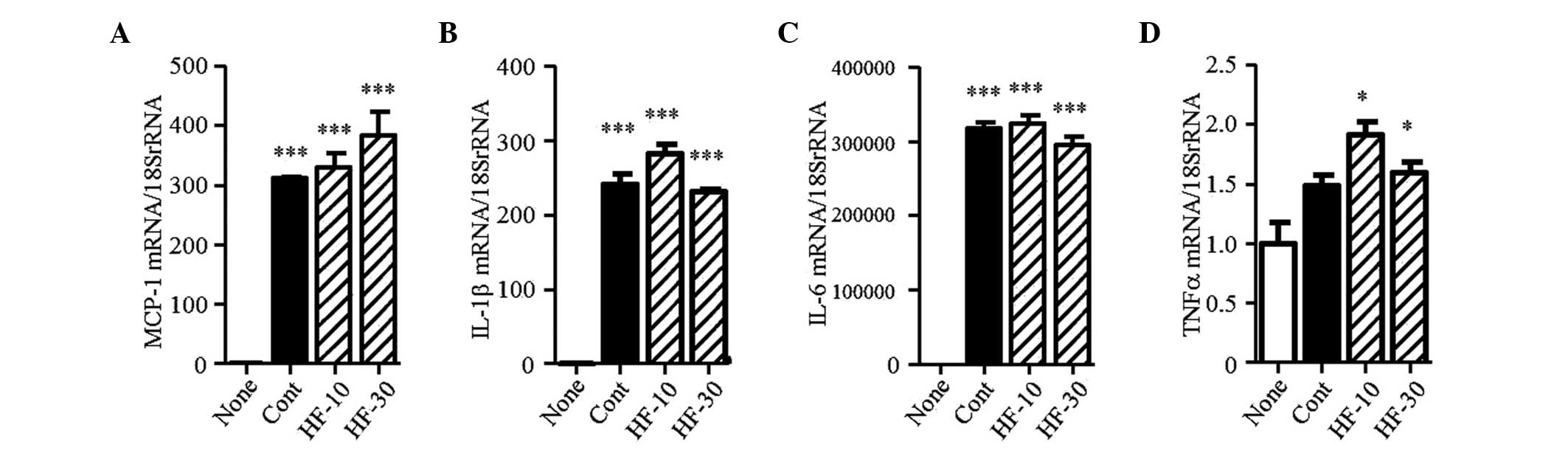 | Figure 9mRNA expression levels of
chemokines/cytokines in RAW264.7 cells. The mRNA expression levels
of (A) MCP-1, (B) IL-1β, (C) IL-6 and (D) TNFα were determined in
RAW264.7 cells. Results are expressed as the mean ± standard error
of the mean (n=3). *P<0.05 and
***P<0.001, vs. none. MCP-1, monocyte chemoattractant
protein-1; IL, interleukin; TNF, tumor necrosis factor; None,
no-stimulation; Cont, lipopolysaccharide stimulation; HF-10 or
HF-30, pretreatment with 10 or 30 µM hydroxyfasudil prior to
lipopolysaccharide stimulation, respectively. |
Discussion
The UUO model is useful for examination of the
mechanisms of tubulointerstitial fibrosis in vivo.
Interstitial inflammation occurs rapidly (2–3 days) and the
subsequent histological changes, including tubular dilation,
tubular atrophy, and fibrosis are observed soon after (~7 days).
These common changes are found in several tubulointerstitial
diseases (25,28–31).
A study involving ROCK1-knockout mice (26) reported no inhibition of
interstitial fibrosis, although fasudil and Y27632 pharmacological
inhibitors inhibit fibrosis in the UUO model (18–20).
To clarify the mechanisms of suppression of the interstitial
fibrosis, the present study investigated the inhibition of ROCK
using the pharmacological ROCK inhibitor, fasudil.
The present study demonstrated that fasudil reduced
renal interstitial fibrosis and attenuated histological changes in
the UUO mice. The results of the immunohistochemical staining and
mRNA expression analysis revealed that fasudil markedly suppressed
the expression of α-SMA in the injured kidney. In the process of
the kidney fibrosis, the number of myofibroblasts producing
extracellular matrix proteins increases in the tubular
interstitium. These cells express a distinctive protein, α-SMA and,
until recently, tubular epithelial cells have been considered the
predominant source of the interstitial myofibroblasts. However,
several studies have reported that transformation to myofibroblasts
occurs in various cells, including epithelial cells, endothelial
cells, bone marrow-derived cells, resident fibroblasts and
pericytes (32–36). In the present study, the NRK-52E
tubular epithelial cell line, and NRK-49F fibroblast cell line were
used to examine the transformation into myofibroblasts and analyze
fibrosis-associated gene expression. In the NRK-49F cells,
hydroxyfasudil attenuated the mRNA and protein expression levels of
α-SMA stimulated by TGF-β1. The upregulated mRNA expression of
collagen I was suppressed by high dose-hydroxyfasudil in the
NRK-49F cells only. These results suggested that fasudil inhibited
TGF-β1-induced transformation and collagen synthesis in the renal
fibroblast cells. In the NRK-52E cells, the mRNA expression levels
of α-SMA increased by TGF-β1 stimulation and were suppressed by
hydroxyfasudil pretreatment. However, the protein expression levels
of α-SMA were not affected by TGF-β1 stimulation. It is possible
that the basal expression level of α-SMA is higher than that of
other cells under the same conditions. Previous in vitro and
in vivo studies have reported that, not only TGF-β1, but the
others, including angiotensin II, aldosterone and high glucose,
induce collagen production in tubular epithelial cells or
fibroblast cells, and ROCK inhibitors reduce collagen production
(16,37,38).
In the NRK-52E and the NRK-49F cells, the mRNA and protein
expression levels of collagen I were markedly increased by TGF-β1
stimulation, but hydroxyfasudil did not suppress them enough. It is
possible that stimuli other than TGF-β1 induce the fibrotic
responses in these cells. In the UUO mice, fasudil attenuated the
TGF-β1 mRNA expression and the activity of downstream, Smad3
phosphorylation. The results suggested that the inhibition of ROCK
activity suppressed the TGF-β-Smad signaling pathway and
subsequently induced fibrotic responses in tubular epithelial cells
and renal fibroblast cells.
It has been reported that damaged tubular cells,
interstitial myofibroblasts and macrophages produce cytokines,
chemokines and growth factors in the UUO kidneys, triggering the
accumulation of interstitial macrophages (39–41).
In the present study, fasudil inhibited the infiltration of
macrophages into the injured kidney. However, the mRNA expression
of MCP-1 was not affected by fasudil administration. In the NRK-49F
cells, hydroxyfasudil did not reduce the TGF-β1-enhanced mRNA
expression of MCP-1, however, in the NRK-52E cells, hydroxyfasudil
markedly inhibited the increase in the mRNA expression of MCP-1
mRNA stimulated by TGF-β1. Myofibroblasts in the injured kidney
consist of several cell types (32–36)
and it is reported that myofibroblasts derived from epithelial
cells comprise a small population (34–36).
In the present study, hydroxyfasudil inhibited the mRNA expression
of MCP-1 in the epithelial NRK-52E cells. Thus, it is likely that
fasudil is able to suppress the MCP-1 production in myofibroblasts
that are derived from epithelial cells. However, these populations
are small in comparison with other myofibroblast groups; therefore,
fasudil may not be able to suppress the expression of MCP-1 in the
overall myofibroblast cell population in the injured kidney.
Several investigators have also reported that renal interstitial
fibrosis can be reduced without inhibition of the mRNA expression
of MCP-1 in UUO mice: however, the mechanism requires clarification
(42–44). Thus, the precise association
between MCP-1 and ROCK in the renal interstitial fibrosis process
remains to be fully elucidated.
Macrophages are categorized into two functional
phenotypes: Classically activated M1 and alternatively activated M2
(45,46). In the first stage of UUO kidney
injury, almost all the infiltrated macrophages exhibit the M1
phenotype (27). To examine the
association between macrophages and ROCK in renal interstitial
fibrosis, the present study assessed the LPS-induced inflammatory
response in RAW264.7 cells. LPS stimulation elevated the mRNA
expression levels of MCP-1, IL-1β, IL-6, TNFα, and iNOS, however,
it was difficult to detect the mRNA expression levels of collagen
and TGF-β1 mRNA (data not shown), which were characteristic of the
M2 phenotype. These gene expression patterns are characteristic for
the phenotype of M1 macrophages (46). Fasudil treatment did not affect the
expression of the genes induced by LPS in the RAW264.7 cells. These
novel results suggested that ROCK may contribute to the
infiltration of monocytes/macrophages into the interstitium of the
injured kidney, rather than affect the expression of inflammatory
cytokines/chemokines.
Taken together, the data of the present study
suggested that the inhibition of ROCK suppressed renal interstitial
fibrosis via the TGF-β-Smad signaling pathway and suppressed the
infiltration of macrophages into the injured kidney. It is likely
that ROCK contributes to the activation of the renal intrinsic
cells and the migration of the extra-renal cells in the progression
of renal interstitial fibrosis.
Abbreviations:
|
ROCK
|
Rho-associated coiled-coil kinase
|
|
UUO
|
unilateral ureteral obstruction
|
Acknowledgments
The authors would like to thank Dr Akiyoshi Fukamizu
and Dr Junji Ishida (Life Science Center, Tsukuba Advanced Research
Alliance, Tsukuba University, Ibaraki, Japan) for their guidance.
The authors would also like to thank Dr Kenji Arakawa, Dr Rikako
Yamauchi and Dr Taku Sato (Mitsubishi Tanabe Pharma Corporation)
for supporting this investigation.
References
|
1
|
Satoh K, Fukumoto Y and Shimokawa H:
Rho-kinase: Important new therapeutic target in cardiovascular
diseases. Am J Physiol Heart Circ Physiol. 301:H287–H296. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hahmann C and Schroeter T: Rho-kinase
inhibitors as therapeutics: From pan inhibition to isoform
selectivity. Cell Mol Life Sci. 67:171–177. 2010. View Article : Google Scholar
|
|
3
|
Olson MF: Applications for ROCK kinase
inhibition. Curr Opin Cell Biol. 20:242–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ming D, Yan BP, Liao JK, Lam YY, Yip GW
and Yu CM: Rho-kinase inhibition: A novel therapeutic target for
the treatment of cardiovascular diseases. Drug Discov Today.
15:622–629. 2010. View Article : Google Scholar
|
|
5
|
Budzyn K, Marley PD and Sobey CG:
Targeting Rho and Rho-kinase in the treatment of cardiovascular
disease. Trends Pharmacol Sci. 27:97–104. 2006. View Article : Google Scholar
|
|
6
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kushiyama T, Oda T, Yamamoto K, Higashi K,
Watanabe A, Takechi H, Uchida T, Oshima N, Sakurai Y, Miura S and
Kumagai H: Protective effects of Rho kinase inhibitor fasudil on
rats with chronic kidney disease. Am J Physiol Renal Physiol.
304:F1325–F1334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishikimi T, Koshikawa S, Ishikawa Y,
Akimoto K, Inaba C, Ishimura K, Ono H and Matsuoka H: Inhibition of
Rho-kinase attenuates nephrosclerosis and improves survival in
salt-loaded spontaneously hypertensive stroke-prone rats. J
Hypertens. 25:1053–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kanda T, Wakino S, Hayashi K, Homma K,
Ozawa Y and Saruta T: Effect of fasudil on Rho-kinase and
nephropathy in subtotally nephrectomized spontaneously hypertensive
rats. Kidney Int. 64:2009–2019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie X, Peng J, Chang X, Huang K, Huang J,
Wang S, Shen X, Liu P and Huang H: Activation of RhoA/ROCK
regulates NF-κB signaling pathway in experimental diabetic
nephropathy. Mol Cell Endocrinol. 369:86–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou H, Li YJ, Wang M, Zhang LH, Guo BY,
Zhao ZS, Meng FL, Deng YG and Wang RY: Involvement of RhoA/ROCK in
myocardial fibrosis in a rat model of type 2 diabetes. Acta
Pharmacol Sin. 32:999–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Zhu W, Tao J, Xin P, Liu M, Li J and
Wei M: Fasudil protect the heart against ischemia-reperfusion
injury by attenuating endoplasmic reticulum stress and modulating
SERCA activity: The differential role for PI3K/Akt and JAK2/STAT3
signaling pathways. PLoS One. 7:e481152012. View Article : Google Scholar
|
|
13
|
Kentrup D, Reuter S, Schnöckel U, Grabner
A, Edemir B, Pavenstädt H, Schober O, Schäfers M, Schlatter E and
Büssemaker E: Hydroxyfasudil-mediated inhibition of ROCK1 and ROCK2
improves kidney function in rat renal acute ischemia-reperfusion
injury. PLoS One. 6:e264192011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matoba K, Kawanami D, Okada R, Tsukamoto
M, Kinoshita J, Ito T, Ishizawa S, Kanazawa Y, Yokota T, Murai N,
et al: Rho-kinase inhibition privents the progression of diabetic
nephropathy by downregulating hypoxia-inducible factor 1α. Kidney
Int. 84:545–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Diah S, Zhang GX, Nagai Y, Zhang W, Gang
L, Kimura S, Hamid MR, Tamiya T, Nishiyama A and Hitomi H:
Aldosterone induced mypfibroblastic transdifferentiation and
collagen gene expression through the Rho-kinase dependent signaling
pathway in rat mesangial cells. Exp Cell Res. 314:3654–3662. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei J, Li Z, Ma C, Zhan F, Wu W, Han H,
Huang Y, Li W, Chen D and Peng Y: Rho kinase pathway is likely
responsible for the profibrotic actions of aldosterone in renal
epithelial cells via inducing epithelial-mesenchymal transition and
extracellular matrix excretion. Cell Biol Int. 37:725–730. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manickam N, Patel M, Griendling KK, Gorin
Y and Barnes JL: RhoA/Rho kinase mediates TGF-β1-induced kidney
myofi-broblast activation through Poldip2/Nox4-derived reactive
oxygen species. Am J Physiol Renal Physiol. 307:F159–F171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagatoya K, Moriyama T, Kawada N, Takeji
M, Oseto S, Murozono T, Ando A, Imai E and Hori M: Y-27632 prevents
tubulointerstitial fibrosis in mouse kidneys with unilateral
ureteral obstruction. Kidney Int. 61:1684–1695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Satoh S, Yamaguchi T, Hitomi A, Sato N,
Shiraiwa K, Ikegaki I, Asano T and Shimokawa H: Fasudil attenuates
interstitial fibrosis in rat kidneys with unilateral ureteral
obstruction. Eur J Pharmacol. 455:169–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takeda Y, Nishikimi T, Akimono K, Matsuoka
H and Ishimitsu T: Benefical effects of a combination of Rho-kinase
inhibitor and ACE inhibitor on tubulointerstitial fibrosis induced
by unilateral ureteral obstruction. Hypertens Res. 33:965–973.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kakimoto T, Kimata H, Iwasaki S, Fukunari
A and Utsumi H: Automated recognition and quantification of
pancreatic islets in Zucker diabetic fatty rats treated with
exendin-4. J Endocrinol. 216:13–20. 2013. View Article : Google Scholar
|
|
22
|
Woessner JF Jr: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this imino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kivirikko KI, Laitinen O and Prockop DJ:
Modifications of a specific assay for hydroxyproline in urine. Anal
Biochem. 19:249–255. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chevalier RL, Forbes MS and Thornhill BA:
Ureteral obstruction as a model of renal interstitial fibrosis and
obstructive nephropathy. Kidney Int. 75:1145–1152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chevalier RL: Obstructive nephropathy:
Towards biomarker discovery and gene therapy. Nat Clin Pract
Nephrol. 2:157–168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen B, Liu X, Fan Y and Qiu J:
Macrophages regulate renal fibrosis through modulating TGFβ
superfamily signaling. Inflammation. 37:2076–2084. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang HC, Zuo Y and Fogo AB: Models of
chronic kidney disease. Drug Discov Today Dis Models. 7:13–19.
2010. View Article : Google Scholar
|
|
28
|
Löpez-Novoa JM, Martinez-Salgado C,
Rodriguez-Peña AB and López-Hernández FJ: Common pathophysiological
mechanisms of chronic kidney disease. Therapeutic perspectives
Pharmacol Ther. 128:61–81. 2010. View Article : Google Scholar
|
|
29
|
Truong LD, Gaber L and Eknoyan G:
Obstructive uropathy. Contrib Nephrol. 169:311–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eddy AA, López-Guisa JM, Okamura DM and
Yamaguchi I: Investigating mechanizes of chronic kidney disease in
mouse models. Pediatr Nephrol. 27:1233–1247. 2012. View Article : Google Scholar
|
|
31
|
Fu P, Liu F, Su S, Wang W, Huang XR,
Entman ML, Schwartz RJ, Wei L and Lan HY: Signaling mechanism of
renal fibrosis in unilateral ureteral obstructive kidney disease in
ROCK1 knockout mice. J Am Soc Nephrol. 17:3105–3114. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ballhause TM, Soldati R and Mertens PR:
Sources of myofibroblats in kidney fibrosis: All answers are
correct, however to different extent! Int Urol Nephrol. 46:659–664.
2014. View Article : Google Scholar
|
|
33
|
Duffield JS: Cellular and molecular
mechanisms in kidney fibrosis. J Clin Invest. 124:2299–2306. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pan SY, Chang YT and Lin SL: Microvascular
pericytes in healthy and diseased kidneys. Int J Nephrol Renovascul
Disease. 7:39–48. 2014.
|
|
35
|
LeBleu VS, Taduri G, O'Connell J, Teng Y,
Cooke VG, Wada C, Sugimoto H and Kalluri R: Origin and function of
myofibroblasts in kidney fibrosis. Nat Med. 19:1047–1053. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jang HS, Kim JI, Jung KJ, Kim J, Han KH
and Park KM: Bone marrow-derived cells play a major role in kidney
fibrosis via proliferation and differentiation in the infiltrated
site. Biochim Biophys Acta. 1832:817–825. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin J, Xie YY, Huang L, Yuan QJ, Mei WJ,
Yuan XN, Hu GY, Cheng GJ, Tao LJ and Peng ZZ: Fluorofenidone
inhibits nicotinamide adeninedinucleotide phosphate oxidase via
PI3K/Akt pathway in the pathogenesis of renal interstitial
fibrosis. Nephrology (Carlton). 18:690–699. 2013.
|
|
38
|
Gu L, Gao Q, Ni L, Wang M and Shen F:
Fasudil inhibits epithelialmyofibroblast transdifferentiation of
human renal tubular epithelial HK-2 cells induced by high glucose.
Chem Pharm Bull (Tokyo). 61:688–694. 2013. View Article : Google Scholar
|
|
39
|
Gande MT, Pèrez-Barriocanal F and
López-Novoa JM: Role of inflammation in tubule-interstitial damage
associated to obstructive nephropathy. J Inflamm (Lond). 7:192010.
View Article : Google Scholar
|
|
40
|
Wang Y and Harris DC: Macrophages in renal
disease. J Am Soc Nephrol. 22:21–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung AC and Lan HY: Chemokines in renal
injury. J Am Soc Nephrol. 22:802–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu N, Tolbert E, Pang M, Ponnusamy MI,
Yan H and Zhuang S: Suramin inhibits renal fibrosis in chronic
kidney disease. J Am Soc Nephrol. 22:1064–1075. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pang M, Ma L, Gong R, Tolbert E, Mao H,
Ponnusamy M, Chin YE, Yan H, Dworkin LD and Zhuang S; A novel STAT3
inhibitor: S31–201. attenuates renal interstitial fibroblast
activation and interstitial fibrosis in obstructive nephropathy.
Kidney Int. 78:257–268. 2010. View Article : Google Scholar
|
|
44
|
Meng XM, Huang XR, Xiao J, Chen HY, Zhong
X, Chung AC and Lan HY: Diverse roles of TGF-β receptor II in renal
fibrosis and inflammation in vivo and in vitro. J Pathol.
227:175–188. 2012. View Article : Google Scholar
|
|
45
|
Anders HJ and Ryu M: Renal
microenvironments and macrophage phenotypes determine progression
or resolution of renal inflammation and fibrosis. Kidney Int.
80:915–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ricardo SD, van Goor H and Eddy AA:
Macrophage diversity in renal injury and repair. J Clin Invest.
118:3522–3530. 2008. View Article : Google Scholar : PubMed/NCBI
|