Introduction
Camels (Camelus romedarius) are important to
the lifestyle of several communities, particularly those of the
Middle East. Furthermore, camels contribute to the economy and food
security of humans by providing milk and meat. It is
well-established that milk is a source of energy, proteins,
vitamins and minerals. In addition to its value as a nutrient
source, milk also has antibiotic properties. The milk of mammals is
protected to various extents against microbial contamination by
natural inhibitory systems, including lactoferrins, lysozymes,
immunoglobulins and free fatty acids (1,2).
Camel milk is reported to have a more marked inhibitory system,
compared with cow milk (1).
Notably, the levels of lysozyme and lactoferrins in camel milk are
two and three times higher than those of cow milk, respectively
(2). Camel milk contains peptides
and proteins, which exhibit biological activities that have
beneficial effects on several bioprocesses, including digestion,
absorption, growth and immunity (3,4).
Furthermore, camel milk can be stored at room temperature for
longer periods of time, compared with the milk from other animals
(5).
Camel milk is used in the treatment of autoimmune
diseases, dropsy, jaundice, splenomegaly, tuberculosis, asthma,
anemia, piles, diabetes and as an antimicrobial (6). In addition, camel milk has antitoxic
effects against cadmium chloride (7,8),
carbon tetrachloride (9),
cisplatin (10) and paracetamol
(11). Camel whey proteins assist
in the prevention of several human diseases (12), and dietary whey supplements may
improve wound healing by increasing glutathione-S-transferase
synthesis and cellular anti-oxidant defense (13). The liver is an important organ
exposed to pathogenicity during microbial infection (7). Camel milk, but not bovine milk,
significantly inhibits HepG2 and MCF7 cell proliferation through
activation of the mRNA expression and activity of caspase-3
(14). Furthermore, camel milk
increases the expression levels of oxidative stress markers,
including heme oxygenase-1, and increases the production of
reactive oxygen species in the two cells (14). Camel milk lysozyme has
bacteriostatic effects against gram-positive bacterial strains and
exerts bactericidal effects against gram-negative strains (1).
Staphylococcus aureus (S. aureus) is a
gram-positive bacteria, which causes numerous infections in humans
and animals (15). S.
aureus can survive for hours to weeks, and even months, on dry
environmental surfaces (15–17).
Similar to S. aureus, Escherichia coli (E.
coli) is a gram-negative microorganism, which causes severe
pathogenicity to the infected host. It has been reported that camel
milk exhibits bacteriostatic effects against E. coli and
Listeria monocytogenes (18). Camel milk is also considered to
have medicinal properties against certain pathogens in the Middle
East (1,2). Therefore, the aim of the present
study was to examine the protective effects of camel milk against
E. coli and S. aureus-induced hepatic pathogencity in
Wistar rats.
Materials and methods
Materials and bacterial strains
E. coli and S. aureus strains were
obtained from Animal Reproduction Research Institute (Alharam Giza,
Egypt). QIAzol for RNA extraction and oligo dT primers were
purchased from Qiagen, Inc. (Valencia, CA, USA). Wistar rats were
purchased from the King Fahd Institute for Scientific Research,
King AbdulAziz University, Jeddah, Saudi Arabia). Solvents and
associated materials were obtained from ADWIA Pharmaceutical Co.
(El Oubor, Egypt). The primers for gene expression analysis were
purchased from Macrogen, Inc., (Seoul, Republic of Korea). The DNA
ladder was purchased from MBI, Fermentas (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The biochemical kit for malondialdehyde
(MDA) was purchased from Bio Diagnostic Company (Dokki, Egypt).
Camel milk, collected from healthy, disease-free Magrabi females
(5–10 years old) of the Magrabi breed, was provided daily from
farms in Turabah (Taif, Saudi-Arabia). All animal procedures were
approved by the Ethical Committee Office of the Dean of Scientific
Affairs of Taif University (Taif, Saudi Arabia).
Camel milk preparation
Camel milk samples were collected daily, early in
the morning, from a camel farm in Turabah, Saudi-Arabia. The milk
was collected from a healthy 4 year-old camel by hand into sterile
screw bottles, and maintained in cool boxes until transported to
the laboratory. The rats were supplemented with unpasteurized camel
milk, which was administered orally at a dose of 100 ml/24 h/cage
(six rats), based on a previous study by Althnaian et al
(19) at a fixed time of 9.00
am.
E. coli preparation
The E. coli strains were isolated from cases
of bovine mastitis grown in brain/heart infusion broth. When the
bacteria were in the logarithmic phase of growth, the suspension
was centrifuged at 15,000 × g for 15 min (Animal Reproduction
Research Institute), the supernatant was discarded, and the
bacteria were re-suspended and diluted in sterile saline (1:1). The
rats were injected intraperitoneally with 1 ml saline containing
2×1010 colony forming units (CFU) of E. coli.
Immediately following bacterial challenge, the rats were maintained
under observation for 7 days.
S. aureus preparation
Preliminary confirmation and phenotypic
investigations were performed, according to standard protocols
(15), using gram staining and
biochemical parameters, including a coagulase test, and were
screened by growth on Baird-Parker selective agar. Following
confirmation, the bacterial culture was cultured in tryptic broth
and incubated overnight. The bacterial culture was then centrifuged
at 15,000 × g for 15 min, and the pellet was resuspended and washed
with sterile phosphate-buffered saline (PBS). The viable bacterial
count was adjusted to ~1×109 CFU/ml. Serial dilution was
performed in PBS to obtain a final concentration of
5×106/0.1 ml bacterial suspension.
Inoculation of E. coli and S.
aureus strains into rats and experimental design
A total of 60 male Wistar rats (4-week-old; 80–100
g) were selected randomly. The rats were exposed to a 12 h
light/dark cycle and provided with access to food and water ad
libitum. The 60 rats were divided into six groups (10
rats/group) with five rats per cage. The control group was fed a
normal diet; the camel milk group was administered with a dose of
100 ml camel milk per six rats, based on a previous study (19); the E. coli group was
intraperitoneally injected with a virulent strain of E. coli
at a dose of 2×1010 CFU/ml/rat (20); the E. coli + camel milk
group was administered with E. coli, as in the E.
coli group following camel milk supplementation; the S.
aureus group was intraperitoneally injected with a virulent
strain of S. aureus at a dose of 1×109 CFU/ml/rat
(21); and the S. aureus +
camel milk group was treated in the same way as the S.
aureus group, following prior camel milk supplementation. The
rats in the E. coli or S. aureus + camel milk groups
were pre-administered with camel milk for 2 weeks prior to pathogen
injection. All animals were maintained under observation for 7
days. At the end of the experimental period (day 8), the rats were
sacrificed by decapitation following overnight fasting and diethyl
ether inhalation. Blood samples (5 – 8 m l/rat) were obtained for
serum extraction by centrifugation at 1,000 × g for 10 min at room
temperature, and liver samples were removed and placed under
aseptic conditions in QIAzol reagent for RNA extraction and gene
expression analyses, and in sterile tubes for total bacterial
count.
Serum MDA measurements
Serum MDA, GPT and GOT were measured using a
commercially available kit prior to spectrophotometric analysis.
The activities of MDA were determined using an ELISA reader at an
optical density (OD) of 532 nm (Absorbance Microplate Reader ELx
800TM BioTek®, BioTek Instruments, Seattle, WA, USA).
For liver biomarkers, Serum levels of GPT and GOT were measured
spectrophotometrically using specific commercial kits
(Biodiagnostic Company, Dokki, Egypt) and assayed, according to the
manufacturer's protocol, as stated in our previous study (22).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis of gene expression
levels
Liver tissues were collected from the rats, flash
frozen in 1 ml QIAzol reagent and subsequently stored at −70°C. The
frozen samples (50–100 mg) were then homogenized using a Polytron
300 D homogenizer (Lauda-Brinkmann, Delran, NJ, USA). Total RNA was
extracted via chloroform extraction, followed by nucleic acid
precipitation using isopropyl alcohol (absolute chloroform). The
pellet was washed with 70% ethanol and re-suspended in molecular
biological grade water (absolute nanopure water).
The RNA (2 µg) was incubated at 65°C for 10
min and was then reverse transcribed using 100 units of Moloney
murine leukemia virus reverse transcriptase (Gibco; Thermo Fisher
Scientific, Inc.), 50 pmol of poly (dT) primer and 20 nmol dNTPs,
in a total volume of 11 µl at 37°C for 1 h. Following
heating at 94°C for 5 min, PCR amplification was performed with 2.5
units Taq polymerase (PerkinElmer, Inc., Waltham, MA, USA),
3 mM MgCl2 and 50 pmol of the forward and reverse
primers specific for the respective genes, in a total volume of 25
µl. The PCR conditions of the genes analyzed are
listed in Table I. The
thermocycling conditions were as follows: Each cycle consisted of
denaturation at 94°C for 1 min, annealing at the gene-specific
temperatures for each gene (Table
I) for 1 min, extension at 72°C for 1 min and final extension
at 72°C for 7 min. The RT-qPCR products were visualized under an
ultraviolet lamp by electrophoresis in 1.5% agarose gel stained
with ethidium bromide. The intensities of the bands were analyzed
densitometrically using the NIG Image program (http://rsb.info.nih.gov/nih-image/).
 | Table IPrimer sequences and polymerase chain
reaction conditions of the of the genes analyzed. |
Table I
Primer sequences and polymerase chain
reaction conditions of the of the genes analyzed.
| mRNA (bp) | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Cycles (n) | Annealing temp
(°C) |
|---|
| Caspase-3
(282) |
ACGGTACGCGAAGAAAAGTGAC |
TCCTGACTTCGTATTTCAGGGC | 30 | 52 |
| Survivin (390) |
CTGATTTGGCCCAGTGTTTT |
TCATCTGACGTCCAGTTTCG | 35 | 52 |
| Bax (600) |
GTCGTCCAGATACTCAGCAT |
CACAGTCGGATATGAGCATC | 35 | 58 |
| TGF-β1 (456) |
TGAGTGGCTGTCTTTTGACG |
TGGTTGTAGAGGGCAAGGAC | 35 | 60 |
| IL-6 (450) |
AGTTGCCTTCTTGGGACTGATGT |
TGCTCTGAATGACTCTGGCTTTG | 35 | 58 |
| GST (575) |
GCTGGAGTGGAGTTTGAAGAA |
GTCCTGACCACGTCAACATAG | 35 | 55 |
| SOD (410) |
AGGATTAACTGAAGGCGAGCAT |
TCTACAGTTAGCAGGCCAGCAG | 33 | 55 |
| GAPDH (309) |
AGATCCACAACGGATACATT |
TCCCTCAAGATTGTCAGCAA | 25 | 52 |
Statistical analysis
The data are expressed as the mean ± standard error
of the mean from five independent rats per group. Statistical
analyses were performed using analysis of variance and Fisher's
post-hoc descriptive tests were performed using SPSS software
(version 11.5) for Windows (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate statistical significance.
Results
Protective effects of camel milk in
Wistar rats
The present study examine the effects of camel milk
on the levels of glutamate pyruvate transaminase (GPT) and
glutamate oxalate transaminase (GOT), and the total bacterial count
following injection with E. coli and S. aureus. The
injection of E. coli and S. aureus induced
significant increases in the expression levels of GPT and GOT due
to the hepatic pathogenicity of the bacterial strains. Prior
supplementation with camel milk decreased the bacterial-induced
upregulation in the expression levels of GPT and GOT. In addition,
the total bacterial counts were higher in the liver tissues of the
E. coli and S. aureus-injected rats, compared with
the control and camel milk groups, and were significantly decreased
in the pathogen injected rats supplemented with camel milk
(Table II).
 | Table IIProtective effects of camel milk,
determined by the expression levels of GPT and GOT in liver
tissues, and the total E. coli and S. aureus count/g
tissue 7 days following exposure to E. coli and S.
aureus in Wistar rats. |
Table II
Protective effects of camel milk,
determined by the expression levels of GPT and GOT in liver
tissues, and the total E. coli and S. aureus count/g
tissue 7 days following exposure to E. coli and S.
aureus in Wistar rats.
| Factor | Control | CM | E. coli | CM + E.
coli | S.
aureus | S. aureus +
CM |
|---|
| GPT (U/l) | 78±8.6 | 63.3±4.4 | 174±9.5a | 98.3±107b | 1517±6.35a | 77±8c |
| GOT (U/l) | 62±7.2 | 64±4.9 | 145±5.5a | 84.3±2.9b | 153±9.3a | 76±12.5c |
| Total E.
coli count | – | – |
4.5×105 |
3.4×105b | – | – |
| Total S.
aureus count | – | – | – | – |
7×105 |
3.6×105c |
Protective effects of camel milk on the
survival rates of Wistar rats injected with E. coli and
S. aureus
The injection of E. coli and S. aureus
led to mortality rates of 60 and 70%, respectively. Camel milk
supplementation induced protective effects, and the survival rates
in the E. coli and S. aureus-injected rats following
camel milk administration were 80 and 70%, respectively (Table III). Notably, the percentage of
camel milk protection from mortality in the E. coli and
S. aureus rats was 40% (Table
III).
 | Table IIISurvival rate and protective effects
of camel milk against E. coli and S. aureus
pathogenicity in Wistar rats. |
Table III
Survival rate and protective effects
of camel milk against E. coli and S. aureus
pathogenicity in Wistar rats.
| Factor | Control | CM | E. coli | CM + E.
coli | S.
aureus | S. aureus +
CM |
|---|
| Rats per group
(n) | 10 | 10 | 10 | 10 | 10 | 10 |
| Rat fatalities
(n) | 0 | 0 | 6 | 2 | 7 | 3 |
| Surviving rats
(n) | 10 | 10 | 4 | 8 | 3 | 7 |
| Survival rate
(%) | 100 | 100 | 40a | 80b | 30a | 70c |
| Camel milk
protection (%) | – | – | – | 40 | – | 40 |
Protective effects of camel milk on E.
coli and S. aureus-induced changes in serum MDA and hepatic
antioxidant genes in Wistar rats
As shown in Fig.
1A, injection with the E. coli and S. aureus
strains induced significant increases in the expression levels of
MDA, marker of oxidative stress. Camel milk supplementation
decreased the expression levels of MDA following E. coli and
S. aureus injection. By contrast, E. coli and S.
aureus decreased the mRNA expression levels of
glutathione-S-transferase (GST; Fig.
1B) and superoxide dismutase (SOD; Fig. 1C), and prior supplementation of
camel milk normalized the decrease in the expression levels of GST
and SOD. Camel milk alone increased the expression levels of GST
and SOD, demonstrating its antioxidant action.
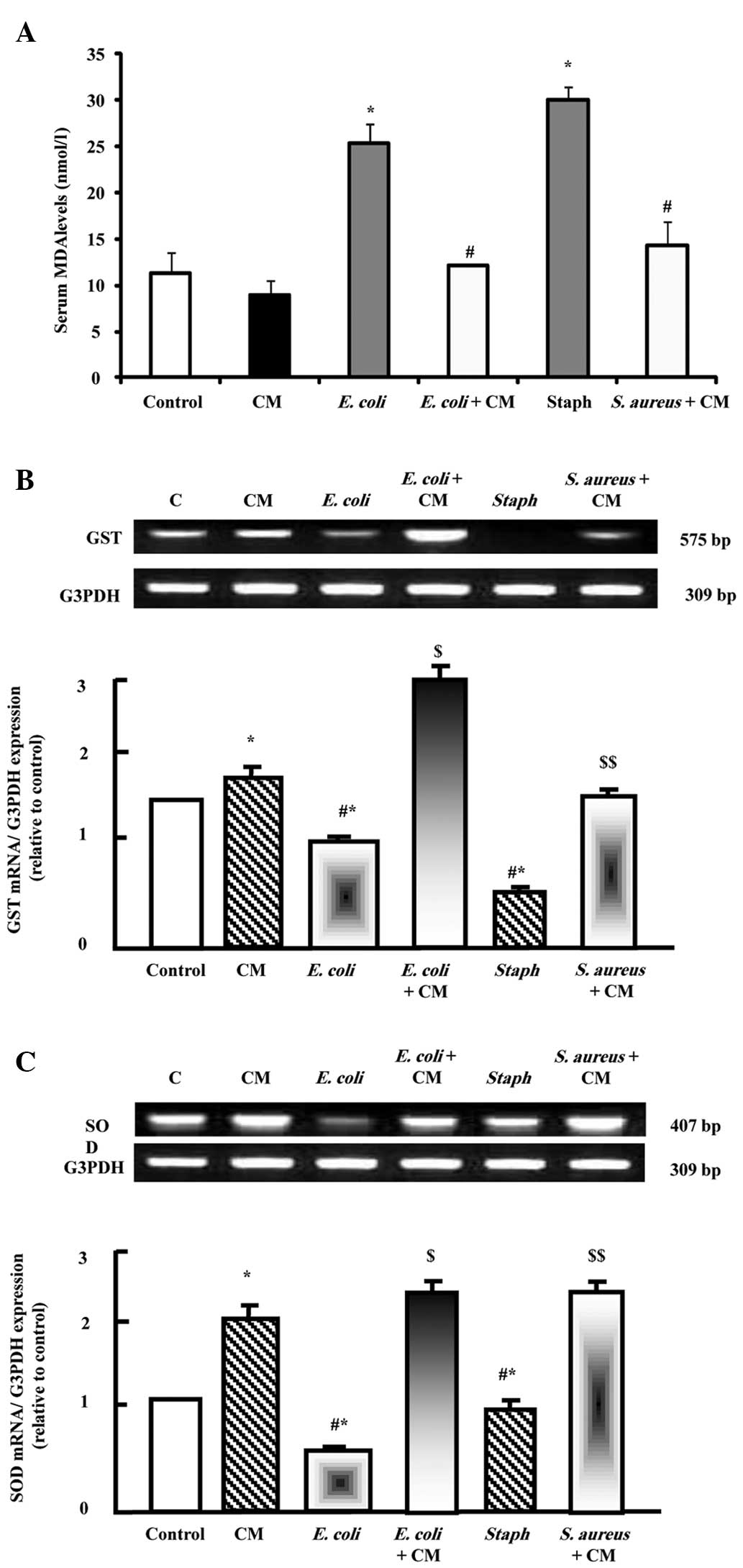 | Figure 1Protective effects of camel milk on
the serum expression levels of (A) MDA, (B) GST and (C) SOD in rats
injected with E. coli and S. aureus either alone or
with camel milk supplementation for 2 weeks prior to pathogen
challenge. Expression levels of MDA were measured
spectrophotometrically. RNA (2 µg) was extracted and reverse
transcription-quantitative polymerase chain reaction analysis was
performed to quantify GST and SOD expression. Data are presented as
the mean ± standard error of the mean for three independent
experiments. *P<0.05, vs. control group;
#P<0.05, vs. CM group; $P<0.05,
vs. E. coli group; $$P<0.05, vs. S.
aureus group. C, control; CM, camel milk; MDA, malondialdehyde;
GST, glutathione-S-transferase; SOD, superoxide dismutase; E.
coli, Escherichia coli; S. aureus, Staphylococcus aureus. |
Protective effects of camel milk on E.
coli and S. aureus-induced changes in mRNA expression levels of
transforming growth factor-ß1 (TGF-ß1) and interleukin-6 (IL-6) in
Wistar rats
As shown in Fig.
2A, camel milk upregulated the expression of TGF-β1. E.
coli and S. aureus also upregulated the expression of
TGF-β1. Supplementation with camel milk with either E. coli
or S. aureus induced additive stimulatory effects on the
expression of TGF-β1. However, camel milk did not affect the
expression of IL-6, whereas the two pathogens upregulated the
expression of IL-6 significantly. Supplementation with camel milk
prior to E. coli and S. aureus injection
downregulated the expression of IL-6.
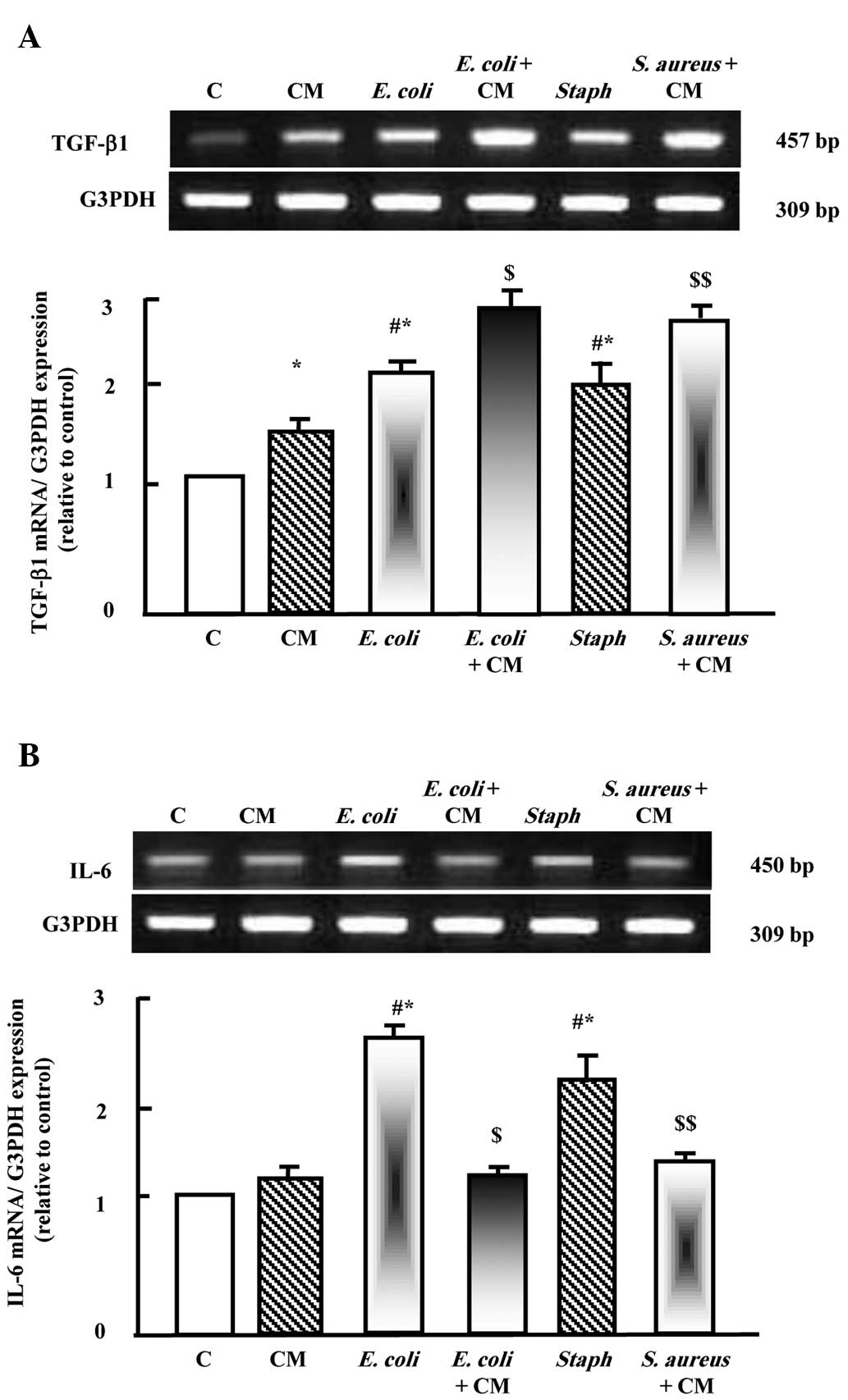 | Figure 2Protective effects of camel milk on
the expression levels of (A) TGF-β1 and (B) IL-6 in liver tissues
of rats injected with E. coli and S. aureus alone, or
with camel milk supplementation for 2 weeks prior to pathogen
challenge. RNA (2 µg) was extracted and reverse
transcription-quantitative polymerase chain reaction analysis was
performed to quantify the expression levels of TGF-β1 and IL-6.
Data are presented as the mean ± standard error of the mean of
three independent experiments. *P<0.05, vs. control
group; #P<0.05, vs. CM group;
$P<0.05, vs. E. coli group;
$$P<0.05, vs. S. aureus group. C, control; CM,
camel milk; TGF-β1, transforming growth factor β1; IL-6,
interleukin 6; E. coli, Escherichia coli; S. aureus,
Staphylococcus aureus. |
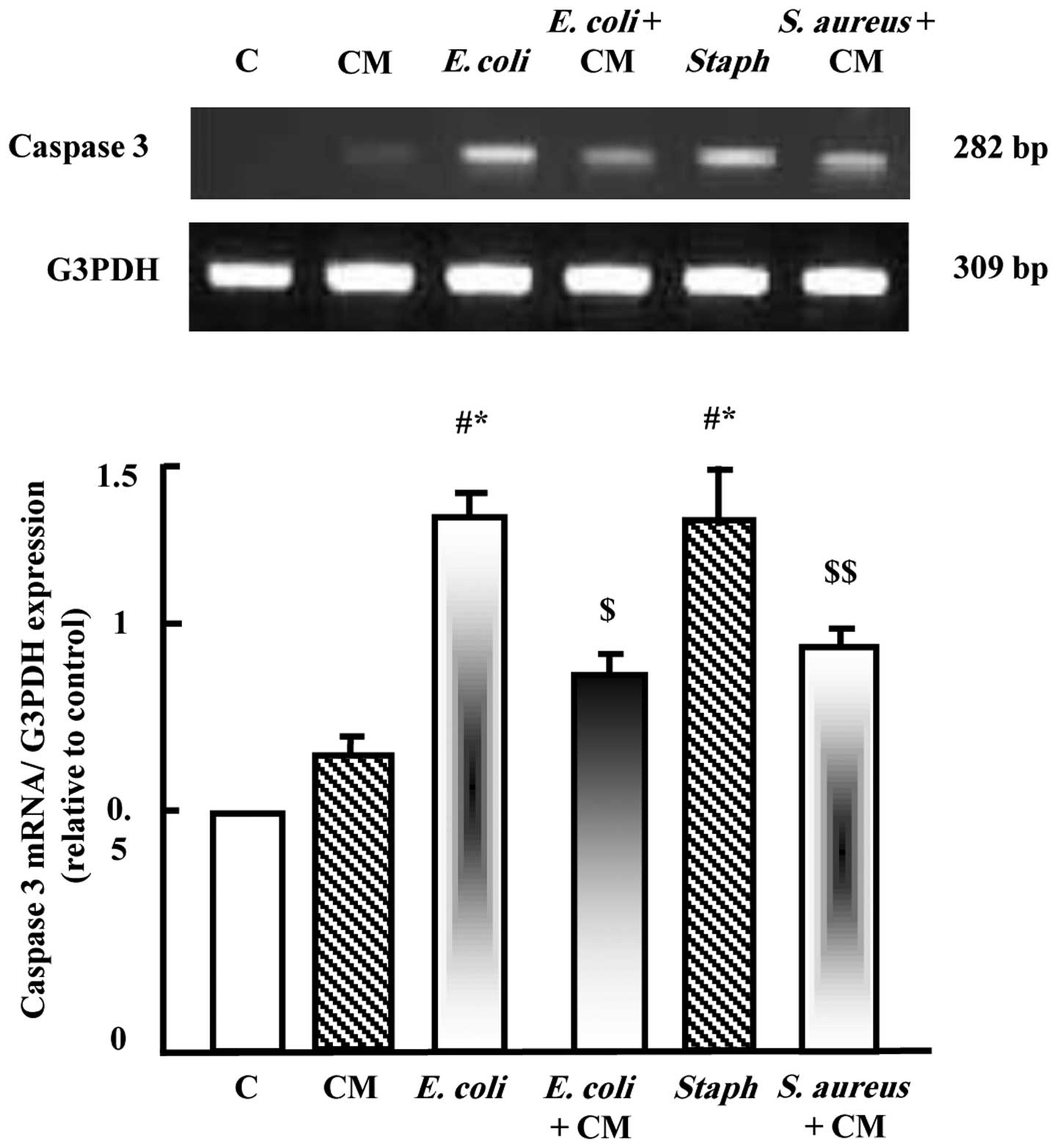
Protective effects of camel milk on E.
coli and S. aureus-induced changes in mRNA expression of caspase-3
in Wistar rats
To examine the effects of camel milk on the
expression of caspase-3, RT-qPCR analysis of liver tissue samples
was performed. As shown in Fig. 3,
camel milk did not significantly alter the expression of caspase-3;
however, E. coli and S. aureus significantly
upregulated the expression of caspase-3 (Fig. 3). Supplementation with camel milk
prior to E. coli or S. aureus injection significantly
reduced the increased expression of caspase-3 induced by the
pathogens.
Protective effects of camel milk
supplementation on E. coli and S. aureus-induced changes in the
mRNA expression levels of B cell lymphoma 2-associated X protein
(Bax) and survivin in Wistar rats
As shown in Fig. 4A and
B, camel milk upregulated the mRNA expression levels of Bax and
survivin. E. coli and S. aureus also significantly
increased the expression levels of Bax and survivin. Prior
supplementation with camel milk resulted in additive stimulatory
effects on the mRNA expression levels of Bax and survivin when
injected with E. coli and S. aureus (Fig. 4A and B).
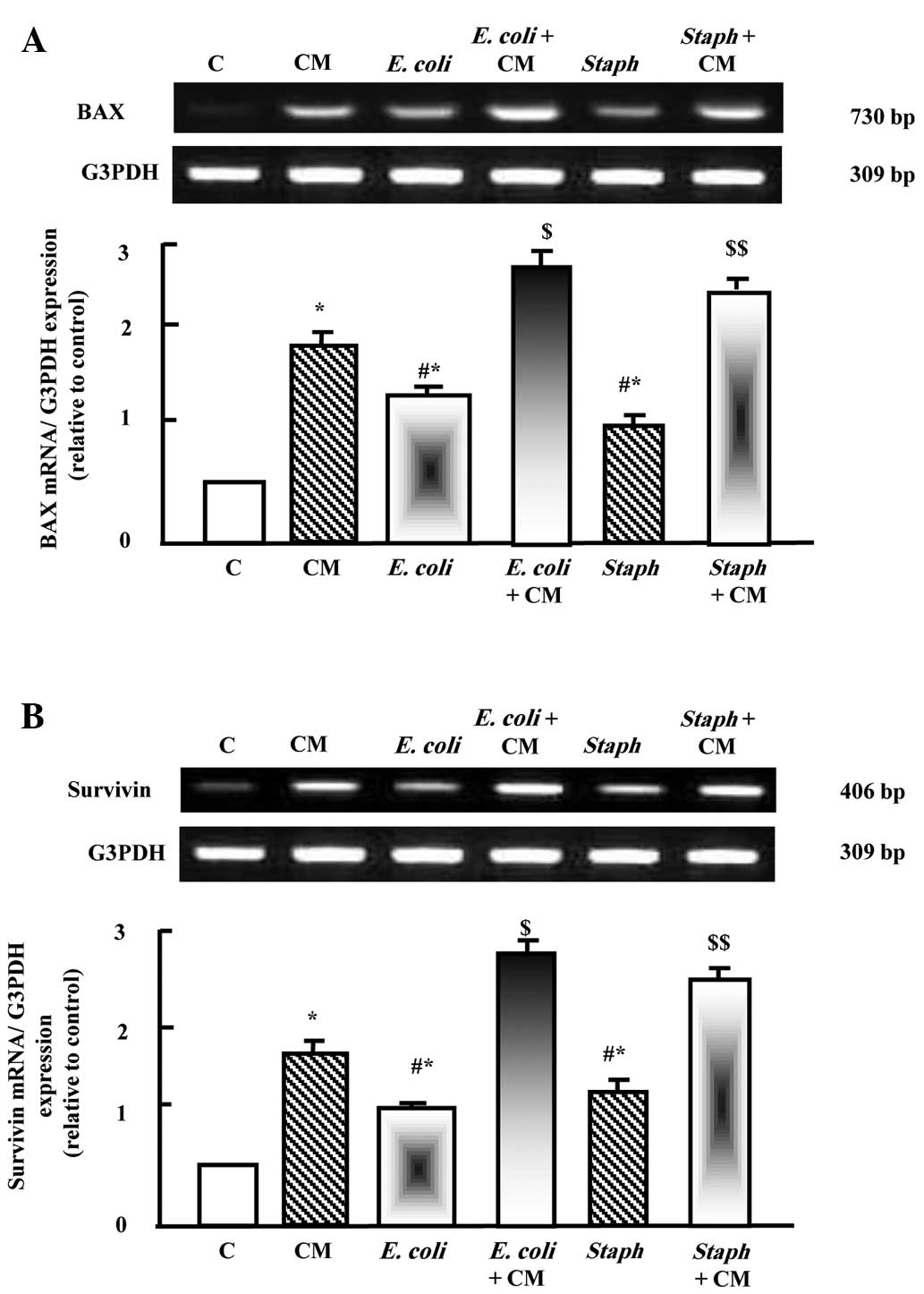 | Figure 4Protective effects of camel milk on
the expression levels of (A) Bax and (B) survivin in liver tissues
of rats injected with E. coli and S. aureus alone, or
with supplementation for 2 weeks prior to pathogen challenge. RNA
(2 µg) was extracted and reverse transcription-quantitative
polymerase chain reaction analysis was performed to quantify the
expression levels of Bax and survivin. Data are presented as the
mean ± standard error of the mean of three independent experiments.
*P<0.05, vs. control group;
#P<0.05, vs. CM group; $P<0.05,
vs. E. coli group; $$P<0.05, vs. S.
aureus group. C, control; CM, camel milk; Bax, B cell
lymphoma 2-associated X protein; E. coli, Escherichia coli; S.
aureus, Staphylococcus aureus. |
Discussion
The present study reported that camel milk
supplementation reversed the increase in oxidative stress induced
by E. coli and S. aureus infection. Furthermore, the
two pathogens induced a decrease in the expression of antioxidants
and affected the expression levels of inflammatory cytokines,
apoptotic, pro-apoptotic and anti-apoptotic genes. These changes
included normalization in the expression levels of antioxidants,
caspase-3, IL-6 and TGF-β. It is well-established that S.
aureus infections can spread through contact with pus from an
infected wound, skin-to-skin contact with an infected person due to
bacteria producing hyaluronidase that degrades tissues and contact
with objects, including towels, sheets, clothing or athletic
equipment, used by an infected individual (16). A large polysaccharide capsule
protects the organism from recognition by the immune defenses in
cows (15). E. coli is
gram-negative microorganism, which causes severe pathogenicity to
the infected host, and it has been reported that camel milk has a
bacteriostatic effect against E. coli and L.
monocytogenes (18).
Oxidative stress initiates apoptosis through
mitochondrial stress caused by free radicals (23,24),
which are indicated by levels of MDA. This involves a balance
between pro-apoptotic and anti-apoptotic proteins, which enhance
the permeability of the mitochondrial outer membrane for the
release of caspase activators (25). Caspase-3 has been identified as an
important contributor to apoptosis, in which activated caspase-3
causes the cell to undergo apoptosis through the cleavage of key
cellular proteins, including cytoskeletal proteins, leading to the
typical morphological changes observed in cells undergoing
apoptosis (25,26), which is counteracted by camel milk
supplementation.
Cytokines are low molecular weight proteins produced
by several types of cell (27) and
exhibit beneficial and pathological effects on target cells.
Imbalanced expression of cytokines has been implicated in the
progression of several diseases (28). During E. coli and S.
aureus pathogenicity, increased expression levels of TGF-β1 and
IL-6 were reported in the present study. The mRNA expression levels
of IL-6 increased following E. coli and S. aureus
injection, and prior supplementation with camel milk normalized
these increases in IL-6 and induced additive effect on TGF-β1
expression. TGF-β1 performs numerous cellular functions, including
the control of cell growth, cell proliferation, cell
differentiation and apoptosis (29). TGF-β1 can be regarded as an early
mediator of the inflammatory response (30). TGF-β1 is one of the major
pro-fibrogenic cytokines in various tissues, and is implicated in
the etiology of pancreatic fibrosis, function of leukocyte
chemotaxis, and fibroblast and smooth muscle cell mitogenesis
(31–33). In the present study, camel milk
regulated the expression levels of TGF-β1 and IL-6, thereby
controlling the inflammation and apoptosis induced by E.
coli and S. aureus injection. Previous studies have
reported that camel milk is the most active milk against E.
coli, S. aureus, Salmonella typhimurium and rotavirus (1,34).
It has also been demonstrated that camel milk, in addition to
secretory immunoglobulin (Ig)A and IgM, also contains numerous
non-antibody components, which possess antiviral activity,
including lactoferrin (34).
Apoptosis is an evolutionary conserved process by
which organisms remove cells that are superfluous, have outlived
their usefulness, or are dangerous for the survival of the organism
(35). The apoptotic process can
occur intracellularly, involving the release of several factors,
including caspase 3 and 6 from mitochondria, which can be activated
by various stressors, and pro-apoptotic proteins, including Bax,
which migrate from the inter-membrane space of the mitochondria
into the cytosol to act as sensors of cell damage or stress
(35,36).
During infection, cytochrome c binds the
adaptor protein, apoptotic protease-activating factor-1, forming a
large multi-protein structure known as the apoptosome (25). The apoptosome then recruits and
activates caspase-9, which in turn activates downstream effector
caspases, including caspases-3 and 7, leading to apoptosis
(25). Under normal conditions,
caspase activity is controlled by a protein family known as
inhibitor of apoptosis proteins, among which is survivin (37). Anti-apoptotic Bcl-2 and Bcl-xL
proteins act to prevent permeabilization of the mitochondrial outer
membrane by inhibiting the action of pro-apoptotic Bax, a cytosolic
protein, located in the mitochondrial membrane (38). It has been reported that caspase-3
inhibits reactive oxygen species production, and is required for
efficient execution of apoptosis (39). The survivin protein acts to inhibit
caspase activation, thereby leading to negative regulation of
apoptosis and/or programmed cell death (40), which was concordant with the
results of the present study. The present study demonstrated that
camel milk upregulated the gene expression of pro-apoptotic Bax, in
order to control and regulate the gene expression of anti-apoptotic
survivin. Camel milk exhibited beneficial effects when supplemented
during E. coli and S. aureus infection.
In conclusion, the present study demonstrated that
camel milk had protective effects against pathogenicity induced by
E. coli and S. aureus in Wistar rats. The protective
effects occurred through the regulation of antioxidant genes, genes
associated with apoptosis/anti-apoptosis, and the expression of
cytokines associated with inflammation and the host defense
mechanism. Future in vitro studies are required to elucidate
the signaling mechanisms underlying the effects of camel milk.
Acknowledgments
The present study was supported by a grant from the
The Deans of Scientific Affairs, Taif University, Kingdom of Saudi
Arabia (grant no. 3281-1-1435).
References
|
1
|
el Agamy EI, Ruppanner R, Ismail A,
Champagne CP and Assaf R: Antibacterial and antiviral activity of
camel milk protective proteins. J Dairy Res. 59:169–175. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kappeler S, Farah Z and Puhan Z:
Alternative splicing of lactophorin mRNA from lactating mammary
gland of the camel (Camelus dromedarius). J Dairy Sci.
82:2084–2093. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yagil R, Saran A and Etzion Z: Camel's
milk: For drinking only? Comp Biochem Physiol A Comp Physiol.
78:263–266. 1984. View Article : Google Scholar
|
|
4
|
Korhonen H and Pihlanto A: Food-derived
bioactive peptides-opportunities for designing future foods. Curr
Pharm Des. 9:1297–1308. 2003. View Article : Google Scholar
|
|
5
|
Omer RH and Eltinay AH: Changes in
chemical composition of Camel's raw milk during storage. Pak J
Nutr. 8:607–610. 2009. View Article : Google Scholar
|
|
6
|
Rao MB, Gupta RC and Dastur NN: Camel milk
and milk products. Indian J Dairy Sci. 23:71–78. 1970.
|
|
7
|
Al-Hashem F: Camel milk protects against
aluminium chloride-induced toxicity in the liver and kidney of
white albino rats. Am J Biochem Biotechnol. 5:98–108. 2009.
View Article : Google Scholar
|
|
8
|
Dallak MA, Bin-Jaliah I, Al-Khateeb MA,
Nwoye LO, Shatoor AS, Soliman HS and Al-Hashem FH: In vivo acute
effects of orally administered hydro-ethanol extract of Catha
edulis on blood glucose levels in normal, glucose-fed hyperglycemic
and alloxan-induced diabetic rats. Saudi Med J. 31:627–633.
2010.PubMed/NCBI
|
|
9
|
Khan AA and Alzohairy M: Hepatoprotective
effects of camel milk against CCl4-induced hepatotoxicity in Rats.
Asian J Biochem. 6:171–180. 2011. View Article : Google Scholar
|
|
10
|
Aff MEM: Effect of camel's milk on
cisplatin-induced nephrotoxicity in swiss albino mice. Am J Biochem
Biotechnol. 6:1472010.
|
|
11
|
Al-Fartosi KG, Khuon OS and Al-Tae HI:
Protective role of camel's milk against paracetamol induced
hepatotoxicity in male rats. Int J Res Pharmaceut Biomed Sci.
2:1795–1799. 2011.
|
|
12
|
Kappeler SR, Heuberger C, Farah Z and
Puhan Z: Expression of the peptidoglycan recognition protein, PGRP,
in the lactating mammary gland. J Dairy Sci. 87:2660–2668. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Velioglu Ogünç A, Manukyan M, Cingi A,
Eksioglu-Demiralp E, Ozdemir Aktan A and Süha Yalçin A: Dietary
whey supplementation in experimental models of wound healing. Int J
Vitam Nutr Res. 78:70–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korashy HM, Maayah ZH, Abd-Allah AR,
El-Kadi AO and Alhaider AA: Camel milk triggers apoptotic signaling
pathways in human hepatoma HepG2 and breast cancer MCF7 cell lines
through transcriptional mechanism. J Biomed Biotechnol.
2012:5931952012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cenci-Goga BT, Karama M, Rossitto PV,
Morgante RA and Cullor JS: Enterotoxin production by Staphylococcus
aureus isolated from mastitic cows. J Food Prot. 66:1693–1696.
2003.PubMed/NCBI
|
|
16
|
Cimolai N: MRSA and the environment:
Implications for comprehensive control measures. Eur J Clin
Microbiol Infect Dis. 27:481–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Curran JP and Al-Salihi FL: Neonatal
staphylococcal scalded skin syndrome: Massive outbreak due to an
unusual phage type. Pediatrics. 66:285–290. 1980.PubMed/NCBI
|
|
18
|
Noreddine B, Majda M, Nargisse B and Kamal
H: Antimicrobial activity of camel's milk against pathogenic
strains of Escherichia coli and Listeria monocytogenes. Int J Dairy
Infect. 5:39–43. 2004.
|
|
19
|
Althnaian T, Albokhadaim I and El-Bahr SM:
Biochemical and histopathological study in rats intoxicated with
carbontetrachloride and treated with camel milk. SpringerPlus.
2:572013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cirioni O, Giacometti A, Ghiselli R,
Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A,
Skerlavaj B, Rocchi M, et al: LL-37 protects rats against lethal
sepsis caused by gram-negative bacteria. Antimicrob Agents
Chemother. 50:1672–1679. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hari Prasad O, Navya A, Vasu D and
Chiranjeevi T: Protective effects of Prosopis juliflora against
Staphylococcus aureus induced hepatotoxicity in rats. Int J Pharm
Biomed Res. 2:172–178. 2011.
|
|
22
|
Soliman MM, Baiyoumi AA and Yassin MH:
Molecular and histopathological study on the ameliorative effects
of curcumin against lead acetate-induced hepatotoxicity and
nephrototoxicity in wistar rats. Biol Trace Elem Res. 167:91–102.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsang WP, Chau SP, Kong SK, Fung KP and
Kwok TT: Reactive oxygen species mediate doxorubicin induced
p53-independent apoptosis. Life Sci. 73:2047–2058. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vecchione A and Croce CM: Apoptomirs:
Small molecules have gained the license to kill. Endocr Relat
Cancer. 17:F37–F50. 2010. View Article : Google Scholar
|
|
26
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Feghali CA and Wright TM: Cytokines in
acute and chronic inflammation. Front Biosci. 2:d12–d26.
1997.PubMed/NCBI
|
|
28
|
Arend WP and Gabay C: Cytokines in the
rheumatic diseases. Rheum Dis Clin North Am. 30:41–67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frieboes RM, Murck H, Maier P, Schier T,
Holsboer F and Steiger A: Growth hormone-releasing peptide-6
stimulates sleep, growth hormone, ACTH and cortisol release in
normal man. Neuroendocrinology. 61:584–589. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Itoh H, Pratt RE and Dzau VJ: Atrial
natriuretic polypeptide inhibits hypertrophy of vascular smooth
muscle cells. J Clin Invest. 86:1690–1697. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aoki H, Ohnishi H, Hama K, Ishijima T,
Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H, et al:
Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and
ERK-dependent pathways in rat pancreatic stellate cells. Am J
Physiol Cell Physiol. 290:C1100–C1108. 2006. View Article : Google Scholar
|
|
32
|
Distler JH, Hirth A, Kurowska-Stolarska M,
Gay RE, Gay S and Distler O: Angiogenic and angiostatic factors in
the molecular control of angiogenesis. Q J Nucl Med. 47:149–161.
2003.PubMed/NCBI
|
|
33
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Conesa C, Sánchez L, Rota C, Pérez MD,
Calvo M, Farnaud S and Evans RW: Isolation of lactoferrin from milk
of different species: Calorimetric and antimicrobial studies. Comp
Biochem Physiol B Biochem Mol Biol. 150:131–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Henry-Mowatt J, Dive C, Martinou JC and
James D: Role of mitochondrial membrane permeabilization in
apoptosis and cancer. Oncogene. 23:2850–2860. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Karst AM and Li G: BH3-only proteins in
tumorigenesis and malignant melanoma. Cell Mol Life Sci.
64:318–330. 2007. View Article : Google Scholar
|
|
37
|
Lavrik IN, Golks A and Krammer PH:
Caspases: Pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reed JC: Bcl-2 family proteins. Oncogene.
17:3225–3236. 1998. View Article : Google Scholar
|
|
39
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sah NK, Khan Z, Khan GJ and Bisen PS:
Structural, functional and therapeutic biology of survivin. Cancer
Lett. 244:164–171. 2006. View Article : Google Scholar : PubMed/NCBI
|