Introduction
The process of chronic renal diseases is typically
accompanied with progressive renal fibrosis and the inhibition of
fibrogenesis appears to be an attractive therapeutic target.
Re-absorption of albumin by renal epithelial cells is important in
the progression of renal tubular fibrosis caused by proteinuria
(1). Mycophenolate mofetil (MMF),
a pro-drug of mycophenolic acid (MPA), is one of the most
frequently used immunosup-pressive drugs for the prophylaxis of
allograft rejection after renal, cardiac or liver transplantation.
It is known that MPA is a potent, selective, non-competitive and
reversible inhibitor of inosine-5′-monophosphate dehydrogenase. MPA
inhibits not only the proliferation of lymphocytes, but also that
of other mesenchymal cells (2–4).
Transforming growth factor beta 1 (TGF-β1) has a central
role in fibrosis. Following combination of TGF-β1 with
its receptor, numerous signaling pathways are activated, including
the Smad signaling pathway and the phosphoinositide-3 kinase
(PI3K)/Akt pathway (5–7). Nuclear factor-κB (NF-κB) is a
transcription factor associated with the production of inflammatory
factors, cell proliferation and apoptosis, which is involved in
numerous processes of inflammatory signal transduction. Activation
of the transcription factor NF-κB is known to drive renal
inflammation and fibrosis (8).
Akt, a member of the serine/threonine protein kinase superfamily
and the PI3K/Akt signaling pathway, has important roles in cell
proliferation, differentiation, metabolism and apoptosis (9).
In spite of previous evidence of albumin-induced
expression of TGF-β1 and NF-κB, this mechanism has
remained to be demonstrated in renal epithelial cells (10). The present study assessed the
effects of MPA on NRK52E rat renal epithelial cell line in order to
test the hypothesis that MPA inhibits albumin-induced expression of
TGF-β1 and activation of NF-κB in renal epithelial cells
through the Akt pathway.
Materials and methods
Chemicals and reagents
The NRK52E normal rat kidney epithelial-derived cell
line was obtained from the American Type Culture Collection
(CRL-1571; Manassas, VA, USA). Dulbecco's modified Eagle's medium,
nutrient mixture F-12 (DMEM/F12; 1:1), fetal bovine serum (FBS) and
trypsin/EDTA solution were purchased from GE Healthcare (Little
Chalfont, UK). TRIzol reagent were purchased from Invitrogen
(Thermo Fisher Scientific, Waltham, MA, USA). Primers were obtained
from Sangon Biological Engineering Technology and Services
(Shanghai, China). Rabbit anti-rat phosphorylated (p)-Akt
monoclonal antibody (cat. no. 4060) and rabbit anti-rat β-actin
monoclonal antibody (cat. no. 4970) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Nuclear proteins
were extracted using the N-Extract kit (Sigma-Aldrich, St. Louis,
MO, USA) and total protein concentration was determined using the
Bio-Rad detergent-compatible protein assay (Bio-Rad Laboratories,
Hercules, CA, USA). Akt inhibitor Ly294002 (9) and MPA were purchased from
Sigma-Aldrich.
Cell culture and reagents
NRK52E cells were maintained in monolayer culture in
75 cm2 Falcon T-flasks (Thermo Fisher Scientific)
containing DMEM/F-12 supplemented with 4% fetal calf serum, 15
mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 20
mmol/l sodium bicarbonate, 0.5 mmol/l sodium pyruvate, 17.5 mmol/l
glucose, streptomycin and penicillin (Invitrogen; Thermo Fisher
Scientific) at 37°C in an incubator with a humidified atmosphere
with 5% CO2 in air. The cells were grown to 40%
confluence, washed with serum and sodium pyruvate-free DMEM/F-12,
and subsequently incubated with MPA or Ly294002. Untreated cells
served as a control.
Experimental groups and treatments
NRK52E cells were divided into the following groups:
Control group (untreated); albumin group (treated with 30 mg/ml
albumin; Sigma-Aldrich); Ly294002 group (treated with 10
µmol/l Ly294002 for 30 min and then with 30 mg/ml albumin);
and the MPA group (30 mg/ml albumin + 10 µmol/l MPA).
Following incubation for 12 h, cells were harvested for the
subsequent assays.
Reverse-transcription polymerase chain
reaction (RT-PCR)
Total RNA was extracted from NRK52E cells using
TRIzol following the manufacturer's instructions. Subsequently, 2
µg total RNA was reverse-transcribed with avian myoblastosis
virus reverse transcriptase (Promega Corporation, Madison, WI,
USA). PCR amplification was then performed; in brief, 50 pmol/l PCR
primers for β-actin and TGF-β1 were added to each
reaction mixture containing 0.2 mmol/l deoxynucleoside
triphosphates (Promega Corporation), 3 mmol/l MgSO4 and
1 U DNA polymerase (Promega Corporation). The sequences of primers
were as follows: TGF-β1 forward,
5′-GGCAGTGGTTGAGCCGTGGA-3′ and reverse, 5′-TGTTGGACAGCTGCTCCACT-3′
(590 bp); β-actin forward, 5′-TCGGACGATATGGAGAAGAT-3′ and reverse,
5′-ATTGCCGATAGTGATGACGT-3′ (240 bp). The PCR cycling conditions
were as follows: Initial denaturation at 94°C for 5 min followed by
30 cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 1 min, and
a final elongation step at 72°C for 10 min. The PCR was conducted
using an LC480 PCR machine (Roche, Basel, Switzerland). The mRNA
expression levels of the target genes were estimated by the optical
density values of the bands in the agarose gels.
Western blot analysis
Following harvesting, cells were lysed in cell lysis
buffer included in the N-Extract kit (Sigma-Aldrich) and the
protein content was determined using a Bradford Protein Assay
(Bio-Rad detergent-compatible protein assay; Bio-Rad Laboratories).
Subsequently, equal amounts of protein (50 µg) were
separated by 12% SDS-PAGE and electrotransferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA).
Following blocking with 5% non-fat milk in phosphate-buffered
saline with 0.05% Tween 20 for 30 min at room temperature, the
membrane was incubated with rabbit anti-rat TGF-β1 monoclonal
antibody (cat. no. 3709; Cell Signaling Technology, Inc.; 1:1,000
dilution) or rabbit anti-rat Akt monoclonal antibody (cat. no.
4685; Cell Signaling Technology, Inc.; 1:2,000 dilution), followed
by incubation with peroxidase-conjugated AffiniPure goat
anti-rabbit IgG, (1:10,000 dilution; cat. no. 111035003; Jackson
ImmunoResearch Inc., West Grove, PA, USA) secondary antibody at
room temperature for 1 h. Blots were then visualized using an
enhanced chemiluminescence detection system (Active Motif,
Carlsbad, CA, USA). Quantification of protein levels was performed
by determining the relative optical density of the protein bands
was using an image analysis system (Image J, version 1.48; National
Institutes of Health, Bethesda, MD, USA).
Electrophoretic mobility shift assay
(EMSA)
Following the indicated treatments, NRK52E cells
were assayed for NF-κB activation using EMSA. Nuclear extracts were
hybridized with [32P]-labeled oligonucleotides
containing the sequence GTTGAGGGGACTTTCCCAGGC from the NF-κB
promoter or a mutated NF-κB sequence TCAACTCCCCTGAAAGGGTCCG in
binding buffer (Rockland Immunochemicals, Inc., Limerick, PA, USA).
These were radiolabeled with [γ32P] adenosine
triphosphate by T4 polynucleotide kinase for 10 min at 37°C. All
reactions were performed in a total volume of 20 µl
containing the binding buffer [10 mM Tris/HCl, pH 7.5, 100 mM NaCl,
1 mM EDTA, 4% (v/v) glycerol, 5 mM dithiothreitol and 100
µg/ml bovine serum albumin (Sigma-Aldrich)]. Each sample
contained 2 µl [32P]-labeled oligonucleotide and
3 µg poly(dI-dC). After incubation for 15 min at room
temperature, samples were electrophoresed on a 5% polyacrylamide
gel/0.25X Tris borate EDTA (pH 8.0) (EMD Millipore). For
competition experiments, unlabeled oligonucleotides were incubated
with extracts for five minutes prior to the addition of
radiolabeled probe. After electrophoresis, gels were dried and the
protein bands were visualized using Immobilon Western HRP substrate
(EMD Millipore) and autoradiographed by exposure to medical X-ray
film. The obtained bands were quantified using the Luminescent
Image Analyzer-LAS 4000 and Image Gauge software, version 3.1
(Fujifilm Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS,
version 17.0 (SPSS, Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard deviation. Significance between groups was
determined using one-way analysis of variance. The Q-test was used
to analyze differences in the mean values between groups. P<0.05
was considered to indicate a statistically significant difference
between values. Each experiment was repeated three times.
Results
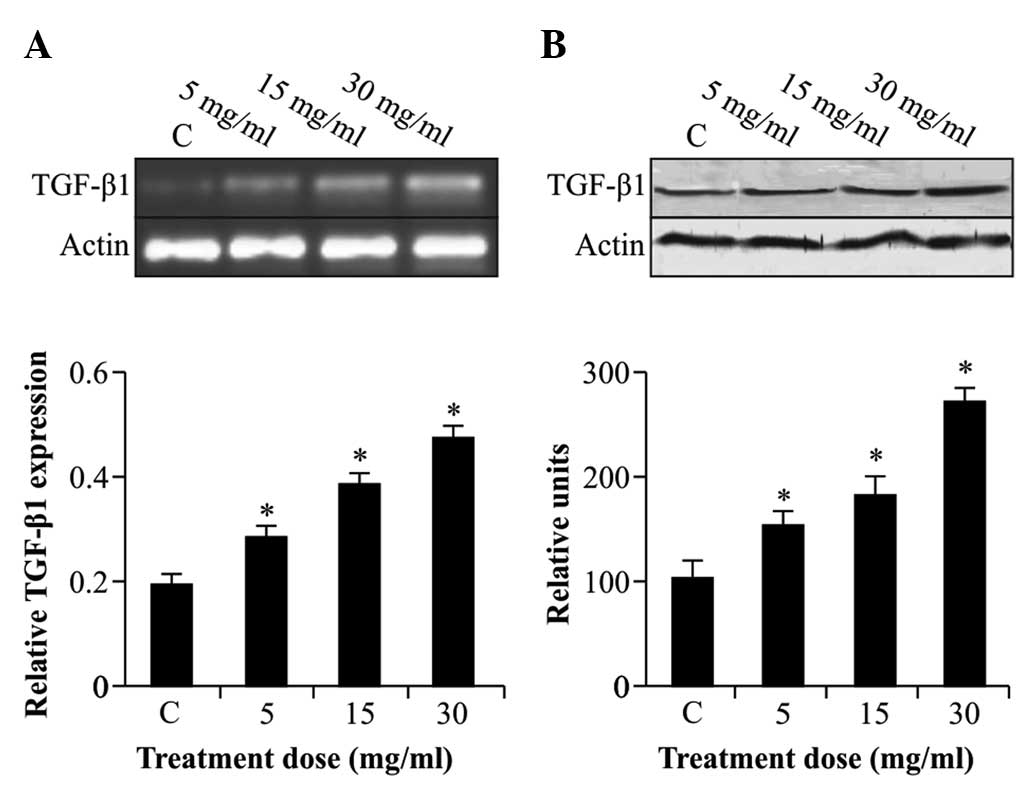
Albumin stimulates the expression of
TGF-β1 mRNA and protein in rat kidney epithelial
cells
Analysis of TGF-β1 showed that NRK52E
cells which were not stimulated with albumin expressed
TGF-β1 mRNA and protein at relatively low levels
(Fig. 1). However,
TGF-β1 expression was significantly enhanced by albumin
(5–30 mg/ml) in a dose-dependent manner (P<0.05).
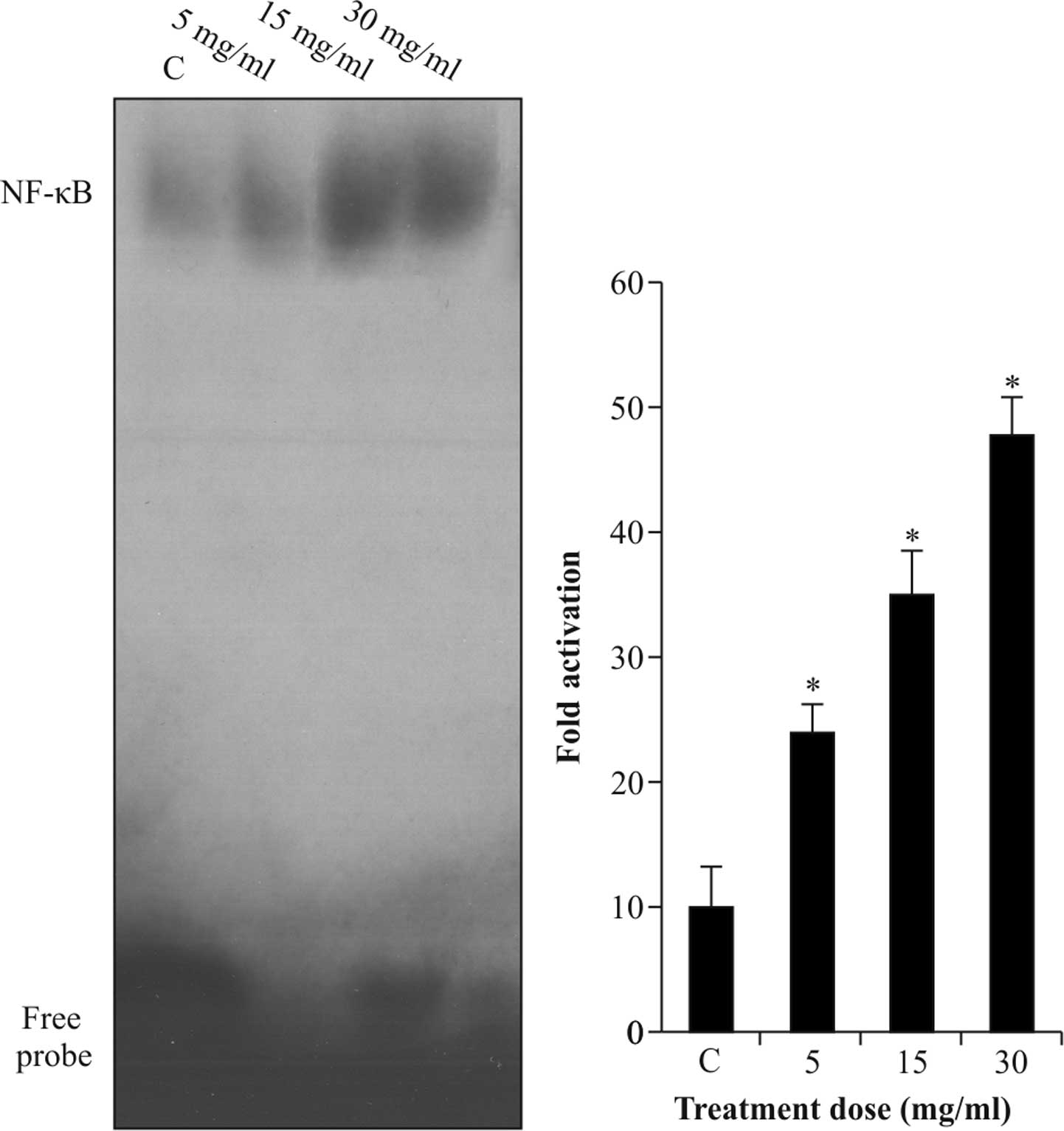
Albumin activates NF-κB protein in rat
kidney epithelial cells
Activation of NF-κB to bind to the promoter region
of TGF-β1 was assessed using an EMSA. As shown in
Fig. 2, NRK-52E cells which were
not stimulated with albumin exhibited relatively low DNA-binding
activity of NF-κB protein, which was enhanced by albumin (5–30
mg/ml) in a dose-dependent manner with increases of up to five-fold
(P<0.05).
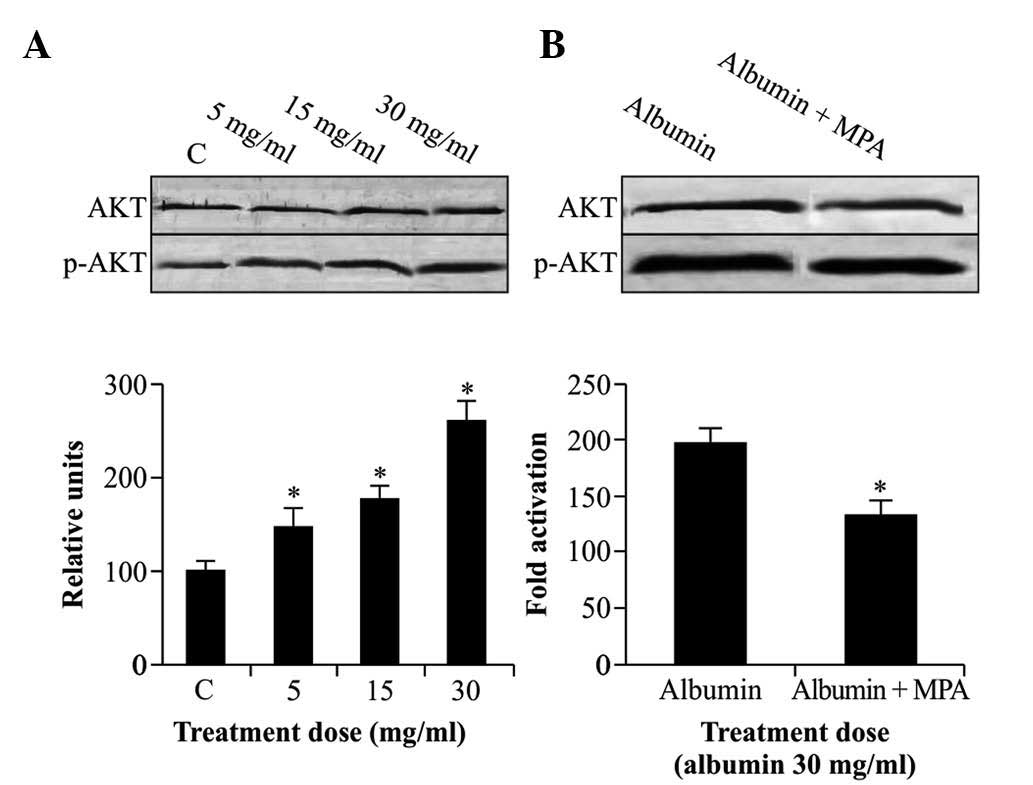
MPA reduces albumin-induced Akt
phosphorylation
Activation of Akt in NRK52E cells was assessed by
western blot analysis using antibodies with specificity for Akt or
activated p-Akt only. As shown in Fig.
3A, albumin (5–30 mg/ml) significantly activated Akt in a
dose-dependent manner following 12 h of incubation. When cells were
starved and treated with albumin (30 mg/ml) for 12 h in the
presence of 10 µmol/l MPA, the phosphorylation of Akt was
significantly inhibited (P<0.05) (Fig. 3B), while no marked effects on the
levels of total Akt protein were observed.
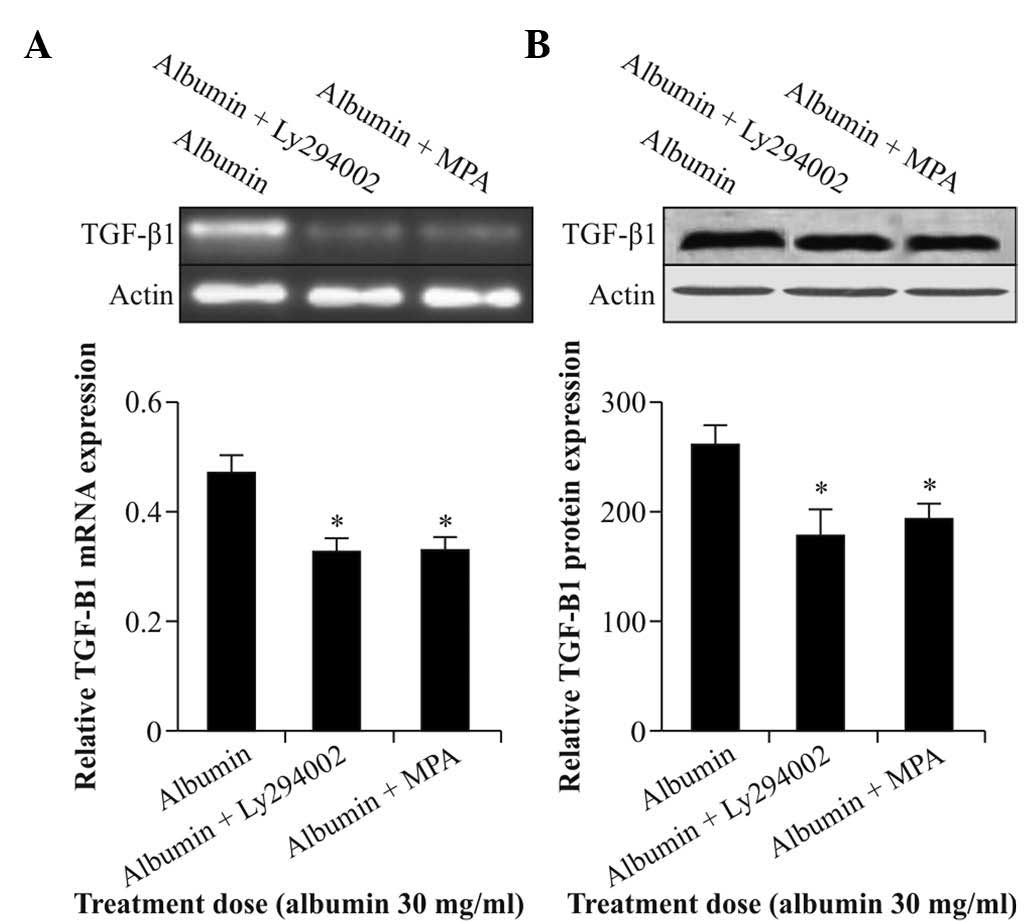
MPA and Akt inhibitor Ly294002 reduce
albumin-induced TGF-β1 expression
To test the involvement of Akt in albumin-induced
TGF-β1 expression in NRK52E cells, the Akt inhibitor
Ly294002 was used. Cells were starved and treated with albumin (30
mg/ml) for 12 h in the presence of 10 µmol/l MPA or 10
µmol/l Ly294002. The results showed that Ly294002 as well as
MPA significantly inhibited the expression of TGF-β1
mRNA and protein (Fig. 4A and B).
As Akt inhibitor Ly294002 was able to inhibit albumin-induced
expression of TGF-β1, it was indicated that the Akt
pathway is involved in albumin-induced expression of
TGF-β1. It is further hypothesized that MPA may also
exert its effects on albumin-induced expression of
TGF-β1 via the Akt pathway, which requires to be
verified in future studies.
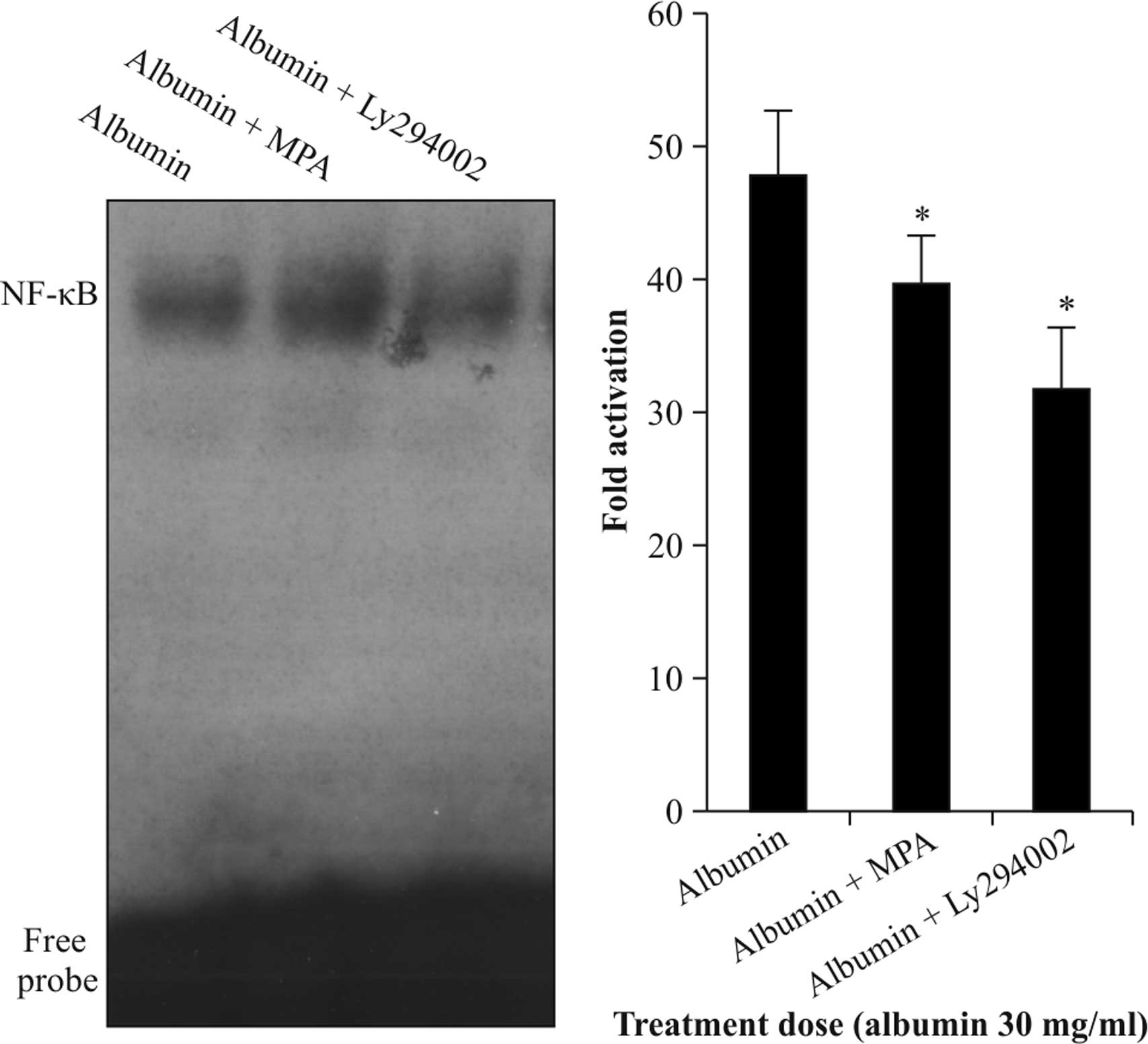
Akt inhibitor Ly294002 and MPA inhibit
albumin-induced NF-κB activation
NRK52E cells were starved and treated with albumin
(30 mg/ml) for 12 h in the presence of 10 µmol/l MPA or
Ly294002. The stimulatory effects of albumin on the DNA-binding
activity of NF-κB were significantly blocked by Ly294002
(P<0.05). MPA also significantly inhibited albumin-induced NF-κB
activation (P<0.05) (Fig. 5).
These results further confirmed that albumin exerts its effects on
NF-κB activity via the Akt pathyway; whether MPA inhibits the
activation of NF-κB by albumin via the Akt pathway requires to be
confirmed in future studies.
Discussion
Re-absorbtion of albumin following proteinuria is
closely correlated with the imbalance of proliferation/apoptosis
and phenotypic differentiation of renal tubular epithelial cells,
as well as the infiltration of inflammatory cells. Injured renal
tubular epithelial cells cultured in vitro have a high rate
of proliferation and secrete large amounts of extracellular matrix,
which results in fibrosis (1).
Simultaneously, the injured renal cells transdifferentiate into
muscular fibroblasts which secrete large amounts of growth factors,
thereby amplifying the local immunoinflammatory reaction and
accelerating the process of fibrosis (11,12).
Proteinuria is a well-known exacerbating factor in renal tubular
interstitial disease (13). Renal
interstitial fibrosis is a common pathological process in
progressive renal diseases, which leads to functional deterioration
of renal cells and eventual loss of renal function (14–17).
MPA, the active metabolite of MMF, is a potent,
non-competitive and reversible inhibitor of
inosine-5′-monophosphate dehydrogenase, the rate-limiting enzyme
for de novo purine synthesis. MPA has an effect on cell
growth and chemokine release of tubular epithelial cells, and these
effects are dependent on the inhibition of cellular guanosine
production. However, limited data are available on the effects of
MPA on renal tubular epithelial cells (4,18).
TGF-β1 is a fibrogenic and inflammatory
cytokine with a central role in the pathogenesis of renal fibrosis
(19). It has been demonstrated
that treatment with TGF-β1 antibody restrained the
function of TGF-β1 to reduce the degree of tubular
fibrosis (20). A further study
showed that intraperitoneal injection of TGF-β induced renal
fibrosis in mice (21). In the
present study, albumin was shown to stimulate the expression of
TGF-β1 in tubular epithelial cells in a dose-dependent
manner, indicating that albumin triggers mechanisms in renal
tubular epithelial cells leading to fibrotic injury. This mechanism
may be the underlying reason for proteinuria causing renal
interstitial fibrosis. Furthermore, the present study demonstrated
that MPA inhibited TGF-β1 expression, thereby
potentially preventing fibrotic injury.
The promoter region of the TGF-β1 gene
contains NF-κB-binding sites. NF-κB is a transcription factor which
consists of a p50 and a p65 sub-unit. NF-κB regulates the
production of pro-inflammatory mediators in cellular inflammation.
Activation of NF-κB drives renal inflammation and fibrosis
(22). In the resting stage, NF-κB
exists in its inactive form in the cytoplasm; however, it becomes
activated when cells are stimulated (23). The present study revealed that
albumin overload can stimulated the binding activity of NF-κB to
the promoter region of TGF-β1. Furthermore, MPA was able
to inhibit albumin-induced activation of NF-κB protein.
Akt is a serine/threonine protein kinase which is
mainly responsible for the initiation of biological signal
transmission by PI3K. Akt is in the central axis of the Akt/PI3K
pathway, with its functions including cell cycle regulation,
induction of apoptosis, and the participation in numerous important
physiological and pathological process, including angiogenesis,
telomerase activity and malignant characteristics of cells
(24). Akt also regulates cell
activation and proliferation through NF-κB (25). It has been reported that Akt
increases the phosphorylation of inhibitor of NF-κB (IκB) and
reduces IκB protein synthesis to activate the NF-κB. Abnormal
activation of Akt has an important role in processes leading to
renal fibrosis (26). The present
study suggested that albumin significantly enhanced the level of
Akt activation in NRK52E cells following l2 h in a dose-dependent
manner. This result suggested that Akt phosphorylation has an
important role in processes of renal tubular epithelial cell
injury. Treatment with Ly294002, a specific inhibitor of Akt,
inhibited the expression of TGF-β1 and activation of
binding of NF-κB to the promoter region of TGF-β1. This
result indicated that albumin induced the synthesis of
TGF-β1 and the activation of NF-κB in tubular epithelial
cells partly through the phosphorylation of Akt.
The present study showed that albumin significantly
increased TGF-β1 expression and NF-κB activation.
Furthermore, MPA and Ly294002 inhibited TGF-β1
expression and NF-κB activation. In addition albumin significantly
enhanced Akt activation, which was inhibited by MPA. The
observation that the Akt inhibitor effectively inhibited
albumin-induced TGF-β1 expression and NF-κB activation
leads to the hypothesis that MPA exerts its anti-fibrotic effects,
at least partially, by inhibiting Akt activation; however, this
remains to be experimentally verified in future studies.
In conclusion, the present study demonstrated that
MPA inhibited albumin-induced TGF-β1 expression and the
binding of NF-κB to the promoter region of TGF-β1,
possibly through inhibiting Akt activation.
Acknowledgments
The current study was supported by the Key
Foundation of Hubei Nature Scientific Funds (grant no.
2013CFB227).
References
|
1
|
Abbate M, Zoja C and Remuzzi G: How does
proteinuria cause progressive renal damage. J Am Soc Nephrol.
17:2974–2984. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waller JR, Brook NR, Bicknell GR, Murphy
GJ and Nicholson ML: Mycophenolate mofetil inhibits intimal
hyperplasia and attenuates the expression of genes favouring smooth
muscle cell proliferation and migration. Transplant Proc.
37:164–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Roos N, Poulalhon N, Farge D, Madelaine I,
Mauviel A and Verrecchia F: In vitro evidence for a direct
antifibrotic role of the immunosuppressive drug mycophenolate
mofetil. J Pharmacol Exp Ther. 321:583–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrova DT, Brandhorst G, Brehmer F, Gross
O, Oellerich M and Armstrong VW: Mycophenolic acid displays
IMPDH-dependent and IMPDH-independent effects on renal fibroblast
proliferation and function. Ther Drug Monit. 32:405–412. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell Res. 19:71–88. 2009.
View Article : Google Scholar
|
|
6
|
Assinder SJ, Dong Q, Kovacevic Z and
Richardson DR: The TGF-beta, PI3K/Akt and PTEN pathways:
Established and proposed biochemical integration in prostate
cancer. Biochem J. 417:411–421. 2009. View Article : Google Scholar
|
|
7
|
Danielpour D: Functions and regulation of
transforming growth factor-beta (TGF-beta) in the prostate. Eur J
Cancer. 41:846–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nowak DE, Tian B, Jamaluddin M, Boldogh I,
Vergara LA, Choudhary S and Brasier AR: RelA Ser276 phosphorylation
is required for activation of a subset of NF-kappaB-dependent genes
by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol
Cell Biol. 28:3623–3638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dummler B and Hemmings BA: Physiological
roles of PKB/Akt isoforms in development and disease. Biochem Soc
Trans. 35:231–235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takase O, Marumo T, Imai N, Hirahashi J,
Takayanagi A, Hishikawa K, Hayashi M, Shimizu N, Fujita T and
Saruta T: NF-kappaB-dependent increase in intrarenal angiotensin II
induced by proteinuria. Kidney Int. 68:464–473. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burton CJ, Harper SJ, Bailey E, Feehally
J, Harris KP and Walls J: Turnover of human tubular cells exposed
to proteins in vivo and in vitro. Kidney Int. 59:507–514. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ,
Kalluri R, Müller GA and Neilson EG: Role of basic fibroblast
growth factor-2 in epithelial-mesenchymal transformation. Kidney
Int. 61:1714–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tareeva IE, Kutyrina IM, Nikolaev Alu,
Lifshits NL and Shvetsov MIu: Ways to inhibit development of
chronic renal failure. Ter Arkh. 72:9–14. 2000.In Russian.
|
|
14
|
Li MX and Liu BC: Epithelial to
mesenchymal transition in the progression of tubulointerstitial
fibrosis. Chin Med J (Engl). 120:1925–1930. 2007.
|
|
15
|
Eddy AA: Molecular basis of renal
fibrosis. Pediatr Nephrol. 15:290–301. 2000. View Article : Google Scholar
|
|
16
|
Klahr S and Morrissey J: Progression of
chronic renal disease. Am J Kidney Dis. 41(3 Suppl 1): S3–S7. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Owen WF Jr: Patterns of care for patients
with chronic kidney disease in the United States: Dying for
improvement. J Am Soc Nephrol. 14(7 Suppl 2): S76–S80. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baer PC, Gauer S, Hauser IA, Scherberich
JE and Geiger H: Effects of mycophenolic acid on human renal
proximal and distal tubular cells in vitro. Nephrol Dial
Transplant. 15:184–190. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qi W, Chen X, Poronnik P and Pollock CA:
Transforming growth factor-beta/connective tissue growth factor
axis in the kidney. Int J Biochem Cell Biol. 40:9–13. 2008.
View Article : Google Scholar
|
|
20
|
Isaka Y, Tsujie M, Ando Y, Nakamura H,
Kaneda Y, Imai E and Hori M: Transforming growth factor-beta 1
antisense oligodeoxynucleotides block interstitial fibrosis in
unilateral ureteral obstruction. Kidney Int. 58:1885–1892. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Samarakoon R, Overstreet JM, Higgins SP
and Higgins PJ: TGF-β1→SMAD/p53/USF2→PAI-1 transcriptional axis in
ureteral obstruction-induced renal fibrosis. Cell Tissue Res.
347:117–128. 2012. View Article : Google Scholar
|
|
22
|
Ma FY, Tesch GH, Ozols E, Xie M, Schneider
MD and Nikolic-Paterson DJ: TGF-β1-activated kinase-1 regulates
inflammation and fibrosis in the obstructed kidney. Am J Physiol
Renal Physiol. 300:F1410–F1421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen ZJ: Ubiquitin signalling in the
NF-kappaB pathway. Nat Cell Biol. 7:758–765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hirai K, Hayashi T, Chan PH, Zeng J, Yang
GY, Basus VJ, James TL and Litt L: PI3K inhibition in neonatal rat
brain slices during and after hypoxia reduces phospho-Akt and
increases cytosolic cytochrome c and apoptosis. Brain Res Mol Brain
Res. 124:51–61. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zdychová J and Komers R: Emerging role of
Akt kinase/protein kinase B signaling in pathophysiology of
diabetes and its complications. Physiol Res. 54:1–16.
2005.PubMed/NCBI
|
|
26
|
Runyan CE, Schnaper HW and Poncelet AC:
The phosphati-dylinositol 3-kinase/Akt pathway enhances
Smad3-stimulated mesangial cell collagen I expression in response
to transforming growth factor-beta1. J Biol Chem. 279:2632–2639.
2004. View Article : Google Scholar
|