Introduction
The autoimmune regulator (Aire) protein is a
transcription factor that is highly expressed in medullary thymic
epithelial cells (mTECs) (1). Aire
is important in deleting autoreactive T cells and inducing
regulatory T cells (Tregs). It maintains central immune tolerance,
preventing autoimmunity by regulating tissue restrictive antigen
(TRA) expression in mTECs of the thymus (2–5). The
mutation or deletion of the Aire gene in humans results in
autoimmune polyendocrine syndrome type I (APS-1) (6), which is primarily characterized by
multiple organ disorders that are mediated by an autoimmune
response. This disorder is also termed autoimmune
polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED).
Patients exhibiting APECED may also develop other disorders,
including Addison's disease, hypoparathyroidism and diabetes. In
addition, APECED patients are susceptible to developing Candida
albicans infections due to immunodeficiency (7,8).
Although primarily expressed in the thymus, Aire is
also detected in dendritic cells (DCs), macrophages and stromal
cells of peripheral tissues, particularly in the blood and the
lymph nodes (1,9,10).
However, the role of Aire at these sites remains to be elucidated.
In addition, the reason for the susceptibility of APECED patients
to C. albicans infection requires further investigation.
Previous studies identified that Aire regulates certain TRAs in
peripheral tissues to maintain peripheral tolerance by deleting
autoreactive T cells, complementary to the action at the thymus
(11,12). Furthermore, alterations in the
antigen-presenting capabilities of DCs and macrophages have been
described in Aire-knockout (KO) mice (13), suggesting that Aire regulates the
immune response. However, other underlying mechanisms by which Aire
regulates tolerance or the immune response in cells of peripheral
tissues cannot be excluded.
Cluster of differentiation (CD)4+ T
helper cells are crucial in regulating the immune response and in
maintaining peripheral tolerance (14,15).
Naive CD4+ T cells may differentiate into distinct
subsets, including T helper (Th)1, Th2, Th17, and Tregs, as well as
Th9 and Th22 (16–19), subsequent to co-stimulation with
and cytokine signals from DCs. These signals contribute
significantly to the development of CD4+ T cell subsets.
Therefore, the present study hypothesized that Aire expression in
DCs is involved in the immune response and in peripheral tolerance
by affecting CD4+ T cell subsets.
In the current study, the effects of cytokines
secreted by the dendritic cell line, DC2.4, which overexpresses
Aire, on the differentiation of CD4+ T cell subsets were
investigated. The results demonstrate that Aire-overexpressing
cells induce Th1 and Th17 differentiation by upregulating
interleukin (IL)-12, IL-6 and transforming growth factor (TGF)-β.
Further analysis indicated that increased phosphorylation of
extracellular signal-regulated kinases (ERK) and p38 may upregulate
the expression of these cytokines in Aire-overexpressing cells.
Materials and methods
Cells and animals
The DC2.4 cell line was obtained from the Shanghai
Cell Research Institute (Shanghai, China) and cultured in 10%
newborn calf serum (Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) in RPMI-1640 (Gibco-BRL; Thermo Fisher Scientific,
Inc.,Waltham, MA, USA). The DC2.4 cells were transfected with
pEGFPC1/Aire or pEGFPC1 plasmids (Takara Bio, Inc., Otsu, Japan),
and stable cell lines were obtained using G418 selection agents
(Sigma-Aldrich, St. Louis, MO, USA), as previously described
(20). A total of 60 male C57BL/J6
mice (4–5 weeks-old; 18–20 g) were purchased from the Experimental
Animal Center of Jilin University (Changchun, China), and all mice
were housed under specific pathogen-free conditions at room
temperature under a 12 h light/dark cycle, and fed with mouse
nutritional food. The present study was approved by the
Institutional Animal Care and Use Committee of Jilin University,
and all mice were treated in accordance with the Guide for the Care
and Use of Laboratory Animals.
Non-contact co-culture of the DC2.4 cell
lines with CD4+ T cells
The mice were sacrificed by cervical dislocation,
then the spleens were removed from the C57BL/J6 mice and gently
dissociated into single-cell suspensions using an aseptic syringe
filled with 0.1 mol/l (pH 7.4) phosphate-buffered saline (PBS).
CD4+ T cells were purified from the spleen cell
suspension using a Mouse CD4+ T Lymphocyte Enrichment
set (BD Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocols. Transwell® Permeable Supports
(3-µm pores, plasma-treated polycarbonate inserts; Corning
Life Sciences, Corning, NY, USA) were utilized for the paracrine,
non-contact cell co-cultures. Aire-overexpressing cells (Aire
cells) or empty vector (control cells) were seeded in 6-well plates
at 2×105 per well in 2 ml media. Once the cells had
attached to the wells, CD4+ T cells (4×106
per well) were added to the top of the Transwell chamber, and
incubated for 48 h at 37°C. Finally, the CD4+ T cells
were harvested for flow cytometry and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analyses.
RNA isolation and RT-qPCR
Total RNA was extracted from the harvested
CD4+ T cells (as described above) or from the Aire and
control DC2.4 cells using RNAiso™ PLUS (Takara Bio, Inc.), and
dissolved in diethylpyrocarbonate-treated water (Takara Bio, Inc.).
The quantity of total RNA was then measured using an Epoch
multi-volume spectrophotometer system (BioTeke Corporation,
Beijing, China). cDNA was synthesized from 1.0 mg total RNA using
reverse transcriptase (Takara Bio, Inc.), Moloney murine leukemia
virus (Takara Bio, Inc.) and oligonucleotides (dT; Takara Bio,
Inc.) in a total volume of 20 ml, according to the manufacturer's
protocols (Takara Bio, Inc.). RT-qPCR was performed using an ABI
PRISM 7300 sequence detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) with SYBR Premix Ex Taq™ II (Takara Bio,
Inc.) following the manufacturer's protocol and using the following
conditions: 95°C for 30 sec, 95°C for 5 sec, and 60°C for 30 sec
for 40 cycles. The results were analyzed according to the
2−∆∆Cq formula. The following primers (Takara Bio, Inc.)
were used: Sense, 5′-GACTTCAACAGCAACTCCCACTC-3′ and antisense,
5′-TAGCCGTATTCATTGTCATACCAG-3′ for GAPDH; sense,
5′-CCCATCCCTTCCCTGTAT-3′ and antisense, 5′-GTCCATTCTCCGTTCTCCA-3′
for T-box transcription factor 21 (T-bet); sense,
5′-TCCAGTCCTCATCTCTTCAC-3′ and antisense,
5′-GTCTCCAGCTTCATGCTATC-3′ for GATA-binding protein 3 (Gata3);
sense, 5′-CCCCTGGAGGTGTCTGATGG-3′ and antisense,
5′-TGTGCTTGGACGAGAACTGGA-3′ for Spi-1 proto-oncogene (PU.1); sense,
5′-AGATTCCAGGTGACTCTGTG-3′ and antisense,
5′-CTGCCCTGTCAGAGTATTTC-3′ for interferon regulatory factor 4
(IRF4); sense, 5′-CCGCTGAGAGGGCTTCAC-3′ and antisense
5′-TGCAGGAGTAGGCCACATTACA-3′ for RAR-related orphan receptor γ
isoform two (RORγt); sense, 5′-GCTACTCCACTTCAGCCACC-3′ and
antisense, 5′-ACTGTCATGCCACTTTCTCC-3′ for aryl hydrocarbon receptor
(AHR); sense, 5′-TACTCGCATGTTCGCCTCTTCA-3′ and antisense,
5′-ATTCATCTACGGTCCACACTGCT-3′ for forkhead box P3 (FoxP3); sense,
5′-ACTTGAACTACGCTACGAGAG-3′ and antisense,
5′-CTTGACTCCGCCTCATCCGGTA-3′ for interleukin-12 subunit β; sense,
5′-GAAACCGCTATGAAGTTCCTCTCTG-3′ and antisense,
5′-GTATCCTCTGTGAAGTCTCCTCTCC-3′ for IL-6; and sense,
5′-GCCCTGGATACCAACTATTGC-3′ and antisense,
5′-GCAGGAGCGCACAATCATGTT-3′ for TGF-β.
Flow cytometry
The cells were collected and counted, and
1×106 cells were suspended in PBS (100 µl total
volume). To compare the different cell subsets, CD4+ T
cells were first incubated with rat anti-mouse CD4+
PE-cyanine 7 (cat. no. 25-0041; 1:160; eBioscience, Inc., San
Diego, CA, USA) on ice for 45 min and subsequently fixed with
Fixation/Permeabilization Concentrate and Diluent (eBio-science,
Inc.) for 1 h. The cells were treated with 0.1% saponin
(Sigma-Aldrich) and rat anti-mouse IL-4-fluorescein isothiocyanate
(cat. no. M100I9-02; 1:200; Tianjin Sungene Biotech, Co., Ltd.,
Tianjin, China), rat anti-mouse IFN-γ PE (cat. no. 12-7311; 1:160;
eBioscience, Inc.), rat anti-mouse IL-17A-PE (cat. no. 12-7177;
1:160; eBioscience, Inc.), rat anti-mouse FoxP3-Alexa Fluor 647
(cat. no. 50-5773; 1:100; eBioscience, Inc.) or rat anti-mouse
IL-9-PE (cat. no. 516404; 1:100; Biolegend, Inc., San Diego, CA,
USA) antibodies at 4°C for 1 h. The cells were washed twice with
PBS and resuspended in 2% paraformaldehyde (Sigma-Aldrich) for
analysis using a BD FACSCalibur™ flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). For phosphorylated (p)-ERK staining, Aire
and control DC2.4 cells were collected and counted using a cell
count board. The fixed cells were stained with anti-mouse p-ERK
(T202/Y204; cat. no. 14-9109; 1:100) and rabbit anti-mouse p-p38
(T180; cat. no. YP0338; 1:100; ImmunoWay Biotechnology, Co.,
Newark, DE, USA) antibodies, followed by staining with a secondary
goat anti-mouse antibody (BD Biosciences).
Cytokine secretion assays
IL-6, IL-12 and TGF-β expression levels in the
cell-free supernatants of the control or Aire-overexpressing DC2.4
cell cultures (1×106 cells/6-well plate in 2 ml for 48
h) were measured using an ELISA kits (eBioscience, Inc.) according
to the manufacturer's protocol.
Antibody blocking
The cytokines secreted from the Aire or control
cells into the supernatants were neutralized with anti-mouse TGF-β
(cat. no. MAB1835; 1:100; R&D Systems, Inc., Minneapolis, MN,
USA), rat anti-mouse IL-6 (cat. no. M100I5-14; 1:20; BioLegend,
Inc.), and rat anti-mouse IL-12 (cat. no. M100I121-14; 1:20;
Tianjin Sungene Biotech) antibodies for 2 h. Freshly isolated
CD4+ T cells were added to the Transwell chamber culture
and incubated for 48 h at 37°C, following which the CD4+
T cells were harvested for fluorescence-activated cell sorting
(FACS) analysis.
Statistical analysis
All experimental data are reported as means ±
experimental standard errors. Statistical analysis was performed
using Student's t-test and P<0.05 was considered to indicate a
statistically significant difference.
Results
Aire-overexpressing DC2.4 cells induce
Th1 and Th17 subsets
The stimulatory signals from DCs influence
CD4+ T cell subsets by regulating the expression levels
of transcription factors. These different CD4+ T cell
subsets perform their functions by secreting various cytokines,
thus, identification can be conducted by analysis of expression
profiles of these master regulators and cytokines. The
transcription factors unique to the CD4+ T cell subsets,
Th1, Th2, Th9, Th17, Th22, and Tregs are T-bet, Gata3, PU.1 and
IRF4, RORγT, AHR, and FoxP3, respectively. Additionally, the unique
cytokines for Th1, Th2, Th9, Th17, and Th22 cells are interferon
(IFN)-γ, IL-4, IL-9, IL-17A and IL-22, respectively (16–19,21).
To examine the effects of Aire cells on the
CD4+ T cell subsets, the messenger (m)RNA expression
levels of the master regulators were detected by RT-qPCR. All the
master regulators were expressed in CD4+ T cells;
however, compared with the control, T-bet (the marker of Th1 cells)
and RORγT (the marker of Th17 cells) were significantly upregulated
in cells co-cultured for 48 h with Aire cells (P<0.05 and
P<0.01; Fig. 1). No significant
differences were noted for the other master regulators, including
Gata3 in the Th2 cells, PU.1 and IRF4 in the Th9 cells, AHR in the
Th22 cells, or FoxP3 in the Tregs. These results indicate that Aire
cells upregulate T-bet and RORγT transcription in CD4+ T
cells.
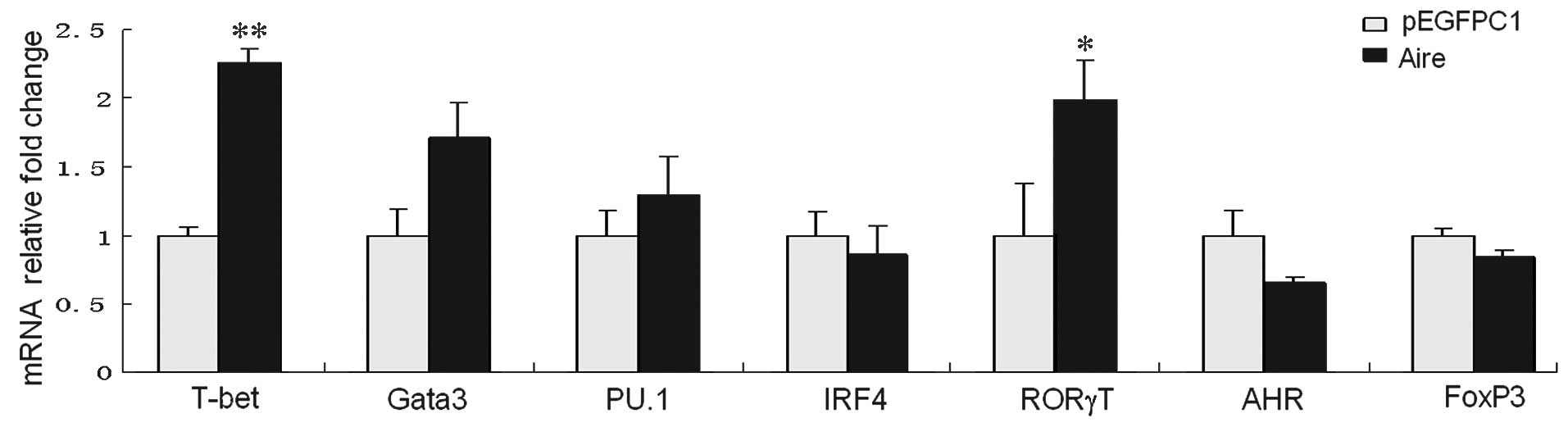 | Figure 1Expression levels of the master
regulators in CD4+ T cells, purified from spleen cells
co-cultured with Aire-overexpressing or control cells submitted to
Transwell assays for 48 h. The mRNA levels were determined using
quantitative reverse transcription-polymerase chain reaction. All
data are shown as the expression levels relative to the expression
of GAPDH and are depicted as the fold change relative to control
cells, normalized to 1. The data are expressed as means ± standard
deviation from between three and five independent experiments.
*P<0.05, **P<0.01 vs. pEGFPC1. T-bet,
T-box transcription factor 21; Gata3, GATA-binding protein 3; PU.1,
Spi-1 proto-oncogene; IRF4, interferon regulatory factor 4; RORγT,
RAR-related orphan receptor gamma isoform 2; AHR, aryl hydrocarbon
receptor; FoxP3, forkhead box P3; CD, cluster of differentiation;
Aire, autoimmune regulator; mRNA, messenger RNA. |
In addition, the cytokines from different
CD4+ T cell subsets were detected by FACS. FoxP3 was
used to identify Tregs, as Tregs do not produce a typical cytokine
(22). The data demonstrate that
the percentages of IFN-γ- and IL-17-expressing cells were higher
than the control cells when the CD4+ T cells were
co-cultured with Aire cells for 48 h. The percentage of cells
expressing FoxP3, IL-4, IL-9, and IL-22 did not significantly
change; however, the percentage of IL-9-producing cells was
increased when co-cultured with Aire cells (Fig. 2A and B). Other transcription
factors may be required for Th9 differentiation, as no differences
in the mRNA levels of the transcription factors, PU.1 or IRF4, were
observed between the Aire and control cells. These results suggest
that Aire has a positive role in inducing Th1 and Th17
development.
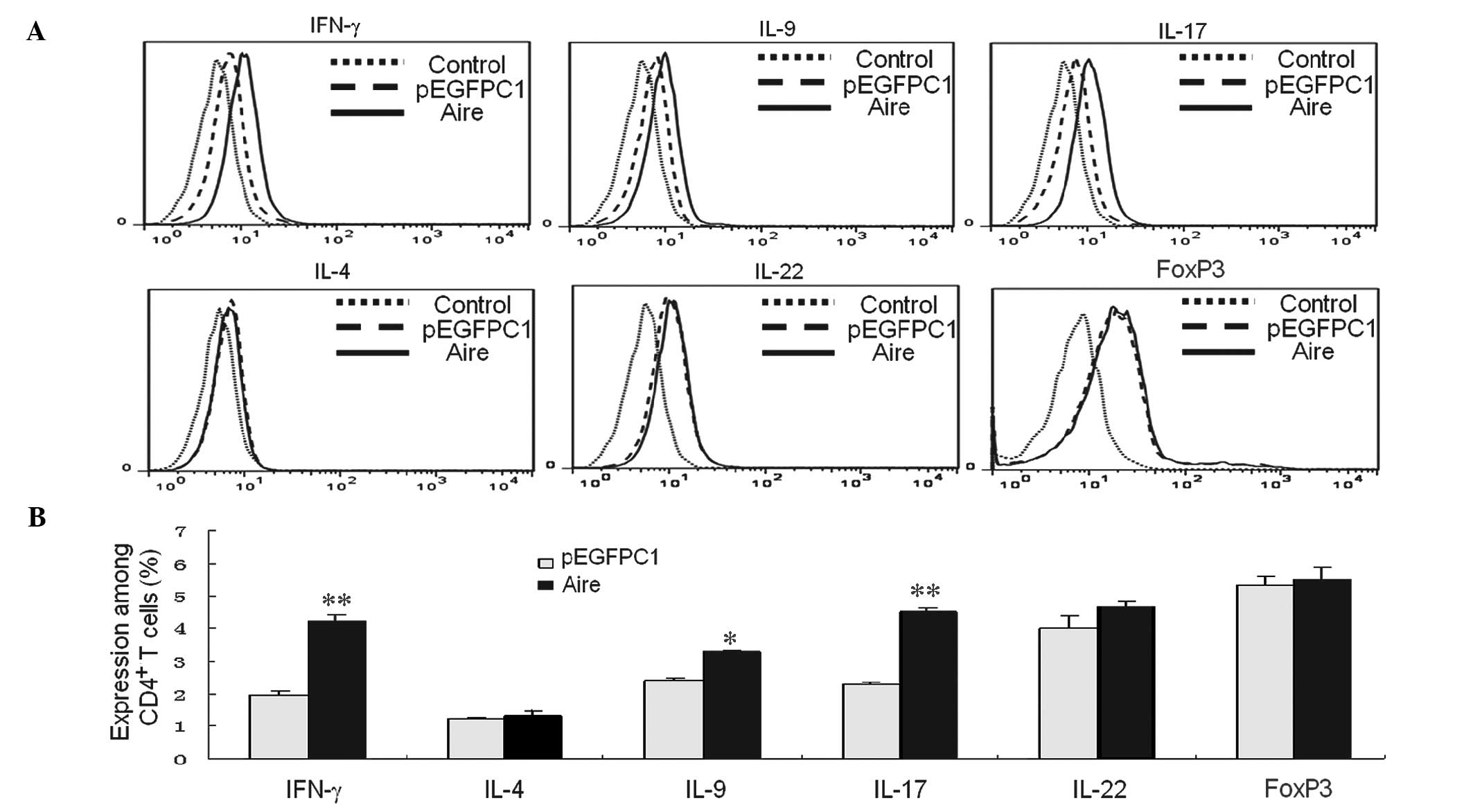 | Figure 2Supernatant of Aire cells affects the
number of CD4+ T cells and their subsets.
CD4+ T cells were purified from spleen cells and
co-cultured with Aire or control cells, and submitted to Transwell
assays for 48 h. (A) The percentages of Tregs and Th1, Th2, Th9,
Th17, and Th22 cells among the CD4+ T cells were
analyzed by fluorescence-activated cell sorting. (B) The emitted
fluorescence was analyzed using FlowJo 7.6 software. The results
from a minimum of three experiments are presented as the percentage
of positive cells ± standard deviation. *P<0.05,
**P<0.05 vs. pEGFPC1. CD, cluster of differentiation;
Th, T helper; IL, interleukin; IFN-γ, interferon γ; FoxP3, forkhead
box P3; Aire, autoimmune regulator. |
Aire-overexpressing DC2.4 cells induce
Th1 and Th17 by upregulating IL-12, IL-6, and TGF-β expression
DCs induce the expression of master regulators in
CD4+ T cells by secreting cytokines, resulting in the
further differentiation of CD4+ T cell subsets from
naïve CD4+ T cells. The expression of T-bet and RORγT in
CD4+ T cells is regulated by IL-12, and IL-6 and TGF-β,
respectively, from DCs (23).
Therefore, to investigate the mechanism underlying the Aire cells
induction of Th1 and Th17 development, the mRNA and protein
expression levels of IL-12, IL-6 and TGF-β in Aire cells were
detected by RT-qPCR and ELISA. As presented in Fig. 3A and B, IL-12, IL-6, and TGF-β mRNA
expression levels were higher in the Aire cells than in the control
cells. In addition, a similar trend in the protein levels of IL-12,
IL-6 and TGF-β in the supernatants of the cultured cells was
demonstrated by the Aire and control cells. These data suggest that
Aire may affect the Th1 and Th17 subsets by regulating the
secretion of cytokines by DCs.
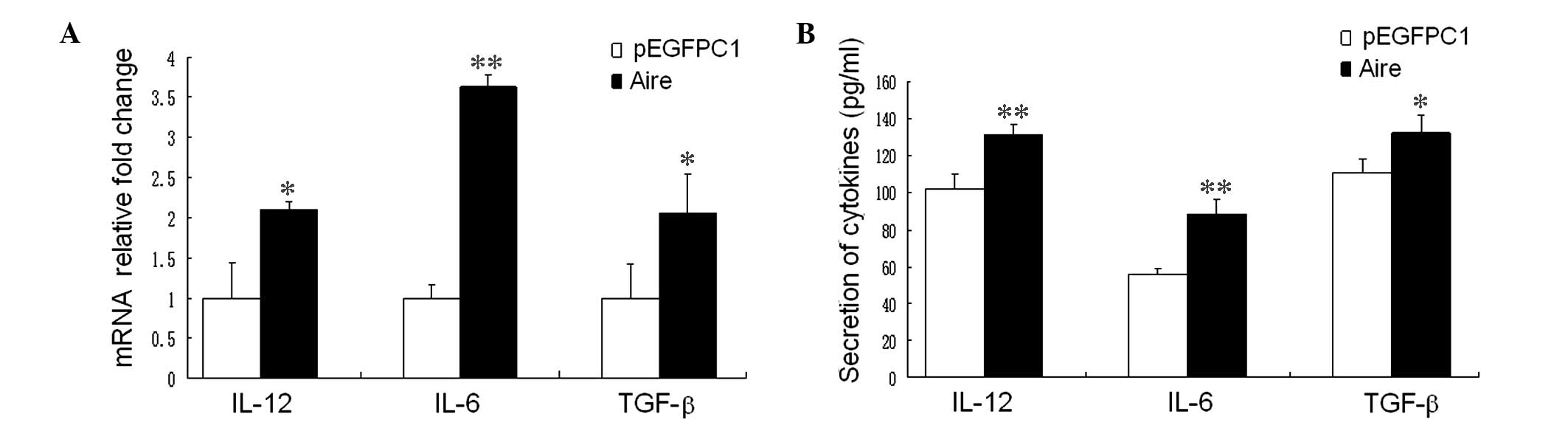 | Figure 3(A) Aire affects the expression
levels of IL-12, IL-6 and TGF-β in DC2.4 cells and the mRNA levels
of IL-12, IL-6 and TGF-β in DC2.4 cells transfected with either
pEGFPC1/Aire or pEGFPC1 plasmids, as detected by quantitative
reverse transcription-polymerase chain reaction. (B)
CD4+ T cells purified from spleen cells were co-cultured
with Aire or control cells and submitted to Transwell assays for 48
h, the supernatants were collected, and IL-12, IL-6 and TGF-β were
detected. The data are expressed as means ± standard deviation of
between three and five independent experiments.
*P<0.05, **P<0.01 vs. pEGFPC1. IL,
interleukin; TGF-β, transforming growth factor β; Aire, autoimmune
regulator; mRNA, messenger RNA; CD, cluster of differentiation. |
To further determine whether the cytokines from Aire
cells are involved in upregulating the Th1 and Th17 subsets,
antibodies against IL-12, IL-6 and TGF-β were used to block the
cytokines in the supernatants of Aire and control cells prior to
co-culture with CD4+ T cells. The expression levels of
these cytokines in CD4+ T cells expressing IFN-γ and
IL-17A were evaluated by FACS following 48 h of co-culture
(Fig. 4). No differences in the
expression levels of these cytokines were observed for
IFN-γ-expressing CD4+ T cells between Aire and control
cells when IL-12 was blocked (Fig. 4A
and C). In addition, no differences were observed for
IL-17A-expressing CD4+ T cells following blocking with
anti-IL-6 or anti-TGF-β antibodies, or a combination of the two
(Fig. 4B and C). These data
demonstrate that Aire cells induce Th1 and Th17 differentiation by
enhancing IL-12, IL-6 and TGF-β secretion.
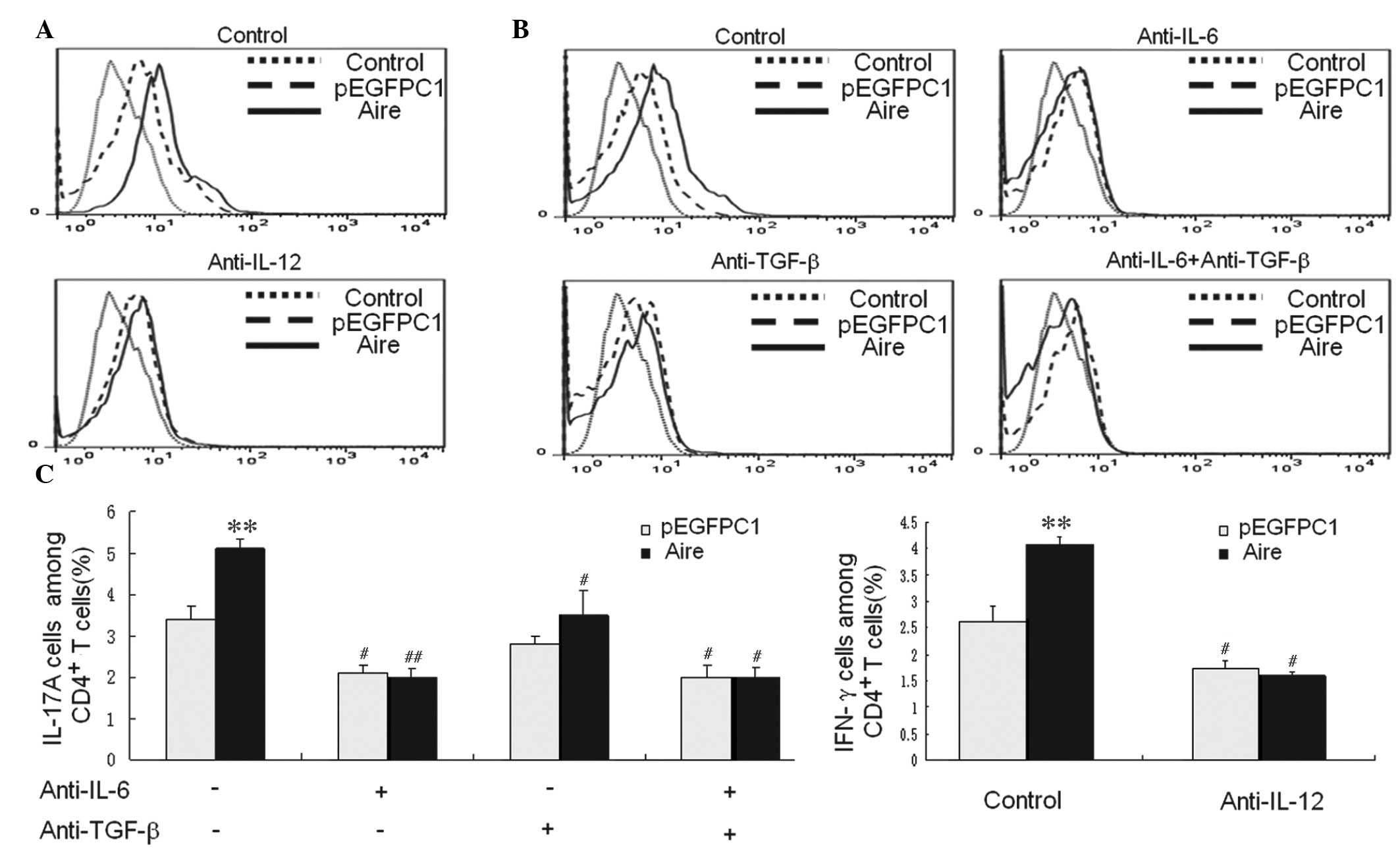 | Figure 4(A) IFN-γ expression levels in
CD4+ T cells were examined by FACS following 48-h
co-culture with Aire or control cells that were either untreated or
preblocked with anti-IL-12 antibody. (B) The IL-17A expression
levels in CD4+ T cells were examined by FACS after a
48-h co-culture with Aire or control cells that were untreated or
preblocked with anti-IL-6 and anti-TGF-β antibodies, either
separately or in combination. (C) The results are presented as the
percentage of positive cells ± standard deviation. Each experiment
was repeated at least three times, and each group was compared with
the pEGFPC1 group. **P<0.01, compared with the
pEGFPC1 group; #P<0.05, ##P<0.01, Aire
or pEGFPC1 groups with antibody blocking compared with Aire or
pEGFPC1 groups with no antibody blocking. IL, interleukin; TGF-β,
transforming growth factor β; CD, cluster of differentiation;
IFN-γ, interferon-γ; FACS, fluorescence-activated cell sorting;
Aire, autoimmune regulator. |
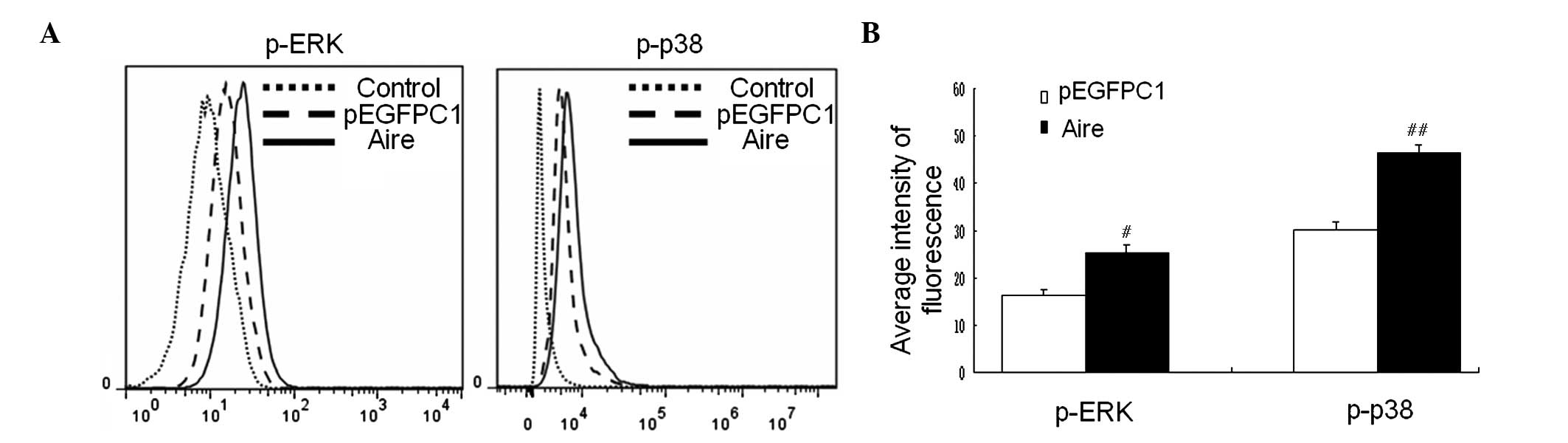
Aire upregulates the expression levels of
IL-12, IL-6 and TGF-β via mitogen-activated protein kinase (MAPK)
signaling
To investigate the signal transduction pathway by
which Aire affects IL-12, IL-6, and TGF-β expression in DCs, the
activities of ERK and p38, which belong to the family of MAPKs
associated with the production of these cytokines, were detected by
FACS (24,25). The results demonstrate that the
phosphorylation levels of ERK and p38 were significantly increased
in Aire cells (P<0.05 and P<0.01; Fig. 5A and B). These data provide an
explanation for the upregulation of IL-12, IL-6 and TGF-β by Aire
in DCs.
Discussion
Aire was first identified in humans as the defective
gene in the monogenic and autosomal recessive disorder, APS-I
(26). The functions of Aire in
the central immune organs, particularly in the thymus, have been
extensively investigated and well defined. Previous studies have
demonstrated that Aire promotes the ectopic expression of
peripheral tissue-specific antigens (PTAs) in mTECs. This imposes
central tolerance via the negative selection of self-reactive T
cells and the induction of Treg cells, which mediate peripheral
immune tolerance (2,3). The functions of Aire in peripheral
tissues remain poorly understood and the subject of debate.
Extrathymic Aire cells (eTACs), which are located in secondary
lymphoid tissue, have been extensively investigated (27). Gardner et al (28) demonstrated that eTACs, as opposed
to mTECs, functionally inactivate CD4+ T cells via the
expression of a distinct and diverse array of self-antigens to
induce peripheral tolerance, providing the first evidence of the
action of Aire in peripheral tissue stromal cells. However, to the
best of our knowledge, the function of Aire in peripheral
hematopoietic cells remains undefined.
APECED presents with autoimmune polyendocrinopathy
and chronic mucocutaneous candidiasis (CMC) (7), which usually results from an
autoimmune response and T cell immunodeficiencies. Aire-expressing
DCs may directly affect CD4+ T cell subsets by
expressing co-stimulatory molecules and by secreting cytokines. The
endocrine disorders in APECED are clearly the result of
autoimmunity (8), while the reason
for the presence of CMC remains unclear. Previous studies have
explained CMC as aberrant adaptive immunity, including a bias of
the T cell repertoire resulting from the inappropriate role of AIRE
as a transcriptional mediator (29). Furthermore, in the present study,
Aire appeared to have little effect on costimulatory molecules in
DC2.4 cells (data not shown). Therefore, the current study
investigated whether Aire-overexpressing DCs affect CD4+
T cell subsets by releasing paracrine cytokines. It was observed
that Aire cells upregulate the mRNA levels of T-bet and RORγT, and
the protein levels of IFN-γ and IL-17A in CD4+ T cells.
This suggests that Aire cells induce Th1 and Th17 subset
differentiation. Previous studies have identified that congenital
errors in IFN-γ cause endemic mycoses and that inborn errors in
IL-17 are associated with CMC (30,31).
Furthermore, the Th1 and Th17 lineages are necessary for pathogen
protection and clearance in various models, including C.
albicans (32). Therefore, the
current study hypothesized that decreased Th1 and Th17
differentiation, IFN-γ and IL-17 generation caused by Aire-KO DCs,
and the presence of autoantibodies targeting key antifungal
cytokines may be one mechanism underlying APECED patient
susceptibility to the Candida infection (33). Previously, Th1 cells were regarded
as the cells that cause the onset and progression of autoimmune
disorders, such as type 1 diabetes (T1D), by producing IFN-γ
(34). However, in-depth studies
have indicated that the Th17 phenotype may be important in
autoimmune disorders (35,36). No differences in IFN-γ or IL-17
expression levels were detected in the blood of APECED patients
when compared with healthy control subjects, suggesting that IFN-γ
and IL-17 are not major reasons for autoimmunity in APECED patients
(33). Conversely, Th17 cells
delayed T1D in non-obese diabetic (NOD) mice treated with
mycobacterial adjuvant (37), and
IFN-γ induction restored normoglycemia in NOD mice (38). Therefore, Aire expression in
peripheral DCs may have a role in the prevention of Candida
infection and autoimmune diseases by promoting Th1 and Th17
differentiation.
The current study demonstrated that Aire cells
promote the differentiation of Th1 and Th17 cells by upregulating
cytokine secretion. IL-12 is generally considered a potent inducer
of Th1 cells (16), and Th17 cells
are induced by the activities of various cytokines, including TGF-β
and IL-6 (21). Results of the
present study are consistent with these reports; the mRNA and
protein levels of TGF-β, IL-6, and IL-12 are increased in
Aire-overexpressing DCs. Further analysis indicated that the
neutralization of IL-12 in the co-culture system abrogated the
differential Th1 subsets between the Aire and control cells,
indicating the importance of IL-12 in the Th1 differentiation
induced by Aire-overexpressing DCs. In addition, TGF-β and IL-6
neutralization decreased Th17 induction, suggesting that TGF-β and
IL-6 are critical in Th17 differentiation induced by
Aire-overexpressing DCs. IL-12 produced by DCs trans-activates
T-bet and induces the differentiation of Th1 cells, which produce
high levels of IFN-γ. Similarly, the transcription factor, RORγt,
which is required for Th17 differentiation and for IL-17A
secretion, is regulated by TGF-β and IL-6 from DCs (23). These findings indicate that Aire
regulates the secretion of cytokines in DCs to affect
CD4+ T cell subgroup differentiation, and regulate the
immune response and tolerance.
The signaling pathway associated with IL-12, IL-6
and TGF-β expression in Aire-transfected DCs was investigated to
observe how Aire regulates cytokine secretion in DCs. p38 in DCs
has been demonstrated to promote IL-6 and TGF-β production, and to
mediate Th17 generation (39). The
increased phosphorylation levels of p38 in the present study may
explain the upregulation of IL-6 and TGF-β mediating Th17
differentiation. Furthermore, ERK activation may promote Th17
generation by increasing IL-6 expression and suppressing Tregs
(40), which is consistent with
the enhanced ERK activation in Aire cells. The polarization of Th1
cells may account for the increased phosphorylation of ERK and p38
in DCs following Aire transfection, as IL-12 secretion is increased
via the activation of p38 and ERK (24). Additional mechanisms may contribute
to this effect; for example, Aire may upregulate IL-12, IL-6 and
TGF-β expression in DCs by activating ERK and p38.
In conclusion, data from the present study
demonstrates that Aire cells induce Th1 and Th17 differentiation by
upregulating cytokines in paracrine cells. This finding may explain
why the genetic mutation of Aire simultaneously results in a
self-tolerance disorder and in susceptibility to C. albicans
infections.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81373127). The
authors would like to thank Dr Xiumei Chi at the Translational
Medical Research Institute of the First Hospital of Jilin
University for assistance with flow cytometry.
References
|
1
|
Suzuki E, Kobayashi Y, Kawano O, Endo K,
Haneda H, Yukiue H, Sasaki H, Yano M, Maeda M and Fujii Y:
Expression of AIRE in thymocytes and peripheral lymphocytes.
Autoimmunity. 41:133–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liston A, Lesage S, Wilson J, Peltonen L
and Goodnow CC: Aire regulates negative selection of organ-specific
T cells. Nat Immunol. 4:350–354. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anderson MS, Venanzi ES, Klein L, Chen Z,
Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist
C and Mathis D: Projection of an immunological self shadow within
the thymus by the aire protein. Science. 298:1395–1401. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abramson J, Giraud M, Benoist C and Mathis
D: Aire's partners in the molecular control of immunological
tolerance. Cell. 140:123–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koh AS, Kuo AJ, Park SY, Cheung P,
Abramson J, Bua D, Carney D, Shoelson SE, Gozani O, Kingston RE, et
al: Aire employs a histone-binding module to mediate immunological
tolerance, linking chromatin regulation with organ-specific
autoimmunity. Proc Natl Acad Sci USA. 105:15878–15883. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cavadini P, Vermi W, Facchetti F, Fontana
S, Nagafuchi S, Mazzolari E, Sediva A, Marrella V, Villa A, Fishcer
A, et al: AIRE deficiency in thymus of 2 patients with Omenn
syndrome. J Clin Invest. 115:728–732. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahonen P, Myllärniemi S, Sipilä J and
Perheentupa J: Clinical variation of autoimmune
polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a
series of 68 patients. N Engl J Med. 322:1829–1836. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perheentupa J: Autoimmune
polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). Horm
Metab Res. 28:353–356. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pöntynen N, Strengell M, Sillanpää N,
Saharinen J, Ulmanen I, Julkunen I and Peltonen L: Critical
immunological pathways are downregulated in APECED patient
dendritic cells. J Mol Med Berl. 86:1139–1152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cohen JN, Guidi CJ, Tewalt EF, Qiao H,
Rouhani SJ, Ruddell A, Farr AG, Tung KS and Engelhard VH: Lymph
node-resident lymphatic endothelial cells mediate peripheral
tolerance via Aire-independent direct antigen presentation. J Exp
Med. 207:681–688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fletcher AL, Lukacs-Kornek V, Reynoso ED,
Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL and
Turley SJ: Lymph node fibroblastic reticular cells directly present
peripheral tissue antigen under steady-state and inflammatory
conditions. J Exp Med. 207:689–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee JW, Epardaud M, Sun J, Becker JE,
Cheng AC, Yonekura AR, Heath JK and Turley SJ: Peripheral antigen
display by lymph node stroma promotes T cell tolerance to
intestinal self. Nat Immunol. 8:181–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramsey C, Hässler S, Marits P, Kämpe O,
Surh CD, Peltonen L and Winqvist O: Increased antigen presenting
cell-mediated T cell activation in mice and patients without the
autoimmune regulator. Eur J Immunol. 36:305–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Asano M, Toda M, Sakaguchi N and Sakaguchi
S: Autoimmune disease as a consequence of developmental abnormality
of a T cell subpopulation. J Exp Med. 184:387–396. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Espinosa V and Rivera A: Cytokines and the
regulation of fungus-specific CD4+ T cell
differentiation. Cytokine. 58:100–106. 2012. View Article : Google Scholar :
|
|
16
|
Guo L, Wei G, Zhu J, Liao W, Leonard WJ,
Zhao K and Paul W: IL-1 family members and STAT activators induce
cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci
USA. 106:13463–13468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kagami S, Rizzo HL, Lee JJ, Koguchi Y and
Blauvelt A: Circulating Th17, Th22, and Th1 cells are increased in
psoriasis. J Invest Dermatol. 130:1373–1383. 2010. View Article : Google Scholar :
|
|
18
|
Kerzerho J, Maazi H, Speak AO, Szely N,
Lombardi V, Khoo B, Geryak S, Lam J, Soroosh P, Van Snick J and
Akbari O: Programmed cell death ligand2 regulates TH9
differentiation and induction of chronic airway hyperreactivity. J
Allergy Clin Immunol. 131:1048–1057. 1057.e1–1057.e2. 2013.
View Article : Google Scholar
|
|
19
|
Sakaguchi S, Sakaguchi N, Asano M, Itoh M
and Toda M: Immunologic self-tolerance maintained by activated T
cells expressing IL-2 receptor alpha-chains (CD25) Breakdown of a
single mechanism of self-tolerance causes various autoimmune
diseases. J Immunol. 155:1151–1164. 1995.PubMed/NCBI
|
|
20
|
Zhu W, Yang W, He Z, Liao X, Wu J, Sun J,
Yang Y and Li Y: Overexpressing autoimmune regulator regulates the
expression of toll-like receptors by interacting with their
promoters in RAW264.7 cells. Cell Immunol. 270:156–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Egawa T, Tillman RE, Naoe Y, Taniuchi I
and Littman DR: The role of the Runx transcription factors in
thymocyte differentiation and in homeostasis of naive T cells. J
Exp Med. 204:1945–1957. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyara M, Yoshioka Y, Kitoh A, Shima T,
Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al:
Functional delineation and differentiation dynamics of human
CD4+ T cells expressing the FoxP3 transcription factor.
Immunity. 30:899–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu J, Yamane H and Paul WE:
Differentiation of effector CD4+ T cell populations (*).
Annu Rev Immunol. 28:445–89. 2010. View Article : Google Scholar
|
|
24
|
Huang G, Wang Y and Chi H: Control of T
cell fates and immune tolerance by p38α signaling in mucosal CD103+
dendritic cells. J Immunol. 191:650–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong Q, Sugiura T, Toyohira Y, Yoshida Y,
Yanagihara N and Karasaki Y: Stimulation of IFN-γ production by
garlic lectin in mouse spleen cells: Involvement of IL-12 via
activation of p38 MAPK and ERK in macrophages. Phytomedicine.
18:309–16. 2011. View Article : Google Scholar
|
|
26
|
Nagamine K, Peterson P, Scott HS, Kudoh J,
Minoshima S, Heino M, Krohn KJ, Lalioti MD, Mullis PE, Antonarakis
SE, et al: Positional cloning of the APECED gene. Nat Genet.
17:393–398. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang R and Turka LA: A breath of fresh
aire. Immunity. 39:427–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gardner JM, Devoss JJ, Friedman RS, Wong
DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et
al: Deletional tolerance mediated by extrathymic Aire-expressing
cells. Science. 321:843–847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kekäläinen E, Tuovinen H, Joensuu J,
Gylling M, Franssila R, Pöntynen N, Talvensaari K, Perheentupa J,
Miettinen A and Arstila TP: A defect of regulatory T cells in
patients with autoimmune polyendocrinopathy-candidiasis-ectodermal
dystrophy. J Immunol. 178:1208–1215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lanternier F, Cypowyj S, Picard C,
Bustamante J, Lortholary O, Casanova JL and Puel A: Primary
immunodeficiencies underlying fungal infections. Curr Opin Pediatr.
25:736–747. 2013.PubMed/NCBI
|
|
31
|
Ling Y and Puel A: IL-17 and infections.
Actas Dermosifiliogr. 105(Suppl 1): 34–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Murdock BJ, Teitz-Tennenbaum S, Chen GH,
Dils AJ, Malachowski AN, Curtis JL, Olszewski MA and Osterholzer
JJ: Early or late IL-10 blockade enhances Th1 and Th17 effector
responses and promotes fungal clearance in mice with cryptococcal
lung infection. J Immunol. 193:4107–4116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kisand K, Lilic D, Casanova JL, Peterson
P, Meager A and Willcox N: Mucocutaneous candidiasis and
autoimmunity against cytokines in APECED and thymoma patients:
Clinical and pathogenetic implications. Eur J Immunol.
41:1517–1527. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I
Definition according to profiles of lymphokine activities and
secreted proteins. J Immunol. 136:2348–2357. 1986.PubMed/NCBI
|
|
35
|
Emamaullee JA, Davis J, Merani S, Toso C,
Elliott JF, Thiesen A and Shapiro M: Inhibition of Th17 cells
regulates autoimmune diabetes in NOD mice. Diabetes. 58:1302–1311.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dolff S, Quandt D, Feldkamp T, Jun C,
Mitchell A, Hua F, Specker C, Kribben A, Witzke O and Wilde B:
Increased percentages of PD-1 on CD4+ T cells is associated with
higher INF-γ production and altered IL-17 production in patients
with systemic lupus erythematosus. Scand J Rheumatol. 43:307–313.
2014. View Article : Google Scholar
|
|
37
|
Nikoopour E, Schwartz JA, Huszarik K,
Sandrock C, Kroughly O, Lee-Chan E and Singh B: Th17 polarized
cells from nonobese diabetic mice following mycobacterial adjuvant
immunotherapy delay type 1 diabetes. J Immunol. 184:4779–4788.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jain R, Tartar DM, Gregg RK, Divekar RD,
Bell JJ, Lee HH, Yu P, Ellis JS, Hoeman CM, Franklin CL and
Zaghouani H: Innocuous IFNgamma induced by adjuvant-free antigen
restores normoglycemia in NOD mice through inhibition of IL-17
production. J Exp Med. 205:207–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gonghua H, Yanyan W, Peter V,
Thirumala-Devi K, Kinya O and Hongbo C: p38α signaling programs
dendritic cells to drive TH17 cell differentiation and autoimmune
inflammation. Nat Immunol. 13:152–161. 2013.
|
|
40
|
Liu H, Yao S, Dann SM, Qin H, Elson CO and
Cong Y: ERK differentially regulates Th17- and Treg-cell
development and contributes to the pathogenesis of colitis. Eur J
Immunol. 43:1716–1726. 2013. View Article : Google Scholar : PubMed/NCBI
|