Introduction
Trichosanthin (TCS), a type I ribosome-inactivating
protein (RIP), is a 27 kDa protein isolated from the root tubers of
the Chinese medicinal herb Tian-Hua-Fen (Trichosanthes
kirilowii) of the Cucurbitaceae family (1). TCS is a traditional Chinese medicine
which has been used as an abortifacient and for the treatment of
hydatidiform moles, malignant hydatidiform moles and ectopic
pregnancies due to its high toxicity to trophoblasts (2,3).
Previous studies suggest that TCS has a broad spectrum of
biological and pharmacological activities including immune
regulatory, antivirus and antitumor activity (1,4,5). Due
to the selective cytotoxicity of TCS to tumor cells, TCS has gained
increased research attention. It was suggested that TCS-treated
cells underwent cell death due to the inhibition of cellular
protein synthesis and the induction of necrosis (3). However, studies have suggested that
TCS exerts antitumor activities by inducing apoptosis in numerous
cell lines, including human choriocarcinoma, breast cancer,
cervical cancer, nasopharyngeal carcinoma, leukemia and lymphoma
cells (5–10). Therefore, TCS has been considered
as a potential novel agent for antitumor treatment.
Apoptosis is an important mode of programmed cell
death that occurs normally during development or as a stress
response, and is an essential mechanism to selectively eliminate
cells in antitumor chemotherapy and radiotherapy (11,12).
Central to the cell suicide program is a cascade of caspases with
caspase-3 being a key terminal executor that in turn is cleaved and
activated by various initiator caspases (13). There are two major pathways of
apoptosis in mammalian cells: The death receptor-initiated
extrinsic pathway and the mitochondria-mediated intrinsic pathway
(13–15). Cross-talk exists between the
extrinsic and intrinsic apoptotic pathways (16). Caspase-8 is the initiator of the
extrinsic pathway and caspase-9 is a key executor of the instrinsic
pathway. The cleavage of either caspase-8 or -9 triggers downstream
effectors, caspase-3, -6 and -7, and poly(ADP-ribose) polymerase-1
(PARP-1), which leads to a series of apoptotic events and
eventually cell death (14,15,17).
A previous study demonstrated that TCS is able to
inhibit the proliferation of Raji and Jurkat cells (10). However, limited types of lymphoma
cell lines have been investigated, and the mechanisms involved in
the TCS-induced apoptosis in these cells remain to be elucidated.
Therefore, the current study screened the potential antitumor
activity of TCS in different types of lymphoma cells and identified
which exhibited the greatest sensitivity to TCS. In these cells,
the effects of TCS on the cell cycle, the induction of apoptosis
and the underlying mechanisms were investigated. The present study
provides an experimental basis for further research, and indicates
that TCS may be a novel antitumor agent for the treatment of
lymphoma.
Materials and methods
Chemicals
TCS was purchased from Shanghai Jinshan
Pharmaceutical Co., Ltd. (Shanghai, China). Dimethyl sulfoxide was
obtained from Sigma-Aldrich (St. Louis, MO, USA) and the Cell
Counting Kit-8 was from Dojindo Molecular Technologies, Inc.
(Kumamoto, Japan). Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) were obtained from Roche Diagnostics GmbH
(Mannheim, Germany). Hoechst 33258 was obtained from Beyotime
Institute of Biotechnology (Haimen, China). The caspase-3,
caspase-7, PARP-1 and glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) primary antibodies were supplied by Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Carbobenzoxy-val-ala-asp-(OMe)-fluoromethylketone (Z-VAD-FMK),
Z-ile-glu(OMe)-thr-asp(OMe)-FMK (Z-IETD-FMK) and
Z-leu-glu(OMe)-his-asp(OMe)-FMK (Z-LEHD-FMK) were obtained from
R&D Systems, Inc. (Minneapolis, MN, USA). TCS was examined
using SDS-PAGE and Coomassie Brilliant Blue staining (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell culture
Raji, Ramos, Namalwa (Burkitt's lymphoma), RL
(follicular lymphoma), Jurkat (T-cell acute lymphoblastic leukemia)
(American Type Culture Collection, Manassas, VA, USA), OCI-ly1,
OCI-ly8, OCI-ly19, SU-DHL-6 (gifts from Professor Xiongzeng Zhu,
Fudan University Shanghai Cancer Center, Shanghai, China),
OCI-ly10, SU-DHL-2 (gifts from Professor Ru Feng, Nanfang Hospital,
Southern Medical University, Guangzhou, China), SU-DHL-4 and
NU-DHL-1 [diffuse large B-cell lymphoma (DLBCL)] (gifts from
Professor Yanhui Liu, Guangdong General Hospital, Guangzhou, China)
cell lines were used in the present study. All cell lines were
grown in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin (GE Healthcare Life Sciences, Logan, UT,
USA) in a 5% CO2 incubator at 37°C.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) assay was used to
measure the sensitivity of the different cell lines to TCS. Cells
were seeded at a density of 6×103 cells/well in 96-well
plates. TCS was added at different concentrations (0.01953125–10.0
µM; 20 µl/well) into the designated wells. Following
69 h of incubation, 8 µl of CCK-8 solution was added to each
well, and the plate incubated for a further 3 h. The absorbance
values were measured at 450 nm using a SpectraMax M5 plate reader
(Molecular Devices LLC, Sunnyvale, CA, USA). The 50% inhibitory
concentration (IC50) values were calculated to construct
the survival curves using the Bliss method (18).
Cell cycle analysis
SU-DHL-2 cells were treated with TCS (0.15, 0.75 and
1.50 µM) for 48 h. The cells were then centrifuged at 377 ×
g for 5 min and washed with 0.01 M phosphate-buffered saline (PBS,
pH 7.4) three times. Subsequently, the cells were fixed with
ice-cold ethanol for a minimum of 18 h. The cells were then washed
with PBS twice and stained with PI solution, which contained 50
µg/ml PI, 100 µg/ml RNase A (KeyGen Biotech Co. Ltd.,
Nanjing, China) and 0.2% Triton X-100 (KeyGen Biotech Co. Ltd.).
The samples were measured using a Gallios flow cytometer (Beckman
Coulter Inc., Brea, CA, USA) and the data were analyzed using
multiflow software (version 2.2; Beckmann Coulter, Inc.).
Annexin V-FITC/PI staining assay
An Annexin V-FITC/PI Apoptosis kit was used
according to the manufacturer's instructions to quantify the
percentage of cells undergoing apoptosis. SU-DHL-2 cells were
incubated for 48 h with TCS (0.03, 0.15 or 0.75 µM) or with
0.75 µM for 3, 6, 12, 24 and 48 h. The Bcl-2/Bcl-xL
inhibitor, ABT-263 (8 µM; Selleckchem, Houston, TX, USA),
was used as the positive control. The cells were washed twice with
cold PBS and resuspended in the binding buffer (Roche Diagnostics
GmbH) at a concentration of 1×106 cells/ml.
Subsequently, 5 µl Annexin V-FITC and 10 µl PI were
added, and the cells were incubated for 5 min at room temperature
in the dark. Following this, 400 µl binding buffer was added
and the cells were analyzed by flow cytometry. The Annexin
V-FITC+/PI− cells were identified as
apoptotic cells, and the Annexin V-FITC+/PI+
cells were identified as necrotic cells. The measurements were
repeated three times for each sample.
Hoechst 33258 staining
The morphological alterations in the TCS-treated
cells were investigated using Hoechst 33258 staining. Following
exposure to TCS (0.03, 0.15 or 0.75 µM) for 48 h, or 0.75
µM for 12, 24 or 48 h, the SU-DHL-2 cells were incubated
with 20 µM Hoechst 33258 for 10 min at room temperature. The
cells were then washed twice with PBS and examined using a confocal
laser scanning microscope (Olympus Fluoview FV1000; Olympus
Corporation, Tokyo, Japan). Apoptotic cells were identified by
Hoechst 33258 staining as exhibiting condensed chromatin and
fragmented nuclei. A minimum of 10 random fields were counted for
each sample.
Western blot analysis
Cells were harvested and rinsed three times with
ice-cold PBS, and total cell lysates were prepared. Cell extracts
were prepared by incubating cells for 30 min on ice with 1X lysis
buffer [PBS with 0.1% sodium dodecyl sulfate (SDS), 1% Nonidet
P-40, 0.5% sodium deoxycholate and 100 mg/ml p-amino
phenylmethylsulfonyl fluoride] with agitation followed by
centrifugation (14,000 × g at 4°C for 20 min). The supernatant
containing the total cell lysates was stored at −80°C prior to
experiments. Cell lysates containing 30 µg total protein
were resolved by SDS polyacrylamide gel electrophoresis (SDS-PAGE;
Bio-Rad Laboratories, Inc.) and transferred onto polyvinylidene
fluoride membranes (Roche Diagnostics GmbH). Membranes were
incubated in blocking buffer [10 mmol/l Tris-HCl (pH 8.0), 150
mmol/l NaCl, 0.1% Tween 20 and 5% skimmed milk] for 2 h at room
temperature, prior to incubation with primary antibodies overnight
at 4°C. The following antibodies were used: Rabbit polyclonal
anti-caspase-7 (cat. no. 9492; 1:1,000 dilution), rabbit monoclonal
anti-caspase-3 (cat. no. 9665; 1:1,000 dilution), rabbit monoclonal
anti-PARP-1 (cat. no. 9532; 1:1,000 dilution) and rabbit monoclonal
anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; cat. no.
2118; 1:5,000 dilution) (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA). Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:2,000 dilution; Cell Signaling Technology, Inc.) for 1
h at room temperature. The protein-antibody complexes were detected
using enhanced chemiluminescence reagents (Cell Signaling
Technology, Inc.) and exposed to X-ray film (Eastman-Kodak Co.,
Rochester, NY, USA).
Statistical analysis
All experiments were repeated a minimum of three
times. The data are presented as the mean ± standard deviation.
Statistical analysis was conducted using SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA) and one-way analysis of
variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
TCS reduces the cell viability of
lymphoma cells
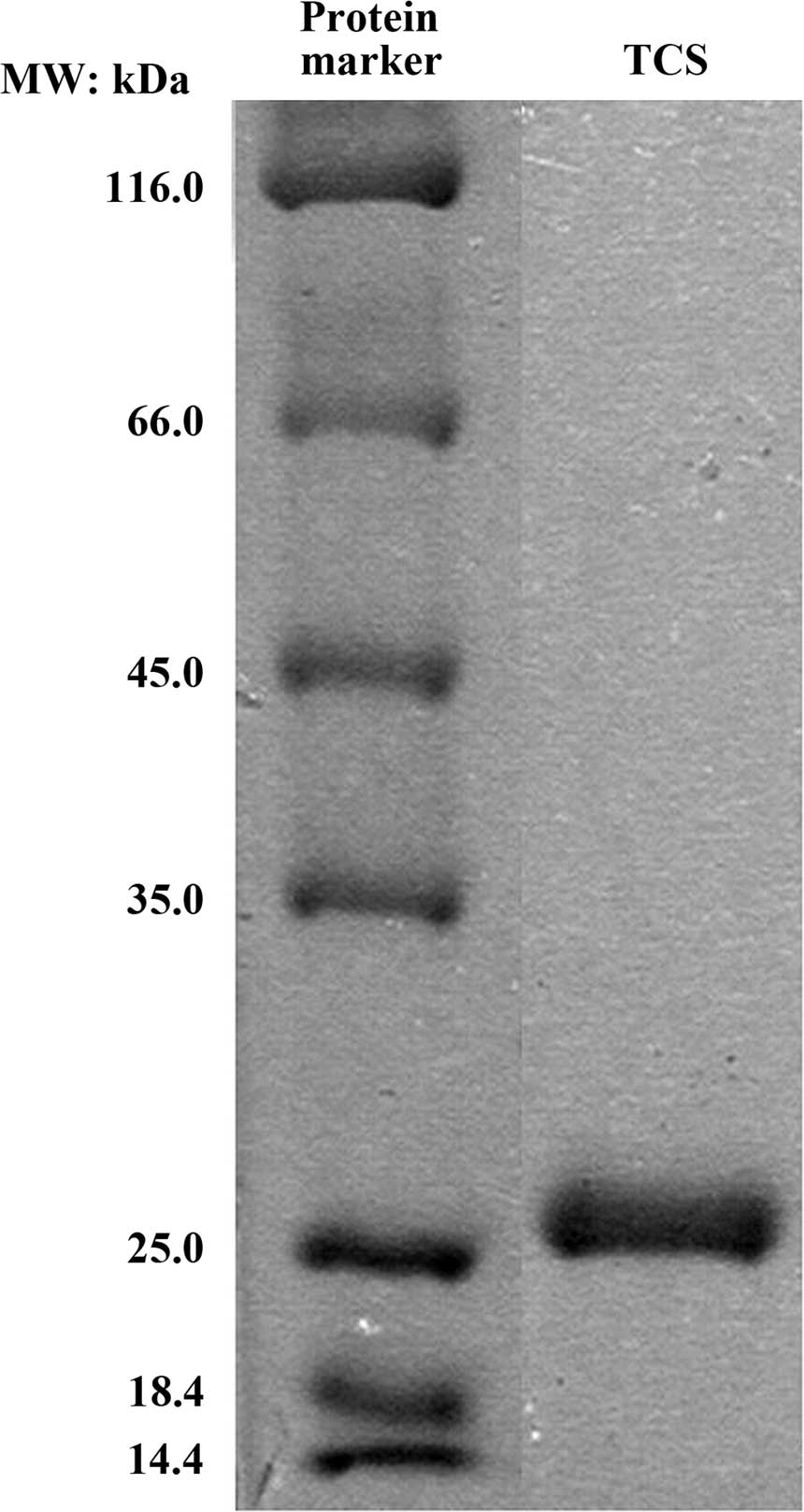
The purity of TCS was examined using SDS-PAGE and
Coomassie Brilliant Blue staining. A single band with a molecular
mass of 27 kDa was observed by SDS-PAGE (Fig. 1), which was in accordance with a
previous study (19). To
investigate the potential anti-tumor activity of TCS on different
types of lymphoma cells, the following thirteen lymphoma cell lines
were selected: Raji, Ramos, Namalwa (Burkitt's lymphoma), OCI-ly1,
OCI-ly8, OCI-ly10, OCI-ly19, SU-DHL-2, SU-DHL-4, SU-DHL-6, NU-DHL-1
[diffuse large B cell lymphoma (DLBCL)], RL (follicular lymphoma)
and Jurkat (T-cell acute lymphoblastic leukemia) cells. The cells
were treated with different concentrations of TCS (ranging from
0.02–10 µM) for 72 h. Cell viability was measured using a
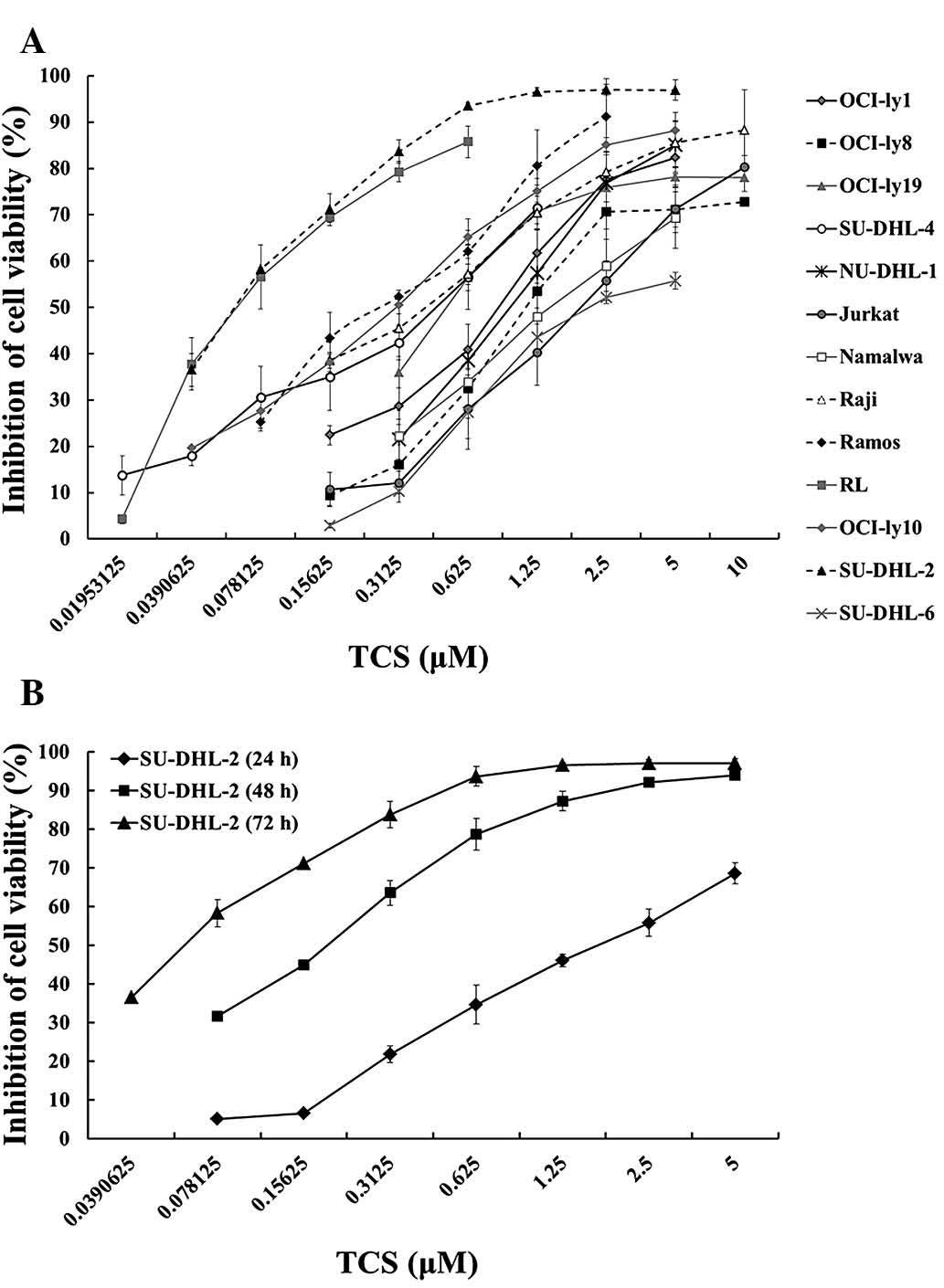
CCK-8 assay. As presented in Fig.
2A, TCS induced a dose-dependent reduction in the viability of
all thirteen tested cell lines with diverse sensitivities.
Following exposure to TCS for 72 h, Jurkat, Namalwa and SU-DHL-6
cells exhibited a lower sensitivity to TCS compared with that of
the other cell lines, with IC50s of 2.20, 2.47 and 1.87
µM, respectively. SU-DHL-2 cells exhibited the greatest
sensitivity to TCS compared with the remaining twelve cell lines.
Based on the IC50 values, SU-DHL-2 cells were selected
for further investigation. In addition, the reduction in viability
mediated by TCS on SU-DHL-2 cells was time-dependent (Fig. 2B). Following 24, 48 and 72 h
treatment with TCS, the IC50s for SU-DHL-2 cells were
1.74, 0.16 and 0.07 µM, respectively.
TCS induces cell cycle arrest in SU-DHL-2
cells
To investigate the molecular mechanisms of the
reduction in viability mediated by TCS, the cell cycle was assessed
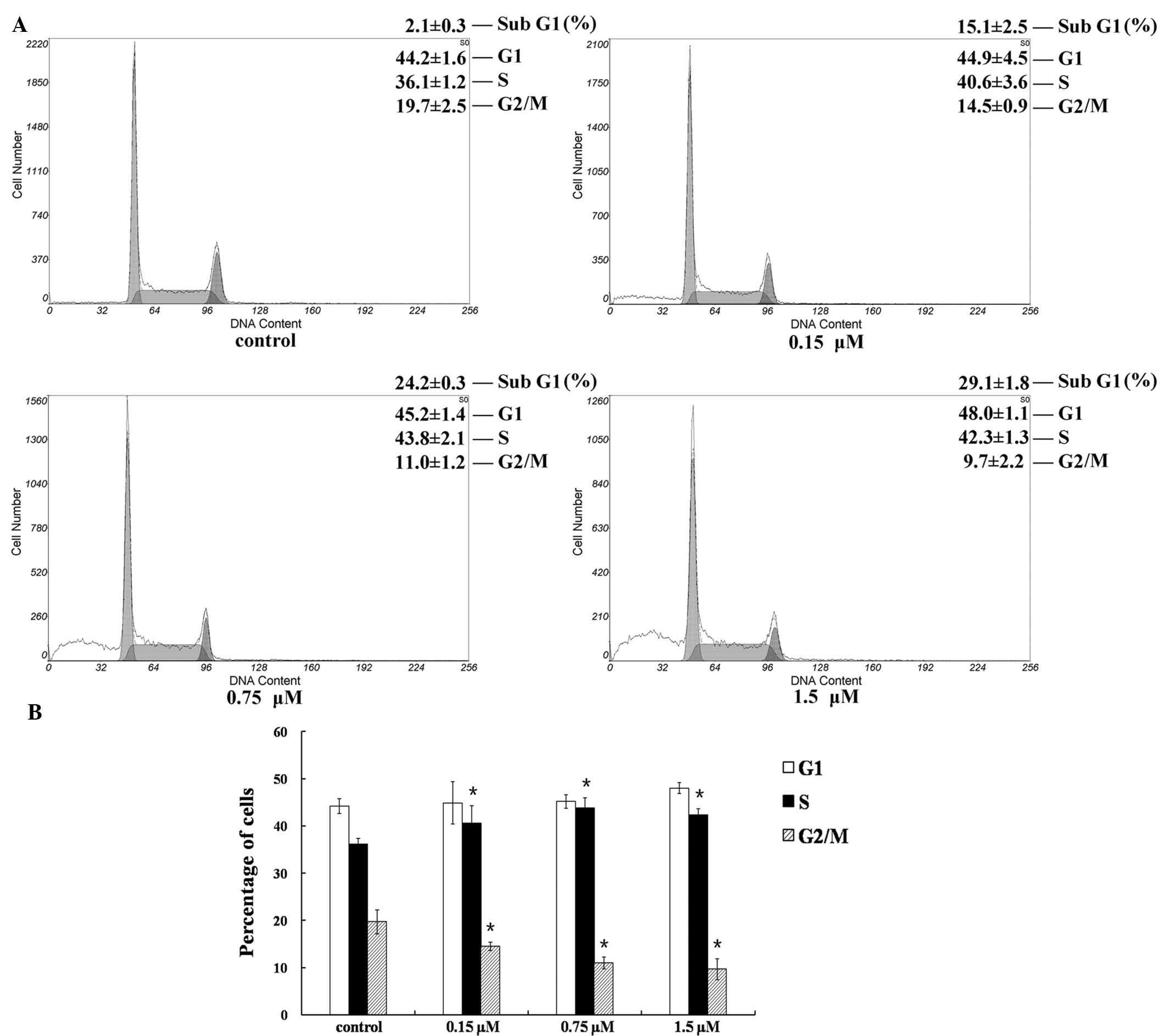
using flow cytometry. Cultured SU-DHL-2 cells were treated with 0,
0.15, 0.75 or 1.50 µM TCS for 24 h and the percentage of
cells in the G1, S and G2/M cell cycle phases
was analyzed. As presented in Fig.
3A, a clear peak appeared in the TCS-treated cells prior to the
G1 peak (sub-G1 population). The
sub-G1 population increased in a dose-dependent manner,
from 2.1–29.1% following treatment with 1.5 µM TCS. In
addition, cell cycle analysis indicated that treatment with TCS
increased the proportion of cells in the S phase, whereas cells in
the G2/M phase were reduced from 19.7 to 9.7%. The
results demonstrated that the reduction in the viability of
SU-DHL-2 cells induced by TCS was associated with cell cycle arrest
at the S to G2/M phase transition (P<0.05; Fig. 3B).
TCS induces apoptosis in SU-DHL-2 cells
in a time- and dose-dependent manner
As the accumulation of cells in the
sub-G1 phase is a potential indicator of apoptosis
(20), it was further investigated
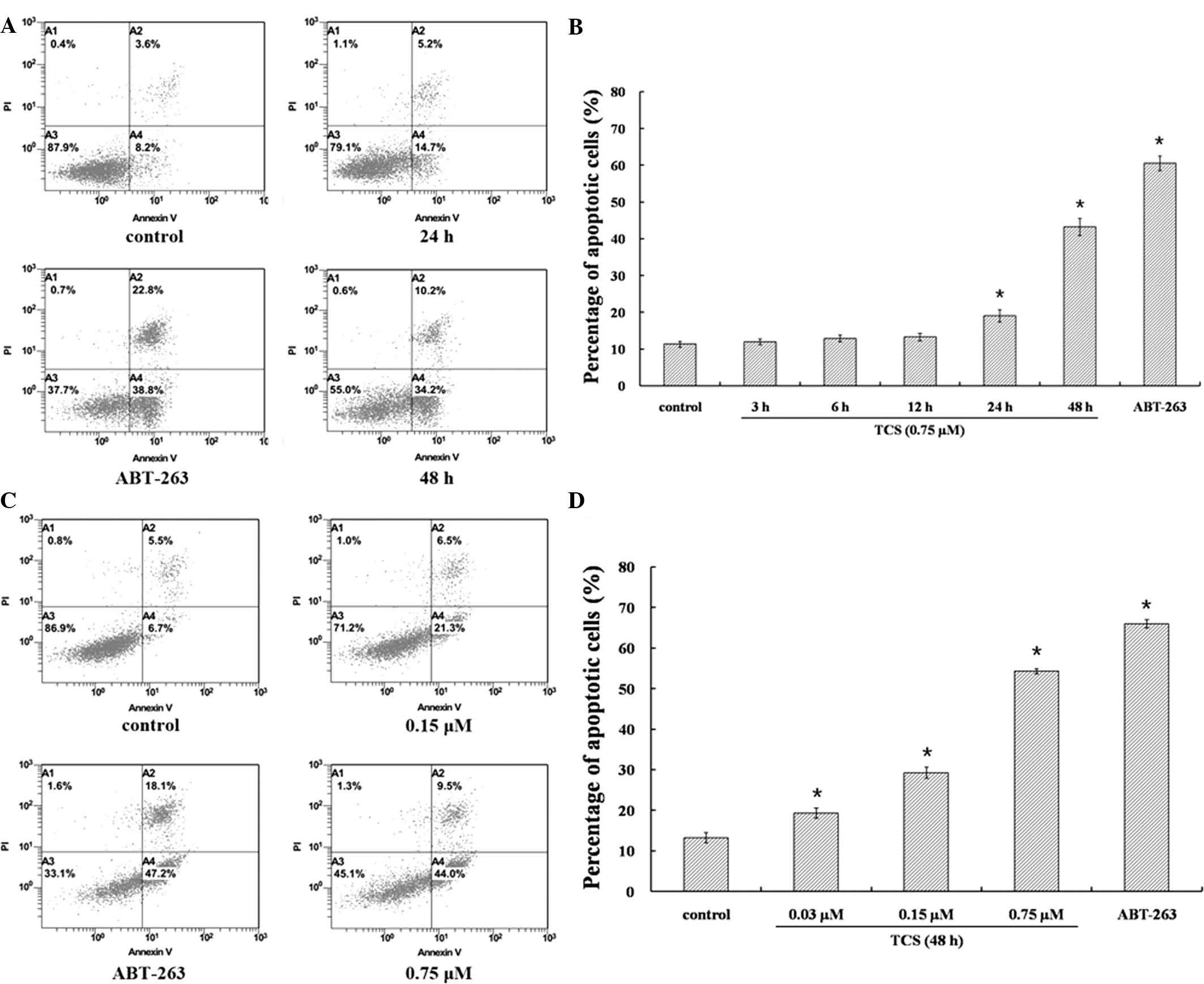
whether TCS induced the apoptosis of SU-DHL-2 cells. SU-DHL-2 cells
were treated with 0.75 µM TCS over different time periods
(3–48 h) or 0, 0.03, 0.15 and 0.75 µM TCS for 48 h. ABT-263
was used as positive control, as it is a Bcl-2/Bcl-xL inhibitor and
apoptosis promoter (21–23). Annexin V-FITC/PI staining was used
to analyze the percentage of apoptotic cells. At 3, 6 and 12 h
following TCS treatment, no significant increase in the apoptotic
rates was observed. However, at 24 and 48 h following TCS
treatment, the apoptotic rates significantly increased from 11.8%
in the non-treated group to 19.9 and 44.4%, respectively, in the
0.75 µM TCS-treated group (Fig.
4A and B). As presented in Fig.
4C, the percentage of apoptotic cells increased from 12.2% in
the non-treated group to 27.8% in the 0.15 µM TCS-treated
group in SU-DHL-2 cells following 48 h treatment. Higher doses of
TCS exerted a greater effect on apoptosis, with 0.75 µM TCS
increasing the percentage of apoptotic cells from 12.2 to 53.5%.
The results demonstrated that TCS dose-dependently increased
apoptosis (P<0.05; Fig. 4D). To
observe the nuclear morphological alterations associated with
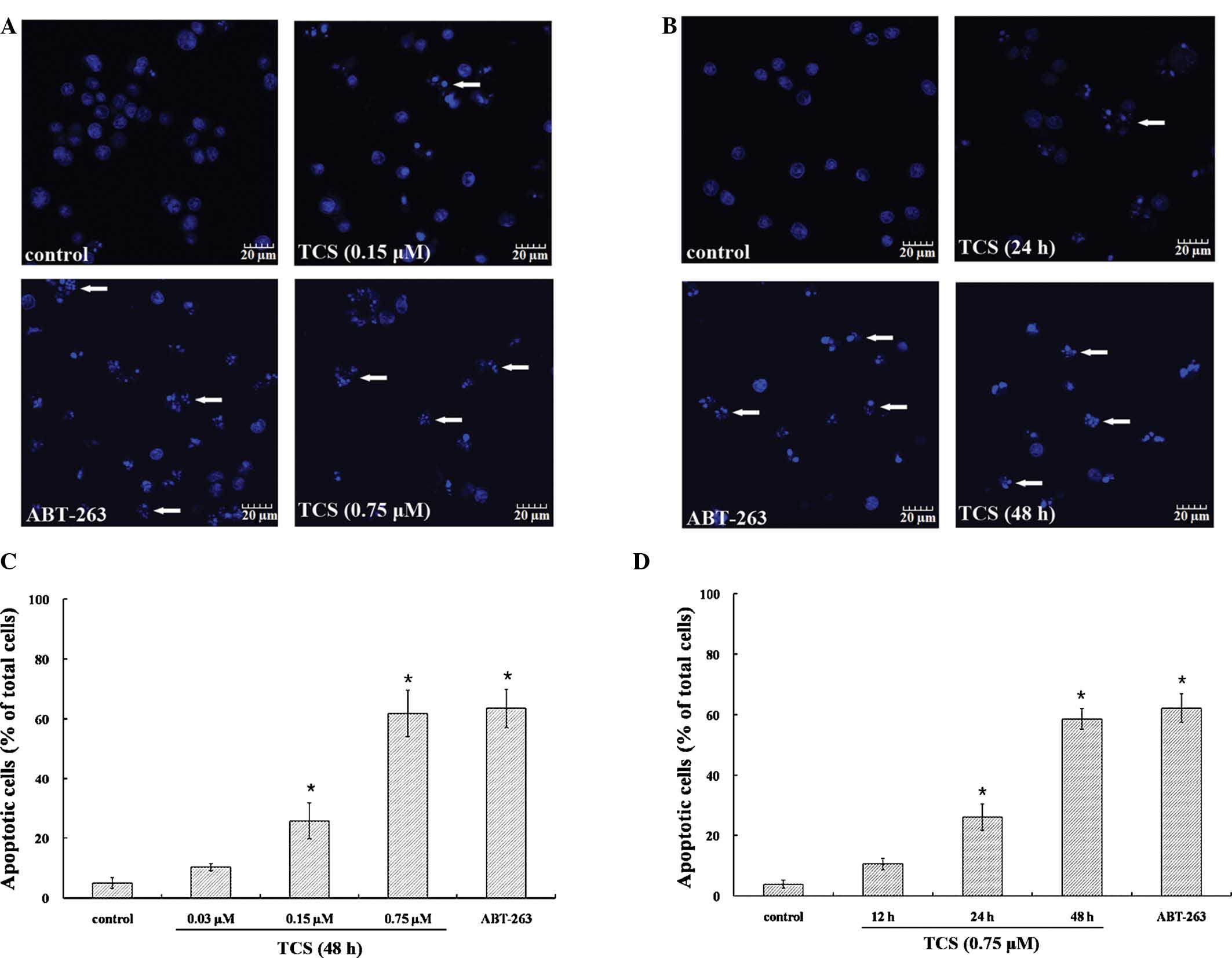
apoptosis in TCS-treated cells, SU-DHL-2 cells were stained with
Hoechst 33258. As presented in Fig. 5A
and B, untreated cells exhibited normal nuclear morphology,
while following treatment with 0.15 µM TCS for 48 h or 0.75
µM TCS for 24 h, the characteristic apoptotic nuclear
condensation and fragmentation were observed. This phenomenon was
time- and concentration-dependent (Fig. 5C and D). Taken together, these data
indicate that TCS induced apoptosis in SU-DHL-2 cells.
Induction of apoptosis through the
activation of caspase-3 and -7, and PARP-1
To investigate the apoptotic mechanisms induced by
TCS, the activities of key apoptosis-associated proteins were
investigated by western blot analysis. SU-DHL-2 cells were treated
with different concentrations (0.03, 0.15 and 0.75 µM) of
TCS for 48 h or 0.75 µM TCS for a range of time points (3–48
h). Subsequently, the protein extracts were prepared and separated
by SDS-PAGE. As presented in Fig.
6A, significant increases in the levels cleaved caspase-3 and
-7, and PARP-1 were observed following treatment with 0.15 and 0.75
µM TCS at 48 h. As the key executioners of apoptosis, the
cleavage and activation of caspase-3 and -7 were increased with the
increase in the concentration of TCS, and the downstream cleavage
of PARP-1 was additionally observed in a concentration-dependent
manner. As shown in Fig. 6B, the
amount of cleaved caspase-3 and -7 as well as PARP-1 gradually
increased following treatment with TCS for 6–48 h, while the levels
of pro-caspase-3 and -7 as well as full-length PARP-1 were reduced
in parallel. Furthermore, the levels of the active fragments were
markedly increased compared with the control (Fig. 6B). Therefore, the results indicate
that TCS induced apoptosis in SU-DHL-2 cells through the
time-dependent sequential activation of caspase-3 and -7, and
PARP-1.
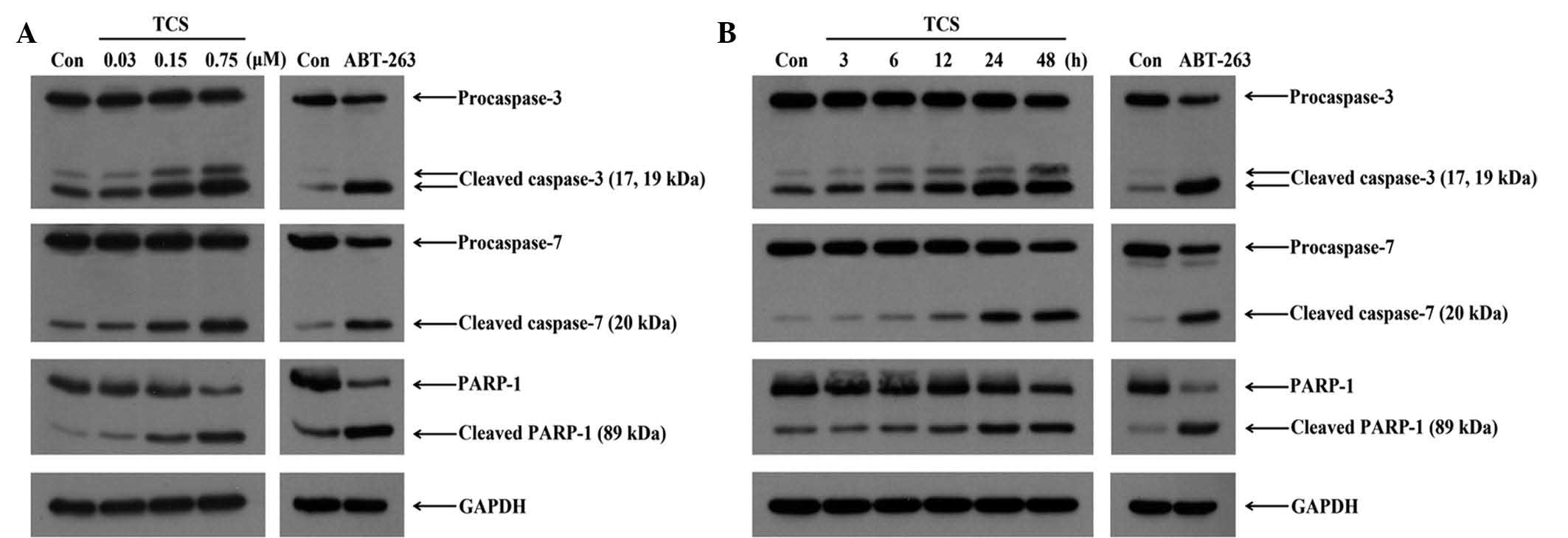 | Figure 6Effect of TCS on the expression of
apoptosis-associated proteins. (A) To examine the dose-dependent
effects, SU-DH-2 cells were treated with TCS (0.03, 0.15 and 0.75
µM) and 8 µM ABT-263 for 48 h. Activated caspase-3
and -7, and cleaved PARP-1 were observed in a dose-dependent
manner. (B) To examine the time-dependent effects, SU-DH-2 cells
were treated with TCS (0.75 µM) for 3, 6, 12, 24 or 48 h.
The activation of caspase-3 and -7, and PARP-1 was maximal at 48 h,
and TCS induced apoptosis in a time-dependent manner. The
experiments were repeated three times with similar results. TCS,
trichosanthin; Con, control; PARP-1, poly (ADP-ribose) polymerase
1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Involvement of various upstream caspase
pathways
Apoptosis is executed by the caspase-8-mediated
extrinsic pathway and/or caspase-9-dependent intrinsic pathway
(24). To investigate whether
these pathways were activated in TCS-induced cell apoptosis,
caspase activity was inhibited using Z-VAD-FMK (pan-caspase
inhibitor), Z-IETD-FMK (caspase-8 inhibitor) and Z-LEHD-FMK
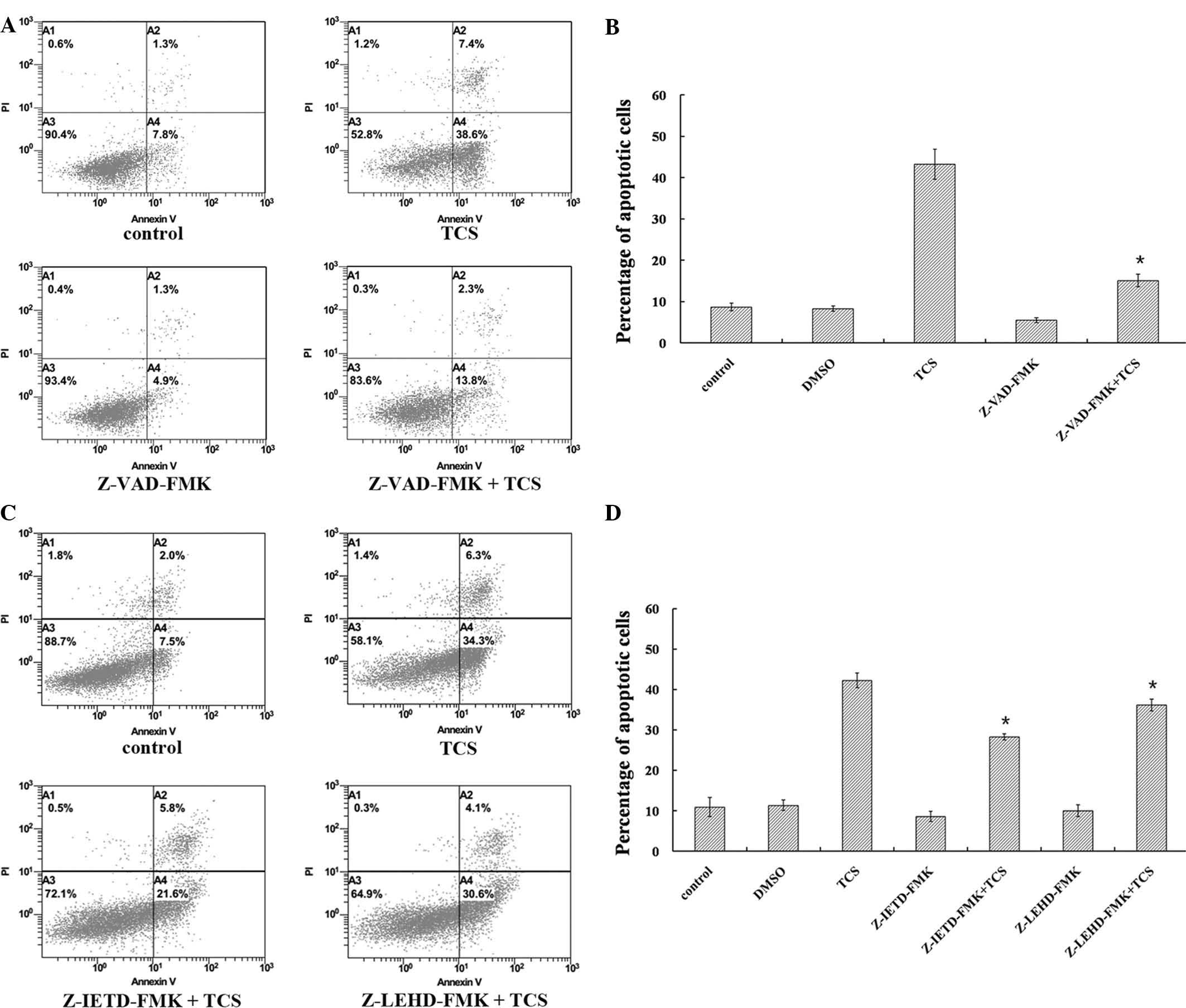
(caspase-9 inhibitor), and the percentage of apoptotic cells was
measured using flow cytometry. SU-DHL-2 cells were treated with
0.75 µM TCS combined with 20 µM Z-VAD-FMK for 48 h,
and the Z-VAD-FMK treatment was observed to markedly inhibit
TCS-induced apoptosis from 46.0 to 16.1%, indicating that
TCS-induced cell death involved caspase activation (P<0.05;
Fig. 7A and B). Notably, treatment
with Z-IETD-FMK (20 µM) or Z-LEHD-FMK (20 µM)
significantly reduced the percentage of TCS-induced apoptotic cells
from 40.6 to 27.4 or 34.7%, respectively (Fig. 7C and D). These results suggest that
the extrinsic and intrinsic apoptotic pathways were involved in
TCS-induced apoptosis.
Discussion
TCS has been used as an anti-inflammatory agent in
traditional Chinese medicine for over a century in China (1,25).
More recently, TCS has been investigated due to its potential
antitumor activity. An advantage of TCS compared with standard
chemotherapy is that TCS has selective toxicity for tumor cells,
with minimal effect on normal cells (4). TCS has been demonstrated to induce
the apoptosis of choriocarcinoma (6), leukemia and lymphoma (4,10),
gastric carcinoma (26), cervical
cancer (5) and hepatoma (27) cells. In the present study, TCS was
demonstrated to exhibit potent antitumor activity toward thirteen
lymphoma cell lines. The cell line with the greatest sensitivity to
TCS was SU-DHL-2. TCS was able to reduce SU-DHL-2 cell viability at
low concentrations. Therefore, SU-DHL-2 cells were selected for
further investigation. In addition, these data demonstrated that
the effects of TCS were dependent on the dose and exposure
duration.
DLBCL, the most common subtype of non-Hodgkin's
lymphoma, has a relatively favorable prognosis. However, despite
attempts to increase the efficacy of conventional chemotherapy
during the past decade, it remains that ~40% of DLBCL patients fail
to respond to treatment with R-CHOP (rituximab, cyclophosphamide,
doxorubicin, vincristine and prednisone)-like regimens (28). Of those patients that are
treatment-resistant or have relapsed, only 30–35% achieve prolonged
progression-free survival with high-dose chemotherapy followed by
autologous stem cell transplantation (29). The present study demonstrated clear
reductions in the viability of SU-DHL-2 cells treated with TCS,
indicating it may be a novel strategy and aid in the improvement of
outcomes for patients with DLBCL who are treatment-resistant or
have relapsed.
To date, the precise mechanisms of TCS-mediated
inhibition of tumor growth remain poorly characterized. Potential
mechanisms involved include the cyclic adenosine monophosphate
signaling pathways (30), caspase
family members and the mitochondrial apoptotic pathway (31), in addition to the regulation of
apoptosis-associated genes and (32) the cytoskeleton (3). To elucidate the mechanisms of action
of TCS, the current study investigated apoptotic induction and cell
cycle arrest at the cellular and molecular levels. Cell cycle
distribution was observed using PI single staining. The percentage
of cells at the sub-G1 phase was increased significantly
in the TCS-treated groups compared with the control group. In
addition, flow cytometric analysis using Annexin V-FITC/PI
indicated that TCS induced early apoptosis in SU-DHL-2 cells in a
dose-dependent manner. It was observed that the cells undergo
apoptotic cell death at 24 and 48 h following TCS treatment (0.75
µM; Fig. 4). When the cells
were incubated with TCS for 48 h, 21.3% of cells were observed to
undergo early stage apoptosis in the 0.15 µM TCS group,
which is significantly greater than the control group (6.7%).
Furthermore, the appearance of apoptotic nuclei was observed in
cells treated with TCS.
In further investigations of apoptosis induced by
TCS, it was observed that TCS-induced apoptosis was associated with
caspase activation. The cleavage of caspase-3 and -7, and PARP-1
was observed in the TCS-treated groups in a dose- and
time-dependent manner. Caspases are cysteine proteases that have
critical roles in the coordination of apoptosis, and cleave target
proteins to execute cell death (13,17,33).
Caspase is a key contributor to the cell disassembly observed in
apoptosis, via the targeting of structural substrates including
nuclear laminins, focal adhesion sites and cell-cell adherence
junctions (34–37). In addition to caspase-3, caspase-7
is activated during the execution phase of apoptosis, and its
functions partially overlap with caspase-3, such that
caspase-3-deficient cells continue to execute apoptosis in the
presence of caspase-7 (38). A
previous study demonstrated that the deficiency of both caspase-3
and caspase-7 is required to entirely prevent the activation of
apoptosis (39). In the current
study, cleavage of both caspase-3 and caspase-7 was observed in
TCS-treated SU-DHL-2 cells.
To investigate the role of the caspase cascade in
TCS-induced cell death, Z-VAD-FMK, a pan-caspase inhibitor, was
used. The ability of TCS to induce apoptosis was inhibited by
Z-VAD-FMK in SU-DHL-2 cells, demonstrating that TCS-induced
apoptosis is caspase-dependent. Caspases are the principal
effectors of apoptosis and are involved in pathways such as the
caspase-8-regulated extrinsic and caspase-9-regulated intrinsic
pathways. The caspase-9 pathway links mitochondrial damage to
caspase activation, and serves as an index of decreased
mitochondrial membrane function. In a previous study, TCS has been
reported to induce apoptosis by the activation of the caspase-8-
and caspase-9-regulated pathways in breast cancer cells (7). To investigate the involvement of
caspase-8 and -9 in TCS-induced apoptosis, SU-DHL-2 cells were
treated with Z-IETD-FMK or Z-LEHD-FMK and co-treated with TCS. The
results demonstrated that Z-IETD-FMK reduced the percentage of
apoptotic cells induced by TCS. However, Z-LEHD-FMK was observed to
reduce the level of apoptotic cells to a lesser extent than
Z-IETD-FMK. Thus, TCS-induced apoptosis of SU-DHL-2 cells is
dependent on caspase-9 and caspase-8, which indicates that TCS
induces apoptosis via the mitochondrial-dependent and the death
receptor pathways, with the caspase-8-mediated extrinsic death
receptor pathway having greater involvement. Further investigation
of these pathways is required to fully elucidate the apoptotic
mechanisms involved. Taken together, these data indicate that the
cell-death inducing activity of TCS is associated with
apoptosis.
Alterations in the regulation of the cell cycle
serves a key role in the growth of numerous types of cancer and is
an important target in cancer therapy (40). G1 phase arrest by TCS
has been previously reported in breast cancer cells (7) and A549 lung cancer cells (41). In the current study, TCS induced an
increase in the percentage of cells in the S phase in SU-DHL-2
cells. For a cell to be able to divide into two daughter cells, the
synthesis and duplication of the DNA are a requirement, and any
abnormality in this would lead to an obstacle in cell cycle
progression. Numerous checkpoints exist to aid cells in the
identification and repair of DNA damage by halting/stalling the
progression through the phases of the cell cycle, including the
G0/G1, S and G2/M checkpoints
(40). The current study observed
arrest at the S to G2/M phase transition of the cell
cycle upon TCS treatment, leading to a halt in cell cycle
progression.
In conclusion, these data indicate that TCS reduced
cell viability in a dose- and time-dependent manner in SU-DHL-2
lymphoma cells. This effect of TCS may be attributed to the
induction of apoptosis and the arrest in the S phase of the cell
cycle in SU-DHL-2 cells. To the best of our knowledge, the current
study demonstrated for the first time that the TCS-induced
apoptosis of SU-DHL-2 cells was associated with the activation of
the extrinsic and intrinsic pathways. However, the precise
mechanisms and the molecular mediators that result in the
initiation of apoptosis remain to be elucidated. Thus, further
studies on TCS as a novel chemotherapeutic agent are required.
Acknowledgments
The current study was supported by Fundamental
Research Funds for the Development of Strategic Emerging Industries
in Shenzhen, China (grant no. JCYJ20120613113228732) and Science
and Technology Research Project of Shenzhen, China (grant no.
JSGG20150512162446307).
References
|
1
|
Shaw PC, Lee KM and Wong KB: Recent
advances in trichosanthin, a ribosome-inactivating protein with
multiple pharmacological properties. Toxicon. 45:683–689. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang XJ and Wang JH: Homology of
trichosanthin and ricin A chain. Nature. 321:477–478. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li M, Li X and Li JC: Possible mechanisms
of trichosanthin-induced apoptosis of tumor cells. Anat Rec
(Hoboken). 293:986–992. 2010. View
Article : Google Scholar
|
|
4
|
Zheng YT, Zhang WF, Ben KL and Wang JH: In
vitro immunotoxicity and cytotoxicity of trichosanthin against
human normal immunocytes and leukemia-lymphoma cells.
Immunopharmacol Immunotoxicol. 17:69–79. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ru QH, Luo GA, Liao JJ and Liu Y:
Capillary electrophoretic determination of apoptosis of HeLa cells
induced by trichosanthin. J Chromatogr A. 894:165–170. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang C, Gong Y, Ma H, An C, Chen D and
Chen ZL: Reactive oxygen species involved in trichosanthin-induced
apoptosis of human choriocarcinoma cells. Biochem J. 355:653–661.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang EF, Zhang CZ, Zhang L, Wong JH, Chan
YS, Pan WL, Dan XL, Yin CM, Cho CH and Ng TB: Trichosanthin
inhibits breast cancer cell proliferation in both cell lines and
nude mice by promotion of apoptosis. PLoS One. 7:e415922012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu F, Wang B, Wang Z and Yu S:
Trichosanthin down-regulates Notch signaling and inhibits
proliferation of the nasopharyngeal carcinoma cell line CNE2 in
vitro. Fitoterapia. 83:838–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang K, Xu J, Huang X, Wu L, Wen C, Hu Y,
Su Y, Chen Y and Zhang Z: Trichosanthin down-regulated p210Bcr-Abl
and enhanced imatinib-induced growth arrest in chronic myelogenous
leukemia cell line K562. Cancer Chemother Pharmacol. 60:581–587.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang YY, Ouyang DY and Zhengx YT:
Mechanism of trichosanthin against human leukemia/lymphoma cells in
vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 15:729–732. 2007.In
Chinese. PubMed/NCBI
|
|
11
|
Herr I and Debatin KM: Cellular stress
response and apoptosis in cancer therapy. Blood. 98:2603–2614.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu W and Kavanagh JJ: Anticancer therapy
targeting the apoptotic pathway. Lancet Oncol. 4:721–729. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dias N and Bailly C: Drugs targeting
mitochondrial functions to control tumor cell growth. Biochem
Pharmacol. 70:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schulze-Osthoff K, Ferrari D, Los M,
Wesselborg S and Peter ME: Apoptosis signaling by death receptors.
Eur J Biochem. 254:439–459. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi Z, Liang YJ, Chen ZS, Wang XW, Wang
XH, Ding Y, Chen LM, Yang XP and Fu LW: Reversal of
MDR1/P-glycoprotein-mediated multidrug resistance by vector-based
RNA interference in vitro and in vivo. Cancer Biol Ther. 5:39–47.
2006. View Article : Google Scholar
|
|
19
|
Fang EF, Ng TB, Shaw PC and Wong RN:
Recent progress in medicinal investigations on trichosanthin and
other ribosome inactivating proteins from the plant genus
Trichosanthes. Curr Med Chem. 18:4410–4417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L, Aaronson SA, Abrams J, Alnemri
ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren
K, Borner C, et al: Guidelines for the use and interpretation of
assays for monitoring cell death in higher eukaryotes. Cell Death
Differ. 16:1093–1107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson MA, Huang D and Roberts A:
Targeting BCL2 for the treatment of lymphoid malignancies. Semin
Hematol. 51:219–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi J, Zhou Y, Huang HC and Mitchison TJ:
Navitoclax (ABT-263) accelerates apoptosis during drug-induced
mitotic arrest by antagonizing Bcl-xL. Cancer Res. 71:4518–4526.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Levesley J, Steele L, Taylor C, Sinha P
and Lawler SE: ABT-263 enhances sensitivity to metformin and
2-deoxyglucose in pediatric glioma by promoting apoptotic cell
death. PLoS One. 8:e640512013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao Q and Shi Y: Apoptosome: A platform
for the activation of initiator caspases. Cell Death Differ.
14:56–65. 2007. View Article : Google Scholar
|
|
25
|
Zhao J, Ben LH, Wu YL, Hu W, Ling K, Xin
SM, Nie HL, Ma L and Pei G: Anti-HIV agent trichosanthin enhances
the capabilities of chemokines to stimulate chemotaxis and G
protein activation, and this is mediated through interaction of
trichosanthin and chemokine receptors. J Exp Med. 190:101–111.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu J, Gao DF, Yan GL and Fan JM: Induced
apoptotic action of recombinant trichosanthin in human stomach
adenocarcinoma MCG803 cells. Mol Biol Rep. 36:1559–1564. 2009.
View Article : Google Scholar
|
|
27
|
Li M, Chen F, Liu CP, Li DM, Li X, Wang C
and Li JC: Dexamethasone enhances trichosanthin-induced apoptosis
in the HepG2 hepatoma cell line. Life Sci. 86:10–16. 2010.
View Article : Google Scholar
|
|
28
|
Vacirca JL, Acs PI, Tabbara IA, et al:
Bendamustine combined with rituximab for patients with relapsed or
refractory diffuse large B cell lymphoma. Ann Hematol. 93:403–409.
2014. View Article : Google Scholar :
|
|
29
|
Friedberg JW and Fisher RI: Diffuse large
B-cell lymphoma. Hematol Oncol Clin North Am. 22:941–952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang P, Yan H and Li JC: CREB-mediated
Bcl-2 expression in trichosanthin-induced Hela cell apoptosis.
Biochem Biophys Res Commun. 363:101–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li J, Xia X, Ke Y, Nie H, Smith MA and Zhu
X: Trichosanthin induced apoptosis in HL-60 cells via mitochondrial
and endoplasmic reticulum stress signaling pathways. Biochim
Biophys Acta. 1770:1169–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang H, Chan H, Wang YY, Ouyang DY, Zheng
YT and Tam SC: Trichosanthin suppresses the elevation of p38 MAPK,
and Bcl-2 induced by HSV-1 infection in Vero cells. Life Sci.
79:1287–1292. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Boland K, Flanagan L and Prehn JH:
Paracrine control of tissue regeneration and cell proliferation by
Caspase-3. Cell Death Dis. 4:e7252013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brancolini C, Lazarevic D, Rodriguez J and
Schneider C: Dismantling cell-cell contacts during apoptosis is
coupled to a caspase-dependent proteolytic cleavage of
beta-catenin. J Cell Biol. 139:759–771. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kook S, Kim DH, Shim SR, Kim W, Chun JS
and Song WK: Caspase-dependent cleavage of tensin induces
disruption of actin cytoskeleton during apoptosis. Biochem Biophys
Res Commun. 303:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View Article : Google Scholar
|
|
37
|
Kothakota S, Azuma T, Reinhard C, Klippel
A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ
and Williams LT: Caspase-3-generated fragment of gelsolin: Effector
of morphological change in apoptosis. Science. 278:294–298. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng TS, Hunot S, Kuida K, Momoi T,
Srinivasan A, Nicholson DW, Lazebnik Y and Flavell RA: Deficiency
in caspase-9 or caspase-3 induces compensatory caspase activation.
Nat Med. 6:1241–1247. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arora S and Tandon S: Achyranthes aspera
root extracts induce human colon cancer cell (COLO-205) death by
triggering the mitochondrial apoptosis pathway and S phase cell
cycle arrest. Scientific World Journal. 2014:1296972014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li CT, Lin CH, Kao TY, Wu MF, Yeh CS, Yeh
KT and Ko JL: The mechanisms of action of Tianhua(™) on antitumor
activity in lung cancer cells. Pharm Biol. 48:1302–1309. 2010.
View Article : Google Scholar : PubMed/NCBI
|