Introduction
Radiation therapy is one of the most effective and
indispensable treatment modalities for patients with cancer, and is
used for the effective control of local disease and for palliative
care (1). In a meta-analysis of
individual patient data from 10,801 women in 17 randomized trials
of radiotherapy following breast-conserving surgery, radiotherapy
reduced the 10 year risk of any loco-regional or distant recurrence
between 35.0 and 19.3%, and reduced the 15 year risk of breast
cancer-associated mortality between 25.2 and 21.4% (2). Improvements in cancer detection and
treatment, and an aging population has resulted in increasing
numbers of individuals living with and surviving from cancer. In
the US, 64% of adults diagnosed with cancer are expected to survive
at least 5 years (3), and the
actuarial survival data of Indian patients with breast cancer with
early stage disease at 10 years is 77% (4). However, the use of radiotherapy is
often associated with normal tissue injury, which includes
immediate and long-term damage to the normal tissues. Chronic side
effects in survivors becomes a serious problem as the number of
individuals treated and their expected survival rates increase
(5). The skeletal system is one of
the important targets for radiation-induced injury. Bone injury
following radiotherapy has been confirmed in epidemiological and
animal studies (6). A study
involving 6,428 postmenopausal women who received radiotherapy
showed that the risk of pelvic fractures was increased by 65–216%
(7). Epidemiological studies have
suggested that decreased bone mass is associated with increased
adiposity with ageing, bone loss and osteoporosis (6). Skeletal complications following
radiotherapy have also been reported in breast, pelvic, brain and
blood cancer; with bone pain, pathological skeletal fracture,
spinal cord compression, decreased survival rates and poor quality
of life being reported (8–10).
Bone is one of the most commonly irradiated normal
tissues, and the irradiation of bone can lead to multiple
morbidities, including fracture and loss of marrow function
(8). However, the underlying
mechanism remains to be elucidated and no preventive or curative
solution for this bone loss has been identified. Previous studies
have suggested that radiotherapy is followed by bone loss, and is
accompanied by increased fat content in bone marrow (9). Jia et al demonstrated that a
single dose of radiation elicited a loss of bone mineral density
(10). At a cellular level,
osteoblasts and adipocytes arise from the same progenitor cells,
bone marrow mesenchymal stem cells (BMSCs), which can differentiate
into multiple cell lineages. Quantitative and qualitative stem cell
defects may underlie the modified number and function of
differentiated cells (11).
Several previous studies have already examined hematopoietic
recovery following irradiation, however, investigation into the
bone marrow microenvironment has received less attention (12–15).
Friedensteinand and Kuralesova first demonstrated that BMSCs
exhibit high proliferation capacity and are able to form bone and
cartilage (16). In addition, as
BMSCs exhibit self-renewal, high proliferative and multiple
differentiation potentials are crucial in bone recovery following
irradiation, maintaining homeostasis with osteogenesis and
adipogenesis under physiological conditions. The proliferation and
growth are balanced with terminal differentiation, and this balance
is essential for the modeling, growth and maintenance of the
skeleton (17,18). A previous study suggested that
BMSCs maturation along the osteoblast lineage comes at the expense
of adipogenesis, and vice versa, with aging (19). The observed inverse association
between bone mass and fat mass in the bone marrow microenvironment
has been hypothesized to be caused by enhanced differentiation of
BMSCs into either the osteoblastic or adipocytic lineages at the
expense of the alternative lineage (20). A study by Justesen et al
supported the hypothesis that, with aging and in osteoporosis,
enhanced adipogenesis is observed in the bone marrow, and that
these changes are inversely correlated with decreased trabecular
bone volume (21). However, other
studies have found no evidence for enhanced adipogenesis with
aging, finding that the adipocyte forming capacity of MSCs was
similar in young and old donors (22,23).
The association between bone and fat formation within the bone
marrow microenvironment is complex and remains an area of active
investigation.
Modern radiation therapy aims to reduce side effects
to a minimum. The ability of the patients to tolerate therapy is
often determined by the potential of stem cells within the marrow
to repair the damage resulting from ionizing radiation and to
repopulate the marrow compartment (24). Therefore, it is important to
investigate the effect of irradiation on the shift in
differentiation between osteoblasts and adipocytes, and the
possible underlying mechanism. The present study aimed to
investigate the effect of irradiation on the proliferation and
differentiation of BMSCs, particularly the effect of osteoblasts
and adipocytes differentiation in vitro, to further
elucidate irradiation induced bone loss disease and cell-based
therapy.
Materials and methods
BMSC isolation and culture
The present study was reviewed and approved by the
Committee for Ethical Use of Experimental Animals at Fudan
University (Shanghai, China). The BMSCs were obtained from three
male 2–4-week-old Sprague-Dawley rats (Department of Experimental
Animals, Fudan University, Shanghai, China), which were housed at
20–26°C with a 16 h light and 8 h dark cycle, and provided ad
libitum food and water. The rats were sacrificed by cervical
dislocation and the animal skeleton was washed in 70% ethanol. The
femurs and tibias were dissected, and muscle and connective tissue
were removed. The end of the tibias and femurs were cut just below
the end of the marrow cavity. A 27-gauge needle, attached to a 10
ml syringe, containing complete media was inserted to flush the
marrow plug out of the cut end of the bone into a dish. The cell
suspension was added to 6 ml Ficoll isolation (Shanghai Hua Jing
Biological High Tech Co., Ltd., Shanghai, China) and centrifuged at
400 × g at 24°C for 30 min. The cotton-like cells were collected at
the interface, and were rinsed twice with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in
low glucose Dulbecco's modified Eagle's medium (L-DMEM; GE
Healthcare Life Sciences, Logan, UT, USA). The whole cells were
resuspended in complete medium containing 10% FBS and 100 U/ml
penicillin/streptomycin (North China Pharmaceutical Co., Ltd.,
Shijiazhuang, China), and were seeded into a 25 cm2
flask for incubation at 37°C in a 5% CO2 incubator. The
non-adherent cells were removed after 2 h by replacing the medium
with fresh complete medium. The medium was replaced every 3 days.
On reaching a confluence of 80–90%, the medium was discarded, and
0.5 ml of 0.25% trypsin (Sigma-Aldrich, St. Louis, MO, USA)/1 mM
ethylenediaminetetraacetic acid (China Pharmaceutical Shanghai
Chemical Reagent Co., Ltd., Shanghai, China) was added for 2 min at
room temperature. The trypsin was neutralized by adding complete
medium. The harvested cells were cultured in a 25 cm2
flask (~1×106 cells/well) at a ratio of 1:2 (25).
Flow cytometry
BMSCs were characterized using flow cytometric
analysis of cell surface markers (CD29, CD34, CD-44 and CD45). The
cells after three passages (P3; ~1×107 cells/well) were
trypsinized and washed with phosphate-buffered saline (PBS), and
were subsequently resuspended in 0.5 ml PBS. Rat polyclonal
anti-CD34-fluorescein isothiocyanate (1:1,000; cat. no. bs-2038R;
FITC; Bioss Biosynthesis Biotechnology Co., Ltd., Beijing, China),
rat monoclonal anti-CD29-FITC (1:1,000; cat. no. 555005; BD
Biosciences, San Jose, CA, USA), rat polyclonal anti-CD-44-FITC
(1:1,000; cat. no. FAB6577G; R&D Systems, Inc., Minneapolis,
MN, USA) and rat anti-CD45-FITC (1:500; cat. no. 554877; BD
Biosciences) antibodies were added separately, followed by 30 min
incubation in the dark at 4°C. The cells were rinsed twice in PBS
at 200 × g for 5 min, following which the cells were washed in 1 ml
PBS and analyzed using a flow cytometer (Gallios; Beckman Coulter,
Brea, CA, USA). At least 1×105 cells were acquired and
analyzed. Unstained cells were used as a control.
Differentiation assay
The P3 cells were trypsinized and seeded at a
density of 5×104/cm2 into 48 well plates for
each group. For osteogenic differentiation: The medium was replaced
with induction medium after 48 h. The osteogenic induction medium
contained 10−8mol/l dexamethasone (Sigma-Aldrich), 10
mmol/l β-glycerophosphate (China Pharmaceutical Shanghai Chemical
Reagent Co., Ltd.), 50 μg/ml ascorbic acid (Sigma-Aldrich),
100 U/ml penicillin/streptomycin and 10% FBS in L-DMEM. The
induction medium was replaced every 3 days. The osteogenic
induction process was performed for 1 week (37°C; 5%
CO2), and the process of was continued for 3 weeks
(37°C; 5% CO2). The induction process of adipogenesis
was performed by alternating between the induction medium,
comprising 10−6 mol/l dexamethasone, 0.5 mmol/l
3-isobutyl-1-methylxanthine (Sigma-Aldrich), 0.1 mmol/l
indomethacin (Sigma-Aldrich), 100 U/ml penicillin and streptomycin
and 10% FBS L-DMEM, and the maintenance medium, comprising L-DMEM
supplemented with 10 μg/ml insulin, 100 U/ml penicillin and
streptomycin and 10% FBS, every 3 days. This process was continued
for 2 weeks.
Irradiation and grouping
The samples were sorted into two groups,
osteogenesis and adipogenesis. Each group of BMSCs was irradiated
following a 24 h incubation using a 137Cs gamma
radiation source (Gammacell-40; MDS Nordion, Inc., Ontario, Canada)
at a single dose.
Cell viability
The BMSCs were seeded into 96-well plates
(5×103cells/well) and incubated in complete medium for
24 h (37°C; 5% CO2). The cells were exposed to various
doses of irradiation (0, 0.25, 0.5, 1, 2, 5 and 10 Gy). The medium
was subsequently removed and 100 μl fresh L-DMEM containing
10% 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) solution (Sigma-Aldrich; 0.5 mg/ml in PBS) was added into
each well. Following 4 h incubation at 37°C, the insoluble formazan
crystals formed were dissolved in 100 μl 10% sodium dodecyl
sulfate (China Pharmaceutical Shanghai Chemical Reagent Co., Ltd.)
for 2 h. The optical density was immediately measured at 570 nm
using a micro-plate reader (Multiskan fc reader; Thermo Fisher
Scientific, Inc.).
Colony forming unit (CFU) assay
Cells in the exponential growth phase were
trypsinized and made into a single cell suspension. The
concentration of the cell suspension was adjusted to 200 cells/ml.
A 5 ml cell suspension was inoculated onto a petri dish (diameter
60 mm), and the cells were evenly dispersed by gentle agitation of
the dish in cross direction. The cells were exposed to different
doses of irradiation (0, 0.5, 1, 2, 5 and 10 Gy) after 24 h. The
culture was terminated after 2 weeks, when visible clones appeared
in the petri dish. The cells were fixed with methanol for 15 min.
Giemsa staining (Amresco LLC, Solon, OH, USA) was performed for 10
min and observed by microscopy (Nikon 80i; Nikon Corporation,
Tokyo, Japan) following air drying.
Cell cycle analysis
The cell cycle was detected using a Cell Cycle and
Apoptosis Analysis kit (cat. no. C1052; Beyotime Institute of
Biotechnology, Haimen, China) following doses of irradiation (0,
0.5, 1, 2, 5 or 10 Gy). Briefly, the cells (~1×106
cells) were trypsinized and made into a single cell suspension. The
cells were subsequently fixed with pre-cooled 70% ethanol at 4°C
for 12 h, prior to incubating with propidium iodide solution (5
μg/ml; Beyotime Institute of Biotechnology) at 37°C for 30
min. Flow cytometric analysis was performed within 24 h. DNA
content and light scattering analyses were performed using software
(Navios™; Beckman Counter).
Alkaline phosphatase (ALP) activity
assay
The BMSCs were seeded into 96-well plates and were
irradiated at different doses (0, 0.5, 1, 5 or 10 Gy) after 24 h,
following which the cells were cultured with osteogenic inductive
medium for 7 and 14 days (37°C; 5% CO2). The measurement
of ALP activity and protein content were performed, as described
previously (26). Briefly, the
cells were lysed with 0.05% Triton X-100 (Sigma-Aldrich) at 4°C for
2 h, and were subsequently lysed by ultrasonication (VCX130PB
Serial; Sonics & Materials, Inc., Newtown, CT, USA) for 10 sec
at 20 kHz three times on ice. A total of 50 μl lysate was
added to 2-amino-2-methyl-1-propanol buffer containing
p-nitrophenyl phosphate (Fluka, Co, Milwaukee, WI, USA) at 37°C for
30 min. The reactions were terminated by adding 50 μl
0.2mol/l NaOH. The absorbance was detected at 405 nm using a
Sunrise microplate reader (Thermo Fisher Scientific, Inc.). The
total proteins were measured using a Bicinchoninic Acid kit
(Beyotime Institute of Biotechnology), according to manufacturer's
protocol. The activity was adjusted to the cell protein and
expressed as U/mg protein.
ALP staining and Oil red O staining
The BMSCs were cultured in 48-well plates
(1×104 cells/well) and irradiated after 24 h. The cells
were subsequently induced with osteogenic or adipogenic induction
medium (37°C; 5% CO2). To estimate osteogenic
differentiation, the cells were rinsed twice with PBS and fixed
with 2.5% glutaraldehyde solution for 5 min. The cells were
subsequently stained using an ALP staining kit, according to the
manufacturer's protocol (Tiangen Biotech Co., Lrd., Beijing,
China). To estimate the adipogenic differentiation, the cells were
fixed with 4% paraformaldehyde (Sigma-Aldrich) solution and rinsed
with PBS. The cells were gently rinsed with 60% isopropanol and the
stained with Oil Red O dye solution (Sigma-Aldrich) for 30 min at
room temperature. Following staining, the cells were visualized and
images were captured using an optical microscope (Nikon 80i).
Simple PCI imaging software (Compix, Inc., Arizona, USA) was used
to count the number and areas of positively stained cells.
Mineralization and alizarin red
staining
The BMSCs were cultured in 48-well plates
(5×104 cells/well) and irradiated after 24 h.
Subsequently, the cells were induced with osteogenic inductive
medium for 3 weeks (37°C; 5% CO2), and the medium was
replaced every 2 days. The cells were fixed with 95% ethanol and
rinsed with PBS. The cells were then stained with 0.2% alizarin red
(pH 8.3; Amresco LLC) for 10 min at room temperature. The numbers
and areas of mineralization nodules were quantified using an
optical microscope (magnification, ×100) and Simple PCI imaging
software.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The BMSCs were seeded into 6-well plates at a
density of 4×105 cells/well and were exposed to the
different doses of irradiation after 24 h. The cells were
subsequently induced with osteogenic or adipogenic induction medium
for 1 week. The total cellular RNA was isolated using an RNAprep
pure cell/bacteria kit (Tiangen Biotech Co., Ltd.), according to
the manufacturer's protocol. Subsequently, 1 μg total RNA
was transcribed into cDNA using a QuantScript RT kit (Tiangen
Biotech Co., Ltd.), according to the manufacturer's protocol. All
PCR primers were supplied by Sangon Biotech Co., Ltd. (Shanghai,
China), and the primer sequences are listed in Table I. Specific transcripts were
quantified by RT-qPCR using a QuantiTect SYBR® Green PCR
kit (Takara Bio, Inc., Tokyo, Japan). A total of 2 μl cDNA,
0.4 μl primers, 10 μl 2X SYBR® Premix
ExTaq™ and 7.2 μl ddH2O were mixed and used to
conduct the reaction with a LightCycler 2.0 Real-Time PCR system
(Roche Diagnostics GmbH, Mannheim, Germany). The 2−ΔΔCq
method was used to calculate gene expression (27). The quantified individual RNA
expression levels were normalized against β-actin. qPCR was
performed as 40 cycles at 94°C for 15 sec, 55°C for 30 sec and 72°C
for 30 sec. At least three independent experiments were
performed.
 | Table IPrimers used in revers
transcription-quantitative polymerase chain reaction analysis. |
Table I
Primers used in revers
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer
(5′-3′) | Reverse primer
(3′-5′) | Product length
(bp) |
|---|
| ALP |
CTGAGCGCACGCGAGCAAC |
GGCGTGGTTCACCCGAGTGG | 116 |
| OCN |
GAACAGACAAGTCCCACAC |
GAGCTCACACACCTCCCTG | 270 |
| RUNX2 |
TGCCACCTCTGACTTCTGC |
GATGAAATGCCTGGGAACTG | 111 |
| PPAR-γ |
ACGGTTGATTTCTCCAGCAT |
GGACGCAGGCTCTACTTTGA | 138 |
| C/EBPα |
GGAGGGACTTAGGGAGTTGG |
GGAAACCTGGCCTGTTGTAA | 146 |
| β-actin |
CACCCGCGAGTACAACCTTC |
CCCATACCCACCATCACACC | 207 |
Western blotting
The protein expression levels of PPAR-γ and RUNX2 in
the different groups were detected using western blot analysis. The
cells were irradiated following incubation for 24 h, and were
induced for 2 weeks. The cells were lysed using 100 μl
radioimmunoprecipitation assay and 1 μl phenylmethylsulfonyl
fluoride (Beyotime Institute of Biotechnology). The samples were
then centrifuged at 20,000 × g for 10 min at 4°C. The supernatant
were obtained and total protein concentration was measured using
the Bicinchoninic Acid kit, according to manufacturer's protocol.
Equal quantities of total protein (20 μg) were subjected to
sodium dodecyl sulfate polyacryl-amide gel electrophoresis (5%
stacking gel and 12% separating gel; Beyotime Institute of
Biotechnology) and transferred onto polyvinylidene fluoride
membrane (Beyotime Institute of Biotechnology). The membrane was
blocked with 5% (w/v) non-fat milk dissolved in PBS-20% Tween and,
followed by incubation with rabbit anti-PPAR-γ (1:1,000; cat. no.
07-466; EMD Millipore, Billerica, MA, USA) and rabbit anti-RUNX2
(1:1,000; cat. no. 8486S; Cell Signaling Technology, Inc., Santa
Cruz, CA, USA). The proteins were visualized by incubating the
membrane with a secondary antibody conjugated to horseradish
peroxidase. Tubulin (Beyotime Institute of Biotechnology) and GAPDH
(Cell Signaling Technology, Inc.) were used as loading controls.
The protein expression levels were quantified by the optical
density ratio of the target protein and loading control using
Quantity One® 4.6.3 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. One-way analysis of variance, followed by a least
significant difference or Dunnett T3 test, was performed to compare
means among multiple groups. Statistical analyses were performed
using SPSS statistical software (IBM, SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
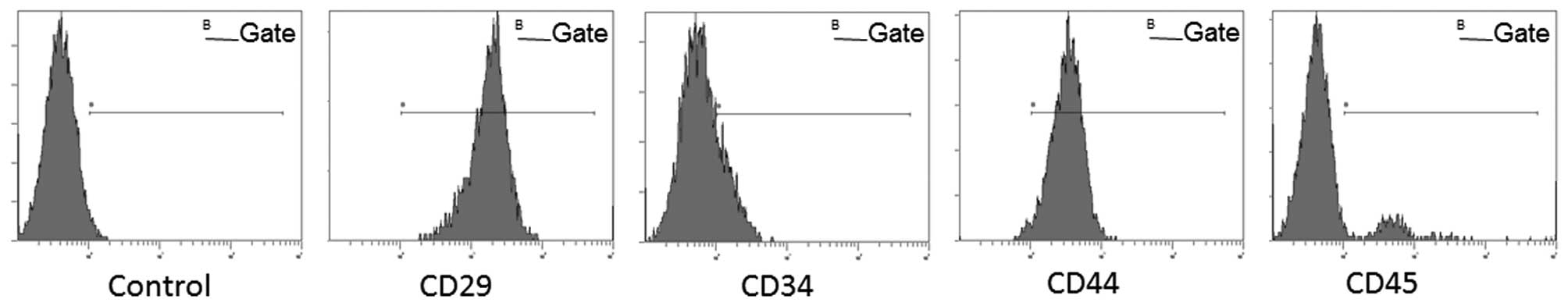
Microscopic morphology and expression of
surface makers in BMSCs
The BMSCs exhibited a fusiform projection,
fibroblast-like, colony growth morphology. Cell surface marker
analysis showed that the BMSCs were positive for CD29 (95.76%) and
CD44 (81.42%), and negative for CD34 (12.70%) and CD45 (26.56%).
Expression profiles of cell-surface markers are qualified as
mesenchymal stem cells (28)
(Fig. 1).
Cell viability
The cell growth curve showed that the cells
exhibited a sustained proliferation state, and entered the
logarithmic phase after 3 days in culture (Fig. 2). The proliferation rate of the
BMSCs was maintained at a high level, even after 14 days in culture
(37°C; 5% CO2). No significant difference was observed
following 9 days of culture with basic culture medium. Cell
viability was marginally lower when cultured for 11 days with
induction medium, however, the difference was not significant.
Therefore, the adipogenic or osteogenic induction medium had no
affect on the cell viability, compared with the basic culture
medium (P>0.05), enabling examination of the differences between
groups without the requirement to consider the inference of
induction medium in the following experiments.
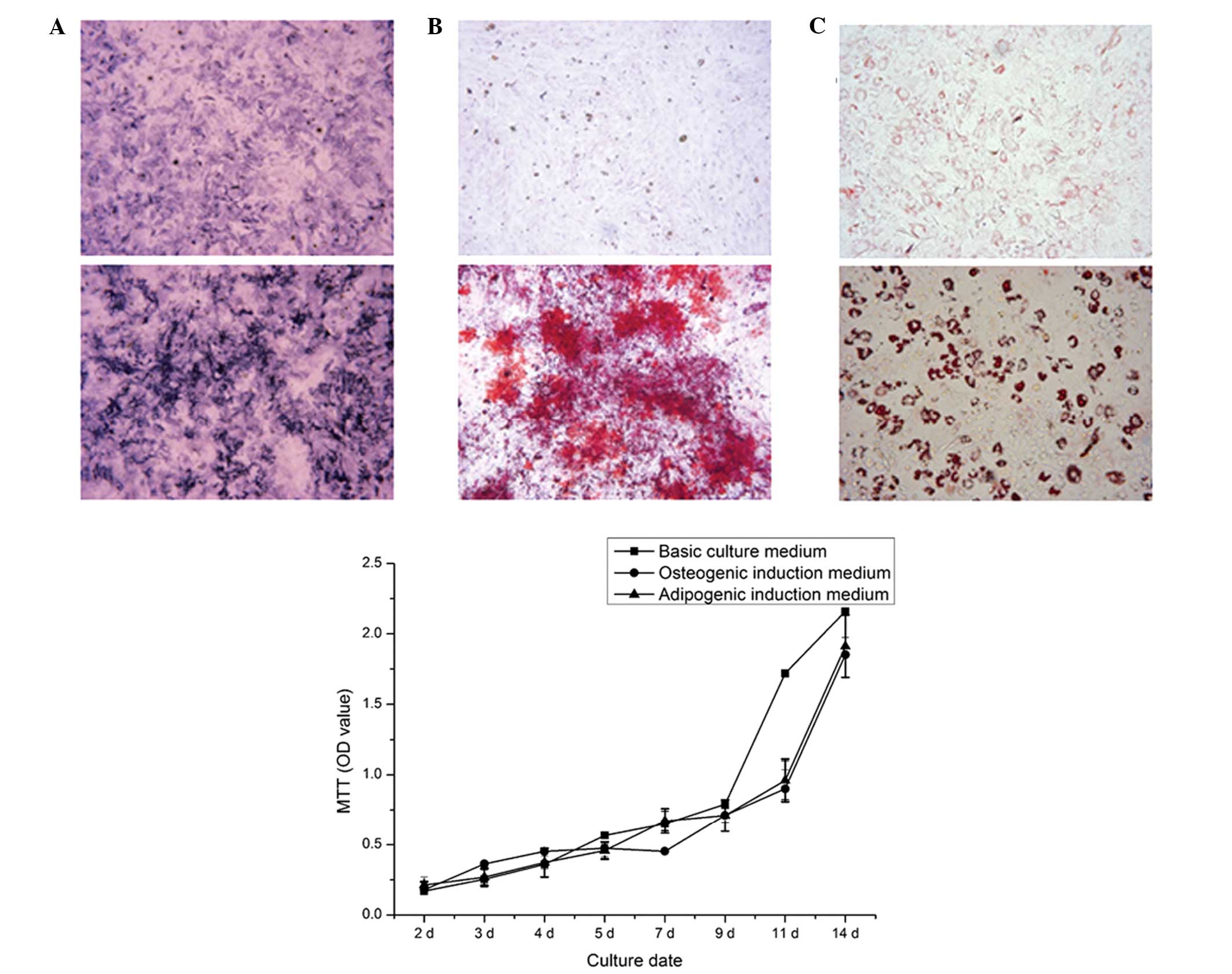 | Figure 2BMSC induction and the viability of
BMSCs cultured with different culture medium over time. (A) Upper
panel, ALP staining of non-induced cultured BMSCs for 7 days
(original magnification, ×40); lower panel, ALP staining of
osteogenic induction of BMSCs for 7 days (original magnification,
×40). Dark blue granules indicate ALP staining. (B) Upper panel,
ARS staining of non-induced cells after 21 days (original
magnification, ×100); lower panel, ARS staining of mineralized
nodules following osteogenic induction of BMSCs for 21 days
(original magnification, ×100). Mineralized nodules stained red.
(C) Upper panel, Oil Red O staining of non-induced BMSCs after 14
days (magnification, ×200); lower panel, Oil Red O staining of
adipogenic induction of BMSCs for 14 days (magnification, ×200).
Fat droplets stained red. Data are expressed as the mean ± standard
deviation. BMSC, bone marrow mesenchymal stem cell; ALP, alkaline
phosphatase; ARS, alizarin red S; MTT,
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; OD,
optical density. |
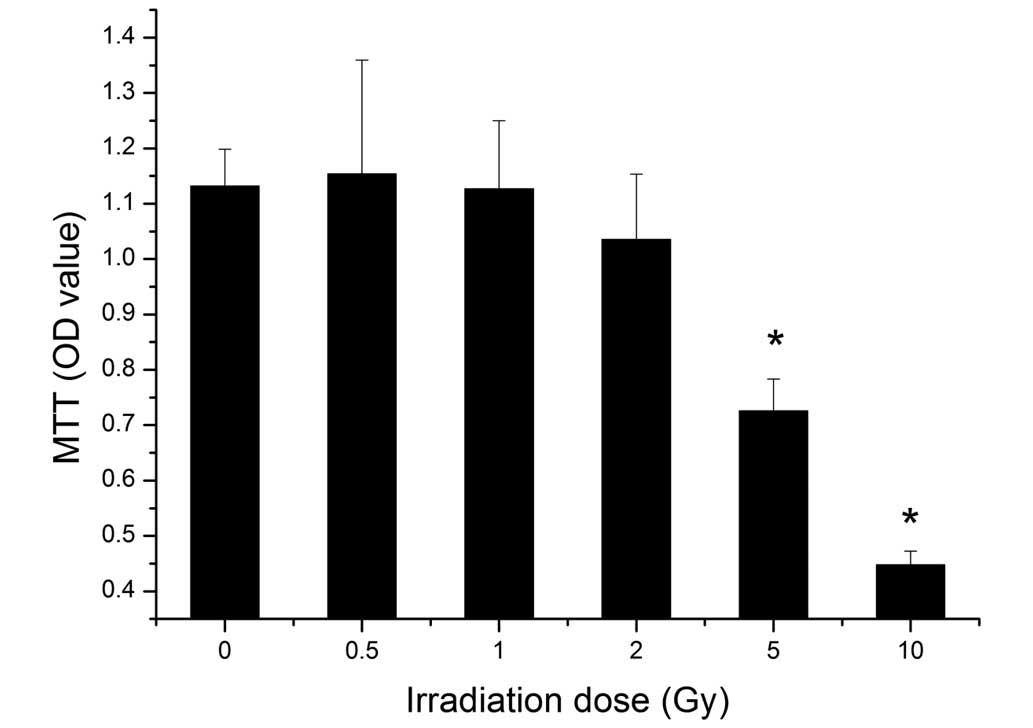
An MTT assay was performed to identify the viability
of the BMSCs following different doses of irradiation. The results
showed that the viability of the BMSCs decreased with increasing
dose. Irradiation at 5 Gy significantly suppressed the cell
viability (P<0.05), and cell viability was decreased further
following exposure to 10 Gy irradiation (Fig. 3).
Cell differentiation
The BMSCs were directly induced to form osteoblasts
in osteogenic induction medium for 7 days. A marked increase in the
number of ALP-positively stained cells was observed, compared with
the non-induced BMSCs. Mineralized nodules formed following
osteogenic induction for 21 days, whereas no mineralized nodules
were observed in the non-induced cells. The Oil red O staining
assay showed that lipid droplets were generated following
adipogenic induction for 14 days (Fig.
2).
CFU assay
The results of the CFU assay showed that the cells
exhibited a high colony formation rate (89.67% in the
non-irradiation group) in vitro. As shown by Giemsa
staining, the numbers of cells in each colony were reduced
following irradiation (Fig. 4).
The number of CFUs reduced as the irradiation dose was increased
for the same duration. Irradiation at 0.5 Gy significantly reduced
the colony formation potential of the BMSCs (P<0.05; Fig. 4).
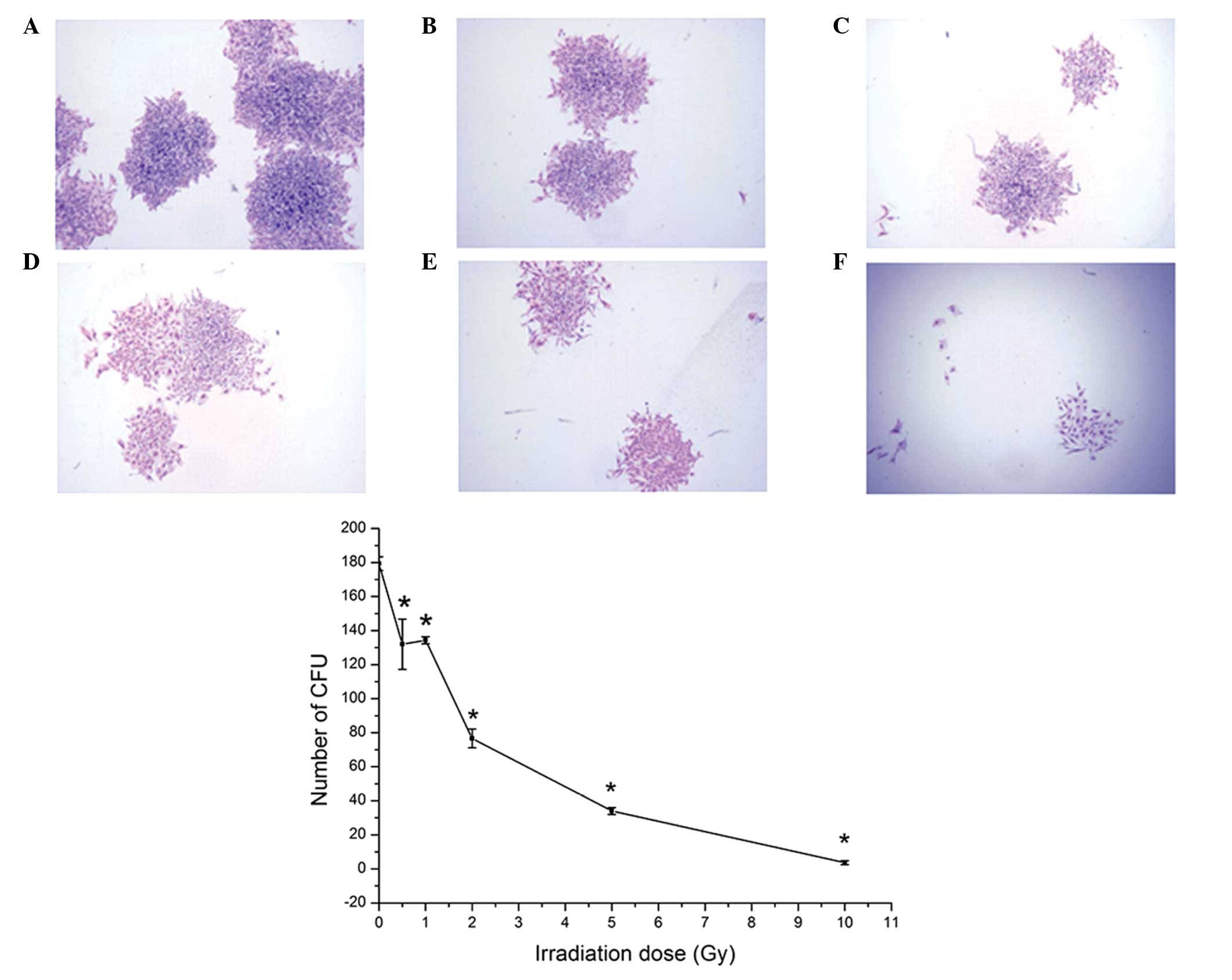 | Figure 4CFUs of BMSCs following irradiation.
Giemsa staining of the BMSCs following irradiation at (A) 0 Gy, (B)
0.5 Gy, (C) 1 Gy, (D) 2 Gy, (E) 5 Gy, (F) 10 Gy. Original
magnification, ×20. The graph shows the number of CFUs following
irradiation with doses of 0, 0.5, 1, 2, 5 and 10 Gy. Data are
expressed as the mean ± standard deviation *P<0.05,
compared with the 0 Gy group. BMSC, bone marrow mesenchymal stem
cell; CFU, colony-forming unit. |
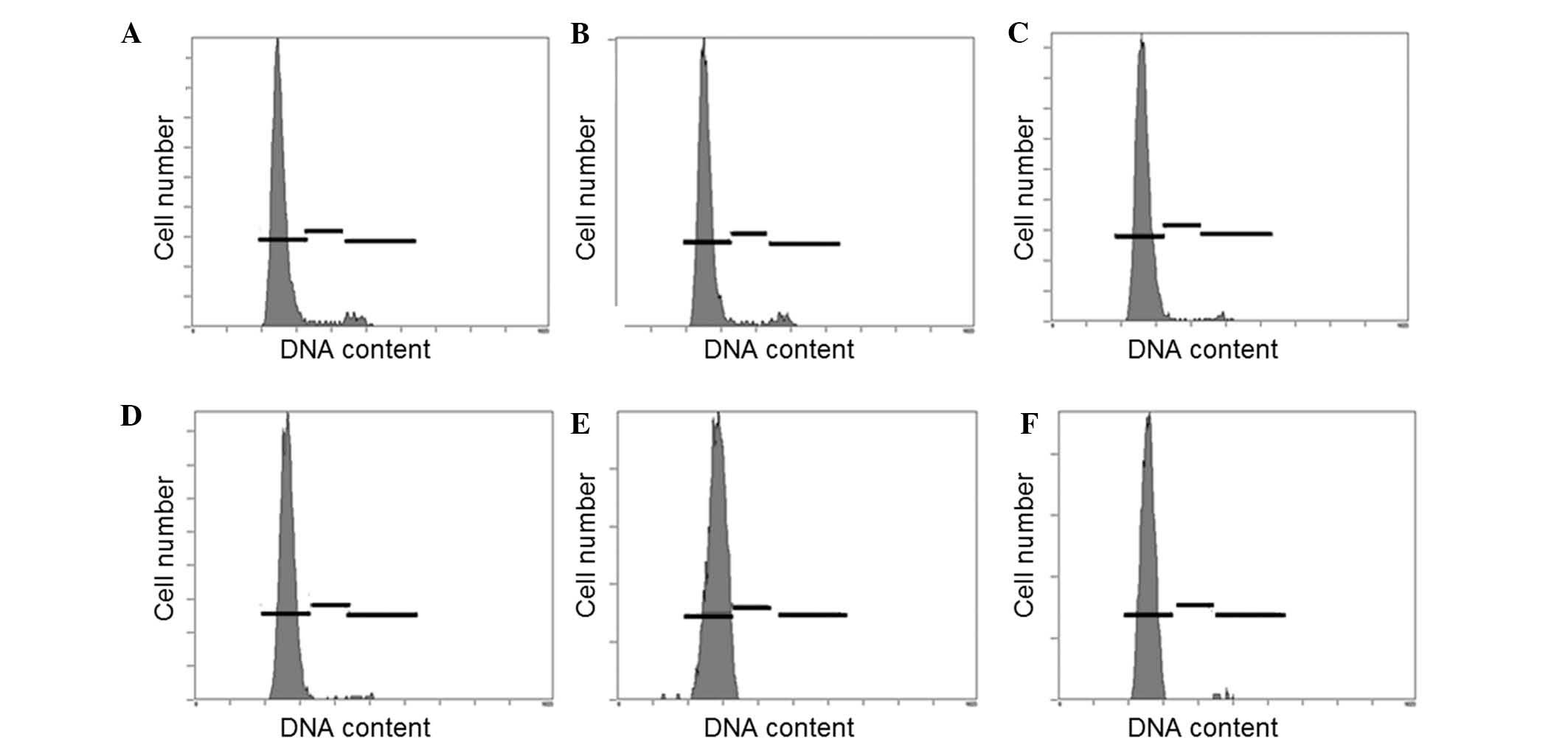
Cell cycle distribution
The results of the present study revealed that BMSC
were predominantly in the G0 stage of the cell cycle. Irradiation
failed to alter the cell cycle, which suggests that changes in cell
proliferation are not achieved through the alteration of cell cycle
progression (Fig. 5).
ALP activity following irradiation
The effect of different doses of irradiation on ALP
activity was determined following 7 days osteogenic induction. The
results showed that all doses (0.5, 1, 5 and 10 Gy) of irradiation
decreased ALP activity (P<0.05), compared with the control
group. Additionally, the ALP activity decreased with increasing
irradiation dose (Table II).
 | Table IIEffect of different doses of
irradiation on ALP activity following 7 days of osteogenic
induction. |
Table II
Effect of different doses of
irradiation on ALP activity following 7 days of osteogenic
induction.
| Irradiation dose
(Gy) | ALP activity (U/mg
protein) | Change in ALP
activity (%) |
|---|
| 0 | 81.500±5.788 | – |
| 0.5 |
73.992±4.174a | ↓9.21 |
| 1 |
56.294±4.983a | ↓30.93 |
| 5 |
43.850±2.560a | ↓46.20 |
| 10 |
39.832±1.520a | ↓51.13 |
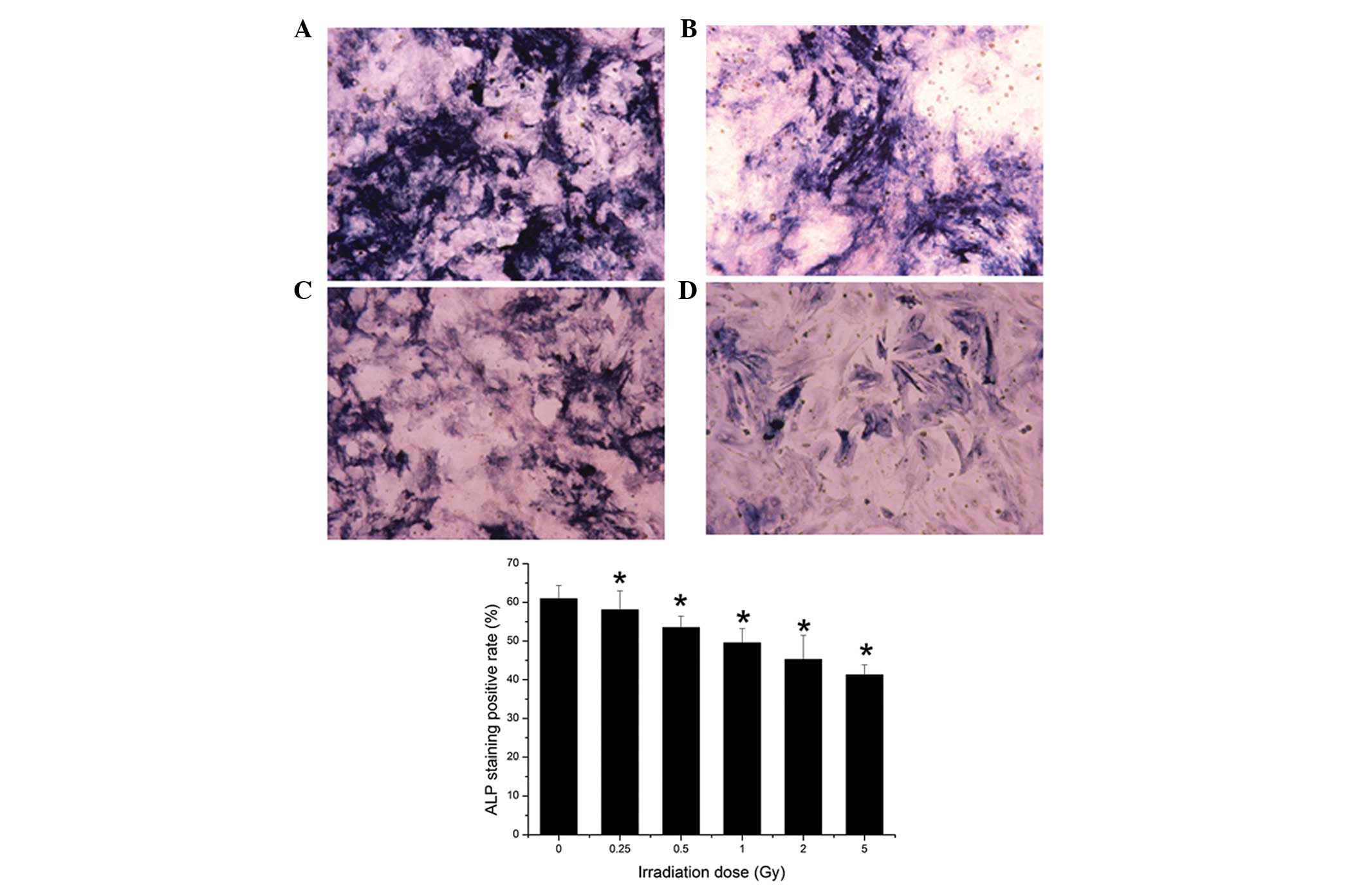
ALP and Oil red O staining
Visualization of the cells under an optical
microscope revealed that both the size of the stained area and
color density decreased as irradiation dose increased. The surface
area of positive ALP staining decreased with increasing irradiation
dose. Irradiation at 0.25 Gy significantly suppressed positive ALP
staining (P<0.05; Fig. 6).
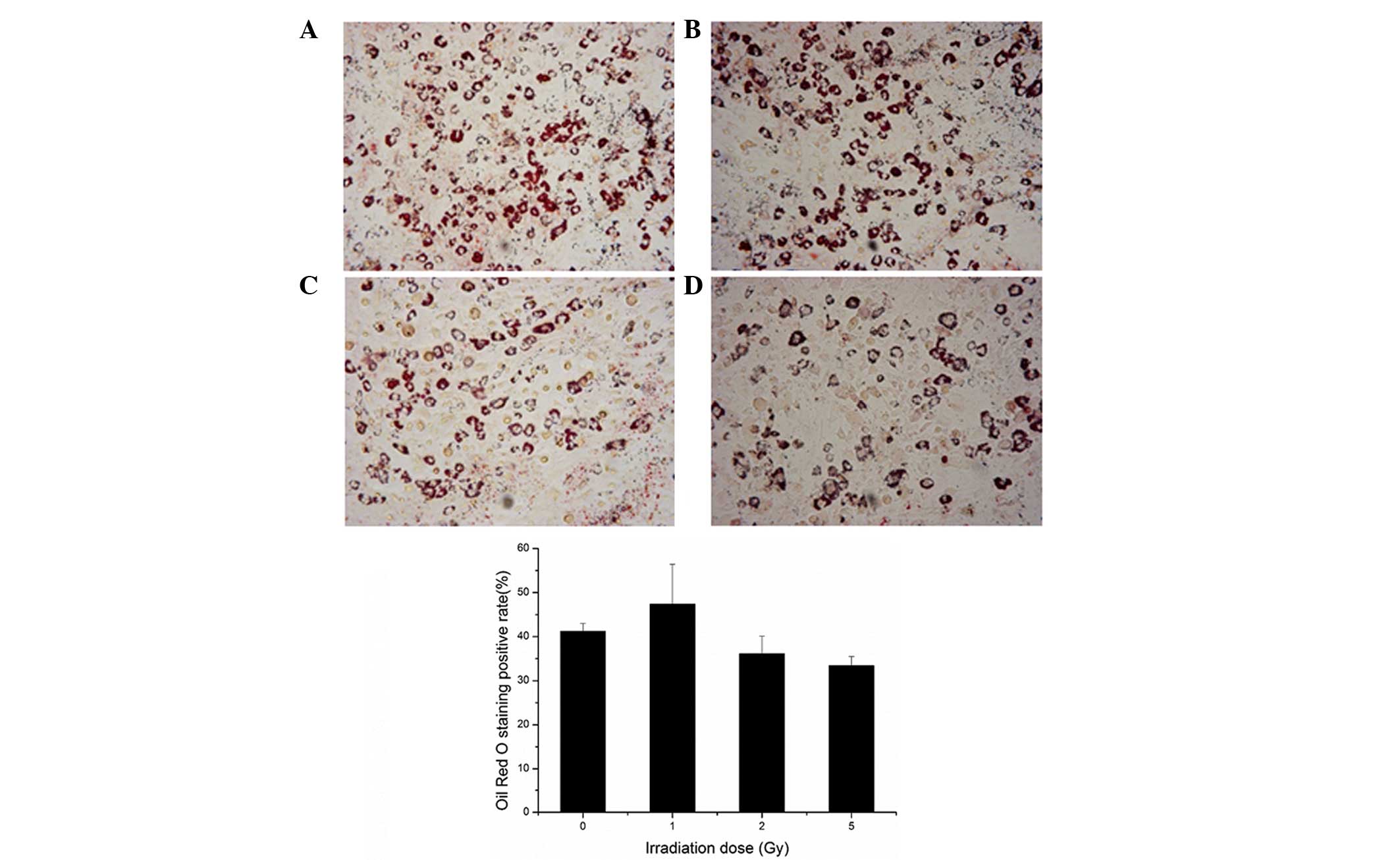
The results of the present study demonstrated that
the rate of positive Oil Red O staining increased marginally
following 0.25, 0.5 or 1 Gy irradiation, and decreased following 2
or 5 Gy irradiation, although no statistical difference was
observed (P>0.05; Fig. 7).
These results indicated that the adipogenic differentiation
potential of the BMSCs was not markedly altered following
irradiation.
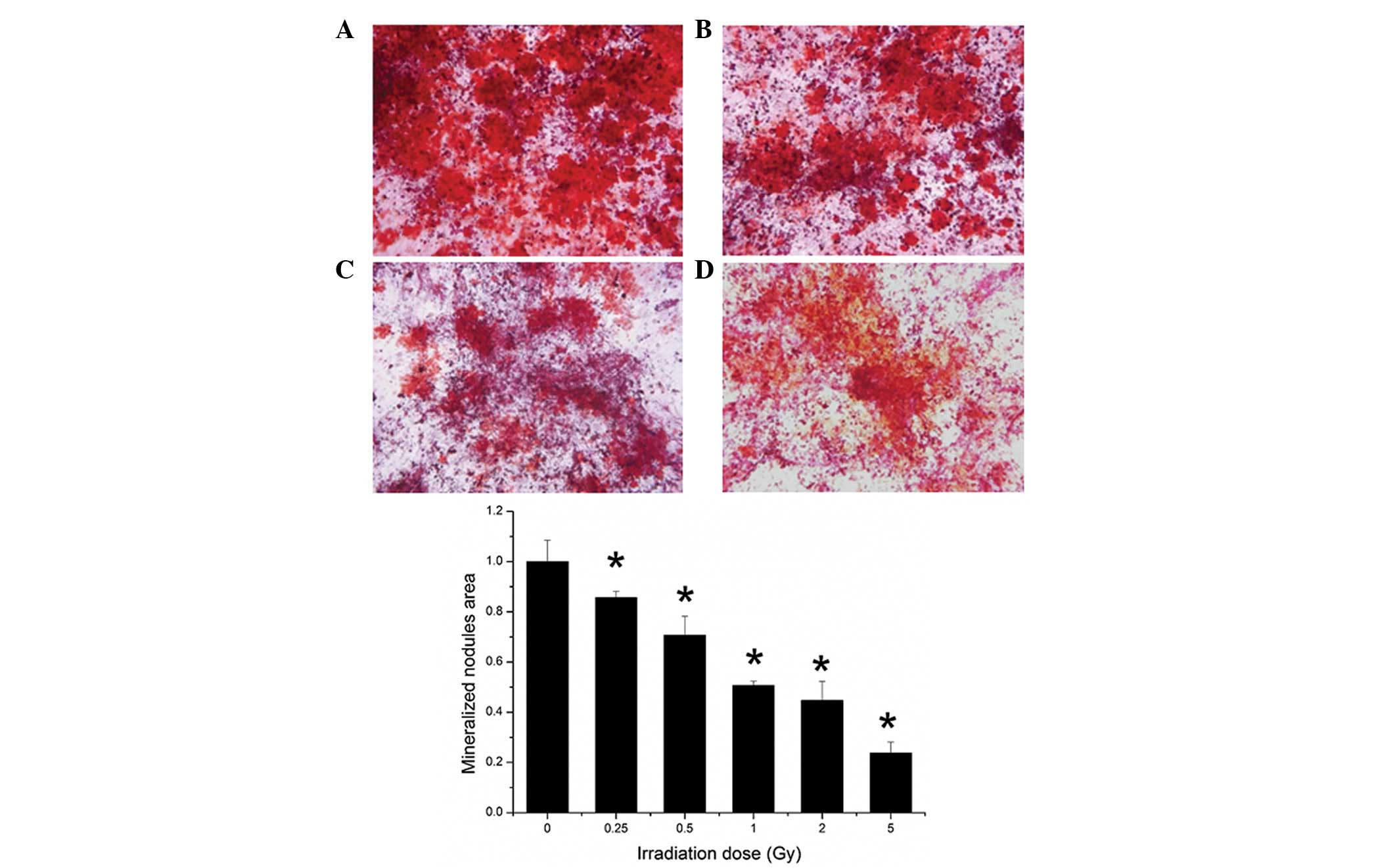
Mineralization and alizarin red
staining
As shown in Fig. 7,
the alizarin red staining of mineralization showed that
γ-irradiation reduced mineralization abilities in vitro.
Quantification of the ARS deposition areas revealed that
irradiation suppressed the mineralization of osteogenesis at all
irradiation doses (Fig. 8).
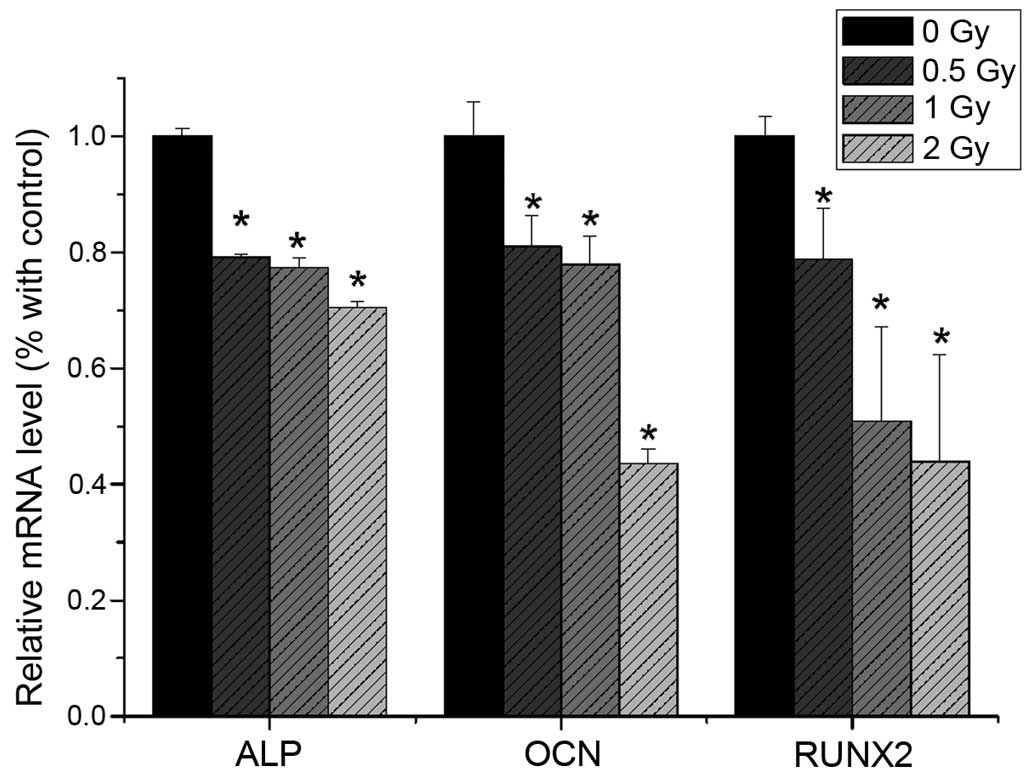
RT-qPCR analysis
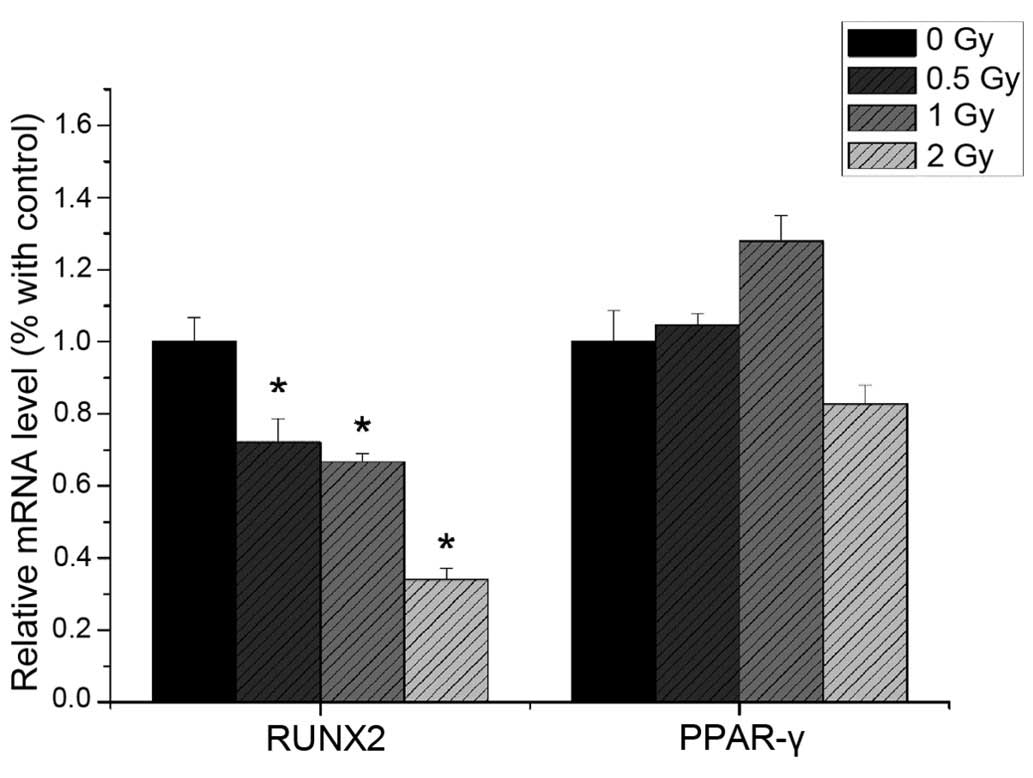
The present study revealed that the mRNA expression
levels of ALP, RUNX2 and OCN were reduced following 0.5 Gy
irradiation (P<0.05), compared with the non-irradiated group.
The expression levels of ALP, RUNX2 and OCN were significantly
decreased with increasing irradiation dose (Fig. 9). The mRNA expression levels of
PPAR-γ and CEBPα were not significantly altered following
irradiation (Fig. 10).
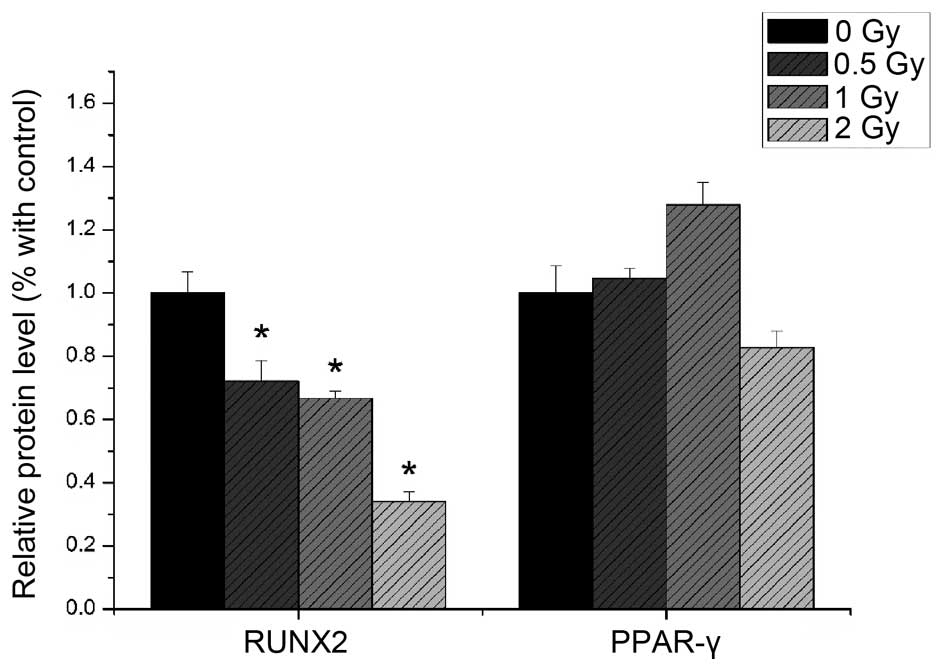
Western blotting
The protein expression levels of RUNX2 and PPAR-γ
were detected using western blotting. As shown in Fig. 10, the protein expression of RUNX2
decreased with increased irradiation dose (P<0.05), whereas the
protein expression of PPAR-γ was not significantly altered by
irradiation (P>0.05; Fig.
11).
Discussion
Osteoporosis and fracture are late effects of
radiotherapy, and present as bone loss, decreased bone strength and
increased fracture rate in cancer survivors, with unknown etiology
and lack of treatment options. A previous study revealed that
radiation can result in loss of trabecular bone (29). Increased trabecular thickness and
separation, and reduced cancellous bone volume fraction,
connectivity density and trabecular number were detected in the
proximal tibia and lumbar vertebra 14 days following 6 Gy
γ-irradiation (30). Thus,
irradiation exposure leads to the destruction of bone architecture,
thereby increasing an individuals lifetime risk of bone loss and
fracture.
In bone healing or distraction osteogenesis,
progenitor cells are involved through successive formation of
fibrous, cartilaginous and osseous tissues (31). BMSCs are considered as the most
suitable cell source for bone tissue engineering due to their
superior osteogenic potential. Adipocytes and osteoblasts originate
from BMSCs, and the balance between adipogenesis and osteogenesis
in BMSCs is reported to modulate the progression of various
diseases, including obesity and osteoporosis (32). The high proliferative capacity of
BMSCs makes them susceptible to damage and injury, altering the
steady-state of the bone marrow environment.
Differentiation into osteoblast and adipocyte
lineages has particular relevance to the maintenance of normal bone
homeostasis. Irradiation can damage the osteogenic activity of
human marrow by suppressing osteoblasts, leading to
post-irradiation bone loss and osteoporosis (33). However, the role of irradiation in
modulating the adipogenic and osteogenic potential remains to be
elucidated.
For over a decade, it has been hypothesized that an
inverse association exists between adipocytes and osteoblasts
within the marrow cavity (34).
Despite substantial data supporting the adverse association between
osteoblasts and adipocytes, studies revealed a more complex
association between bone and fat tissue volume in human and animal
models in vivo (35).
The present study hypothesized that radiation
therapy alters the osteogenic and adipogenic differentiation
potentials of BMSCs. To confirm this hypothesis, the Ficoll
technique was used to isolate cells, as a previous study suggested
that the Ficoll technique may be suitable for the isolation of
multi-potent BMSCs (36). The
cells obtained exhibited high proliferation potential under basic
culture medium and in induction medium, and the cell surface makers
were suitable for qualification as stem cells. Following induction,
BMSCs can successfully differentiate into osteoblasts and
adipocytes in vitro.
The results of the present study demonstrated that 2
Gy irradiation reduced cell viability. This was concordant with a
previous study, which demonstrated that persistent injury in the
stem cell population can be induced by relatively small doses, and
that the threshold total dose in mice is ~1.5 Gy, determined using
fractionated whole-body irradiation (37). The CFU assay in the present study
showed that 0.5 Gy irradiation decreased cell proliferation,
suggesting that the BMSCs were relatively sensitive to irradiation
in vivo or in vitro.
Normal cells are permanently held in a state in
which their continued existence depends on a tight balance between
survival and death signals. In a normal cell, the accumulation of
DNA damage leads to cell cycle arrest, during which the potential
for repair is assessed. If the extent of the damage exceeds the
capacity to repair without leaving residual genetic abnormality,
the balance of survival and death signals tips, and the cell
activates its apoptotic signaling pathway leading to cell death
(38). The results of the present
study showed no significant cell cycle arrest following different
dose of irradiation, therefore further investigation of the
mechanism is required.
The results of the ALP and Oil red O staining assay
indicated that irradiation may have a suppressive effect on
osteogenic differentiation of the BMSCs, however, it had no marked
suppressive or enhancing effect on adipogenic differentiation.
However, the relative ratio of osteogenesis and adipogenesis was
increased, therefore, further examination of the gene and protein
expression levels in the process of BMSCs differentiation was
performed.
The developmental fate of BMSCs is largely
determined by the expression of specific groups of transcription
factors to drive the differentiation of uncommitted precursors down
a specific lineage. Expression of the RUNX2 and osterix
transcription factors are the predominant determinants for the
osteogenic differentiation of BMSCs (39). In addition, the peroxisome
proliferator activated receptor-γ (PPAR-γ) transcription factor and
the CCAAT/enhancer-binding protein family, are key factors driving
the adipogenesis differentiation of BMSCs (40). In the present study, the expression
levels of RUNX2, ALP and OCN were decreased following irradiation,
indicating that irradiation suppressed osteogenic differentiation
at the early and late stages of differentiation. Therefore, the
proliferation of pre-osteoblasts and the formation of osteoid were
inhibited, as regulated by the osteogenic differentiation of BMSCs,
resulting in an imbalance of bone formation.
PPAR-γ, also termed the glitazone receptor or
7nuclear receptor subfamily 1, group C, member 3, is a
ligand-activated transcription factor, which belongs to the type II
nuclear hormone receptor superfamily and functions as a heterodimer
with a retinoid X receptor by binding to PPAR-γ responsive
elements. PPAR-γ is important in adipocyte differentiation.
Adipogenesis commitment of MSCs is determined by the expression
and/or activation of the PPAR-γ transcription factor (41). In the present study, no significant
changes in the gene and protein expression levels of PPAR-γ were
observed following irradiation. Therefore, the present study does
not support the hypothesis that decreased bone volume and increased
adipose tissue following radiotherapy is the result of
irradiation-induced alterations in the cellular compositions of
osteoblasts and adipocytes in BMSCs. Although these results differ
from the results of previous studies, certain studies support the
results of the present study. For example, Justesen et al
(42) reported no evidence for
enhanced adipogenesis with aging, as the adipocyte forming capacity
of BMSCs was similar between younger and older donors (42).
A possible explanation of the results of the present
study is that adipogenesis and osteogenesis can be regulated
independently. In support of this hypothesis, further experiments
are required to demonstrate the specific mechanisms of lipid
metabolism and bone metabolism.
Bone marrow post-irradiation syndrome seriously
affects quality of life in individuals following tumor treatment.
Therefore, investigating the mechanisms underlying bone injury and
recovery can provide novel insights into MSC differentiation and
the treatment of bone loss diseases to reduce the risk of
fracture.
Acknowledgments
The present study was sponsored by the Shanghai
Natural Science Fund (grant no. 14ZR1401600) and Shanghai Municipal
Commission of Health (grant no. 2013ZYJB0801).
References
|
1
|
Jeremic B: Radiation therapy. Hematol
Oncol Clin North Am. 18:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darby S, McGale P, Correa C, Taylor C,
Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et
al Early Breast Cancer Trialists' Collaborative Group (EBCTCG):
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer survivorship - United States,
1971–2001. MMWR Morb Mortal Wkly Rep. 53:526–529. 2004.
|
|
4
|
Dinshaw KA, Budrukkar AN, Chinoy RF, Sarin
R, Badwe R, Hawaldar R and Shrivastava SK: Profile of prognostic
factors in 1022 Indian women with early-stage breast cancer treated
with breast-conserving therapy. Int J Radiat Oncol Biol Phys.
63:1132–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agrawal S: Late effects of cancer
treatment in breast cancer survivors. South Asian J Cancer.
3:112–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williams HJ and Davies AM: The effect of
X-rays on bone: A pictorial review. Eur Radiol. 16:619–633. 2006.
View Article : Google Scholar
|
|
7
|
Baxter NN, Habermann EB, Tepper JE, Durham
SB and Virnig BA: Risk of pelvic fractures in older women following
pelvic irradiation. JAMA. 294:2587–2593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Green DE and Rubin CT: Consequences of
irradiation on bone and marrow phenotypes, and its relation to
disruption of hematopoietic precursors. Bone. 63:87–94. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coquard R: Late effects of ionizing
radiations on the bone marrow. Cancer Radiother. 1:792–800. 1997.
View Article : Google Scholar
|
|
10
|
Jia D, Gaddy D, Suva LJ and Corry PM:
Rapid loss of bone mass and strength in mice after abdominal
irradiation. Radiat Res. 176:624–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodríguez JP, Astudillo P, Ríos S and Pino
AM: Involvement of adipogenic potential of human bone marrow
mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res
Ther. 3:208–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asano S: Current status of hematopoietic
stem cell transplantation for acute radiation syndromes. Int J
Hematol. 95:227–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao L, Luo Y and Zhou D: Hematopoietic
stem cell injury induced by ionizing radiation. Antioxid Redox
Signal. 20:1447–1462. 2014. View Article : Google Scholar :
|
|
14
|
Christensen DM, Iddins CJ and Sugarman SL:
Ionizing radiation injuries and illnesses. Emerg Med Clin North Am.
32:245–265. 2014. View Article : Google Scholar
|
|
15
|
Heylmann D, Rödel F, Kindler T and Kaina
B: Radiation sensitivity of human and murine peripheral blood
lymphocytes, stem and progenitor cells. Biochim Biophys Acta.
1846.121–129. 2014.
|
|
16
|
Friedenstein A and Kuralesova AI:
Osteogenic precursor cells of bone marrow in radiation chimeras.
Transplantation. 12:99–108. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Peng LP, Wu N and Li LP:
Development of bone marrow mesenchymal stem cell culture in vitro.
Chin Med J (Engl). 125:1650–1655. 2012.
|
|
18
|
Bidwell JP, Alvarez MB, Hood MJ and
Childress P: Functional impairment of bone formation in the
pathogenesis of osteoporosis: The bone marrow regenerative
competence. Curr Osteoporos Rep. 11:117–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bethel M, Chitteti BR, Srour EF and Kacena
MA: The changing balance between osteoblastogenesis and
adipogenesis in aging and its impact on hematopoiesis. Curr
Osteoporos Rep. 11:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
James AW, Shen J, Khadarian K, Pang S,
Chung G, Goyal R, Asatrian G, Velasco O, Kim J, Zhang X, et al:
Lentiviral delivery of PPARγ shRNA alters the balance of
osteogenesis and adipogenesis, improving bone microarchitecture.
Tissue Eng Part A. 20:2699–2710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Justesen J, Stenderup K, Ebbesen EN,
Mosekilde L, Steiniche T and Kassem M: Adipocyte tissue volume in
bone marrow is increased with aging and in patients with
osteoporosis. Biogerontology. 2:165–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Post S, Abdallah BM, Bentzon JF and Kassem
M: Demonstration of the presence of independent pre-osteoblastic
and pre-adipocytic cell populations in bone marrow-derived
mesenchymal stem cells. Bone. 43:32–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gimble JM, Zvonic S, Floyd ZE, Kassem M
and Nuttall ME: Playing with bone and fat. J Cell Biochem.
98:251–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nicolay NH, Lopez PR, Debus J and Huber
PE: Mesenchymal stem cells - A new hope for radiotherapy-induced
tissue damage? Cancer Lett. 366:133–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soleimani M and Nadri S: A protocol for
isolation and culture of mesenchymal stem cells from mouse bone
marrow. Nat Protoc. 4:102–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu J, Zhu G, Chen X, Shao C and Gu S:
Combined effects of γ-irradiation and cadmium exposures on
osteoblasts in vitro. Environ Toxicol Pharmacol. 33:149–157. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen X, Zhu G, Gu S, Jin T and Shao C:
Effects of cadmium on osteoblasts and osteoclasts in vitro. Environ
Toxicol Pharmacol. 28:232–236. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Siclari VA, Zhu J, Akiyama K, Liu F, Zhang
X, Chandra A, Nah HD, Shi S and Qin L: Mesenchymal progenitors
residing close to the bone surface are functionally distinct from
those in the central bone marrow. Bone. 53:575–586. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wernle JD, Damron TA, Allen MJ and Mann
KA: Local irradiation alters bone morphology and increases bone
fragility in a mouse model. J Biomech. 43:2738–2746. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Turner RT, Iwaniec UT, Wong CP,
Lindenmaier LB, Wagner LA, Branscum AJ, Menn SA, Taylor J, Zhang Y,
Wu H, et al: Acute exposure to high dose γ-radiation results in
transient activation of bone lining cells. Bone. 57:164–173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tewarie RDSN, Hurtado A, Grotenhuis JA and
Oudega M: Bone marrow stromal cell survival, migration, and
differentiation following acute and delayed transplantation into
the moderately contused adult rat thoracic spinal cord. Cell Res.
18:S1002008. View Article : Google Scholar
|
|
32
|
Chen Q, Shou P, Zhang L, Xu C, Zheng C,
Han Y, Li W, Huang Y, Zhang X, Shao C, et al: An
osteopontin-integrin interaction plays a critical role in directing
adipogenesis and osteogenesis by mesenchymal stem cells. Stem
Cells. 32:327–337. 2014. View Article : Google Scholar :
|
|
33
|
Cao X, Wu X, Frassica D, Yu B, Pang L,
Xian L, Wan M, Lei W, Armour M, Tryggestad E, et al: Irradiation
induces bone injury by damaging bone marrow microenvironment for
stem cells. Proc Natl Acad Sci USA. 108:1609–1614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gimble JM, Zvonic S, Floyd ZE, Kassem M
and Nuttall ME: Playing with bone and fat. J Cell Biochem.
98:251–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abdallah BM and Kassem M: New factors
controlling the balance between osteoblastogenesis and
adipogenesis. Bone. 50:540–545. 2012. View Article : Google Scholar
|
|
36
|
Agata H, Yamazaki M, Uehara M, Hori A,
Sumita Y, Tojo A and Kagami H: Characteristic differences among
osteogenic cell populations of rat bone marrow stromal cells
isolated from untreated, hemolyzed or Ficoll-treated marrow.
Cytotherapy. 14:791–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hendry JH: The cellular basis of long-term
marrow injury after irradiation. Radiother Oncol. 3:331–338. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ashkenazi A and Dixit VM: Apoptosis
control by death and decoy receptors. Curr Opin Cell Biol.
11:255–260. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng Y, Wu S, Zhou H, Bi X, Wang Y, Hu Y,
Gu P and Fan X: Effects of a miR-31, Runx2, and Satb2 regulatory
loop on the osteogenic differentiation of bone mesenchymal stem
cells. Stem Cells Dev. 22:2278–2286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Zhang N, Huang X, Xu J, Fernandes
JC, Dai K and Zhang X: Dexamethasone shifts bone marrow stromal
cells from osteoblasts to adipocytes by C/EBPalpha promoter
methylation. Cell Death Dis. 4:e8322013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Viccica G, Francucci CM and Marcocci C:
The role of PPARγ for the osteoblastic differentiation. J
Endocrinol Invest. 33(Suppl): 9–12. 2010.
|
|
42
|
Justesen J, Stenderup K, Eriksen EF and
Kassem M: Maintenance of osteoblastic and adipocytic
differentiation potential with age and osteoporosis in human marrow
stromal cell cultures. Calcif Tissue Int. 71:36–44. 2002.
View Article : Google Scholar : PubMed/NCBI
|