Introduction
It is a challenge for dentists to save dental pulp
in patients with pulp disease without resorting to root canal
therapy. Pulp disease is not a potentially fatal disease, however
markedly affects the quality of life of those affected, and
recovering damaged pulp remains to be a challenge for dentists.
Formation of tertiary dentin to maintain pulp vitality is a key
odontoblast response to dental pulp injury (1,2). At
present, root canal therapy is a one of the main treatment
modalities used for the majority of pulp diseases, which involves
the removal of all pulp tissue and leaves an empty tooth without a
nervous or nutritional supply. Therefore, there is a requirement to
explore novel therapeutic means to retain the healthy teeth intact,
without root canal therapy subsequent to the occurrence of pulp
disease.
To efficiently recover damaged pulp, angiogenesis is
a key step. Pulp is enriched vascularized tissue that protects
against frequent inflammatory insults (3). Injured pulp cells secrete angiogenic
growth factors to stimulate angiogenesis, which precedes reparative
dentine formation (4). The dentine
matrix contains angiogenic growth factors released from the matrix
subsequent to injury to stimulate reparative responses in the
dentine pulp complex (5).
Angiogenesis is important for successful tissue regeneration,
repair and healing; without adequate blood supply, tissue
regeneration cannot be accomplished and necrotic or scar tissues
are subsequently formed (6).
Vascular endothelial growth factor (VEGF) is the
most potent angiogenic and vasculogenic factor involved in tertiary
dentin formation. VEGF, an endothelium-specific secreted protein,
serves an important role in angiogenesis (7). The VEGF family includes VEGF-A, -B,
-C and -D, and these VEGFs have been reported to be expressed in
human dental pulp, serving autocrine and paracrine roles in local
blood vessels and immune cells (8,9).
Certain bacteria have been observed to upregulate VEGF-A (10), however severe inflammation can
result in a reduction of the number of blood vessels and VEGF-A
expression levels (8). A previous
study identified that pulpal stem cells secrete VEGF during
activation (11), and VEGF has
been identified to induce proliferation and differentiation of
human pulp cells into odontoblasts (12). These facts suggest that VEGF may be
a useful growth factor in the repair of damaged pulp and dentin
(12). The current study aimed to
evaluate whether VEGF can be used in the treatment and prevention
of dental pulp diseases.
Materials and methods
Cell culture
Normal exfoliated human deciduous incisors were
collected from children aged 6–8 years old with the informed
consent of patients and their parents, under the approved
guidelines of China's bioethics law. The protocol was approved by
the Research Ethics Review Committee of the School and Hospital of
Stomatology, Jilin University (Changchun, China). The pulp was
separated from a remnant crown and root and was washed with high
glucose-Dulbecco's modified Eagle's medium (H-DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 1,000
units/ml penicillin and 1,000 µg/ml streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc.). The pulp was then cut
into ~0.5 × 0.5 × 0.5 mm tissue sections using sterilized eye
scissors, placed into a 1.5 ml tube and digested in a solution with
3 mg/ml collagenase type I (Invitrogen; Thermo Fisher Scientific,
Inc.) and 4 mg/ml dispase (Invitrogen; Thermo Fisher Scientific,
Inc.) for 15 min at 37°C in 5% CO2. The human dental
pulp cells (hDPCs) were pelleted by centrifugation at 82 × g for 5
min at room temperature. The cell pellet was then re-suspended with
H-DMEM with 20% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.), 100 units/ml penicillin and 100 µg/ml
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequent to the first passage (~10–15 days), the FBS
concentration was reduced to 10%. For all experiments in the
current study, hDPCs from the third passage were used.
Adenoviral vector preparation
In the present study, subsequent to the deletion of
the first generation of early-transcribed gene 1, replication
deficient adenovirus serotype 5 vectors, AdCMV-enhanced green
fluorescent protein (EGFP) and AdCMV-hVEGF, were used (Clontech
Laboratories, Inc., Mountainview, CA, USA). The two vectors were
propagated in 293 cells, purified by CsCl (Sigma-Aldrich, St.
Louis, MO, USA) gradient centrifugation using an Optima L-90K
Ultracentrifuge (Beckman Coulter, Inc., Brea, CA, USA) and SW41
rotor (Beckman Coulter, Inc.) at 151,000 × g for 19 h at room
temperature. They were dialyzed against 4 l dialysis buffer
containing 4% glycerol, 40 mM Tris (pH 7.4) and 1 mM
MgCl2 (Invitrogen; Thermo Fisher Scientific, Inc.) for 4
h at 4°C and then were stored in aliquots at −80°C for later use.
Vector titers were determined by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) using transgene-specific
primers.
Adipogenic induction assay in vitro
The hDPCs were seeded at 105 cells/well
in H-DMEM in 6-well plates and incubated at 37°C in a humidified 5%
CO2 atmosphere for 36 h. A total of 0.5 mM
isobutylmethylxanthine, 1 µM dexamethasone, 10 mg/l insulin
(Sigma-Aldrich) and 200 µM indomethacin (Qinghai Dadi
Pharmaceutical Industry Co., Ltd., Xining, China) were then added
and the cells were cultured for 5 weeks. The cell culture medium
was replaced every 3 days. On day 35, cells were fixed with 70%
ethanol for 1 h, washed with phosphate-buffered saline (PBS) once,
stained with Oil Red O (Sigma-Aldrich) for 1 h at 37°C, washed
again and observed using an Olympus IX71 (Olympus Corporation,
Tokyo, Japan).
Cell transduction efficiency with
AdCMV-EGFP in vitro
The hDPCs were seeded at 5×104 cells/well
in 12-well plates and incubated at 37°C in a humidified 5%
CO2 atmosphere for 36 h. Subsequently, these cells were
transduced with the recombinant adenovirus vector encoded with
EGFP, AdCMV-EGFP, at 0, 4, 8 or 10 multiplicity of infection
(MOI)/cell. A total of 1, 3, 5 and 7 days post-transduction, cells
were directly observed under the Olympus IX71, transduction
efficiency was calculated and the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay (Bio-Tek Instruments, Inc., Winooski, VT, USA)
was performed.
VEGF expression by RT-PCR
The hDPCs were seeded at a density of 105
cells/well in 6-well plates and were cultured for 36 h. The cells
were then transduced using AdCMV-EGFP or AdCMV-hVEGF at 10
MOI/cell. On day 3, total RNA was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (1
µg) underwent a reverse transcription reaction using the
PrimeScript® RT Reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd., Dalian, China) to synthesize cDNA. The
hVEGFF1 (5′-AGAAGGAGGAGGGCAGAATC-3′) and hVEGFB1
(5′-AATGCTTTCTCCGCTCTG-3′) primers were used for the PCR reaction.
RT reaction mixture (1 µl) was used for the RT-PCR reaction.
PCR assays were performed in Premix Taq™ (Takara Taq™ version 2.0
plus dye; Takara Biotechnology Co., Ltd.) using the Gene
Amp® PCR System 9700 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) under the following conditions: 94°C for 5 min,
then 30 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30
sec, followed by extension at 72°C for 5 min. β-actin was used as
the internal control. The PCR products were separated using 2%
agarose gel electrophoresis, and were imaged with the Molecular
Imager® Gel Doc™ XR System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Alizarin red S staining and alkaline
phosphatase (ALP) activity assays in vitro
The hDPCs were seeded at 105 cells/well
in 6-well plates or at 103 cells/well in 96-well plates,
and were cultured for 36 h, then transduced using AdCMV-EGFP or
AdCMV-hVEGF at 10 MOI/cell. One day post-transduction, culture
medium was replaced with H-DMEM supplemented with 10% FBS, 10 mM/l
sodium β-glycerol phosphate (Sigma-Aldrich), 50 mg/l L-ascorbic
acid (Sigma-Aldrich) and 10−8 M/l dexamethasone
(Sigma-Aldrich) to induce mineralization. On days 14 and 28, cells
in the 6-well plates were washed with PBS and fixed in 95% ethanol
at 4°C for 30 min, then stained with 0.1% Alizarin red S
(Sigma-Aldrich) at 37°C for 30 min and washed with dH2O
five times.
The hDPCs in 96-well plates on days 3, 7, 14 and 21
were used to detect cell ALP activity using the ALP substrate
(Sigma-Aldrich) and plates were read with an ELx800 Absorbance
Reader (Bio-Tek Instruments, Inc.) at a wavelength of 520 nm
according to the manufacturer's instructions.
RT-qPCR in vitro
Total RNA was extracted as described above on days
3, 7 and 14 by TRIzol. Total RNA (6 µg) was used for the
reverse transcription reaction using the PrimeScript® RT
Reagent kit with gDNA Eraser. RT reaction mixture (1 µl) was
used for qPCR. Primers and probes for bone morphogenetic protein 2
(BMP2), runt-related transcription factor 2 (Runx2), ALP, collagen
type Iα (Col 1α), bone sialoprotein (BSP), Sp7, dentin matrix
acidic phosphoprotein 1 (DMP1), osteocalcin (OCN) and dentin
sialophosphoprotein (DSPP) were obtained from Thermo Fisher
Scientific, Inc. All qPCR assays were performed using the MX3005P
system (Agilent Technologies, Inc., Santa Clara, CA, USA) using
TaqMan® Universal PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) under the following conditions:
50°C for 2 min, 95°C for 10 min, then 40 cycles of 95°C for 15 sec
and 60°C for 1 min. β-actin was used as the internal control.
In vivo animal assays
The animal experimental protocol was approved by the
Institutional Animal Care and Use Committee and the Ethics
Committee of the Faculty of Dentistry, Jilin University (Changchun,
China). A total of 30 upper first molars from 15 specific
pathogen-free male Wistar rats (~200 g, 2 months old) were used in
the current study (Animal Experimental Center of Jilin University).
Rats were anesthetized with ketamine (60 mg/kg; Jiangsu Hengrui
Medicine Co., Ltd., Lianyungang, China) and xylazine (8 mg/kg;
Sigma-Aldrich). The coronal enamel and dentin of the first molar
were carefully removed from the occlusal surface with stainless
steel burs under water cooling to expose the pulp using an
endodontic microscope (OPMI® pico; Carl Zeiss AG,
Oberkochen, Germany). Subsequent to washing the pulp with saline, a
gelatin sponge containing AdCMV-EGFP or AdCMV-hVEGF at
1.25×104 MOI/0.25 µl was applied to the exposed
pulp surface on the left or right side. The two adenoviral vectors
were then released and transduced into adjacent dental pulp cells.
The occlusal cavities were sealed with GC Fuji IX GP (GC
Corporation, Tokyo, Japan). On days 3, 7 and 14 subsequent to
surgery, animals were anesthetized with ketamine (60 mg/kg) and
xylazine (8 mg/kg) and euthanized by intracardiac perfusion with a
4% paraformaldehyde buffered solution (Beijing Chemical Works,
Beijing, China). The upper molars were excised and fixed with 4%
paraformaldehyde for two days, decalcified in 10%
ethylenediaminetetraacetic acid (EDTA; Beijing Chemical Works,
Beijing, China) for three months at room temperature, then rinsed
in water, dehydrated in a series of increasing concentrations of
alcohol, embedded in paraffin and cut into 3 µm sections
using a rotary micro-tome (RM2245; Leica Biosystems, Buffalo Grove,
IL, USA). Sections were stained with hematoxylin and eosin
(Ameresco, Inc., Framingham, MA, USA) or immunohistochemical
staining.
Immunohistochemical analysis
Subsequent to deparaffinization, the sections were
treated with compound enzyme digestive juice (0.125% trypsin + 0.1%
pepsin + 0.01% EDTA; Wuhan Boster Biological Technology, Ltd.,
Wuhan, China) for 20 min and then with antigen retrieval solution
(Wuhan Boster Biological Technology, Ltd.) for 10 min. The
immunohistochemistry was performed according to the instructions of
UltraSensitive™ SP (mouse/rabbit) immunohistochemistry kit
(Maixin-Bio, Fuzhou, China). The anti-rat mouse monoclonal DMP1-C-8
G 10.3 and anti-rat mouse monoclonal anti-DSP-2C12.3 antibodies
(donated by Dr Chunlin Qin, Baylor College of Dentistry, Texas
A&M University Health Science Center, Dallas, TX, USA)
(13,14) at a dilution of 1:1,000 used as the
primary antibodies (15). The
sections were then observed under a microscope (Olympus BX51TF;
Olympus Corporation).
Statistical analysis
Results were presented as the mean ± standard error.
A paired t-test was used to determine the statistical significance.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of hDPCs from exfoliated
deciduous teeth
In the current study, hDPC culture was established
following Miura's method (16).
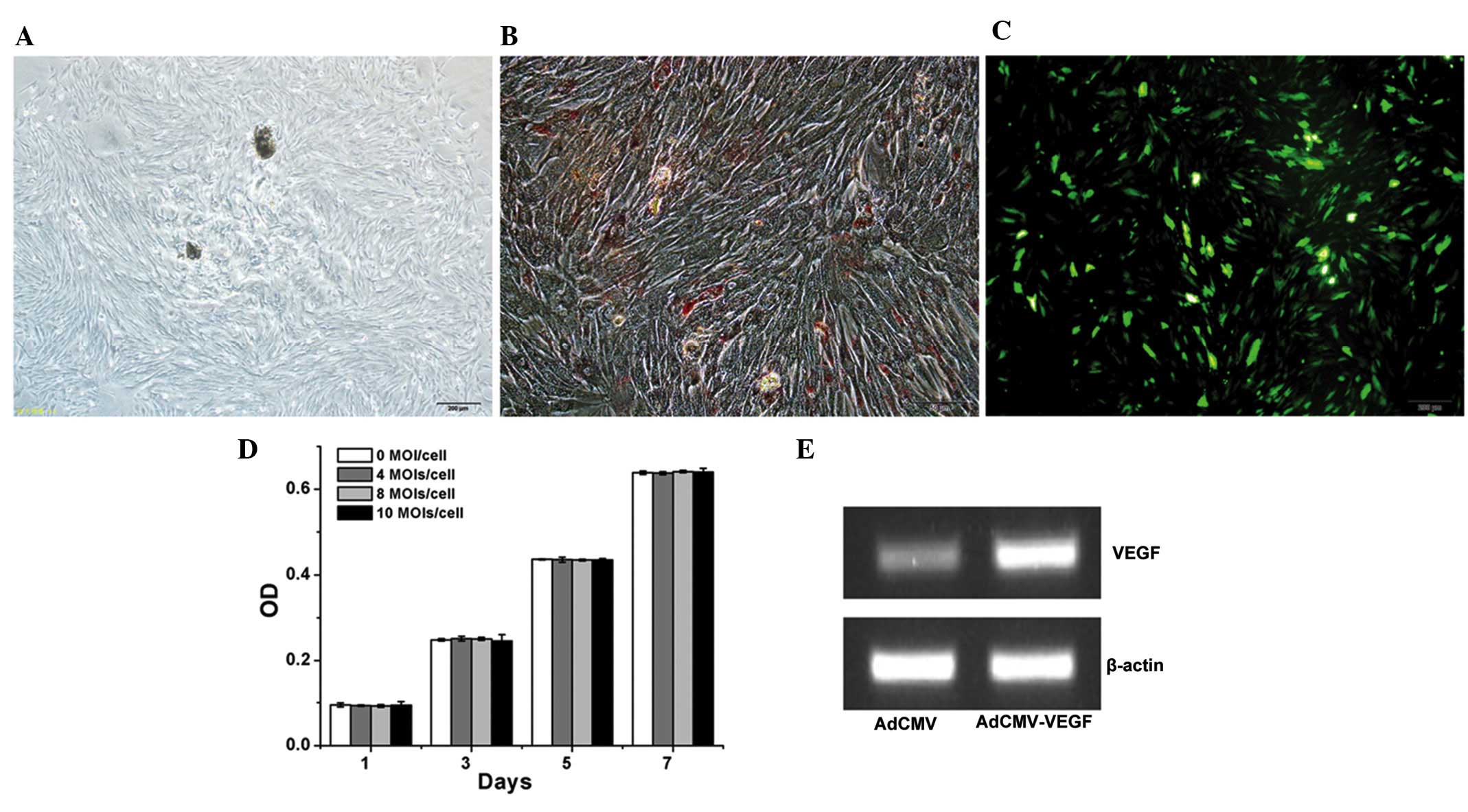
Fig. 1A presents the general
morphology of hDPCs on day 3 under the inverted microscope. In
general, hDPCs were cultured for 10–15 days, then passaged three
times (5 days/passage). Cells from the third passage were used for
the experiments. When the cells were cultured in 0.5 mM
isobutylmethylxanthine, 1 µM dexamethasone, 10 mg/l insulin
and 200 µM indomethacin for 5 weeks, certain cells were
observed to be Oil Red O positive (Fig. 1B), which indicates that adipogenic
differentiation had occurred.
To evaluate the transduction efficiency of the
adenoviral vector, hDPCs were transduced with AdCMV-EGFP at 10
MOI/cell. Approximately 70% of the hDPCs were identified as being
EGFP-positive on day 3 (Fig. 1C).
Subsequently, MTT assays were conducted in order to assess whether
the adenoviral vector affected the proliferation of hDPCs. Fig. 1D demonstrates that the adenoviral
vector, AdCMV-EGFP, did not influence the proliferation of hDPCs
from 0 to 10 MOI/cell. VEGF expression was also measured, and was
observed to be increased subsequent to transduction of the hDPCs
with AdCMV-hVEGF at 10 MOI/cell (Fig.
1E). This indicated that these adenoviral vectors could be used
to transduce these cells.
Effects of hVEGF on hDPCs
differentiation
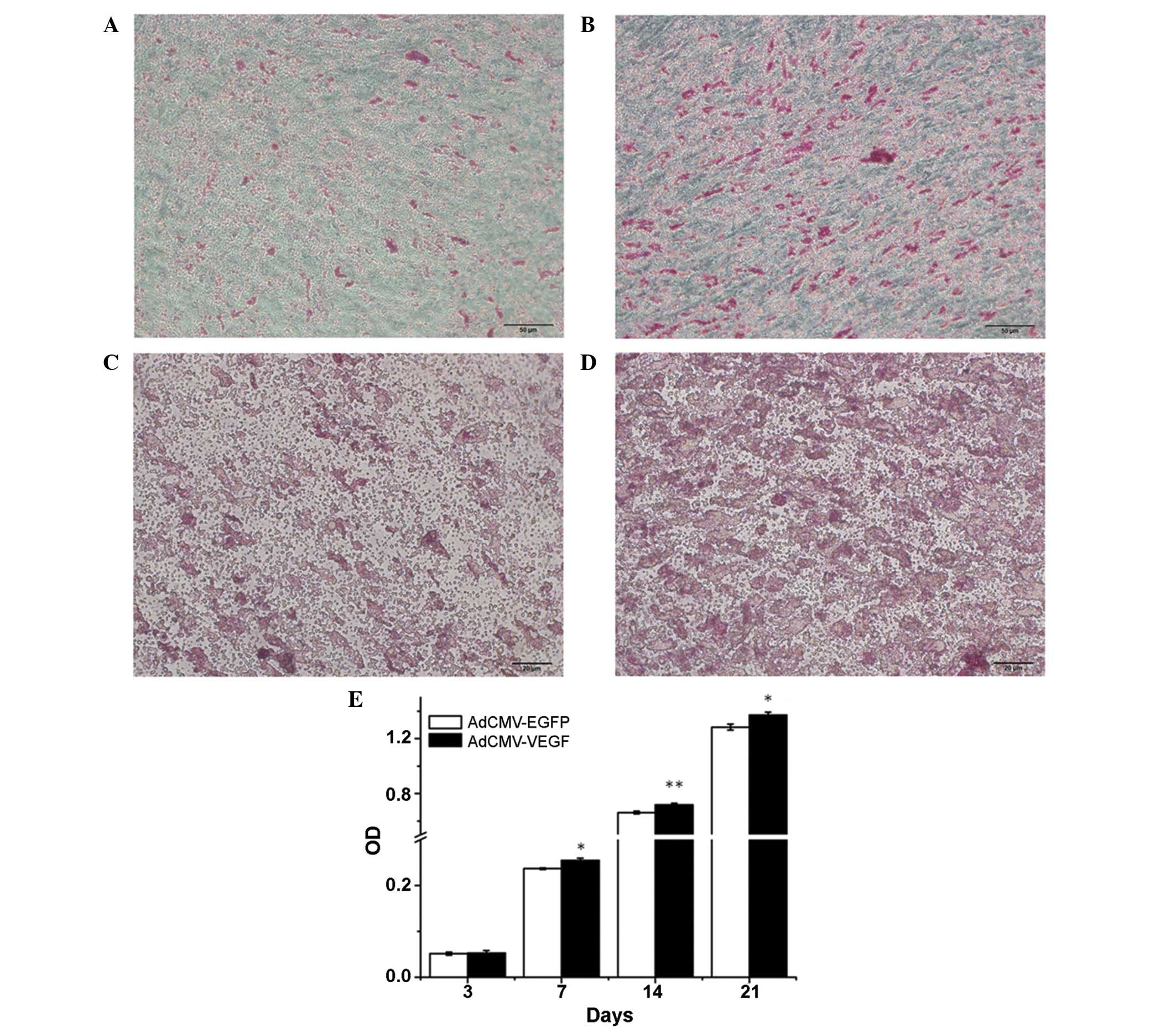
Alizarin red S staining and ALP activity were the
two indicators used to monitor the differentiation of hDPCs in the
culture media in the current study. Data demonstrated that calcium
deposition occurred in the hDPCs, whereas the AdCMV-hVEGF-treated
group had a significantly greater number of Alizarin red S-positive
cells or mineralized nodules on days 14 and 28 compared with that
of the AdCMV-EGFP control group (Fig.
2A–D). hVEGF was identified to significantly increase ALP
activity 7 days post-transduction (Fig. 2E). These results demonstrate that
VEGF is able to promote the mineralization and differentiation of
hDPCs.
Effects of hVEGF on gene expression
following mineralization induction
To further understand the effects of hVEGF on the
differentiation of hDPCs at the molecular level, RT-qPCR assays
were conducted to quantitatively evaluate gene expression of nine
osteogenic/odontogenic gene markers: Runx2, ALP, Col 1α, SP7, DMP1,
DSPP, BMP2, BSP and OCN. Data in Fig.
3 indicates that hVEGF significantly increased the expression
of these genes when compared with the AdCMV-EGFP group on days 7
and 14, particularly on day 7. This indicates that VEGF is able to
affect osteoblasts/odontoblasts.
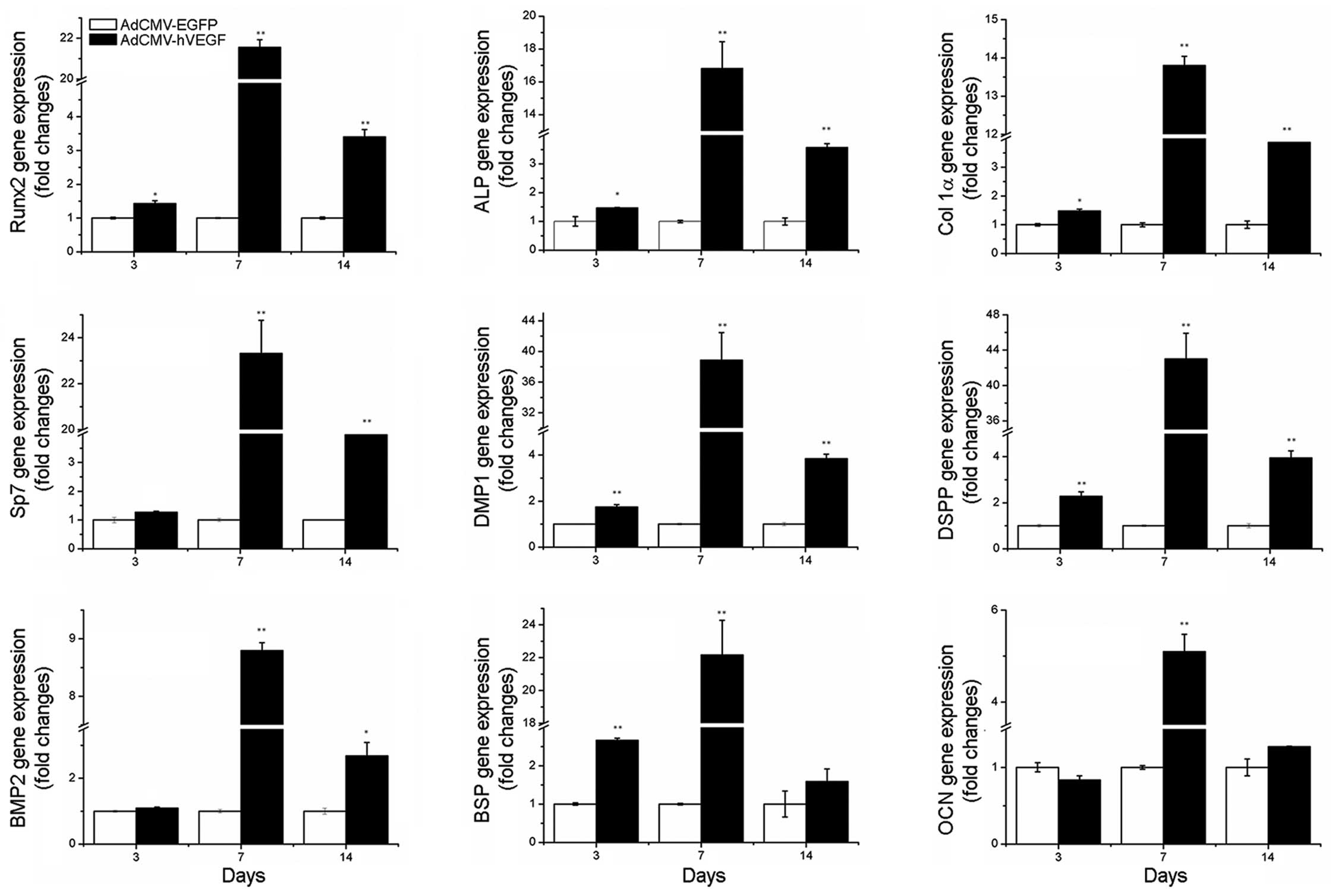 | Figure 3Gene expression profiles of hDPCs
transduced with AdCMV-EGFP or AdCMV-hVEGF 3, 7 or 14 days
post-transduction. Data are presented as the mean ± standard error.
The assays were repeated three times. *P<0.05 and
**P<0.01 vs. AdCMV-EGFP. hDPCs, human dental pulp
cells; EGFP, enhanced green fluorescent protein; hVEGF, human
vascular endothelial growth factor; Runx2, runt-related
transcription factor 2; ALP, alkaline phosphatase; Col 1α, collagen
type Iα; DMP1, dentin matrix acidic phosphoprotein 1; DSPP, dentin
sialophosphoprotein; BMP2, bone morphogenetic protein 2; BSP, bone
sialoprotein; OCN, osteocalcin. |
Direct effects of hVEGF on reparative
dentin formation in vivo
The above-mentioned in vitro data
demonstrated that VEGF was able to affect differentiation and
mineralization of hDPCs. Subsequently, in vivo assays to
investigate whether hVEGF directly affects pulp cells in
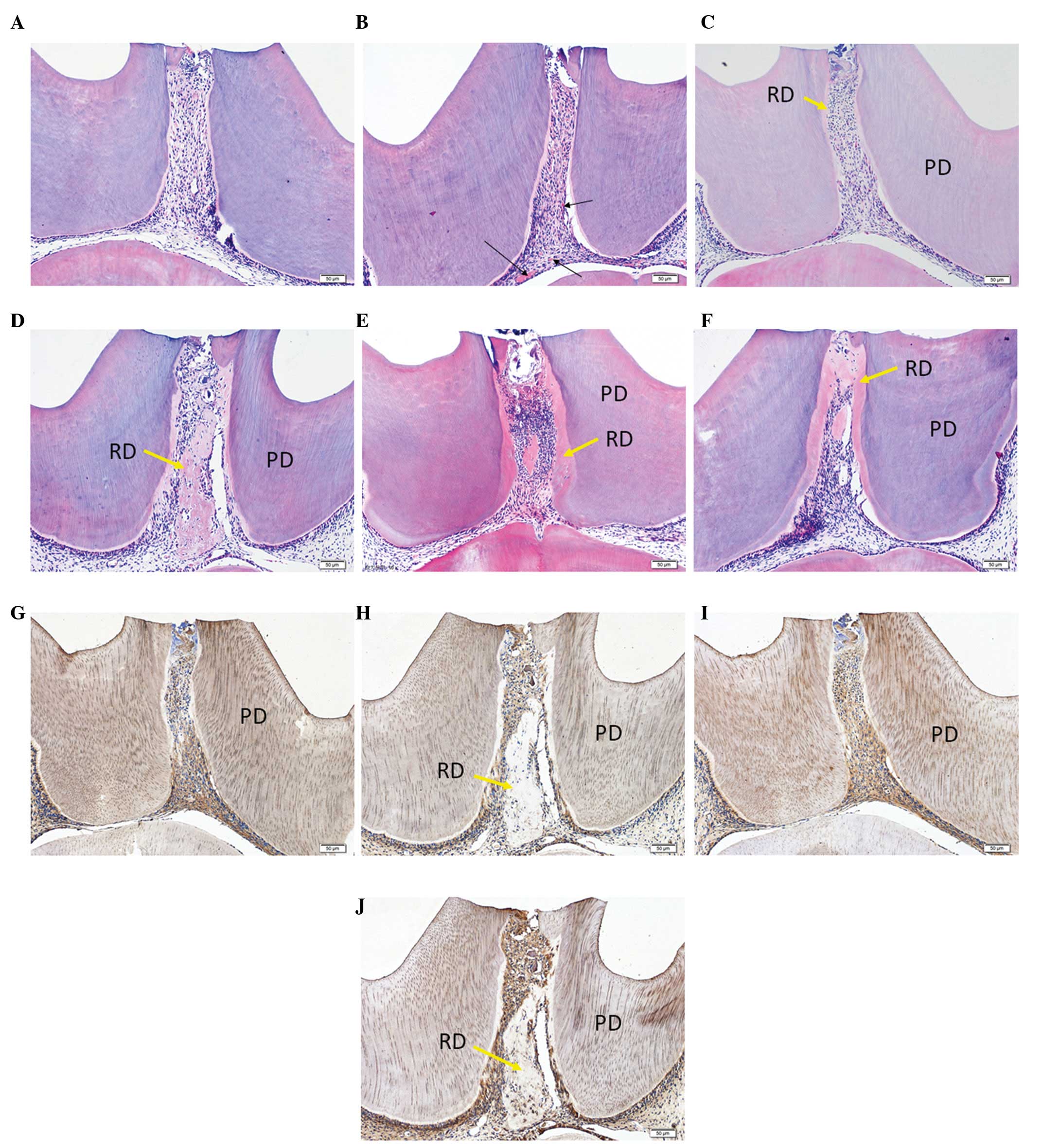
vivo were undertaken. The collected data demonstrated that the
AdCMV-hVEGF-treated groups had a marked increase in the number of
blood vessels in the dental pulp compared with the
AdCMV-EGFP-treated group on days 3, 7 and 14 (Fig. 4A–F). The results additionally
identified that hVEGF was able to increase the volume of reparative
dentin compared with the AdCMV-EGFP-treated groups on days 7 and 14
(Fig. 4C–F). In addition, it was
observed by immunohistochemical staining that the
AdCMV-hVEGF-treated groups exhibited stronger DMP1 and DSP-positive
pulp cells than in the AdCMV-EGFP-treated groups (Fig. 4G–J), which indicated that the pulp
cells actively proliferate in AdCMV-hVEGF-treated dental pulp.
Discussion
The body's vascular system supports the critical
functions of supplying cells and tissues with nutrients and
clearing away waste products. Vascular permeability is markedly
increased in cases of acute and chronic inflammation such as those
in pulpitis (17,18). Endotoxins produced by cariogenic
bacteria stimulate VEGF expression in dental pulp cells (19), and VEGF is a key regulator in the
response to pulp injury resulting in increases in vascular
permeability and angiogenesis during the healing process (10,20).
The results of the current study demonstrated that VEGF is able to
promote proliferation and differentiation of pulp cells in
vitro and in vivo.
A previous study reported that stem cells from human
exfoliated deciduous teeth expressed the membrane-bound VEGF
receptors (VEGFR)-1 and -2, CD31 and vascular endothelial-cadherin
(11). An additional study
reported that VEGF was able to stimulate proliferation and increase
ALP in human pulp cells (8). It
has been widely reported that the gene expression levels of Runx-2
and ALP serve important roles in the early and middle stages of
differentiation during bone formation, whereas expression of BSP,
OPN and OCN serve critical roles in the late stages of osteoblast
differentiation (21). The DSPP
gene produces two key noncollagenous dentin proteins: Dentin
sialoprotein and dentin phosphoprotein, which are essential for
dentin mineralization (22,23).
DMP-1 is predominantly expressed in odontoblasts and is a candidate
gene for dentinogenesis imperfecta (24). Notably, the data of the current
study demonstrated that VEGF may promote the mineralization and
differentiation of hDPCs (Fig. 2)
and markedly increase gene expression of Runx2, ALP, Col 1α, SP7,
DMP1, DSPP, BMP2, BSP and OCN in pulp cell culture in vitro
(Fig. 3). Therefore, the data of
the present study suggests that VEGF is able to enhance
osteoblast/odontogenic differentiation and mineralization of hDPCs
in vitro.
Reparative dentin is a type of tertiary dentin
(reactionary and reparative dentin), which functionally responds to
severe injury and is formed by replacement odontoblasts (25). Angiogenesis serves a key role in
tissue regeneration due to the requirements of nutrient supply and
waste removal for the functioning of a vascular network (26). Pulp tissue normally only receives
blood supply from one end, at the root apex, therefore, dental pulp
tissue is vulnerable to damage, infection and the development of
irreversible pulpitis (26).
Applying angiogenic growth factors locally has been suggested to
increase local angiogenesis at the site of dental tissue repair
subsequent to tooth root fracture (27). Numerous studies have applied
different strategies for the promotion of angio-genesis for their
disease model (28–30). Mullane et al (31) demonstrated that recombinant hVEGF
(165) was able to enhance the neovascularization of human dental
pulp. In vivo data from the current study additionally
demonstrated that hVEGF may increase proliferation of dental pulp
and promote neovascularization and formation of reparative dentin
in the dental pulp.
In conclusion, the current study demonstrates that
hVEGF has positive influences on proliferation, differentiation,
mineralization, neovascularization and formation of reparative
dentin of dental pulp tissue in vitro and in vivo.
The data collected strongly suggest that hVEGF has clinical
therapeutic potential for the treatment of pulp diseases. The
current study additionally suggests that a gene therapy strategy
may be useful for treatment of dental pulp diseases. As a next
step, hVEGF and inhibitors of inflammation will be used in order to
investigate whether it is possible to treat reversible and
irreversible pulpitis, with a particular focus on irreversible
pulpitis.
Acknowledgments
The authors would like to thank Dr Chunlin Qin
(Baylor College of Dentistry, Texas A&M University Health
Science Center, Dallas, TX, USA) for the donation of the DMP1 and
DSP antibodies and Ms. Cindy Clark (NIH Library Editing Service,
Bethesda, MD, USA) for reviewing the manuscript. The current study
was supported by the Science and Technology Development Projects of
Jilin Province (grant no. 20140204018SF), Jilin Provincial Health
Department Research Projects (grant no. 2012S017), the Fundamental
Research Project of the Central Universities (grant no.
450060491132), the 2013 Human Resources and Social Security
Development Postdoctoral Research Projects of Jilin Province (grant
no. 20130419431) and the National Natural Science Foundation of
China (grant no. 81271111).
References
|
1
|
Couve E, Osorio R and Schmachtenberg O:
Reactionary dentinogenesis and neuroimmune response in dental
caries. J Dent Res. 93:788–793. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farges JC, Joffre A and Magloire H:
Response of odontoblastic and pulpal cells to carious lesions. C R
Seances Soc Biol Fil. 187:582–595. 1992.In French.
|
|
3
|
Bjørndal L and Mjör IA: Pulp-dentin
biology in restorative dentistry. Part 4: Dental
caries-characteristics of lesions and pulpal reactions.
Quintessence Int. 32:717–736. 2001.
|
|
4
|
Tran-Hung L, Laurent P, Camps J and About
I: Quantification of angiogenic growth factors released by human
dental cells after injury. Arch Oral Biol. 53:9–13. 2008.
View Article : Google Scholar
|
|
5
|
Roberts-Clark DJ and Smith AJ: Angiogenic
growth factors in human dentine matrix. Arch Oral Biol.
45:1013–1016. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Madeddu P: Therapeutic angiogenesis and
vasculogenesis for tissue regeneration. Exp Physiol. 90:315–326.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hood JD, Meininger CJ, Ziche M and Granger
HJ: VEGF upregulates ecNOS message, protein, and NO production in
human endothelial cells. Am J Physiol. 274:H1054–H1058.
1998.PubMed/NCBI
|
|
8
|
Artese L, Rubini C, Ferrero G, Fioroni M,
Santinelli A and Piattelli A: Vascular endothelial growth factor
(VEGF) expression in healthy and inflamed human dental pulps. J
Endod. 28:20–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Virtej A, Løes S, Iden O, Bletsa A and
Berggreen E: Vascular endothelial growth factors signalling in
normal human dental pulp: A study of gene and protein expression.
Eur J Oral Sci. 121:92–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soden RI, Botero TM, Hanks CT and Nör JE:
Angiogenic signaling triggered by cariogenic bacteria in pulp
cells. J Dent Res. 88:835–840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai VT, Zhang Z, Dong Z, Neiva KG,
Machado MA, Shi S, Santos CF and Nör JE: SHED differentiate into
functional odontoblasts and endothelium. J Dent Res. 89:791–796.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsushita K, Motani R, Sakuta T,
Yamaguchi N, Koga T, Matsuo K, Nagaoka S, Abeyama K, Maruyama I and
Torii M: The role of vascular endothelial growth factor in human
dental pulp cells: Induction of chemotaxis, proliferation and
differentiation and activation of the AP-1-dependent signaling
pathway. J Dent Res. 79:1596–1603. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang B, Maciejewska I, Sun Y, Peng T, Qin
D, Lu Y, Bonewald L, Butler WT, Feng J and Qin C: Identification of
full-length dentin matrix protein 1 in dentin and bone. Calcif
Tissue Int. 82:401–410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baba O, Qin C, Brunn JC, Jones JE, Wygant
JN, McIntyre BW and Butler WT: Detection of dentin sialoprotein in
rat periodontium. Eur J Oral Sci. 112:163–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moses KD, Butler WT and Qin C:
Immunohistochemical study of small integrin-binding ligand,
N-linked glycoproteins in reactionary dentin of rat molars at
different ages. Eur J Oral Sci. 114:216–222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: Stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagy JA, Benjamin L, Zeng H, Dvorak AM and
Dvorak HF: Vascular permeability, vascular hyperpermeability and
angiogenesis. Angiogenesis. 11:109–119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heyeraas KJ and Berggreen E: Interstitial
fluid pressure in normal and inflamed pulp. Crit Rev Oral Biol Med.
10:328–336. 1999. View Article : Google Scholar
|
|
19
|
Botero TM, Shelburne CE, Holland GR, Hanks
CT and Nör JE: TLR4 mediates LPS-induced VEGF expression in
odontoblasts. J Endod. 32:951–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Botero TM, Son JS, Vodopyanov D, Hasegawa
M, Shelburne CE and Nör JE: MAPK signaling is required for
LPS-induced VEGF in pulp stem cells. J Dent Res. 89:264–269. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mangano C, Paino F, d'Aquino R, De Rosa A,
Iezzi G, Piattelli A, Laino L, Mitsiadis T, Desiderio V, Mangano F,
et al: Human dental pulp stem cells hook into biocoral scaffold
forming an engineered biocomplex. PLoS One. 6:e187212011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng JQ, Luan X, Wallace J, Jing D,
Ohshima T, Kulkarni AB, D'Souza RN, Kozak CA and MacDougall M:
Genomic orga-nization, chromosomal mapping and promoter analysis of
the mouse dentin sialophosphoprotein (Dspp) gene, which codes for
both dentin sialoprotein and dentin phosphoprotein. J Biol Chem.
273:9457–9464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
MacDougall M, Gu TT and Simmons D: Dentin
matrix protein-1, a candidate gene for dentinogenesis imperfecta.
Connect Tissue Res. 35:267–272. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki S, Sreenath T, Haruyama N,
Honeycutt C, Terse A, Cho A, Kohler T, Müller R, Goldberg M and
Kulkarni AB: Dentin sialo-protein and dentin phosphoprotein have
distinct roles in dentin mineralization. Matrix Biol. 28:221–229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mitsiadis TA and Rahiotis C: Parallels
between tooth development and repair: Conserved molecular
mechanisms following carious and dental injury. J Dent Res.
83:896–902. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang GT: Pulp and dentin tissue
engineering and regeneration: Current progress. Regen Med.
4:697–707. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin H, Thomas HF and Chen J: Wound healing
and revascularization: A histologic observation of experimental
tooth root fracture. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 81:26–30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanematsu A, Yamamoto S, Ozeki M, Noguchi
T, Kanatani I, Ogawa O and Tabata Y: Collagenous matrices as
release carriers of exogenous growth factors. Biomaterials.
25:4513–4520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peters MC, Polverini PJ and Mooney DJ:
Engineering vascular networks in porous polymer matrices. J Biomed
Mater Res. 60:668–678. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Q, Chen RR, Shen Y, Mooney DJ,
Rajagopalan S and Grossman PM: Sustained vascular endothelial
growth factor delivery enhances angiogenesis and perfusion in
ischemic hind limb. Pharm Res. 22:1110–1116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mullane EM, Dong Z, Sedgley CM, Hu JC,
Botero TM, Holland GR and Nör JE: Effects of VEGF and FGF2 on the
revascularization of severed human dental pulps. J Dent Res.
87:1144–1148. 2008. View Article : Google Scholar : PubMed/NCBI
|