Introduction
Epidermal growth factor receptor (EGFR) is a member
of the ErbB family of receptor tyrosine kinases (1), and EGFR activation by ligand binding
stimulates multiple signals, including phosphatidylinositol
3-kinase (PI3K)/AKT, mitogen-activated protein kinases (MAPKs) and
nuclear factor-κB pathways, ultimately resulting in cellular
proliferation, survival, angiogenesis invasion and metastasis
(2,3). Abnormal protein activity and/or
expression levels of EGFR have been correlated with the etiology of
several types of human cancer, including colorectal cancer
(4,5), non-small cell lung cancer (6,7),
breast cancer (8,9), head and neck squamous cell carcinoma
(10,11), pancreatic cancer (12) and brain cancer (13). EGFR-targeted therapy has been
validated in human colorectal cancer (14), and the chemotherapeutics include
pharmacological agents, including cetuximab (Erbitux), which is an
EGFR inhibitor (15). EGFR acts to
bind an EGFR-selective ligand activated by epidermal growth factor
(EGF), transforming growth factor-α, amphiregulin or neuregulin,
finally leading to cell proliferation, invasion and the inhibition
of apoptosis (15–17).
Tetrandrine is a bisbenzylisoquinoline alkaloid,
which is isolated from the dried root of Stephania tetrandra
of the Menispermaceae family (18,19).
Tetrandrine has been shown to have broad pharmacological actions
(20–22). Several reports have indicated that
tetrandrine presents potent anticancer effects on multiple cancer
cells in vitro (23–29).
Tetrandrine retards Wnt/β-catenin signaling and inhibits tumor
growth of HCT116 human colorectal cancer (24). Furthermore, the apoptosis of cells
in human hepatocellular carcinoma caused by tetrandrine is mediated
through the production of reactive oxygen species and the
repression of AKT activity (30).
Our previous study revealed that tetrandrine induces apoptotic and
autophagic cell death in SAS human oral cancer cells (31). Wu et al (32) demonstrated that tetrandrine
inhibits cell proliferation, invasion and migration by suppressing
the levels of A disintegrin/metalloprotease 17, phosphorylated
(p)-EGFR and p-AKT in U87 glioblastoma cells. Therefore, evidence
suggests that the suppression of EGFR-PI3K/AKT signaling may
contribute to the tetrandrine-induced anticancer activities of
inhibition of cell migration and invasion. In the present study, it
was demonstrated that tetrandrine inhibited EGF-induced HT29 cell
invasion and migration through stimulating the phosphorylation of
EGFR, sequentially inactivating the PI3K/AKT cascade, repressing
MAPK/extracellular signal-regulated protein kinase (ERK)-mediated
signaling and reducing MMP-2 and MMP-9 signals.
Materials and methods
Chemicals and reagents
In the present study, tetrandrine, EGF and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetra-zolium bromide
(MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), penicillin-streptomycin and trypsin-EDTA were purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Anti-p-EGFR (Y845) (2231; 1:1,000), anti-p-EGFR (Y992) (2235;
1:1,000), anti-p-EGFR (Y1068) (2234; 1:1,000),
anti-p-phos-phoinositide-dependent kinase-1 (PDK1; 3031; 1:1,000),
anti-p-PI3K (4228; 1:1,000), anti-p-AKT (S308) (13038; 1:1,000),
anti-p-AKT (S473) (4060; 1:1,000), anti-p-ERK (Thr202/Tyr204)
(4370; 1:1,000), anti-p-c-Jun N-terminal kinase (JNK;
Thr183/Tyr185) (4668; 1:1,000) and anti-p-p38 (Thr180/Tyr182)
(4511; 1:1,000) antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Antibodies against EGFR
(sc-03, 1:500), PI3K (sc-423, 1:500), AKT (sc-8312, 1:500), ERK
(sc-135900, 1:500), JNK (sc-571, 1:500), p38 (sc-7972, 1:500) and
β-actin (sc-1616, 1:5,000), as well as horseradish peroxidase
(HRP)-conjugated secondary antibodies (goat anti-mouse IgG-HRP,
sc-2031, 1:10,000; donkey anti-goat IgG-HRP, sc-2033, 1:10,000; and
goat anti-rabbit IgG-HRP, sc-2030, 1:10,000) were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
The HT29 human colorectal adenocarcinoma cell line
was cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin
and 100 µg/ml streptomycin, and incubated in a humidified
incubator with 5% CO2 at 37°C (33).
Cell invasion assay
The membrane of each Transwell insert was coated
with Matrigel (BD BioCoat BD Matrigel Invasion Chamber; BD
Biosciences, Bedford, MA, USA), according to the manufacturer's
protocol. The HT29 cells (2×104) were seeded onto the
upper chamber of the insert in 0.5 ml complete DMEM per Transwell,
containing 100 ng/ml EGF and either 0.5, 1 or 2 µM of
tetrandrine for 48 h at 37°C. The number of invaded cells was
analyzed, as previously described (34).
Cell migration assay
The HT29 cells (2×104) were seeded into a
Transwell insert (BD Biosciences) and incubated with 100 ng/m EGF
and either 0.5, 1 or 2 µM of tetrandrine for 48 h at 37°C.
The number of migrated cells were counted, as described previously
by Lu et al (35).
Determination of cell viability using an
MTT assay
The HT29 cells (2×104) were seeded into
the 96-well plate and were incubated with EGF (100 ng/ml) and
tetrandrine (0, 0.5, 1 or 2 µM). Following incubation for 48
h at 37°C, MTT solution (0.5 mg/ml) was added for an additional 4
h, and the formazan crystals were dissolved by 200 µl of
dimethyl sulfoxide (Sigma-Aldrich), as described previously
(35). The cytotoxicity was
determined as previously described (35), with the value of the untreated
control sample set as 100%.
Gelatin zymography assay
The HT29 cells (1×105) were seeded into a
12-well plate and were exposed to EGF (100 ng/ml) and various
concentrations of tetrandrine (0, 0.5, 1 or 2 µM) for 48 h
at 37°C. The conditioned media were collected, and the samples were
separated by electrophoresis on an 8% SDS-polyacrylamide gel with
0.1% gelatin (Sigma-Aldrich). Subsequently, the gel was incubated
in zymogen developing buffer (Sigma-Aldrich), containing 50 mM Tris
(pH 7.5), 200 mM NaCl, 5 mM CaCl2, 1 µM
ZnCl2 and 0.02% Brij-35, overnight at 37°C. The bands
corresponding to activity were stained with 0.5% Coomassie
Brilliant blue G-250 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), and the band of gelatinolytic activity was determined using
NIH Image J software, version 1.47 (National Institutes of Health,
Bethesda, MA, USA), as described previously (35,36).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) analysis
The HT29 cells (1×107 cells in a 75
cm2-flask) were exposed to 0.5, 1 and 2 µM of
tetrandrine and EGF (100 ng/ml) for 48 h at 37°C prior to total RNA
being extracted using a Qiagen RNeasy Mini kit (Qiagen, Valencia,
CA, USA). cDNAs were synthesized from each RNA sample, as
previously reported (37,38). Subsequent qPCR for each sample was
performed using an Applied Biosystems 7300 Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's instructions. The cDNAs were
mixed with 2X SYBR Green PCR Master mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) and the following primers, according to
the manufacturer's protocol (Sigma-Aldrich): MMP-2, forward
5′-CCCCAGACAGGTGATCTTGAC-3′ and reverse 5′-GCTTGCGAGGGAAGAAGTTG-3′;
MMP-9, forward 5′-CGCTGGGCTTAGATCATTCC-3′ and reverse
5′-AGGTTGGATACATCACTGCATTAGG-3′; and GAPDH, forward
5′-ACACCCACTCCTCCACCTTT-3′ and reverse TAGCCAAATTCGTTGTCATACC-3′.
Each transcript was calculated relative to the housekeeping gene,
GAPDH.
Immunoblotting analysis
The HT29 cells cells (1×107 cells in a 75
cm2-flask) were treated with EGF (100 ng/ml) and exposed
to 0.5, 1 and 2 µM of tetrandrine for 48 h at 37°C.
Following treatment, the whole cell lysate was collected, and
immunoblotting was performed to determine the protein expression
levels, as detailed by Chen et al (36). The protein signals were detected
using an Immobilon Western Chemiluminescent HRP Substrate kit
(Merck Millipore, Billerica, MA, USA) and Bio-MAX MR X-ray film
(Eastman Kodak, Rochester, NY, USA), as previously described
(37,38).
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance followed by Student's
t-test using SPSS software, version 12.0 (SPSS, Inc., Chicago, IL,
USA) was used to compare the differences. P<0.05 was considered
to indicate a statistically significant difference.
Results
Tetrandrine inhibits EGF-induced HT29
cell invasion and migration
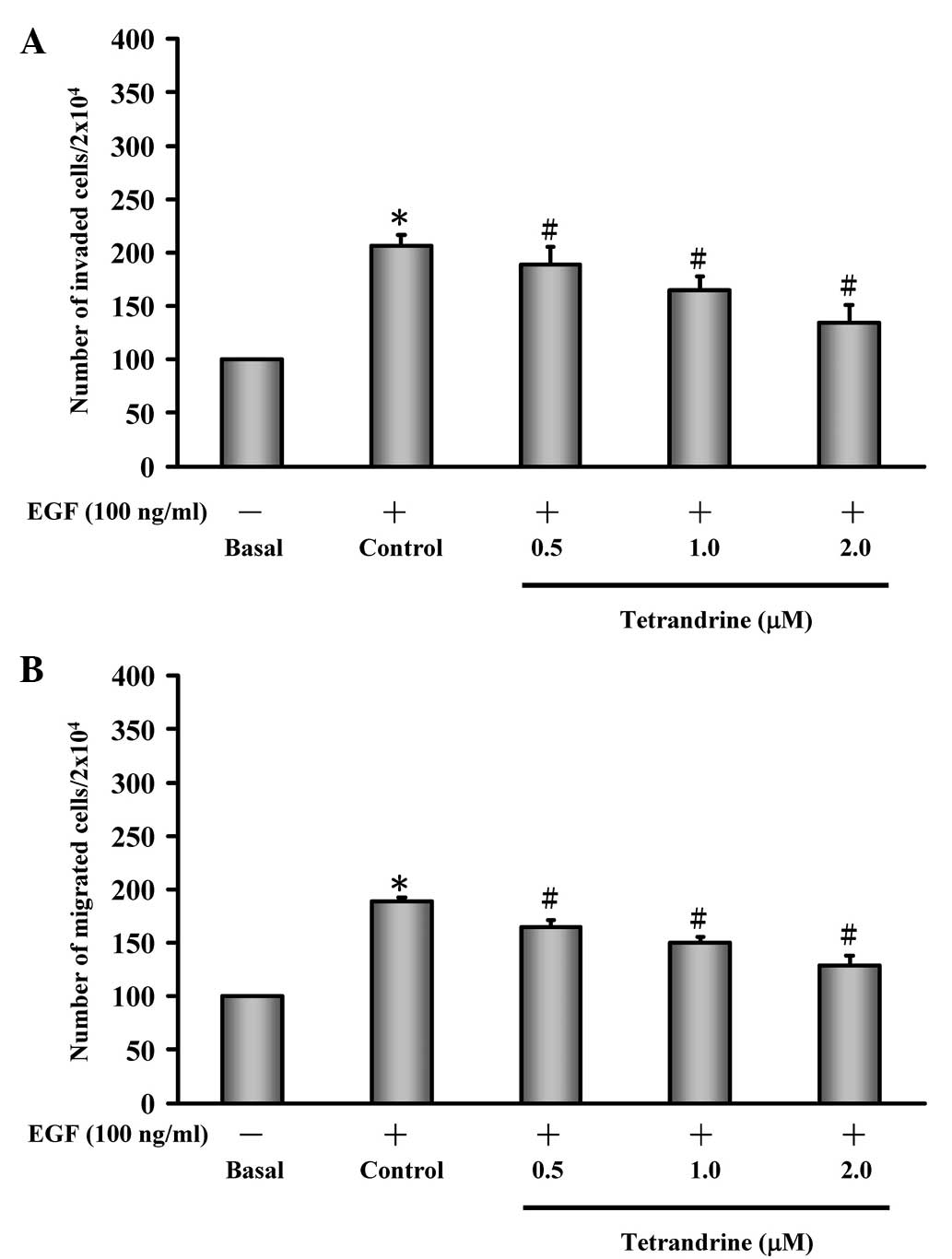
To determine the effects of tetrandrine on
EGF-induced HT29 cells, the abilities of cell invasion and
migration was investigated. EGF induction increased the invasion of
the HT29 cells, when compared with the untreated control cells
(basal), and treatment of the EGF-induced HT29 cells with
tetrandrine decreased cell invasion in a concentration-dependent
manner (Fig. 1A). In addition, EGF
stimulated HT29 cell migration, whereas tetrandrine decreased
EGF-induced migration of the HT29 cells in a
concentration-dependent manner (Fig.
1B).
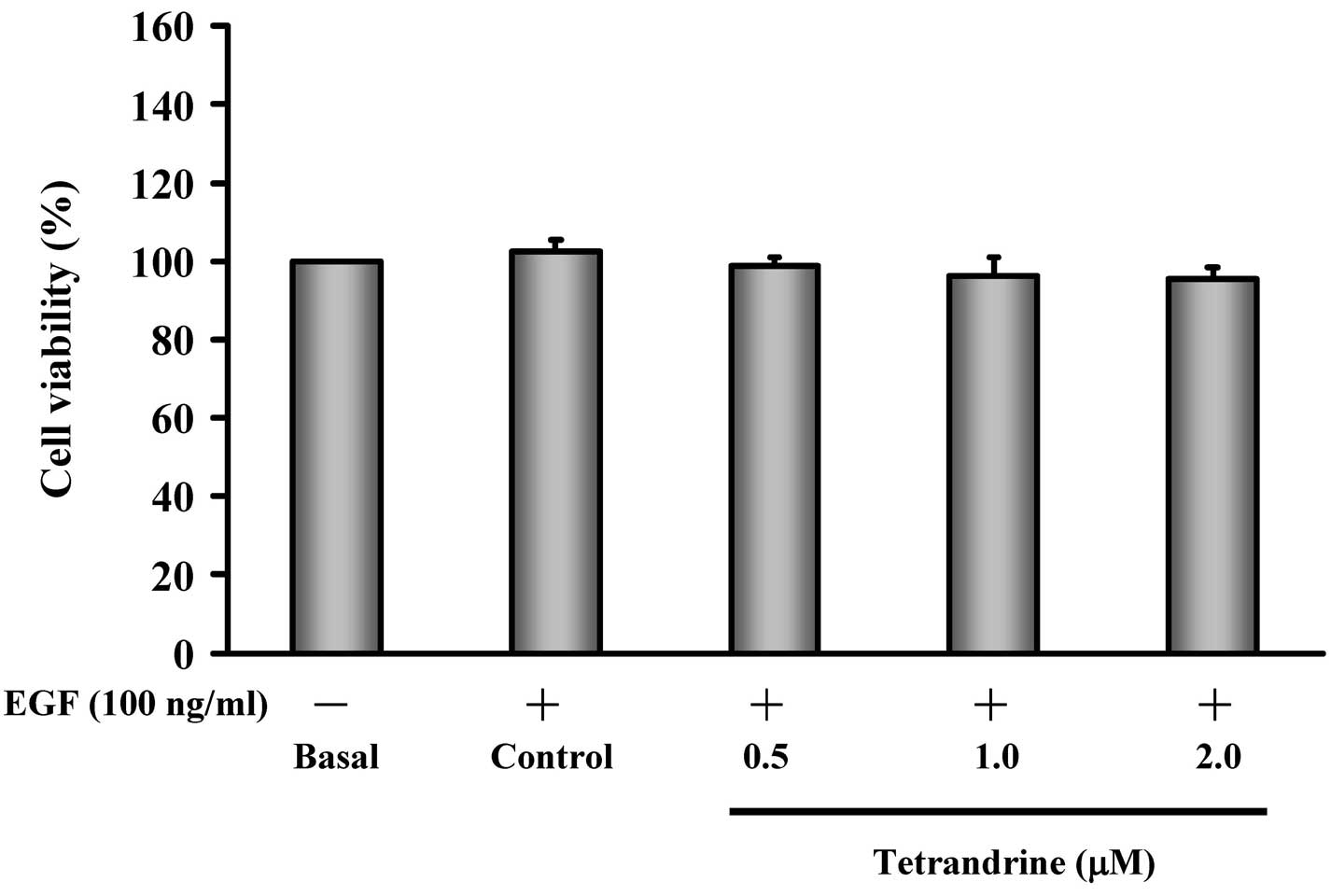
Exposure to low concentrations of
tetrandrine has no effect on the viability of EGF-induced HT29
cells
To determine whether the inhibited invasion and
migration of EGF-induced HT29 cells following exposure to
tetrandrine was the result of cytotoxic effects, the present study
assessed HT29 cell viability following tetrandrine exposure. The
EGF-induced HT29 cells were exposed to various concentrations (0,
0.5, 1 and 2 µM) of tetrandrine. The results demonstrated
that tetrandrine at 0.5–2 µM was not cytotoxic towards the
EGF-induced HT29 cells (Fig.
2).
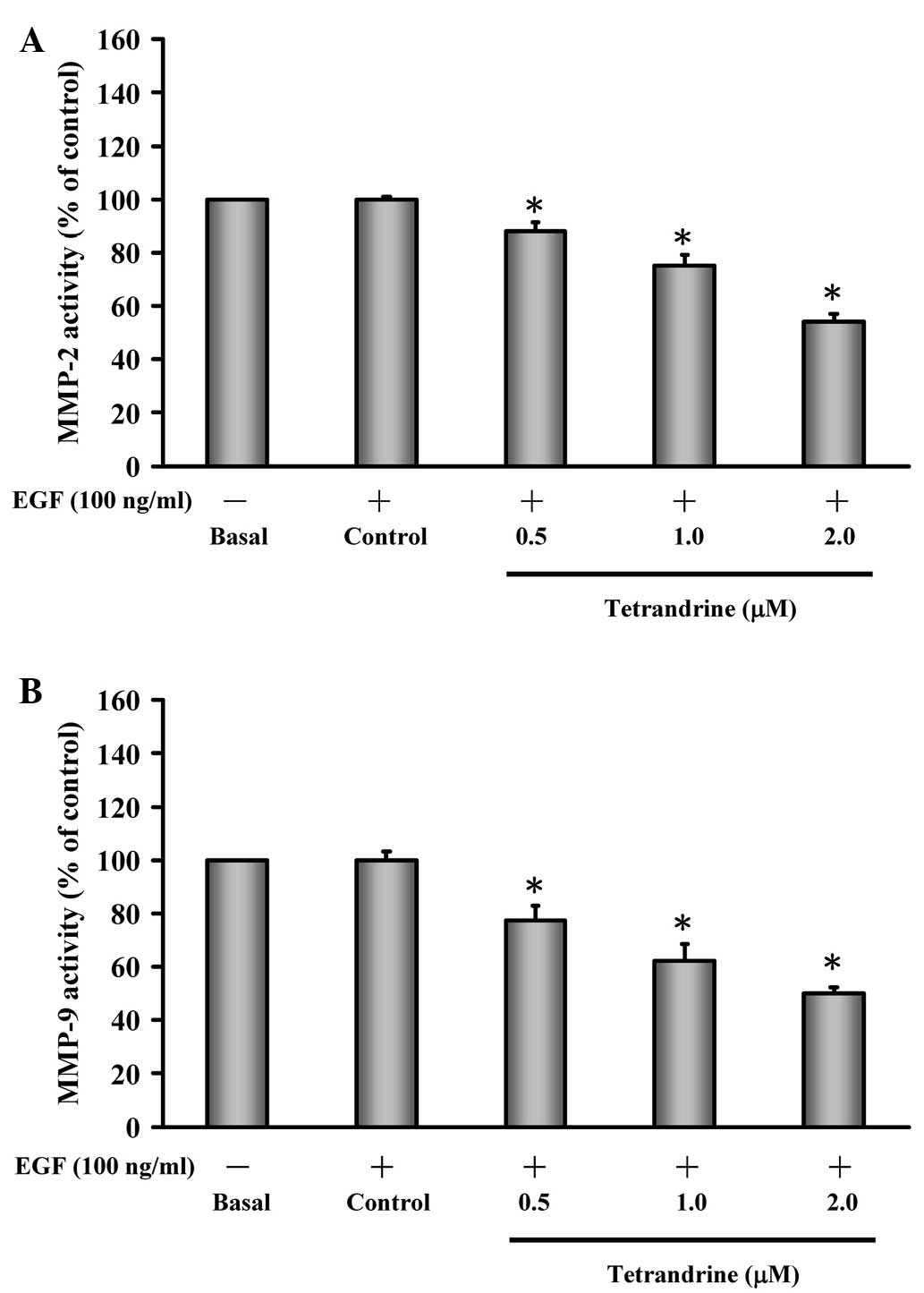
Tetrandrine inhibits the enzymatic
activities of MMP-2 and MMP-9 in EGF-induced HT29 cells
It has been documented that MMP-2 (gelatinase A) and
MMP-9 (gelatinase B) are detected in the invasion and metastasis of
colorectal cancer (39), which is
closely associated with the malignant potential of tumor invasion
and migration (40). Therefore, in
the present study, EGF-induced HT29 cells were treated with or
without tetrandrine (0.5, 1 and 2 µM) and the enzymatic
activities of MMP-2/-9 were assessed. Treatment of the EGF-induced
cells with tetrandrine reduced the gelatinase activity of MMP-2
(Fig. 3A) and MMP-9 (Fig. 3B), and these effects were
dose-dependent.
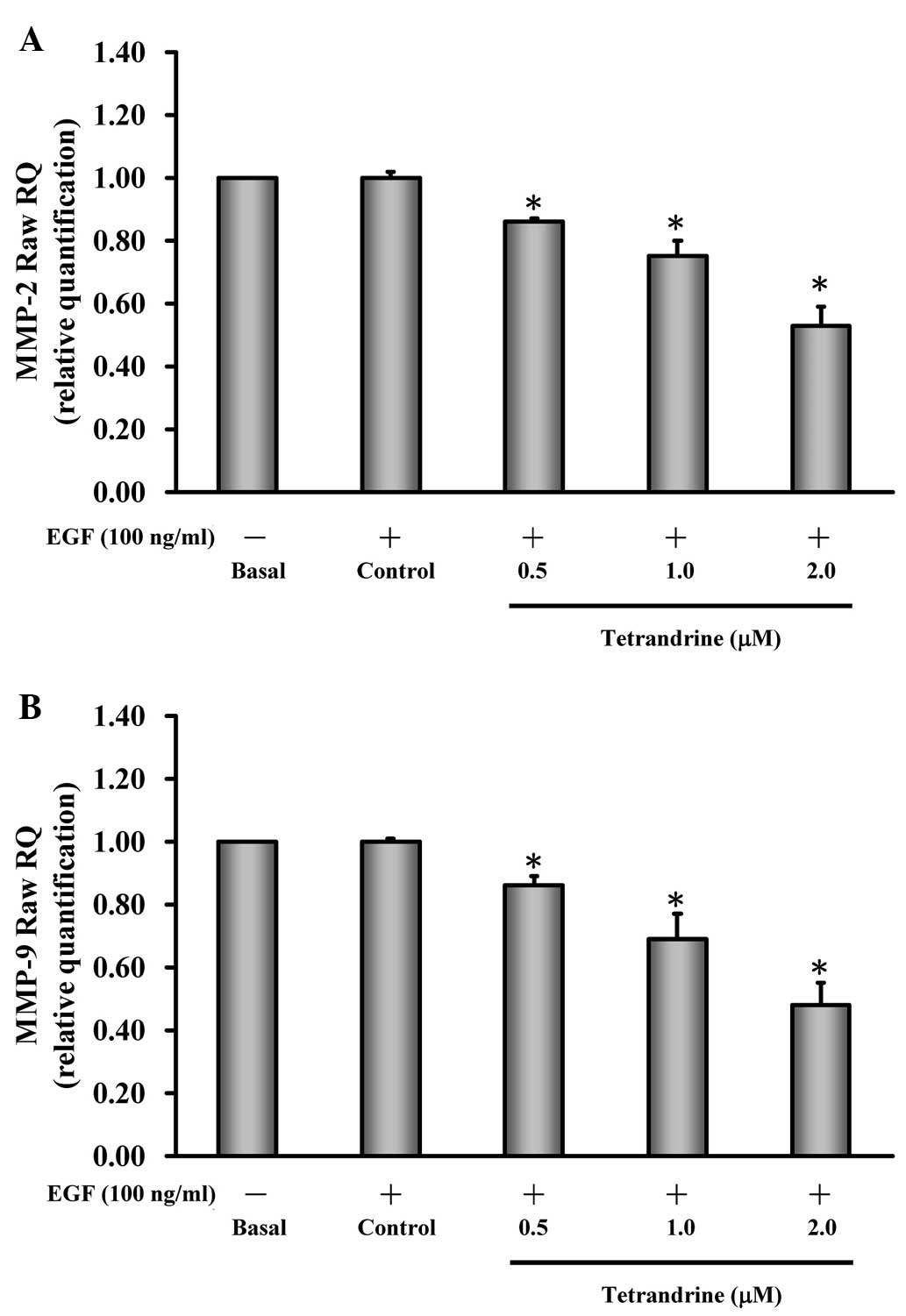
Tetrandrine reduces the gene expression
levels of MMP-2 and MMP-9 in EGF-induced HT29 cells
The present study further investigated whether the
suppression of MMP-2 and MMP-9 occurred at the transcriptional
level. Prior to EGF induction, the cells were incubated with or
without 0.5, 1 and 2 µM of tetrandrine, and the gene
expression levels of MMP-2 and MMP-9 were determined. The data
demonstrated that tetrandrine decreased the mRNA expression levels
of MMP-2 (Fig. 4A) and MMP-9
(Fig. 4B) in a dose-dependent
manner. Based on these findings, it was inferred that the two
gelatinases (MMP-2 and MMP-9) contributed to EGF-induced invasion
and anti-metastatic effect of HT29 cells.
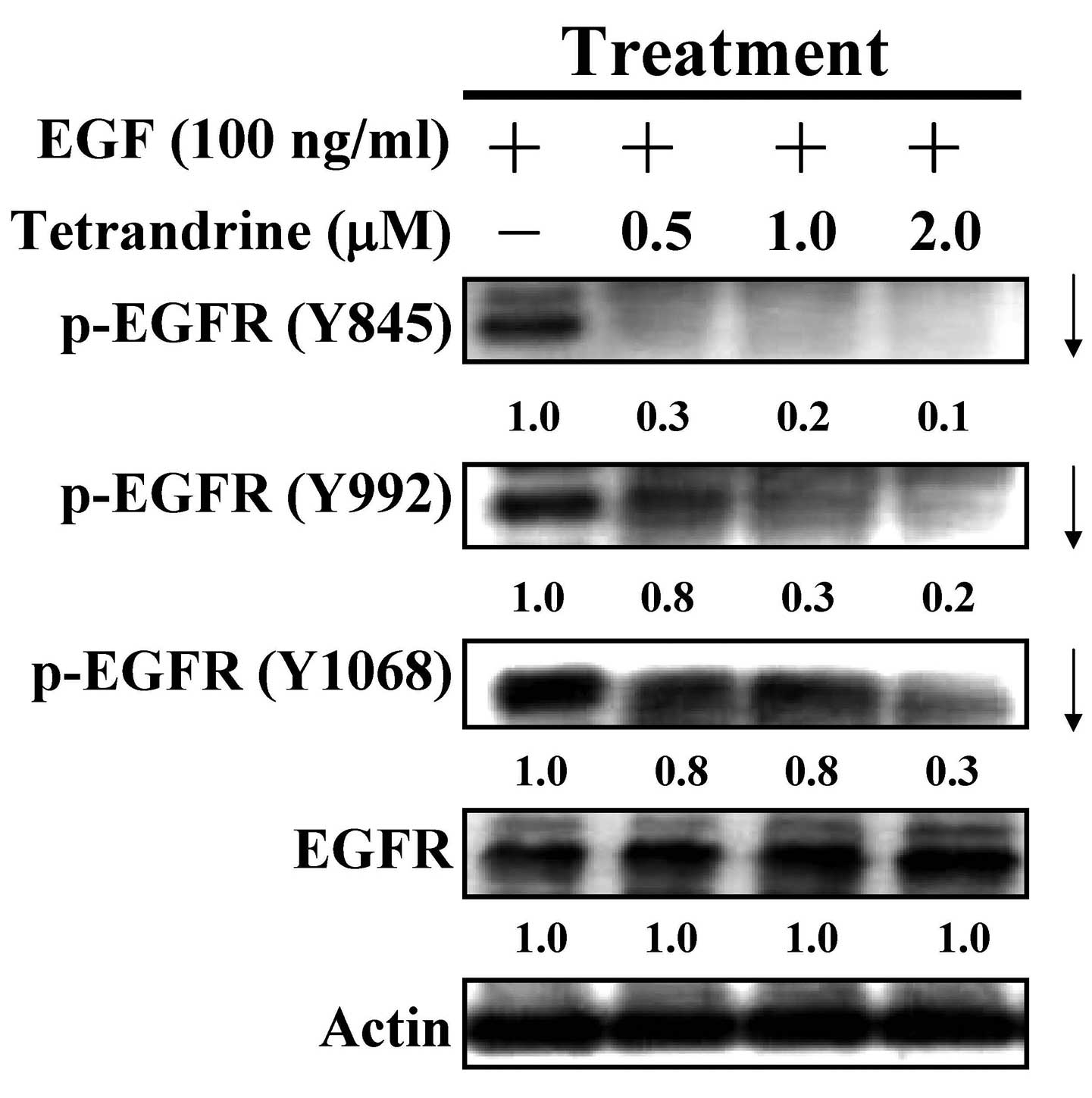
Tetrandrine inhibits the activation of
EGFR
It is well reported that the activation of EGF and
its cognate receptor, EGFR, can modulate cell proliferation,
survival, invasion and metastasis through activating the
autophosphorylation of EGFR and stimulating PI3K/AKT and MAPKs
signaling (2,3). In the present study, the effects of
tetrandrine on the activation (tyrosine phosphorylation) of EGFR
were examined in EGF-induced HT29 cells. Tetrandrine at 0.5, 1 and
2 µM led to dose-dependent attenuation of the tyrosine
phosphorylation of EGFR on the sites of Y845, Y992 and Y1068 in the
treated HT29 cells (Fig. 5). The
data indicated that tetrandrine inhibited the activation of EGFR in
EGF-induced HT29 cells.
Tetrandrine retards the phosphorylation
of PDK1, PI3K and AKT in EGF-induced HT29 cells
To understand the mechanism by which tetrandrine
alters the downstream signaling, the present study further examined
the PI3K/AKT pathway. The data demonstrated that tetrandrine (0.5,
1 and 2 µM) decreased the protein phosphorylation of PDK1
and PI3K (p85), and reduced the levels of p-AKT on S308 and S473 in
the EGF-induced cells (Fig. 6). No
effects on the protein levels of PI3K and AKT were observed
following tetrandrine challenge. These findings showed that
downregulation of EGFR activation caused by tetrandrine treatment
was mediated through PI3K/AKT signaling in the EGF-induced HT29
cells.
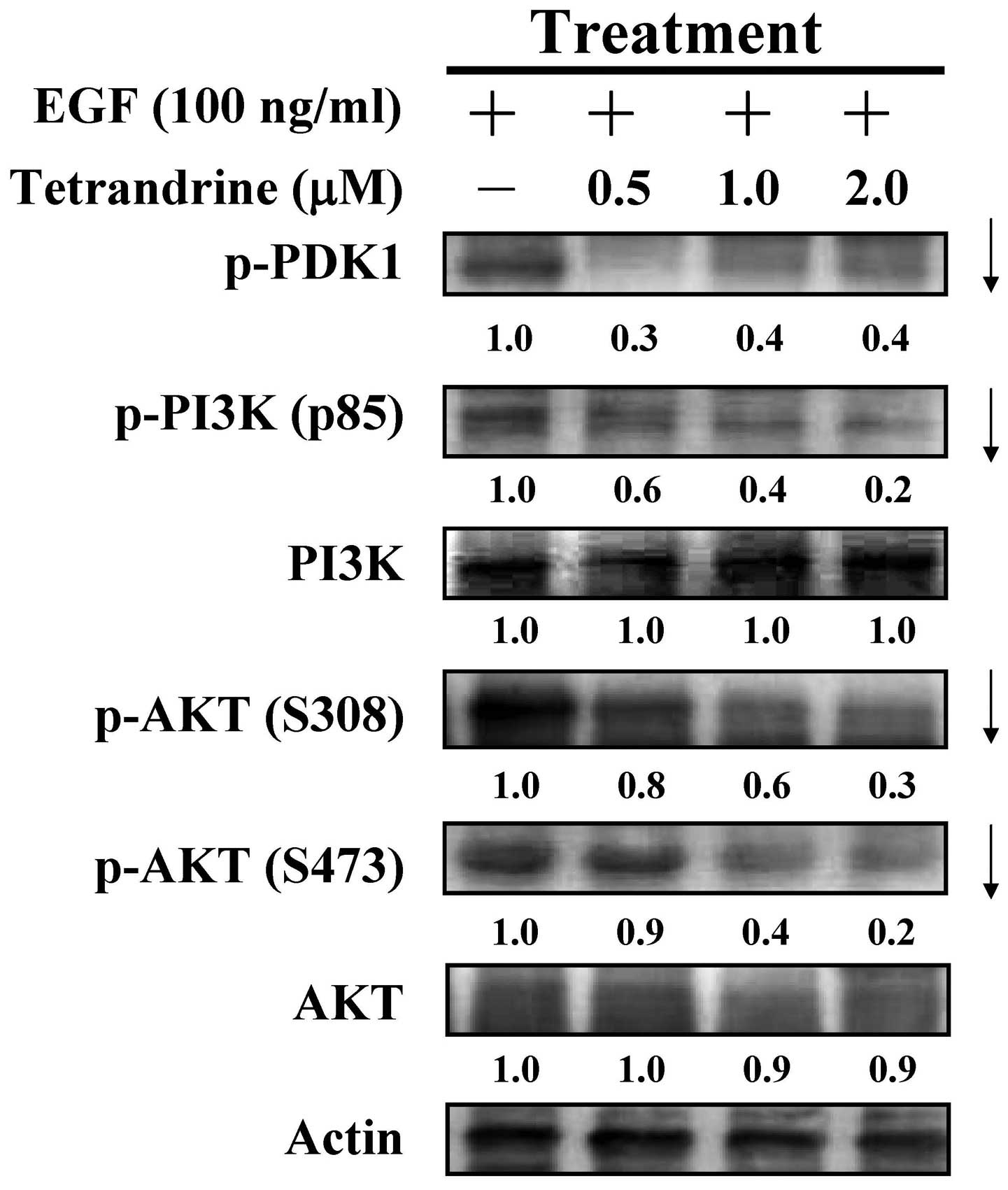 | Figure 6Effects of tetrandrine on the
PI3K/AKT pathway in EGF-stimulated HT29 cells. Following
stimulation with 100 ng/ml EGF, the cells were treated with 0.5, 1
and 2 µM of tetrandrine for 48 h, and cell lysates were
subjected to western blotting for detection of the protein levels
of p-PDK1, p-PI3K (p85), PI3K, p-AKT (S308), p-AKT (S473) and AKT.
Each band was normalized to Actin. EGF, epidermal growth factor;
PI3K, phosphatidylinositol 3-kinase; PDK1,
phosphoinositide-dependent kinase 1; p-, phosphorylated. |
Tetrandrine affects the MAPK/ERK pathway
in EGF-induced HT29 cells
In an attempt to determine the effects of
tetrandrine on downstream of EGFR activation, the MAPK (p38, JNK
and ERK) pathways were examined in the tetrandrine-exposed cells.
Tetrandrine at 0.5, 1 and 2 µM reduced the phosphorylation
of ERK. However, no effects were observed on the protein expression
levels of the p38, JNK and ERK of the MAPK signaling (Fig. 7), indicating that the MAPK/ERK
signaling pathway was suppressed by tetrandrine in the EGF-induced
HT29 cells.
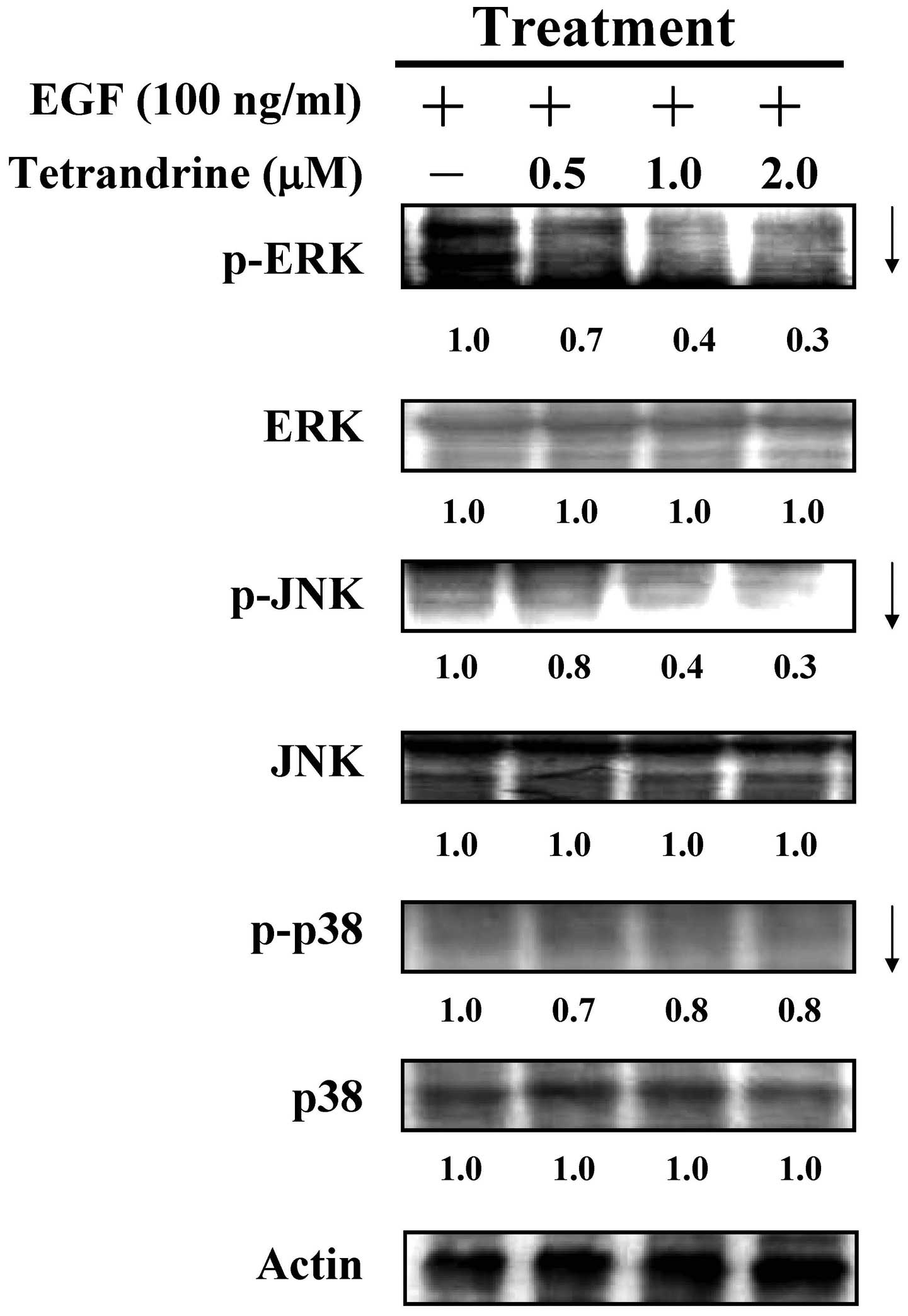 | Figure 7Effects of tetrandrine on the MAPK
pathway in HT29 cells following EGF-induction. The cells, which
were pretreated with or without 100 ng/ml EGF, were treated with
0.5, 1 and 2 µM of tetrandrine for 48 h and lysed prior to
immunoblotting. The whole-cell lysates were examined to determine
the expression levels of p-ERK, ERK, p-JNK, JNK, p-p38 and p38.
Actin served as an internal control. EGF, epidermal growth factor;
MAPK, mitogen-activated protein kinase; JNK, c-Jun N-terminal
kinase; p-, phosphorylated. |
Discussion
Colorectal cancer is the third most common cause of
cancer-associated mortality (4,5), and
selectively targeting the tumor offers a novel strategy for
developing novel colorectal anticancer agents (15), as the treatment for colorectal
cancer remains unsatisfactory. It has been shown that tumor cell
apoptosis caused by tetrandrine exhibits inhibitory effects on
various types of tumor cell (24–29).
The results of the present study indicated that tetrandrine had no
cytotoxic effect on the HT29 cells, however, the anti-metastatic
effect of tetrandrine on EGF-induced HT29 cancer cells was
observed, and the underlying molecular signaling was evaluated.
Tetrandrine treatment exerted an inhibitory effect of HT29 cell
migration and invasion in the EGF-induced HT29 cells (Fig. 1). Based on these findings, the
present study is the first, to the best of our knowledge, to report
the effects of tetrandrine on human colorectal cancer cells.
A study by Tsai et al reported that the
dysregulation of EGFR was associated with colorectal cancer in
Taiwan (41). In addition, the
expression levels of EGFR, HER2 and HER3 were determined in primary
tumors of colorectal cancer cells, and corresponded with lymph node
metastases and liver metastases (42,43).
The dysregulation of human EGFR pathways by the overexpression or
constitutive activation promote s tumor processes, angiogenesis and
metastasis in several types of human cancer (4–13).
Previous studies have demonstrated that tetrandrine inhibits cell
metastasis in 4T1 breast cancer cells (18) and CT26 colorectal cancer cells
in vivo (25). In the
latter, tetrandrine-treated BALB/c mice were found to exhibit fewer
metastases, compared with vehicle-treated mice, and no acute
toxicity or marked changes in body weight were observed (25). The results of the present study
indicated that the EGF-induced invasion of HT29 cells was
suppressed by tetrandrine through the inactivation of EGFR and
downstream molecules, including suppression of the phosphorylation
cascade of PI3K, PDK1 and AKT, and the reduction of p-ERK, which
was in agreement with a previous study on lung cancer cells
(44).
MMP-2 and MMP-9 are responsible for degradation of
the extracellular matrix and facilitating the spread and metastasis
of tumor cells in colorectal cancer (39,40).
In the present study, tetrandrine suppressed the activities and
mRNA expression levels of MMP-2 and MMP-9 in the EGF-induced HT29
cells (Figs. 3 and 4). Therefore, tetrandrine retarded the
metastatic effects of EGFR-overexpressed HT29 cells by reducing
MMP-2 and MMP-9, and the phosphorylation of EGFR.
The MAPK pathway is a major downstream signaling
regulated by EGFR (45). The
present study also demonstrated that the levels of p-ERK decreased
following tetrandrine treatment in the HT29 cells, which is
contradictory to a previous report (46). It has been reported that
tetrandrine induces cell autophagy through the activation of MAPK
(47). However, the in
vitro system in the present study presented the possibility of
tetrandrine-triggered autophagy in addition to the suppression of
invasion and migration. The data of the present study demonstrated
that tetrandrine had inhibitory effects on invasion and mobility in
EGF-induced HT29 cells at 1–2 µM (Fig. 1). This evidence suggests that
tetrandrine repressed the invasion and metastasis of HT29 cells by
activating MAPK and downstream signaling to drive the expression of
specific genes. However, it is necessary to be elucidated for the
further detailed mechanism in tetrandrine-treated colon cancer
cells in vitro.
Taken together, the present study demonstrated that
tetrandrine is a promising chemotherapeutic agent with
anti-metastatic effects, including the inhibition of migration and
invasion, in HT29 human colorectal cancer cells. These findings
suggest that tetrandrine may be a potential candidate for the
treatment of human colorectal cancer.
References
|
1
|
Segatto O, Anastasi S and Alemá S:
Regulation of epidermal growth factor receptor signalling by
inducible feedback inhibitors. J Cell Sci. 124:1785–1793. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lemmon MA, Schlessinger J and Ferguson KM:
The EGFR family: Not so prototypical receptor tyrosine kinases.
Cold Spring Harb Perspect Biol. 6:a0207682014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gibson S, Tu S, Oyer R, Anderson SM and
Johnson GL: Epidermal growth factor protects epithelial cells
against fas-induced apoptosis. Requirement for akt activation. J
Biol Chem. 274:17612–17618. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Augustine TA, Baig M, Sood A, Budagov T,
Atzmon G, Mariadason JM, Aparo S, Maitra R and Goel S: Telomere
length is a novel predictive biomarker of sensitivity to anti-EGFR
therapy in metastatic colorectal cancer. Br J Cancer. 112:313–318.
2015. View Article : Google Scholar
|
|
5
|
Möller Y, Siegemund M, Beyes S, Herr R,
Lecis D, Delia D, Kontermann R, Brummer T, Pfizenmaier K and
Olayioye MA: GFR-targeted TRAIL and a Smac mimetic synergize to
overcome apoptosis resistance in KRAS mutant colorectal cancer
cells. PLoS One. 9:e1071652014. View Article : Google Scholar
|
|
6
|
Umeguchi H, Sueoka-Aragane N, Kobayashi N,
Nakamura T, Sato A, Takeda Y, Hayashi S, Sueoka E and Kimura S:
Usefulness of plasma HGF level for monitoring acquired resistance
to EGFR tyrosine kinase inhibitors in non-small cell lung cancer.
Oncol Rep. 33:391–396. 2015.
|
|
7
|
Iommelli F, De Rosa V, Gargiulo S, Panico
M, Monti M, Greco A, Gramanzini M, Ortosecco G, Fonti R, Brunetti A
and Del Vecchio S: Monitoring reversal of MET-mediated resistance
to EGFR tyrosine kinase inhibitors in non-small cell lung cancer
using 3′-deoxy-3′-[18F]-fluorothymidine positron emission
tomography. Clin Cancer Res. 20:4806–4815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Madden JM, Mueller KL, Bollig-Fischer A,
Stemmer P, Mattingly RR and Boerner JL: Abrogating phosphorylation
of eIF4B is required for EGFR and mTOR inhibitor synergy in
triple-negative breast cancer. Breast Cancer Res Treat.
147:283–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin Y, Han B, Chen J, Wiedemeyer R,
Orsulic S, Bose S, Zhang X, Karlan BY, Giuliano AE, Cui Y and Cui
X: FOXC1 is a critical mediator of EGFR function in human
basal-like breast cancer. Ann Surg Oncol. 21(Suppl 4): S758–S766.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fung C, Zhou P, Joyce S, Trent K, Yuan JM,
Grandis JR, Weissfeld JL, Romkes M, Weeks DE and Egloff AM:
Identification of epidermal growth factor receptor (EGFR) genetic
variants that modify risk for head and neck squamous cell
carcinoma. Cancer Lett. 357:549–556. 2015. View Article : Google Scholar
|
|
11
|
Yoshikawa M, Tsuchihashi K, Ishimoto T,
Yae T, Motohara T, Sugihara E, Onishi N, Masuko T, Yoshizawa K,
Kawashiri S, et al: xCT inhibition depletes CD44v-expressing tumor
cells that are resistant to EGFR-targeted therapy in head and neck
squamous cell carcinoma. Cancer Res. 73:1855–1866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan Y, Zheng M, Zhong L, Yang J, Zhou S,
Qin Y, Xiang R, Chen Y and Yang SY: A preclinical evaluation of
SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a
therapeutic agent against pancreatic cancer. Mol Cancer Ther.
14:407–418. 2015. View Article : Google Scholar
|
|
13
|
Mak KS, Gainor JF, Niemierko A, Oh KS,
Willers H, Choi NC, Loeffler JS, Sequist LV, Shaw AT and Shih HA:
Significance of targeted therapy and genetic alterations in EGFR,
ALK, or KRAS on survival in patients with non-small cell lung
cancer treated with radiotherapy for brain metastases. Neuro Oncol.
17:296–302. 2015. View Article : Google Scholar
|
|
14
|
Wang X, Zuo D, Chen Y, Li W, Liu R, He Y,
Ren L, Zhou L, Deng T, Wang X, et al: Shed syndecan-1 is involved
in chemotherapy resistance via the EGFR pathway in colorectal
cancer. Br J Cancer. 111:1965–1976. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Loupakis F, Cremolini C, Fioravanti A,
Orlandi P, Salvatore L, Masi G, Schirripa M, Di Desidero T,
Antoniotti C, Canu B, et al: EGFR ligands as pharmacodynamic
biomarkers in metastatic colorectal cancer patients treated with
cetuximab and irinotecan. Target Oncol. 9:205–214. 2014. View Article : Google Scholar
|
|
16
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar
|
|
17
|
Miyagawa S, Katsu Y, Watanabe H and Iguchi
T: Estrogen-independent activation of erbBs signaling and estrogen
receptor alpha in the mouse vagina exposed neonatally to
diethylstilbestrol. Oncogene. 23:340–349. 2004. View Article : Google Scholar
|
|
18
|
Gao JL, Ji X, He TC, Zhang Q, He K, Zhao
Y, Chen SH and Lv GY: Tetrandrine suppresses cancer angiogenesis
and metastasis in 4T1 tumor bearing mice. Evid Based Complement
Alternat Med. 2013:2650612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mei L, Chen Y, Wang Z, Wang J, Wan J, Yu
C, Liu X and Li W: Synergistic antitumor effects of tetrandrine and
chloroquine combination therapy in human cancer: A potential
antagonistic role for p21. Br J Pharmacol. 172:2232–2245. 2015.
View Article : Google Scholar
|
|
20
|
Zhao H, Luo F, Li H, Zhang L, Yi Y and Wan
J: Antinociceptive effect of tetrandrine on LPS-induced
hyperalgesia via the inhibition of IKKβ phosphorylation and the
COX-2/PGE2 pathway in mice. PLoS One. 9:e945862014.
View Article : Google Scholar
|
|
21
|
Chang DM, Kuo SY, Lai JH and Chang ML:
Effects of anti-rheumatic herbal medicines on cellular adhesion
molecules. Ann Rheum Dis. 58:366–371. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Yu B, Zhang XQ, Sheng ZF, Li SJ,
Wang ZJ, Cui XY, Cui SY and Zhang YH: Tetrandrine, an
antihypertensive alkaloid, improves the sleep state of
spontaneously hypertensive rats (SHRs). J Ethnopharmacol.
151:729–732. 2014. View Article : Google Scholar
|
|
23
|
Yoo SM, Oh SH, Lee SJ, Lee BW, Ko WG, Moon
CK and Lee BH: Inhibition of proliferation and induction of
apoptosis by tetrandrine in HepG2 cells. J Ethnopharmacol.
81:225–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
25
|
Chang KH, Liao HF, Chang HH, Chen YY, Yu
MC, Chou CJ and Chen YJ: Inhibitory effect of tetrandrine on
pulmonary metastases in CT26 colorectal adenocarcinoma-bearing
BALB/c mice. Am J Chin Med. 32:863–872. 2004. View Article : Google Scholar
|
|
26
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar
|
|
27
|
Liu W, Zhang J, Ying C, Wang Q, Yan C,
Jingyue Y, Zhaocai Y, Yan X, Heng-Jun S and Lin J: Tetrandrine
combined with gemcitabine and cisplatin for patients with advanced
non-small cell lung cancer improve efficacy. Int J Biomed Sci.
8:28–35. 2012.PubMed/NCBI
|
|
28
|
Chen B, Yin L, Cheng J, Ding J, Gao C, Sun
Y, Zhao G, Wang J, Bao W, Xia G, et al: Effect of D,
L-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol and
tetrandrine on the reversion of multidrug resistance in K562/A02
cells. Hematology. 16:24–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wan J, Liu T, Mei L, Li J, Gong K, Yu C
and Li W: Synergistic antitumour activity of sorafenib in
combination with tetrandrine is mediated by reactive oxygen species
(ROS)/akt signaling. Br J Cancer. 109:342–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu C, Gong K, Mao X and Li W: Tetrandrine
induces apoptosis by activating reactive oxygen species and
repressing akt activity in human hepatocellular carcinoma. Int J
Cancer. 129:1519–1531. 2011. View Article : Google Scholar
|
|
31
|
Huang AC, Lien JC, Lin MW, Yang JS, Wu PP,
Chang SJ and Lai TY: Tetrandrine induces cell death in SAS human
oral cancer cells through caspase activation-dependent apoptosis
and LC3-I and LC3-II activation-dependent autophagy. Int J Oncol.
43:485–494. 2013.PubMed/NCBI
|
|
32
|
Wu Z, Wang G, Xu S, Li Y, Tian Y, Niu H,
Yuan F, Zhou F, Hao Z, Zheng Y, et al: Effects of tetrandrine on
glioma cell malignant phenotype via inhibition of ADAM17. Tumour
Biol. 35:2205–2210. 2014. View Article : Google Scholar
|
|
33
|
Lai KC, Lu CC, Tang YJ, Chiang JH, Kuo DH,
Chen FA, Chen IL and Yang JS: Allyl isothiocyanate inhibits cell
metastasis through suppression of the MAPK pathways in epidermal
growth factor-stimulated HT29 human colorectal adenocarcinoma
cells. Oncol Rep. 31:189–196. 2014.
|
|
34
|
Chen YY, Chiang SY, Lin JG, Ma YS, Liao
CL, Weng SW, Lai TY and Chung JG: Emodin, aloe-emodin and rhein
inhibit migration and invasion in human tongue cancer SCC-4 cells
through the inhibition of gene expression of matrix
metalloproteinase-9. Int J Oncol. 36:1113–1120. 2010.PubMed/NCBI
|
|
35
|
Lu CC, Yang JS, Chiang JH, Hour MJ,
Amagaya S, Lu KW, Lin JP, Tang NY, Lee TH and Chung JG: Inhibition
of invasion and migration by newly synthesized quinazolinone MJ-29
in human oral cancer CAL 27 cells through suppression of MMP-2/9
expression and combined down-regulation of MAPK and aKT signaling.
Anticancer Res. 32:2895–2903. 2012.PubMed/NCBI
|
|
36
|
Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya
S, Lin YC and Yang JS: Phenethyl isothiocyanate suppresses
EGF-stimulated SAS human oral squamous carcinoma cell invasion by
targeting EGF receptor signaling. Int J Oncol. 43:629–637.
2013.PubMed/NCBI
|
|
37
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840.2310–2320. 2014.
|
|
38
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peng ZH, Wan DS, Li LR, Chen G, Lu ZH, Wu
XJ, Kong LH and Pan ZZ: Expression of COX-2, MMP-2 and VEGF in
stage II and III colorectal cancer and the clinical significance.
Hepatogastroenterology. 58:369–376. 2011.PubMed/NCBI
|
|
40
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tsai WC, Lin CK, Lee HS, Chen A, Nieh S,
Yu CP, Wu CC, Jao SW and Jin JS: Discordance between EGFR
expression and clinicopathologic parameters of colorectal
adenocarcinoma in Taiwan. Chin J Physiol. 55:352–360. 2012.
View Article : Google Scholar
|
|
42
|
Wei Q, Shui Y, Zheng S, Wester K, Nordgren
H, Nygren P, Glimelius B and Carlsson J: EGFR, HER2 and HER3
expression in primary colorectal carcinomas and corresponding
metastases: Implications for targeted radionuclide therapy. Oncol
Rep. 25:3–11. 2011.
|
|
43
|
Rigopoulos DN, Tsiambas E, Lazaris AC,
Kavantzas N, Papazachariou I, Kravvaritis C, Tsounis D, Koliopoulou
A, Athanasiou AE, Karameris A, et al: Deregulation of
EGFR/VEGF/HIF-1a signaling pathway in colon adenocarcinoma based on
tissue microarrays analysis. J BUON. 15:107–115. 2010.PubMed/NCBI
|
|
44
|
Wang Y, Liu W and Lin H: Effect and
significance of tetrandrine on epidermal growth factor and its
receptor in the lung of congenital diaphragmatic hernia rat model.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 20:1109–1113. 2006.In
Chinese. PubMed/NCBI
|
|
45
|
Nyati MK, Morgan MA, Feng FY and Lawrence
TS: Integration of EGFR inhibitors with radiochemotherapy. Nat Rev
Cancer. 6:876–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dang Y, Xu Y, Wu W, Li W, Sun Y, Yang J,
Zhu Y and Zhang C: Tetrandrine suppresses
lipopolysaccharide-induced microglial activation by inhibiting
NF-κB and ERK signaling pathways in BV2 cells. PLoS One.
9:e1025222014. View Article : Google Scholar
|
|
47
|
Gong K, Chen C, Zhan Y, Chen Y, Huang Z
and Li W: Autophagy-related gene 7 (ATG7) and reactive oxygen
species/extracellular signal-regulated kinase regulate
tetrandrine-induced autophagy in human hepatocellular carcinoma. J
Biol Chem. 287:35576–35588. 2012. View Article : Google Scholar : PubMed/NCBI
|