Introduction
Atherosclerosis is now recognized as a chronic
inflammatory disease of the vascular wall (1). Cell-cell and cell-matrix adhesion
have important roles in the formation of atherosclerotic lesions
(2). As the disease progresses,
vascular smooth muscle cells (VSMCs) undergo phenotypic
transformation and become activated to secrete pro-inflammatory
cytokines and monocyte chemoattractant protein 1, and express cell
adhesion molecules that promote leukocyte recruitment, migration
and differentiation (3).
Accumulating evidence implied that vascular cell adhesion
molecule-1 (VCAM-1) is upregulated in VSMCs of atherosclerotic
lesions (4–6). Furthermore, in cultured VSMCs,
interleukin-1 (IL-1) and tumor necrosis factor (TNF-α) induced
VCAM-1 and intercellular adhesion molecule-1 (ICAM-1) expression as
well as monocyte adhesion to VSMCs (7,8).
Therefore, preventing the expression of these adhesion molecules on
VSMCs may be a promising therapeutic approach for
atherosclerosis.
Compelling evidence has revealed that certain
natural products, particularly those from medicinal plants, may
represent an ideal source to develop safe and effective agents for
the management of atherosclerosis. Stereocalpin A, an active
component of the Antarctic lichen Ramalina terebarata,
prevented the induction of the expression of adhesion molecules in
a concentration-dependent manner after stimulation with an
inflammatory cytokine (9).
Sulforaphane, a compound naturally occurring in Brassica
oleracea var. italica (broccoli) and numerous other cruciferous
vegetables, was also shown to inhibit the expression of
TNF-α-induced adhesion molecules in VSMCs (10).
Matrine is a major active component of Sophora
flavescens roots, which are used to treat inflammatory
diseases, including enteritis and hepatitis (11,12).
Besides its anti-inflammatory activity, matrine has been shown to
affect the cardiovascular system. A previous study reported that
matrine inhibits VSMC proliferation via upregulation of the p53/p21
signaling pathway (13). However,
the effects of matrine on the expression of adhesion molecules in
VSMCs have remained elusive. The purpose of the present study was
to investigate the effects of matrine on adhesion molecule
accumulation in TNF-α-stimulated human aortic smooth muscle cells
(HASMCs) as well as the underlying mechanisms of action. The
results demonstrated that matrine suppressed TNF-α-induced adhesion
molecule expression through the inhibition of mitogen-activated
protein kinase (MAPK) and nuclear factor (NF)-κB signaling pathways
and intracellular reactive oxygen species (ROS) production in
HASMCs.
Materials and methods
Materials
Matrine (purity, >99%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). Dulbecco's modified Eagle's medium,
fetal bovine serum (FBS) and Lipofectamine Plus were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
pGL3-NF-κB vector and the luciferase assay system were provided by
Promega (Madison, WI, USA), and the pCMV-β-gal vector was obtained
from Lonza (Walkersville, MD, USA). Antibodies against ICAM-1
(mouse monoclonal) and VCAM-1 (mouse monoclonal) were purchased
from R&D Systems, Inc. (Minneapolis, MN, USA) and antibodies
against the inhibitor of NF-κB (IκB-α; rabbit polyclonal), p65
(rabbit polyclonal), c-Jun N-terminal kinase (JNK; rabbit
polyclonal), phospho-JNK (p-JNK; rabbit polyclonal), extra-cellular
signal-regulated kinase (ERK; rabbit monoclonal), p-ERK (rabbit
polyclonal), p38 (rabbit polyclonal), p-p38 (rabbit polyclonal),
Akt, p-Akt, lamin A (rabbit polyclonal) and β-actin (rabbit
polyclonal) were purchased from Abcam Inc. (Cambridge, MA, USA).
PCR primers and PCR premix were purchased from Bioneer Corporation
(Daejeon, South Korea). phosphate-buffered saline (PBS),
tris-buffered saline (TBS) and Tween 20 were purchased from
Sigma-Aldrich. (St. Louis, MO, USA).
Cell culture
HASMCs were purchased from Clonetics Corp. (San
Diego, CA, USA) and cultured in DMEM medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 100 IU/ml penicillin, 100 mg/ml
streptomycin, and 5% FBS (Invitrogen; Thermo Fisher Scientific,
Inc.) with 2 ng/ml basic fibroblast growth factor, 10 ng/ml
recombinant human epidermal growth factor and 5 µg/ml
insulin (all from Sigma-Aldrich).
Cell viability assay
The effects of matrine on the proliferation of
HASMCs were determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. HASMCs were seeded onto 96-well plates (1×104
cells/well) and subsequently treated with various concentrations of
matrine (0, 10, 50 and 100 µg/ml). Following 72 h of
incubation, MTT (0.5 mg/ml; Sigma-Aldrich) was added to each well.
After incubation for 4 h, the supernatant was removed and 0.1%
dimethylsulfoxide (Sigma-Aldrich) was added to dissolve the
formazan crystals. The absorbance at 550 nm was measured using a
microplate reader 3350 (Bio-Rad Laboratories, Hercules, CA, USA)
and the percentage of viable cells compared with that in the
control group was calculated.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
RNA extraction was performed with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Subsequently, RNA was
reverse-transcribed in a 20-ml reaction system using an Advantage
RT kit (Clontech Laboratories, Inc., Palo Alto, CA, USA) according
to the supplier's recommended protocol. mRNA levels were quantified
by RT-qPCR using SYBRGreen Master Mix (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using a 30 ng template in a 20 µl
reaction mixture. For PCR amplification, the following primers were
used: VCAM-1 forward, 5′-CAA AGG TGG ATC AGA TTC AAG-3′ and
reverse, 5;-GGT GAG CAT TAT CAC CCA GAA-3′; ICAM-1 forward, 5′-CAA
AGG TGG ATC AGA TTC AAG-3′ and reverse, 5;-GGT GAG CAT TAT CAC CCA
GAA-3′; GAPDH forward, 5′-CAA AGG TGG ATC AGA TTC AAG-3′ and
reverse, 5;-GGT GAG CAT TAT CAC CCA GAA-3′. The PCR cycling program
was 95°C for 3 min, followed by 28 cycles of 95°C for 20 sec, 60°C
for 20 sec and 72°C for 15 sec, and a final extension at 72°C for 5
min. The levels of individual gene mRNA transcripts were initially
normalized to the control β-actin. Subsequently, the differential
expression of these genes was analyzed using the 2−ΔΔCq
method (14).
Western blot analysis
HVSMCs were cultured and treated with matrine as
described above and subsequently subjected to western blot
analysis. After treatment, the cells were washed twice with PBS and
suspended in 70 µl of Buffer A [10 mM HEPES (pH 7.9), 1.5 mM
MgCl2, 10 mM KCl, 0.5 mM DTT, 0.5 mM PMSF and Protease
Inhibitor Cocktail (Sigma-Aldrich)] and incubated on ice. After 15
min, 0.5% Nonidet P (NP)-40 was added to lyse the cells, which were
vortexed for 1 sec. Then, cytosolic cell extracts were obtained
after centrifuging at 1500 × g for 10 min at 4°C. The collected
nuclei were resuspended in 50 µl of Buffer C [20 mM HEPES
(pH 7.9), 1.5 mM MgCl2, 420 mM NaCl, 0.2 mM EDTA, 25%
v/v glycerol, 0.5 mM PMSF and Protease Inhibitor Cocktail] and
incubated on ice for 20 min with intermittent agitation. Nuclear
cell extracts were recovered after centrifugation for 10 min at
13,000 × g at 4°C. The protein concentration in the cell extracts
was then determined using the Bradford protein dye reagent (Bio-Rad
Laboratories, Inc.). Proteins (30 µg/lane) were separated on
10% sodium dodecyl sulfate polyacrylamide gels (Bio-Rad
Laboratories) and transferred onto polyvinylidene difluoride
membranes (Millipore, Billerica, MA, USA). Membranes were blocked
with 5% non-fat milk in TBST buffer (50 mM Tris, pH 7.5, 250 mM
NaCl, 0.1% Tween 20) and incubated with the appropriate primary
antibodies overnight at 4°C. Subsequent to washing the membranes
three times with phosphate-buffered saline, 5 min per wash,
containing 0.1% (v/v) Tween 20, membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies
(MyBioSource, Inc., San Diego, CA, USA) for 1 h, followed by
visualization of the antibodies using enhanced chemiluminescence
detection reagents. The blots were developed using an enhanced
chemiluminescence kit (GE Healthcare Life Sciences, Chalfont, UK).
Semi-quantitative determination of protein levels was performed
using Image-Pro Plus software (NIH Image J 1.61; Media Cybernetics,
Rockville, MD, USA).
Transfection and reporter assays
The cells (1×105 cells/ml) were plated
into each well of a 6-well plate. The cells were transiently
co-transfected with the plasmids, pGL3-NF-κB and pCMV-β-gal using
Lipofectamine Plus according to the manufacturer's protocol.
Briefly, a transfection mixture containing 0.5 µg pGL3-NF-κB
and 0.2 µg pCMV-β-gal was mixed with the Lipofectamine Plus
reagent and added to the cells. After 4 h, the cells were
pretreated with matrine, and then lysed with 200 µl of lysis
buffer (24 mM Tris-HCl (pH 7.8), 2 mM dithiotreitol, 2 mM EDTA, 10%
glycerol, and 1% Triton X-100) and 10 µl of cell lysates
were used for luciferase activity assay. The luciferase and
β-galactosidase activities were determined. The values shown
represent an average of three independent transfections, which were
normalized with β-galactosidase activity. Each transfection was
performed in triplicate and experiments were repeated three
times.
Assessment of ROS levels
ROS levels were determined according to the method
of a previous study (15).
5,6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
(CMH2DCFDA; Molecular Probes, Eugene, OR, USA) was used
to determined intracellular ROS levels using flow cytometry.
Following pre-treatment of HASMCs (3×106 cells/ml) with
various concentrations of matrine for 2 h, cells were incubated
with TNF-α (10 ng/ml) for 4 h. The cells were then stained with 5
µM CMH2-DCFDA for 15 min at 37°C. The cells were
kept in the dark on ice and at least 10,000 cells for each sample
were analyzed using a Becton Dickinson FACSCalibur (BD Biosciences,
San Jose, CA, USA). Changes in the levels of intracellular ROS are
expressed as a percentage of TNF-α-stimulated, matrine-untreated
cells.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Data from different groups were compared using a
Student's t-test or one-way analysis of variance followed by
Dunnett's test. Statistical analysis was performed using SPSS
(version 12.5S; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
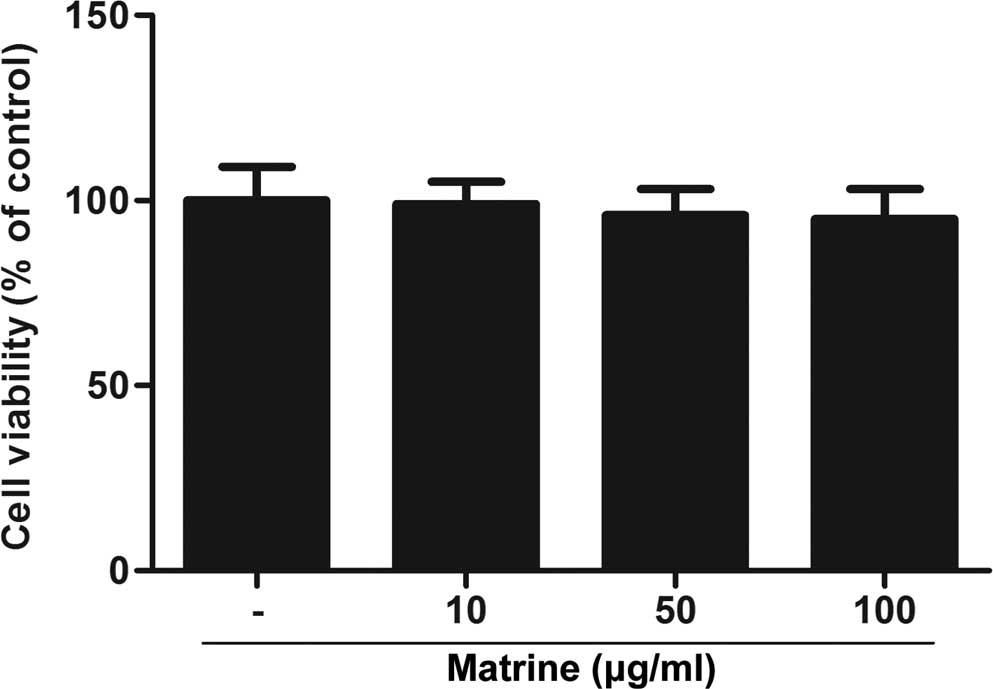
Matrine does not affect HAMSC
viability
To examine the effect of matrine on cell viability,
HASMCs were treated with various concentrations of matrine for 8 h
and subjected to an MTT assay. As shown in Fig. 1, no significant cytotoxicity of
matrine was observed. These observations indicated that matrine had
no effect on the viability of HASMCs.
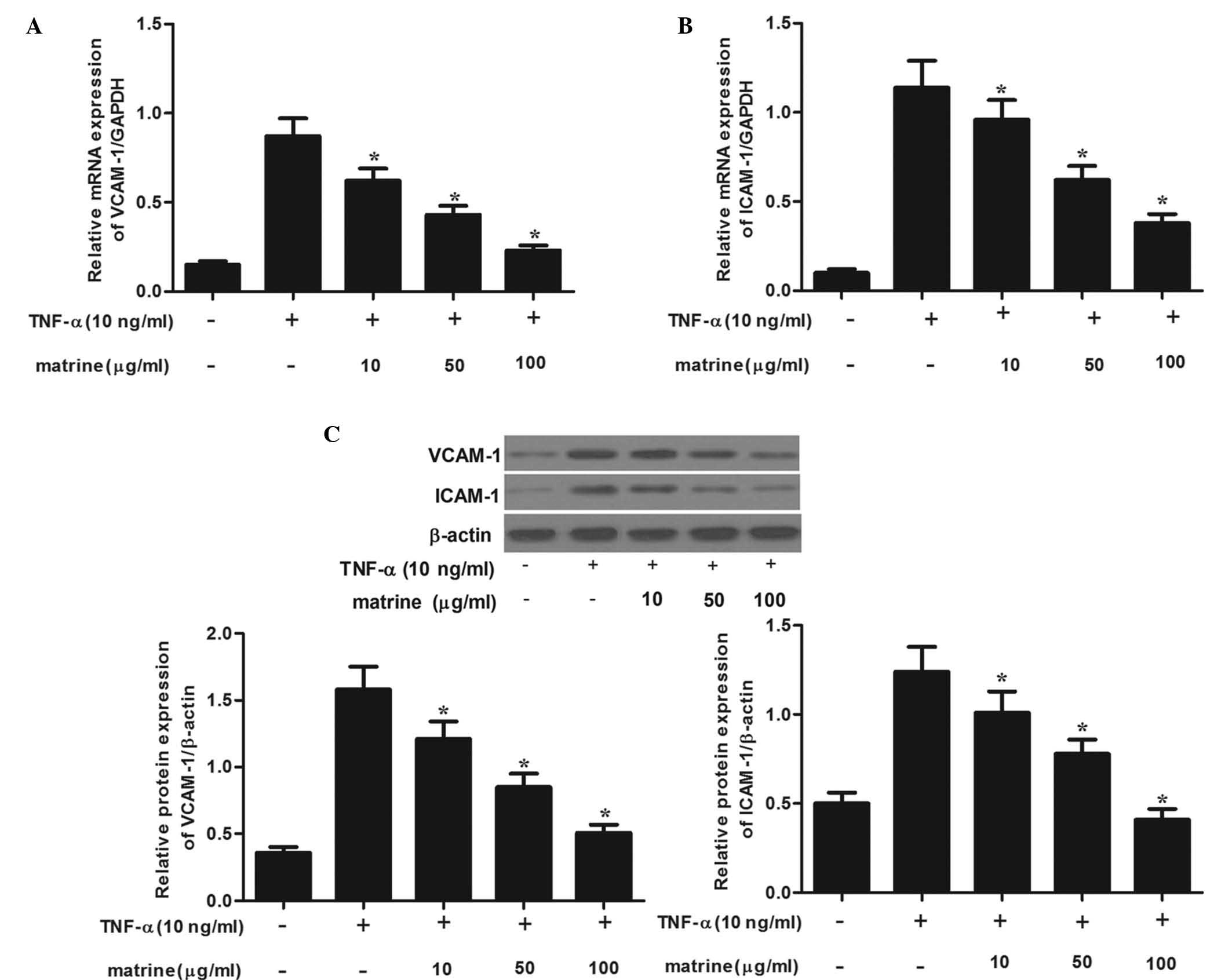
Matrine inhibits TNF-α-mediated induction
of adhesion molecules in HAMSCs
To examine whether matrine affects TNF-α-mediated
induction of adhesion molecules, HASMCs were pre-treated with
various concentrations of matrine for 2 h, followed by stimulation
with TNF-α (10 ng/ml) and subjected to RT-qPCR analysis. As shown
in Fig. 2A and B, treatment with
TNF-α induced the mRNA expression of VCAM-1 and ICAM-1 on HASMCs.
However, matrine significantly inhibited TNF-α-induced mRNA
expression of VCAM-1 and ICAM-1 in a concentration-dependent
manner. Consistent with these results, western blot analysis showed
that matrine obviously suppressed TNF-α-induced protein expression
of VCAM-1 and ICAM-1 in a concentration-dependent manner (Fig. 2C). These results suggested that
matrine effectively blocks TNF-α-induced expression of VCAM-1 and
ICAM-1.
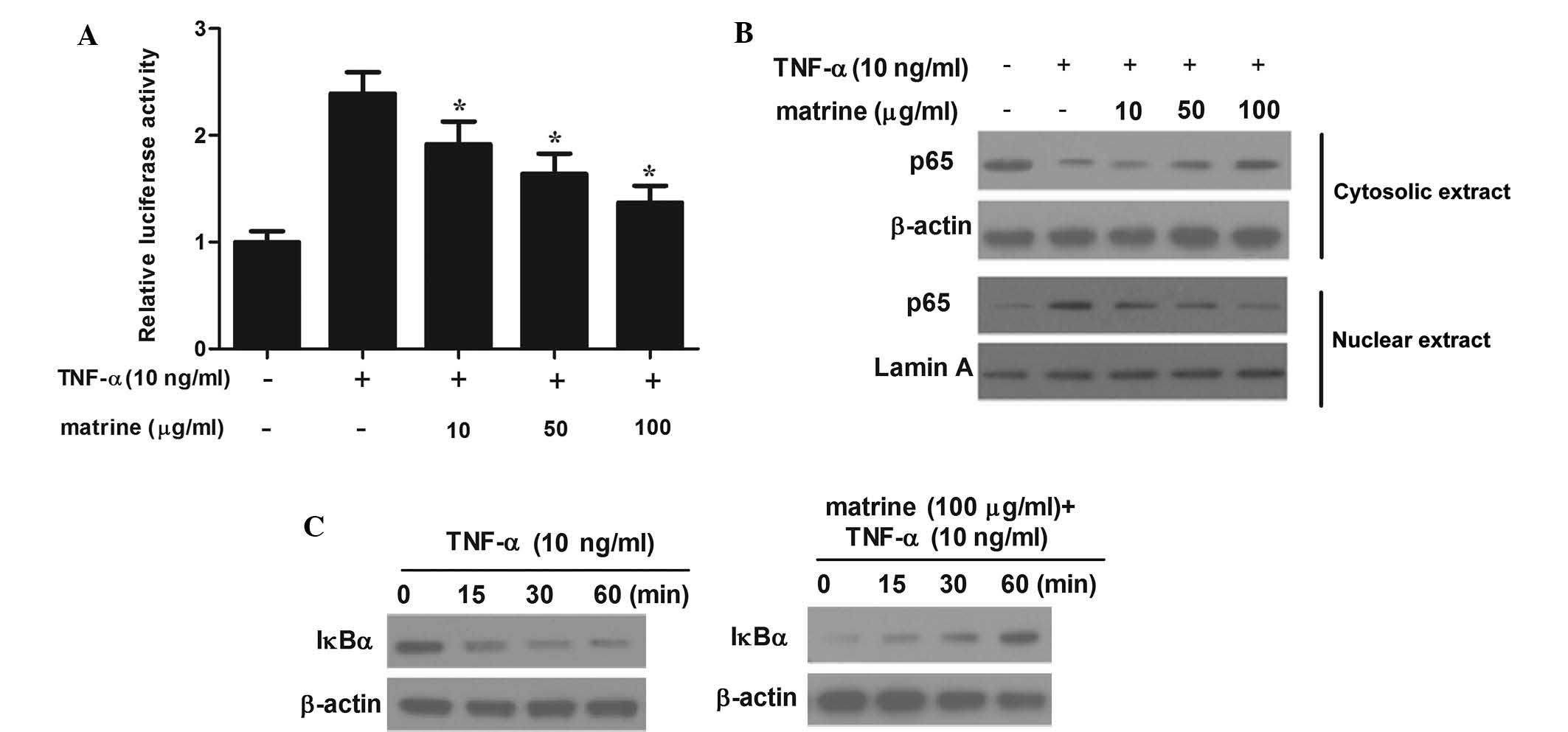
Matrine inhibits TNF-α-induced NF-κB
activation in HAMSCs
Activation of NF-κB is linked with the development
of vascular damage; furthermore, transcription factors are known to
mediate the expression of adhesion molecules (16). Therefore the present study examined
the effects of matrine on NF-κB-mediated transcriptional
activation. HASMCs were treated with various concentrations of
matrine for 2 h and subsequently stimulated with TNF-α for 4 h.
Transcriptional activation assays were then employed to determine
whether matrine affects NF-κB-dependent transcription. Stimulation
with TNF-α obviously increased luciferase activity, while matrine
significantly prevented this effect (Fig. 3A). Furthermore, the expression of
NF-κB p65 protein was detected by western blot analysis to clarify
the inhibitory action of matrine. As shown in Fig. 3B, pre-treatment of HASMCs with
matrine significantly decreased the nuclear levels of NF-κB p65,
while simultaneously increasing its cyto-solic levels. Furthermore,
the effects of matrine on IκB protein in TNF-α-stimulated HASMCs
were determined. TNF-α caused a significant degradation of IκBα at
30 min, which was inhibited by matrine (Fig. 3C). These results suggested that
matrine impedes TNF-α-induced NF-κB activation.
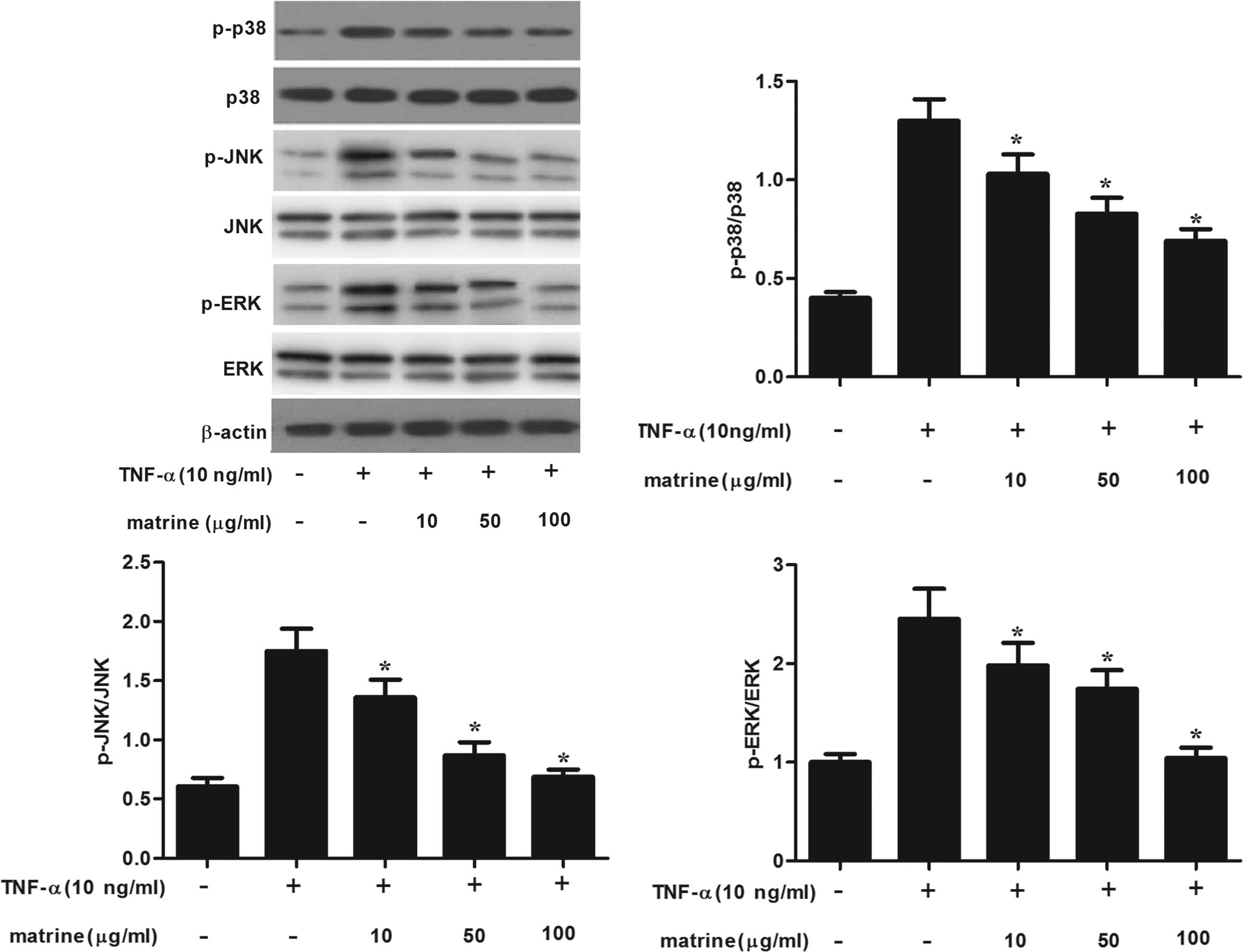
Matrine inhibits TNF-α-induced MAPK
activation in HAMSCs
MAPK signaling is involved in the regulation of
adhesion-molecule expression (16). Therefore, the present study
investigated the effects of matrine on TNF-α-induced
phosphorylation of MAPKs in HAMSCs. As shown in Fig. 4, TNF-α significantly increased the
activation of p38/MAPK, ERK1/2 and JNK in matrine-untreated cells.
However, matrine concentration-dependently inhibited TNF-α-induced
phosphorylation of MAPKs in HAMSCs.
Matrine reduces ROS production in
TNF-α-stimulated HAMSCs
As it has been reported that TNF-α-induced ROS
production activates NF-κB in vascular cells, the present study
investigated the effect of matrine on the production of
TNF-α-induced ROS production in HAMSCs (17). HAMSCs were pre-treated with matrine
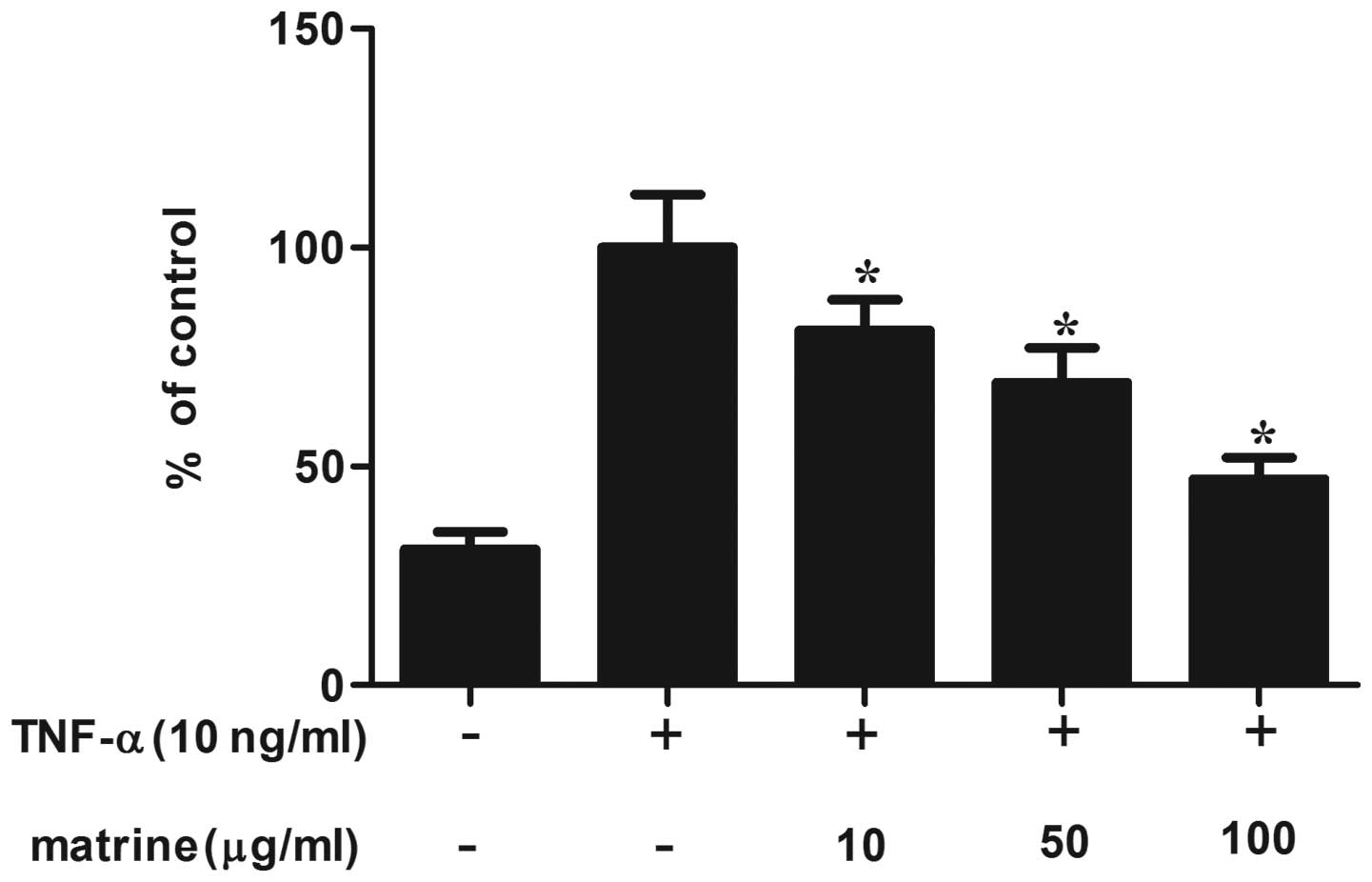
for 2 h and then stimulated with TNF-α. As shown in Fig. 5, matrine significantly reduced the
production of TNF-α-induced ROS in a concentration-dependent
manner. The production of ROS was reduced to ~50% by the highest
concentration of matrine (100 µg/ml).
Discussion
Cytokines such as IL-1 and TNF-α have been shown to
induce the expression of cellular adhesion molecules VCAM-1 and
ICAM-1 in atherosclerosis (18).
VSMCs express VCAM-1 and ICAM-1, which are prominent in the fibrous
caps of advanced atherosclerotic plaques (19). Therefore, pharmacological agents
that inhibit the expression of these adhesion molecules have are
potential drugs for inhibiting atherosclerosis. The present study
showed that matrine inhibits the expression of VCAM-1 and ICAM-1 in
TNF-α-stimulated HASMCs via the suppression of ROS production as
well as NF-κB and MAPK pathway activation.
A previous study showed that the expression of
adhesion molecules, including VCAM-1 and ICAM-1, is increased in
coronary atheroscleorotic tissue (20). In addition, inflammatory cytokines,
including IL-1β and TNF-α, increase the expression of VCAM-1 and
ICAM-1 (21,22). The present study demonstrated that
TNF-α significantly upregulated VCAM-1 and ICAM-1 expression in
HAMSCs. These results were consistent with those of earlier
published studies. However, matrine prevented the TNF-α-induced
expression of VCAM-1 and ICAM-1. The present study showed that
matrine has inhibitory effects on adhesion molecule expression in
HASMCs stimulated with TNF-α.
In all vascular cells implicated in the development
of atherosclerosis, inflammatory mediators stimulate NF-κB
activation (23-25). In quiescent cells, due to its
association with IκB, NF-κB is localized to the cytoplasm and
unable to translocate to the nucleus (26). However, IκB is phosphorylated,
ubiquitinated and subsequently degraded via the proteasome pathway
in lipopolysaccharide- and cytokine-activated cells, which
facilitates the nuclear translocation of NF-κB, where it initiates
the transcription of numerous genes, including pro-inflammatory
cytokines, cell adhesion molecules and chemokines (27,28).
The results of the present study demonstrated that matrine
decreased TNF-α-induced NF-κB activation through inhibition of IκB
kinase activation and subsequent IκBα degradation. Collectively,
these results revealed that the inhibitory effects of matrine on
the expression of adhesion molecules is, at least partially,
mediated through the suppression of NF-κB activation.
In addition to NF-κB, the MAPK signaling pathway
also has a major role in diseases associated with vascular
remodeling, as it regulates cell adhesion, proliferation, apoptosis
and migration (29–31). TNF-α-activated signaling pathways
may also include MAPKs, which may mediate the resulting
inflammatory responses (32). To
better identify the effects of matrine on the MAPK signaling
pathway, the present study assessed its impact on the levels of
total and phosphorylated p38/MAPK, JNK and ERK1/2 in TNF-α-treated
HASMCs. The results revealed that the TNF-α-induced phosphorylation
of these MAPKs was significantly reduced by matrine in a
concentration-dependent manner. Therefore, it was indicated that
matrine downregulated adhesion molecule expression induced with
TNF-α through inhibition of MAPK activation.
ROS serve as secondary messengers, which activate
multiple signaling pathways, including NF-κB and MAPKs, leading to
the induction of numerous downstream genes with essential roles in
the physiology and pathophysiology of vascular cells (27,33).
In addition, the expression of adhesion molecules has been shown to
be stimulated via the NF-κB signaling pathway, which was activated
by induction of ROS (34). The
results of the present study demonstrated that matrine
significantly and concentration-dependently decreased the ROS
production induced by TNF-α. These findings indicated that in
TNF-α-treated HASMCs, matrine inhibits the activation of NF-κB and
MAPKs via suppressing ROS production.
In conclusion, the results of the present study
suggested that matrine reduced the expression of VCAM-1 and ICAM-1
in HASMCs stimulated with TNF-α via the suppression of ROS
production and consequently of NF-κB and MAPK pathway activation.
Therefore, matrine was indicated to be an effective
anti-inflammatory agent with potential therapeutic use for
preventing the advancement of atherosclerotic lesions.
References
|
1
|
Montecucco F and Mach F: Atherosclerosis
is an inflammatory disease. Semin Immunopathol. 31:1–3. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pollard TD and Borisy GG: Cellular
motility driven by assembly and disassembly of actin filaments.
Cell. 112:453–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P and Li H: Vascular cell adhesion
molecule-1 and smooth muscle cell activation during atherogenesis.
J Clin Invest. 92:538–539. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Cybulsky MI, Gimbrone Ma-Jr and
Libby P: An atherogenic diet rapidly induces VCAM-1, a
cytokine-regulatable mononuclear leukocyte adhesion molecule, in
rabbit aortic endothelium. Arterioscl Thromb. 13:197–204. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Brien KD, Allen MD, McDonald TO, Chait
A, Harlan JM, Fishbein D, McCarty J, Ferguson M, Hudkins K,
Benjamin CD, et al: Vascular cell adhesion molecule-1 is expressed
in human coronary atherosclerotic plaques. Implications for the
mode of progression of advanced coronary atherosclerosis. J Clin
Invest. 92:945–951. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Teupser D, Thiery J, Haas U, Stein O,
Stein Y and Seidel D: Expression of vascular cell adhesion
molecule-1 (VCAM-1) in the aortae of hypercholesterolemic rabbits
with high (HAR) and low (LAR) atherosclerotic response.
Atherosclerosis. 128:157–164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Braun M, Pietsch P, Felix S and Baumann G:
Modulation of intercellular adhesion molecule-1 and vascular cell
adhesion molecule-1 on human coronary smooth muscle cells by
cytokines. J Mol Cell Cardiol. 27:2571–2579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thorne SA, Abbot SE, Stevens CR, Winyard
PG, Mills PG and Blake DR: Modified low density lipoprotein and
cytokines mediate monocyte adhesion to smooth muscle cells.
Atherosclerosis. 127:167–176. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byeon HE, Park BK, Yim JH, Lee HK, Moon
EY, Rhee DK and Pyo S: Stereocalpin A inhibits the expression of
adhesion molecules in activated vascular smooth muscle cells. Int
Immunopharmacol. 12:315–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JY, Park HJ, Um SH, Sohn EH, Kim BO,
Moon EY, Rhee DK and Pyo S: Sulforaphane suppresses vascular
adhesion molecule-1 expression in TNF-α-stimulated mouse vascular
smooth muscle cells: Involvement of the MAPK, NF-κB and AP-1
signaling pathways. Vascul Pharmacol. 56:131–141. 2012. View Article : Google Scholar
|
|
11
|
Tan HR and Zhang BH: Experimental study of
the anti-inflammatory effect of matrine. Zhong Xi Yi Jie He Za Zhi.
5:108–110. 1985.In Chinese.
|
|
12
|
Long Y, Lin XT, Zeng KL and Zhang L:
Efficacy of intramuscular matrine in the treatment of chronic
hepatitis B. Hepatobiliary Pancreat Dis Int. 3:69–72.
2004.PubMed/NCBI
|
|
13
|
Zhu P, Chen JM, Chen SZ, Zhang C, Zheng
SY, Long G, Chen J, Zhou ZL, Fan RX, Fan XP, et al: Matrine
inhibits vascular smooth muscle cell proliferation by modulating
the expression of cell cycle regulatory genes. Acta Pharmacol Sin.
31:1329–1335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banning A, Schnurr K, Böl GF, Kupper D,
Müller-Schmehl K, Viita H, Ylä-Herttuala S and Brigelius-Flohé R:
Inhibition of basal and interleukin-1-induced VCAM-1 expression by
phospholipid hydroperoxide glutathione peroxidase and
15-lipoxygenase in rabbit aortic smooth muscle cells. Free Radic
Biol Med. 36:135–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SR, Kwak JH, Kim HJ and Pyo S:
Neuroprotective effects of kobophenol A against the withdrawal of
tropic support, nitrosative stress, and mitochondrial damage in
SH-SY5Y neuroblastoma cells. Bioorg Med Chem Lett. 17:1879–1882.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JY, Park HJ, Um SH, Sohn EH, Kim BO,
Moon EY, Rhee DK and Pyo S: Sulforaphane suppresses vascular
adhesion molecule-1 expression in TNF-α-stimulated mouse vascular
smooth muscle cells: involvement of the MAPK, NF-κB and AP-1
signaling pathways. Vascul Pharmacol. 56:131–141. 2012. View Article : Google Scholar
|
|
17
|
Byeon HE, Um SH, Yim JH, Lee JH and Pyo S:
Ohioensin F suppresses TNF-α-induced adhesion molecule expression
by inactivation of the MAPK, Akt and NF-κB pathways in vascular
smooth muscle cells. Life Sci. 90:396–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kasper HU, Schmidt A and Roessner A:
Expression of the adhesion molecules ICAM, VCAM, and ELAM in the
arterio-sclerotic plaque. Gen Diagn Pathol. 141:289–294.
1996.PubMed/NCBI
|
|
20
|
Yang PY, Rui YC, Lu L, Li TJ, Liu SQ, Yan
HX and Wang HY: Time courses of vascular endothelial growth factor
and intercellular adhesion molecule-1 expressions in aortas of
athero-sclerotic rats. Life Sci. 77:2529–2539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F, Yu W, Hargrove JL, Greenspan P,
Dean RG, Taylor EW and Hartle DK: Inhibition of TNF-alpha induced
ICAM-1, VCAM-1 and E-selectin expression by selenium.
Atherosclerosis. 161:381–386. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen YH, Lin SJ, Chen JW, Ku HH and Chen
YL: Magnolol attenuates VCAM-1 expression in vitro in
TNF-alpha-treated human aortic endothelial cells and in vivo in the
aorta of cholesterol-fed rabbits. Br J Pharmacol. 135:37–47. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Martin R, Hoeth M, Hofer-Warbinek R and
Schmid JA: The transcription factor NF-kappaB and the regulation of
vascular cell function. Arterioscler Thromb Vasc Biol. 20:E83–E88.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Browatzki M, Schmidt J, Kübler W and
Kranzhöfer R: Endothelin-1 induces interleukin-6 release via
acctivation of the transcription factor NF-kappaB in human vascular
smooth muscle cells. Basic Res Cardiol. 95:98–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Csiszar A, Smith K, Labinskyy N, Orosz Z,
Rivera A and Ungvari Z: Resveratrol attenuates TNF-alpha-induced
activation of coronary arterial endothelial cells: Role of
NF-kappaB inhibition. Am J Physiol Heart Circ Physiol.
291:H1694–H1699. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baeuerle PA and Baltimore D: I kappaB: A
specific inhibitor of the NF-kappaB transcription factor. Science.
242:540–546. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Baeuerle PA and Henkel T: Function and
activation of NF-kappaB in the immune system. Annu Rev Immunol.
12:141–179. 1994. View Article : Google Scholar
|
|
28
|
Ledebur HC and Parks TP: Transcriptional
Regulation of the intercellular adhesion molecule-1 gene by
inflammatory cytokines in human endothelial cells essential roles
of a variant kappaB site and p65 homodimers. J Biol Chem.
270:933–943. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kingsley K, Huff JL, Rust WL, Carroll K,
Martinez AM, Fitchmun M and Plopper GE: ERK1/2 mediates PDGF-BB
stimulated vascular smooth muscle cell proliferation and migration
on laminin-5. Biochem Biophys Res Commun. 293:1000–1006. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajesh M, Mukhopadhyay P, Hasko G, Huffman
J, Mackie K and Pacher P: CB2 cannabinoid receptor agonists
attenuate TNF-alpha-induced human vascular smooth muscle cell
proliferation and migration. Br J Pharmacol. 153:347–357. 2008.
View Article : Google Scholar
|
|
31
|
Li M, Liu Y, Dutt P, Fanburg BL and Toksoz
D: Inhibition of serotonin-induced mitogenesis, migration, and ERK
MAPK nuclear translocation in vascular smooth muscle cells by
ator-vastatin. Am J Physiol Lung Cell Mol Physiol. 293:L463–L471.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ju JW, Kim SJ, Jun CD and Chun JS: p38
Kinase and c-Jun N-terminal kinase oppositely regulates tumor
necrosis factor alpha-induced vascular cell adhesion molecule-1
expression and cell adhesion in chondrosarcoma cells. IUBMB Life.
54:293–299. 2002. View Article : Google Scholar
|
|
33
|
Griendling KK, Sorescu D, Lassègue B and
Ushio-Fukai M: Modulation of protein kinase activity and gene
expression by reactive oxygen species and their role in vascular
physiology and pathophysiology. Arterioscl Thromb Vasc Biol Vas.
20:2175–2183. 2000. View Article : Google Scholar
|
|
34
|
Qin P, Tang X, Elloso MM and Harnish DC:
Bile acids induce adhesion molecule expression in endothelial cells
through activation of reactive oxygen species, NF-kappaB and p38.
Am J Physiol-Heart Circ Physiol. 291:H741–H747. 2006. View Article : Google Scholar
|