Introduction
Leukemia is one of the leading causes of malignant
cancer-related mortality in males and females, and despite the
efforts made to improve its treatment and diagnosis, the mortality
rates remain high to date (1).
Currently, the predominant therapeutic regimens for the treatment
of leukemia are chemotherapy-based, due to the fact that the
majority of the adult patients with leukemia are incompatible for
bone marrow transplantation. Chemotherapy-based treatments,
however, are associated with severe side effects (2,3), and
therefore, finding novel, safe and effective therapeutic regimens
for leukemia is imperative. Traditional Chinese medicines are used
for the treatment of various diseases, and a number of these agents
have been considered as potential agents for cancer treatment or
prevention (4–7).
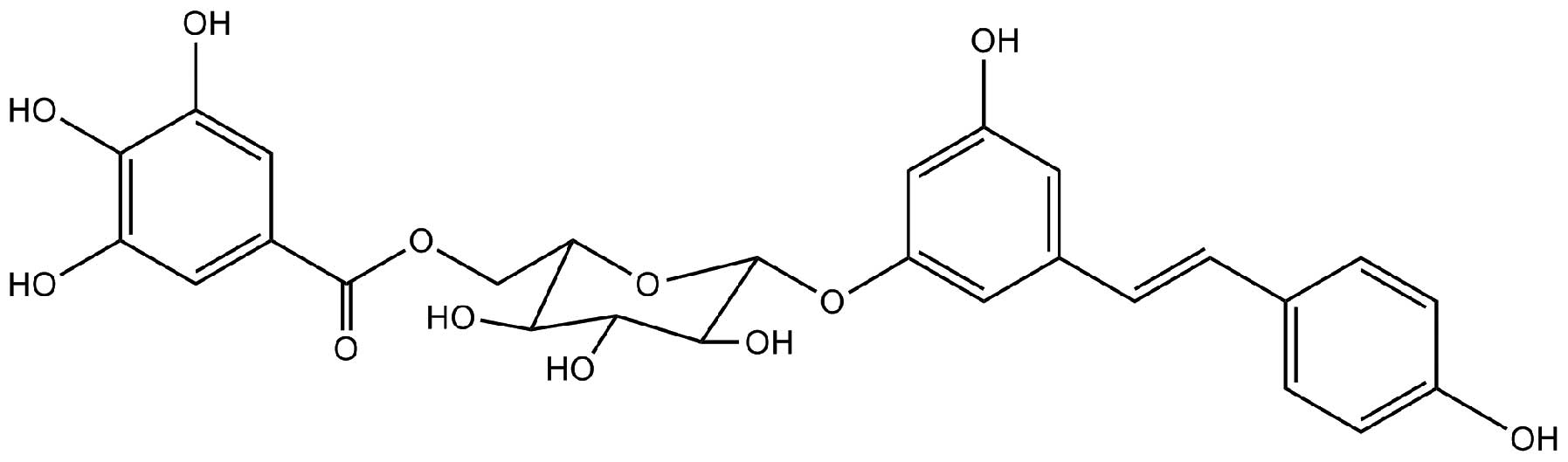
Resveratrol-4-O-D-(2′-galloyl)-glucopyranoside (REG)
is one of the predominant stilbene glycosides isolated from
Polygonum cuspidatum (8).
No pharmacological activity of REG has been reported so far,
however, preliminary experiments demonstrated potential anticancer
effects in vivo. The present study investigated the effect
of REG on leukemia cells, and the findings demonstrated that it
exerts marked anticancer effects on leukemia through the induction
of apoptosis.
Materials and methods
Chemicals and reagents
Resveratrol-4-O-D-(2′-galloyl)-glucopyranoside
(Fig. 1) was purchased from
Dingrui Chemical Ltd. (Shanghai, China). Cell Counting kit-8
(CCK-8) was obtained from Dojindo Biochem (Shanghai, China),
dimethyl sulfoxide (DMSO) from Sigma-Aldrich (St. Louis, MO, USA),
and RPMI 1640 media and fetal bovine serum (FBS) from Invitrogen,
Thermo Fisher Scientific, Inc., (Waltham, MA, USA). Monoclonal
rabbit antibodies against β-actin (cat. no. 49701), cytochrome
c (cat. no. 4280s), cleaved (c)-caspases-3 (cat. no. 9664s)
and -9 (cat. no. 9509s), B-cell lymphoma 2 (Bcl-2; cat. no. 2870s)
and Bcl-2-associated protein x (Bax; cat. no. 5023s) were purchased
from Cell Signaling Technology Inc. (Denver, MA, USA). An Annexin
V-fluorescein isothiocyanate (FITC) propidium iodide (PI) kit was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
All other chemicals used in the present study were of analytical
grade.
Animals
Nude mice (n=20; age, 5–6 weeks) were obtained from
the Shanghai SLRC Laboratory Animal Company (Shanghai, China). The
mice were maintained at 21±1°C under a 12-h light/dark cycle with
access to standard food pellets and water ad libitum. All
animal treatments were conducted in strict accordance with the
international ethical guidelines and the Guide for the Care and Use
of Laboratory Animals by the National Institutes of Health
(9). The experiments were approved
by the Animal Experimentation Ethics Committee of Fudan University
(Shanghai, China).
Cell culture and CCK-8 assay
HL-60, Jurkat and U937 human leukemia cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The cells were cultured in RPMI-1640 medium supplemented
with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich). Cells were sub-cultured until they reached the
logarithmic growth phase at 7°C in 5% CO2/95% air. Then,
the CCK assay was performed to evaluate the anti-proliferative
activity of REG on leukemia cell lines as previously described
(10).
Flow cytometry for the detection of
apoptosis
HL-60 cells were harvested, washed with
phosphate-buffered saline and stained using a commercial Annexin
V-FITC/PI kit according to the manufacturer's protocol. Cell
apoptosis was detected by flow cytometric analysis on a
FACSCalibur™ flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA). The percentage of cells undergoing early apoptosis was
indicated by Annexin V-positivity and PI-negativity, and that of
cells undergoing late apoptosis was indicated by Annexin
V-positivity and PI-positivity.
Western blot analysis
Following total protein extraction from cells using
mammalian protein extraction reagents (Beyotime Institute of
Biotechnology), the protein concentration was determined using the
Enhanced Bicinchoninic acid Protein Assay Reagent (Beyotime
Institute of Biotechnology). Subsequently, 40 µg protein
samples were separated by 12% sodium dodecyl sulfate polyacrylamide
gel electrophoresis (Beyotime Institute of Biotechnology), and
transferred onto polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% fat-free
dry milk in 1X Tris-buffered saline containing 0.05% Tween 20 for 2
h, and incubated with monoclonal primary antibodies (diluted
1:1,000) at 4°C for 10 h. The membrane was washed with TBST buffer
[Sangon Biotech Co., Ltd., Shanghai, China; 20 M Tris-HCl (pH 7.4),
150 M NaCl and 0.1% Tween 20 and protein bands were incubated with
goat anti-rabbit horseradish peroxidase-conjugated monoclonal
antibody (diluted, 1:2,000; cat. no. A0208; Beyotime Institute of
Biotechnology) for 2 h at room temperature, and subsequently
detected by chemiluminescence (Beyotime Institute of Biotechnology)
using a ChemiDoc™ XRS imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). To measure protein loading, antibodies directed
against β-actin were used.
Xenograft assay in vivo
The mice were divided into the REG treatment and
control groups (n=8/group). All mice received a subcutaneous
injection of HL-60 cells (2×106/mouse) in the right
flank. When the tumors grew to ~2–3 mm in diameter, the REG
treatment group received an intraperitoneal injection of REG (40
mg/kg/day) and the control group received an equal volume of
solvent control (0.5% DMSO). The mice from the two groups remained
under observation for 15 days, and the tumor sizes were measured
every 5 days. The tumor diameters were determined using a Vernier
caliper (Bangsheng Equipment, Shanghai, China), and the tumor
volumes were then calculated according to the formula (11): Volume = (width2 ×
length)/2. All the animals were sacrificed by anesthesia with 50
mg/kg pentobarbital sodium (Sigma-Aldrich) immediately after 15
days of observation, and the tumors tissues were collected and
homogenized for western blot analysis.
Statistical analysis
The significant differences between the groups were
determined using Student's t-test and analyzed by SPSS software for
Windows (version 18.0; SPSS, Inc., Chicago, IL, USA). The results
are presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
REG exhibits an anti-proliferative effect
on leukemia cell lines
HL-60, Jurkat and U937 leukemia cell lines were
treated with REG at concentrations of 5-160 µg/ml, and the
cytotoxic activity of REG was evaluated using a CCK-8 assay. The
results indicated that REG exerted a marked anti-proliferative
effect on the three leukemia cell lines in a concentration and
time-dependent manner (Fig. 2).
The half maximal inhibitory concentration (IC50) values
of REG for HL-60, Jurkat and U937 ell lines were 38.4, 49.1 and
48.2 µg/ml, respectively. These results indicated that REG
can significantly inhibit proliferation in leukemia cells in a
concentration- and time-dependent manner. In addition, the HL-60
cell line was selected for the rest of the experiments as REG
exhibited the most marked anti-proliferative effect on this cell
line.
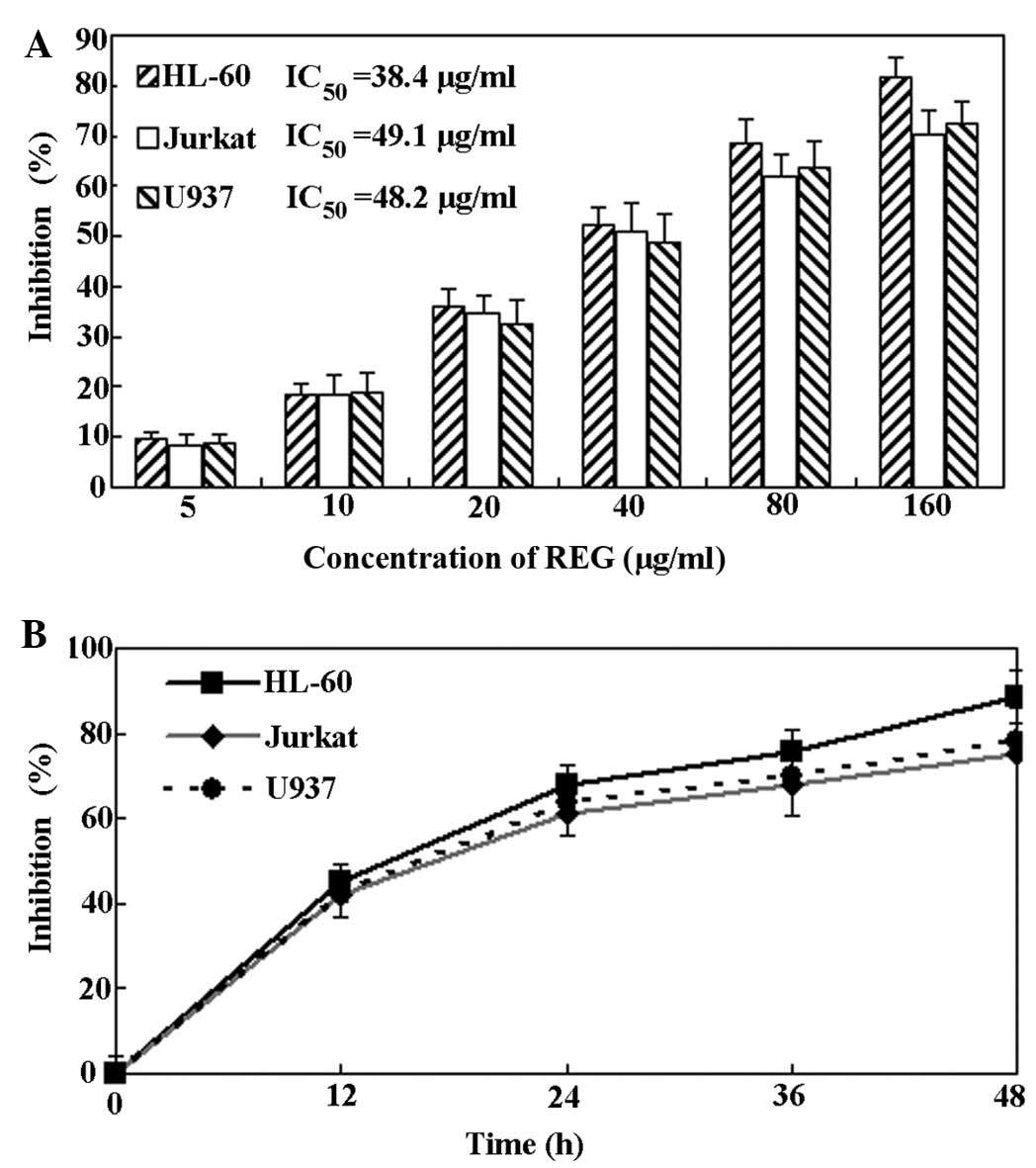 | Figure 2Anti-proliferative activity of REG on
leukemia cells. (A) Concentration-dependent effect of REG on
leukemia cell proliferation. Cell lines were treated with
low-to-high concentrations of REG (5, 10, 20, 40, 80 and 160
µg/ml) for 24 h, and then a CCK-8 assay was performed and
the values calculated. (B) Time-dependent effect of REG on leukemia
cells. Cell lines were treated with REG (80 µg/ml) for 12,
24 and 48 h, and then a CCK-8 assay was performed. Data are
presented as the mean ± standard deviation (n=4). REG,
resveratrol-4-O-D-(2′-galloyl)-glucopyranoside; CCK-8, cell
counting kit-8; IC50, half maximal inhibitory
concentration values. |
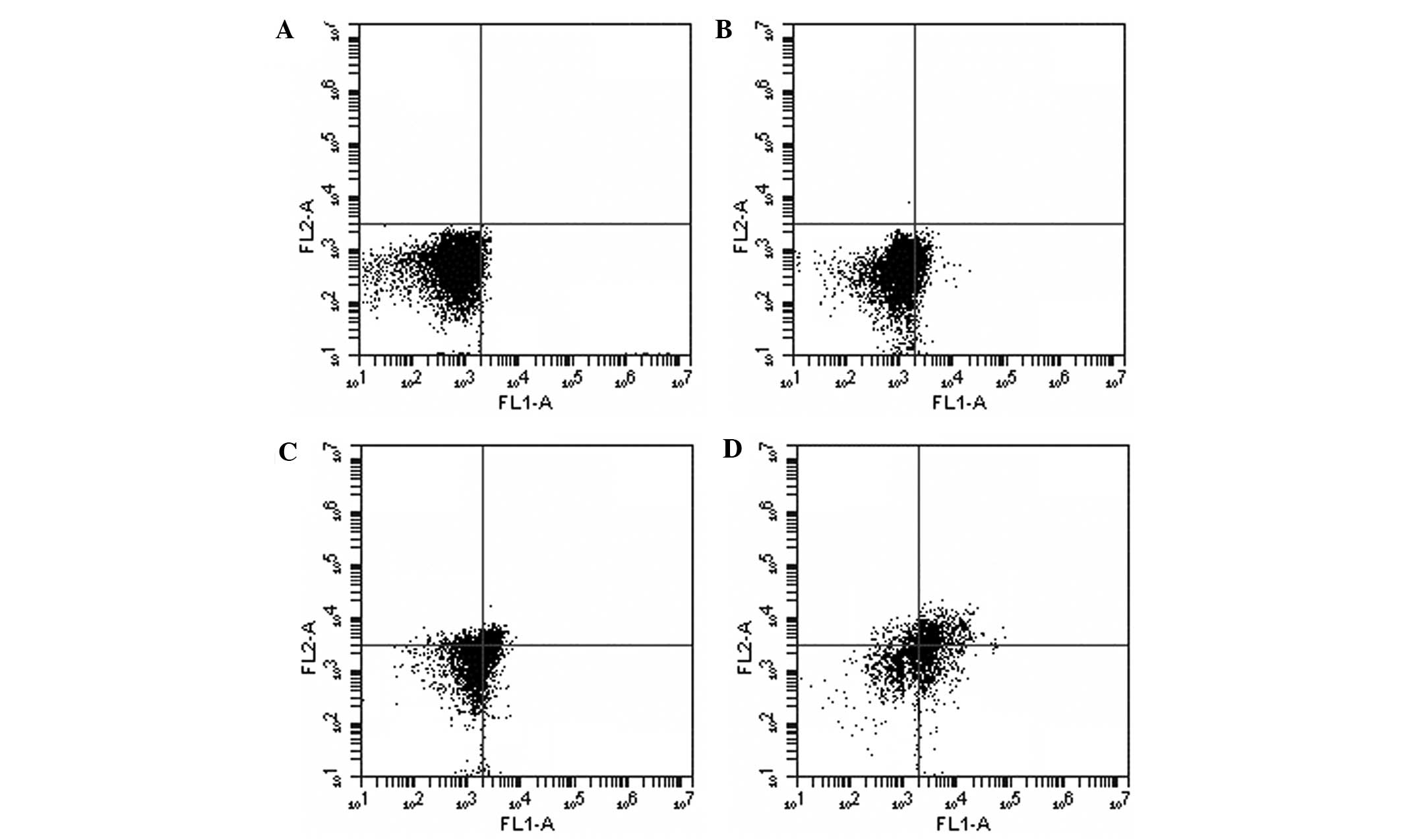
Flow cytometric analysis results
In order to confirm whether the anti-proliferative
effect of REG on leukemia cells was associated with the induction
of apoptosis, flow cytometric analysis was performed. As shown in
Fig. 3, following exposure to REG
at concentrations of 20, 40 and 80 µg/ml for 24 h, a
concentration-dependent increase in apoptosis was observed. Thus,
these results demonstrated that the cytotoxic activity of REG on
leukemia cells in vitro may be associated with the induction
of apoptosis.
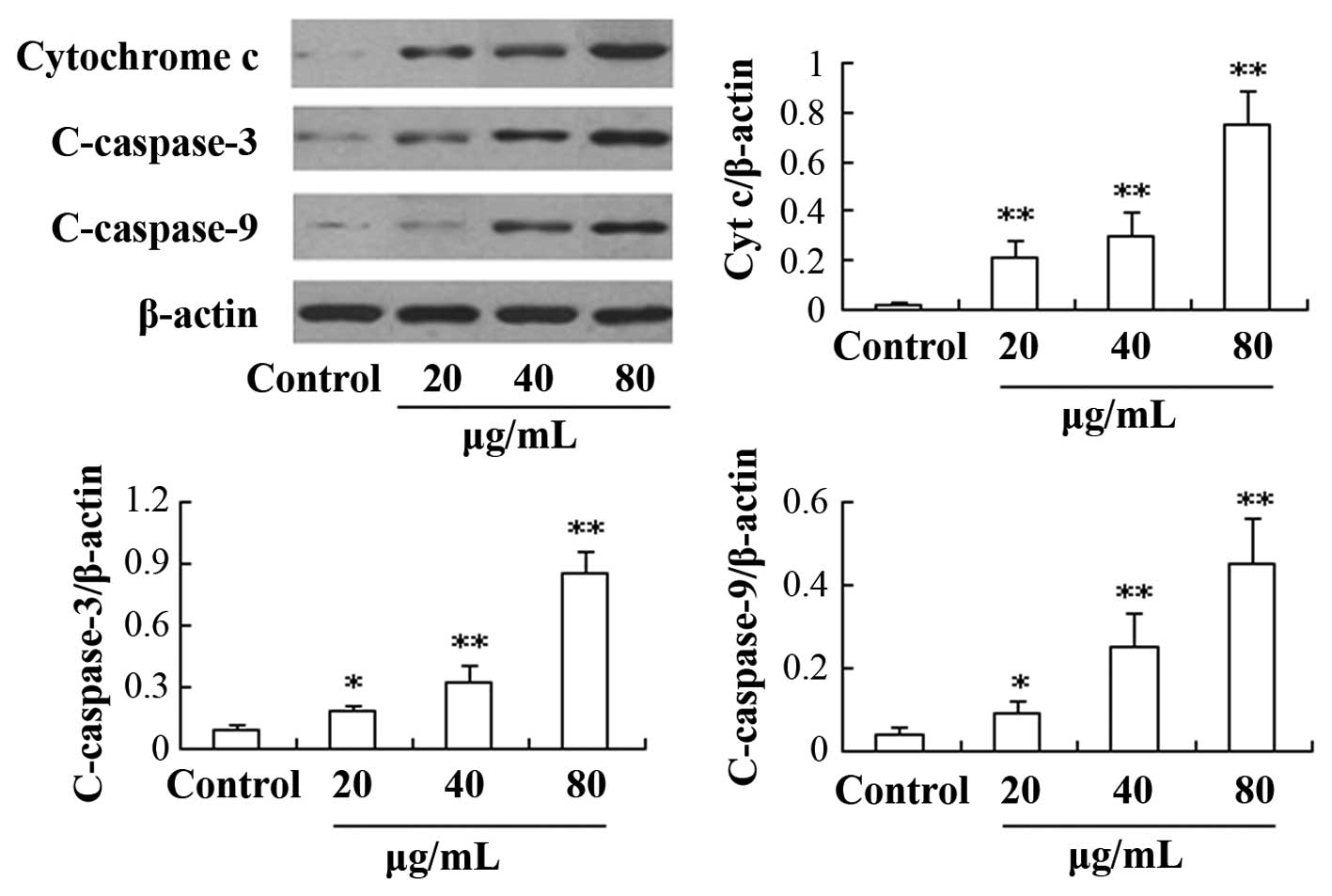
Effect of REG on the expression of
caspase and Bcl-2 family proteins
In order to investigate the mechanism underlying the
effect of REG, the levels of certain apoptosis-related proteins
were detected. As shown in Fig. 4,
the expression of cytochrome c, and c-caspases-3 and -9
proteins was found to be significantly upregulated in a
concentration-dependent manner in the REG treatment group compared
with the control group (P<0.05). Furthermore, the expression
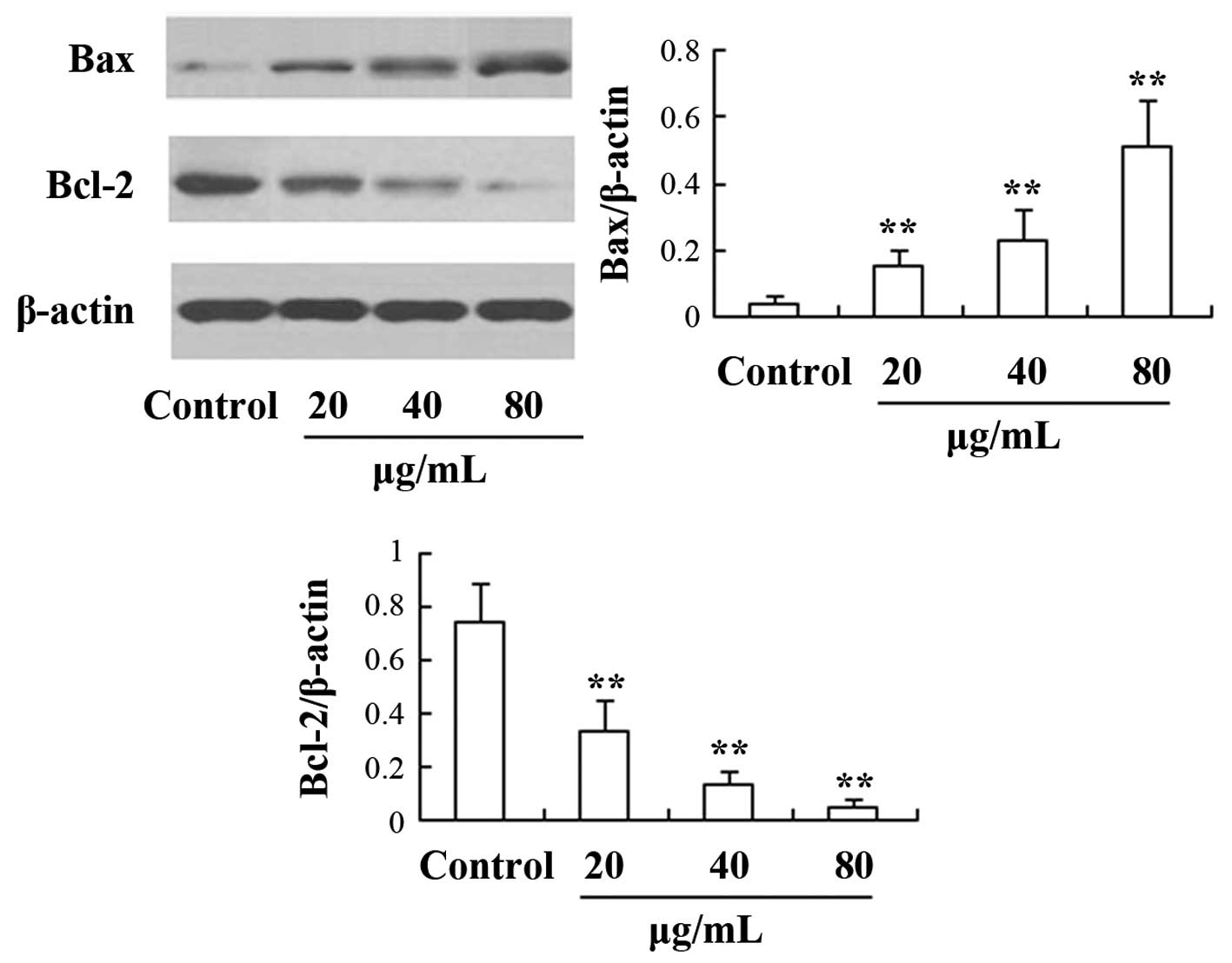
levels of proteins from the Bcl-2 family were also determined in
the present study (Fig. 5). The
results showed that the Bcl-2 expression was significantly
downregulated, whereas the expression of Bax was significantly
upregulated compared with the control group (P<0.05).
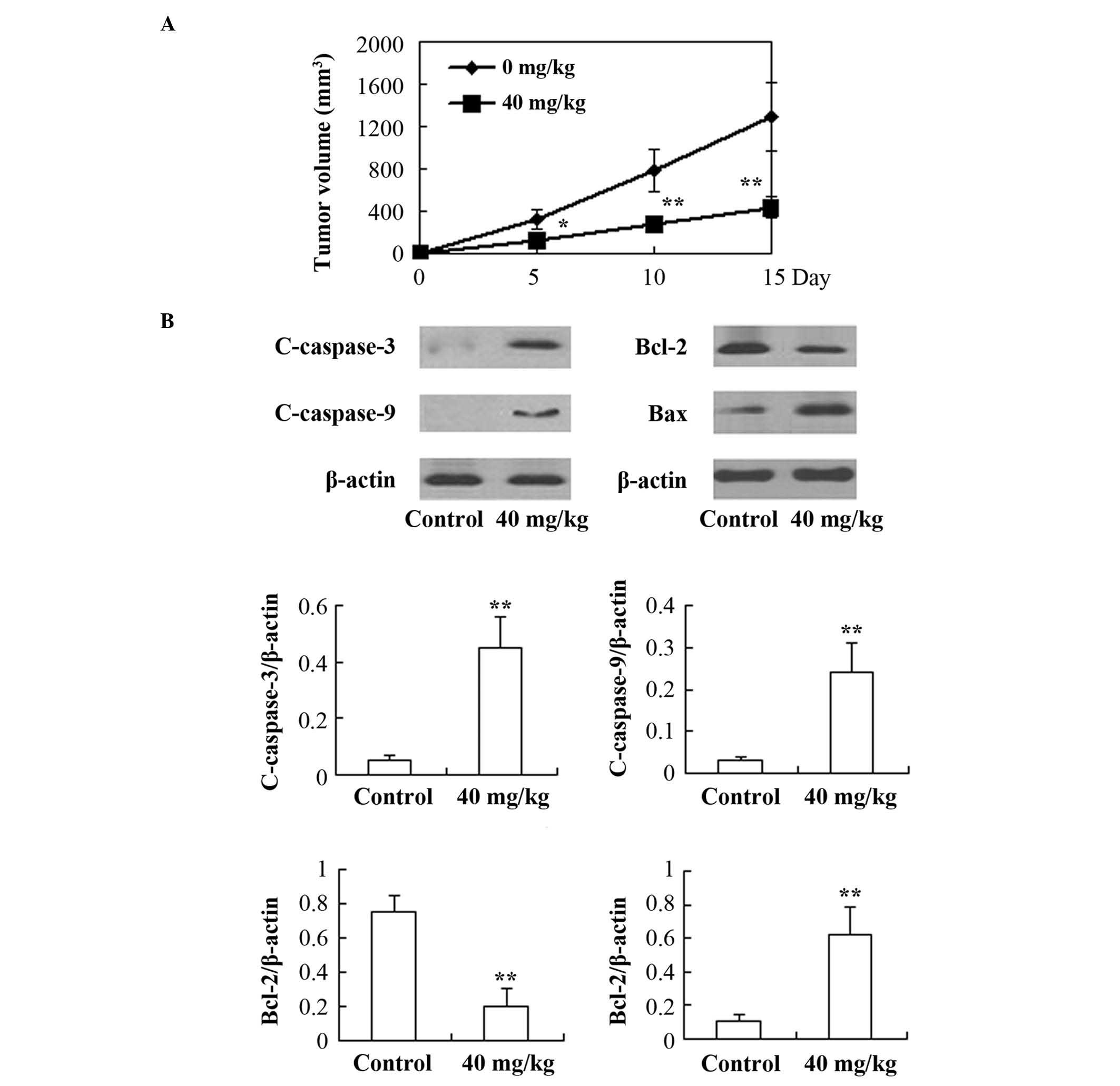
In vivo anticancer effects of REG
on the HL-60 xenograft model in nude mice
As shown in Fig. 6,
following treatment with REG (40 mg/kg/day for 15 days), a
significant inhibitory effect on tumor growth was observed during
the 15-day observation period (P<0.05) compared with the control
group. Furthermore, the expression of caspases-3 and -9, Bax and
Bcl-2 proteins in the tumor tissues was examined. The results
showed that the expression of c-caspases-3 and -9, and Bax
increased significantly, whereas the expression of Bcl-2 was found
to be clearly decreased in the REG-treated mice compared with that
in the control group (P<0.01).
Discussion
To the best of our knowledge, no studies have been
conducted regarding the antitumor effect of REG on leukemia. To the
best of our knowledge, the present study demonstrated for the first
time that REG exerts a marked antileukemia activity, and that the
underlying mechanism may be associated with the
mitochondria-mediated apoptosis pathway.
It is known that apoptosis is a desirable pathway
for killing cancer cells, and numerous studies have aimed to
identify apoptosis-inducing agents (12–14).
Mitochondria-mediated apoptosis has been reported to be an
important apoptosis pathway (15).
The release of cytochrome c into the cytosol from the
mitochondria is the initial step in the induction of apoptosis, and
the apoptosis-associated caspase proteins, such as caspase-9. It
has been reported that cytosolic cytochrome c can activate
procaspase-9 by binding to the apoptotic peptidase activating
factor-1 in the presence of deoxyadenosine triphosphate, resulting
in the activation of caspase-9 (15,17).
The activation of caspase-9 often leads to the activation of
caspase-3. Caspase proteins are a family of cysteine proteases that
are activated in the execution phase of apoptosis. Caspase-3 is a
crucial signaling protein of the mitochondria-mediated apoptosis
pathway, and is considered to be the most important 'executioner'
protein; therefore, the activation of caspase-3 is usually
considered to be a biochemical hallmark of apoptosis (18,19).
In the present study, it was demonstrated that REG
treatment can significantly increase the cytochrome c
expression levels in the cytoplasm. In addition, the key caspase
proteins, caspases-3 and -9, were also upregulated following
exposure to REG. These results indicated that the anticancer
activity of REG in leukemia cells could be associated with the
mitochondria-mediated apoptosis pathway.
The Bcl-2 family of proteins is also considered to
exhibit a key role in the regulation of the mitochondria-mediated
apoptosis pathway. Bax is one of the main pro-apoptotic proteins of
the Bcl-2 family, whereas Bcl-2 is one of the main anti-apoptotic
proteins. Furthermore, the ratio of Bax/Bcl-2 is a key factor in
the induction of mitochondrial-mediated apoptosis (3,20).
The results of the present study showed that Bcl-2 protein
expression was markedly downregulated following REG treatment. By
contrast, the expression level of Bax was found to be significantly
increased following treatment with REG; therefore, the relative
ratio of Bax/Bcl-2 was significantly elevated by REG treatment. In
addition, the results of the in vivo experiment were
consistent with the results obtained in vitro, suggesting
that the anticancer activity of REG in leukemia is associated with
the mitochondria-mediated apoptosis pathway.
In conclusion, the present study demonstrated that
REG is an effective and reliable antileukemia agent, and that the
underlying mechanism may be associated with the induction of the
caspase-3 cascade, as well as the downregulation of the
anti-apoptotic Bcl-2 family proteins. Findings from the current
study indicated that REG exerts marked antitumor effects on
leukemia cell lines, which may suggest the compound has potential
clinical use as a therapeutic agent; however, further investigation
of the mechanism underlying the effects of REG is required.
Acknowledgments
The present study was supported by grants from the
National Science & Technology Pillar Program during the 12th
Five-year Plan Period (grant no. 2012BAI37B01) and the subtopic of
Shanghai Committee of Science and Technology (grant no.
12DZ1941803).
References
|
1
|
Siegel R, Ma J, Zou ZH and Jemal A: Cancer
Statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burnett AK, Goldstone A, Hills RK,
Milligan D, Prentice A, Yin J, Wheatley K, Hunter A and Russell N:
Curability of patients with acute myeloid leukemia who did not
undergo transplantation in first remission. J Clin Oncol.
31:1293–1301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hseu YC, Lee CC, Chen YC, Kumar KJ, Chen
CS, Huang YC, Hsu LS, Huang HC and Yang HL: The anti-tumor activity
of Antrodia salmonea in human promyelocytic leukemia (HL-60) cells
is mediated via the induction of G1 cell-cycle arrest
and apoptosis in vitro or in vivo. J Ethnopharmacol. 153:499–510.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen HJ, Lo YC and Chiang WC: Inhibitory
effects of adlay bran (Coix lachrymajobi L. var.ma-yuen Stapf) on
chemical mediator release and cytokine production in rat basophilic
leukemia cells. J Ethnopharmacol. 141:119–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lansky EP, Paavilainen HM, Pawlus AD and
Newman RA: Ficus spp. (fig): Ethnobotany and potential as
anticancer and anti-inflammatory agents. J Ethnopharmacol.
119:195–213. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li JW and Vederas JC: Drug discovery and
natural products: End of an era or an endless frontier? Science.
325:161–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ovadje P, Chatterjee S, Griffin C, Tran C,
Hamm C and Pandey S: Selective induction of apoptosis through
activation of caspase-8 in human leukemia cells (Jurkat) by
dandelion root extract. J Ethnopharmacol. 133:86–91. 2011.
View Article : Google Scholar
|
|
8
|
Peng W, Qin RX, Li XL and Zhou H: Botany,
phytochemistry, pharmacology, and potential application of
Polygonum cuspidatum Sieb.et Zucc.: A review. J Ethnopharmacol.
148:729–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th ed.
National Academy Press; Washington, D.C.: 2010
|
|
10
|
Ngamwongsatit P, Banada PP, Panbangred W
and Bhunia AK: WST-1-based cell cytotoxicity assay as a substitute
for MTT-based assay for rapid detection of toxigenic Bacillus
species using CHO cell line. J Microbiol Methods. 73:211–215. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su YT, Chang HL, Shyue SK and Hsu SL:
Emodin induces apoptosis in human lung adenocarcinoma cells through
a reactive oxygen species-dependent mitochondrial signaling
pathway. Biochem Pharmacol. 70:229–241. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao N, Budhraja A, Cheng SP, Yao H, Zhang
Z and Shi XL: Induction of apoptosis in human leukemia cells by
grape seed extract occurs via activation of c-Jun NH2-terminal
kinase. Clin Cancer Res. 15:140–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JG, Pen W, Yi J, Wu YB, Chen TQ and Wu
JZ: Chemical composition, antimicrobial activity against
Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the
essential oil from Toona sinensis (A. Juss.) Roem. leaves. J
Ethnopharmacol. 154:198–205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hellwig CT, Passante E and Rehm M: The
molecular machinery regulating apoptosis signal transduction and
its implication in human physiology and pathophysiologies. Curr Mol
Med. 11:31–47. 2011. View Article : Google Scholar
|
|
16
|
Jiang X and Wang X: Cytochrome C-mediated
apoptosis. Annu Rev Biochem. 73:87–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou H, Yang R, Hao J, Wang J, Sun C, Fesik
SW, Wu JC, Tomaselli KJ and Armstrong RC: Regulation of the
Apaf-1/caspase-9 apoptosome by caspase-3 and XIAP. J Biol Chem.
278:8091–8098. 2003. View Article : Google Scholar
|
|
18
|
Twiddy D and Cain K: Caspase-9 cleavage,
do you need it? Biochemical J. 405:e1–e2. 2007. View Article : Google Scholar
|
|
19
|
Woo HJ, Jun do Y, Lee JY, Woo MH, Yang CH
and Kim YH: Apoptogenic activity of
2α,3α-dihydroxyurs-12-ene-28-oic acid from Prunella vulgaris var.
lilacina is mediated via mitochondria-dependent activation of
caspase cascade regulated by Bcl-2 in human acute leukemia Jurkat T
cells. J Ethnopharmacol. 135:626–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XK, Xu MY, Xu GS, Zhang YL and Xu ZX:
In vitro and in vivo antitumor activity of scutebarbatine A on
human lung carcinoma A549 cell lines. Molecules. 19:8740–8751.
2014. View Article : Google Scholar : PubMed/NCBI
|