Introduction
Retinal pigmented epithelial cells (RPEs) comprise a
single layer of epithelial cells, which is located between the
light-sensing photoreceptor cells and the choriocapillaris
(1). RPEs, which are highly
metabolically active, are sensitive to oxidative stress when
retinal cells are exposed to high levels of reactive oxygen species
(ROS) (2). Abnormal RPEs are
regarded as an important contributor to age-related macular
degeneration (AMD), a disease that results in irreversible visual
blindness in the elderly population (3,4).
Although the pathogenic mechanism underlying the progression of AMD
is currently unknown, previous studies have suggested that the
pathogenesis of AMD is associated with oxidative stress-induced
cumulative damage to RPEs, which occurs in response to excessive
production of ROS or abnormal ROS homeostasis (5,6).
Therefore, the identification of strategies that protect RPEs from
oxidative stress-induced damage, thus preventing or delaying the
progression of AMD, is required.
Consumer preference for natural products and
functional foods, which are considered to be largely harmless, has
recently been increasing (7). With
regards to targeting AMD, novel drugs with antioxidant and natural
properties have garnered much attention. The present study focused
on allicin, which is a defense molecule present in garlic (8). Allicin is a component of garlic that
exerts several biological activities. It has previously been
reported that allicin exerts antimicrobial, immune-modulatory and
anticancer activity (9).
Furthermore, allicin exerts antioxidant effects at the
physiological level when administered at lower doses (9). The well-known protective effects of
allicin against oxidative stress have been shown to target
cardiovascular diseases, which are frequently correlated with
oxidative stress (10,11). In addition, allicin exerts
cardioprotective effects via activation of redox-sensitive
transcription factors, including nuclear factor erythroid 2-related
factor 2 (Nrf2) (12). Allicin has
also been demonstrated to exert beneficial cardiovascular effects
by preventing cardiac hypertrophy, hyperlipidemia and platelet
aggregation (13). In vivo,
allicin has been shown to attenuate fatty streaks, which are
associated with atherosclerosis, in mice fed a high fatty acid diet
(14). Allicin not only protects
against cardiovascular diseases, but also protects against various
other age-related diseases, including neurodegenerative diseases
with age-related cognitive and memory deficits. Li et al
(15) demonstrated that allicin
markedly improved ageing-induced cognitive dysfunction via the
activation of Nrf2 signaling pathways. Therefore, allicin may be
considered a potential candidate for the treatment of cognitive
deficits in aging and Alzheimer's disease (15). The present study hypothesized that
allicin may counteract other age-related diseases, such as AMD, due
to its high antioxidant effect and potential as a therapy for the
treatment of cardiovascular diseases and Alzheimer's disease.
The aim of the present study was to explore whether
allicin could protect ARPE-19 cells from hydrogen peroxide
(H2O2)-induced damage, and to determine the
molecular mechanism underlying the effects of allicin on
H2O2-stimulated ARPE-19 cells. Briefly,
H2O2 was used to stimulate ARPE-19 cells, and
to establish a cellular model of AMD. Subsequently, the effects of
allicin on H2O2-stimulated ARPE-19 cells were
detected, and further studies were performed to determine which
biological effects of allicin were dominant in the
H2O2-stimulated ARPE-19 cells.
Materials and methods
Cell culture
The ARPE-19 human RPE cell line was purchased from
the American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle medium (DMEM)
supplemented with 10% heat-inactivated fetal bovine serum (FBS)
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
µg/ml streptomycin and 100 U/ml penicillin (CSPC
Pharmaceutical Group Ltd., Shijiazhuang, China) at 37°C in an
atmosphere containing 5% CO2. The cells were passaged
every 3 days once grown to ~90% confluence.
Measurement of cell viability
ARPE-19 cells were grown to 80% confluence, and were
treated with H2O2 (250 or 500 µM;
Sigma-Aldrich, St. Louis, MO, USA) for 12 or 24 h following
supplementation with allicin (5, 10, 20 or 40 µg/ml; 98%
purity; Shaanxi Ciyuan Biotech Co., Ltd., Xian, China) for 4 h.
Cells were harvested after the appropriate treatments. Cell
viability was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-di-phenyl tetrazolium bromide
(MTT) assay (Sigma-Aldrich). Briefly the cells were incubated with
50 µl MTT at 37°C for 4 h. Subsequently, the MTT-containing
medium was discarded and 200 µl dimethyl sulfoxide was added
to the cells, in order to dissolve the formazan crystals. Optical
densities (OD) were measured at 450 nm using a VersaMax microplate
reader (Molecular Devices, Sunnyvale, CA, USA). Viability was
calculated as OD value/cell number; untreated ARPE-19 cells
(control group) were denoted as 100% viable.
Measurement of oxidative stress
An Intracellular ROS Assay kit (Cell Biolabs, Inc.,
San Diego, CA) was used to detect the intracellular ROS levels.
Briefly, 100 µl 2′,7′-dichlorodihydro fluorescein diacetate
(DCFH-DA) was added to the culture media of the ARPE-19 cells for
30 min at 37°C. Subsequently, the cells were washed twice with
phosphate-buffered saline (PBS). DCFH fluorescence of the cell
lysate was measured at an excitation wavelength of 485 nm and an
emission wavelength of 535 nm using a FLUOstar optima (BMG LABTECH,
Inc., Cary, NC, USA), and was quantified using ImageJ version 1.41
software (National Institutes of Health, Bethesda, MD, USA). The
average fluorescence intensity was analyzed from five fields for
each treatment. The relative fluorescence intensity was expressed
as a percentage increase with reference to the intensity of the
normal control cells. The glutathione (GSH)/glutathione disulfide
(GSSG) ratio and malondialdehyde (MDA) concentrations were assessed
using the GSH/GSSG Assay kit (Beyotime Institute of Biotechnology,
Nantong, China) and the MDA Assay kit (Nanjing Jiancheng Biological
Engineering Institute, Nanjing, China), according to the
manufacturer's protocols. The superoxide dismutase (SOD) activity
was measured using the SOD Assay kit (Nanjing Jiancheng Biological
Engineering Institute), according to the manufacturer's protocols
(16).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the ARPE-19 cells using
the RNeasy kit (Qiagen, Inc., Valencia, CA), and first-strand cDNA
was synthesized using reverse transcription (RT) reagents (Takara
Bio, Inc., Otsu, Japan) and SuperScript III (Invitrogen; Thermo
Fisher Scientific, Inc.). RT was conducted in a total volume of 10
µl containing 1 µl RNA, which had been treated with 1
µl RNase inhibitor (Promega Corporation, Madison, WI, USA).
RT-qPCR was performed using an Applied Biosystems 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and SYBR
Premix Ex Taq kit (Takara Bio, Inc.). The PCR cycling
conditions were as follows: 95°C for 10 min, followed by 45 cycles
at 95°C for 10 sec, 57°C for 30 sec and extension at 75°C for 10
sec, and a final 5 min extension step. The primer sequences used
were as follows: NADPH oxidase 4 (NOX4), forward
tatccagccttccgttggtt, reverse ctgaggtacagctggatgttga; Nrf2, forward
gagaagtaccaggaggcgttg, reverse gagccttggacaccaacagat;
glyceraldehyde 3-phosphate dehydrogenase (GAPDH), forward
ggagtcaacggatttggtc and reverse ggaatcattggaacatgtaaac (Sangon
Biotech Co., Ltd., Shanghai, China). GAPDH was used as an internal
control. Data were analyzed using the 2−∆∆Cq method of
relative quantification (17).
Western blotting
The ARPE-19 cells were harvested at times
appropriate to the indicated treatments. The cells were washed
twice with PBS and were then lysed in order to extract the
proteins. The nuclear proteins were extracted as previously
described (18). Subsequently, the
protein samples were isolated using a Protein Extraction kit
(Qiagen GmbH, Hilden, Germany) and were quantified with a Bio-Rad
Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
protein samples (30 µg) were then separated by 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and were
transferred to nitrocellulose membranes (EMD Millipore, Bedford,
MA, USA). The membranes were blocked for non-specific binding using
2% bovine serum albumin (Sigma-Aldrich) at 4°C overnight.
Subsequently, the membranes were incubated with primary antibodies
at 4°C overnight, and appropriate horseradish-peroxidase-conjugated
secondary antibodies at room temperature for 1 h (1:500; cat. no.
MA1-10371; Thermo Fisher Scientific, Inc.). Signals were acquired
using the Enhanced Chemiluminescence Detection system (SuperSignal
West Femto; Pierce; Thermo Fisher Scientific, Inc.). Western
blotting images from three independent experiments were scanned and
quantified using ImageJ version 1.41 software (National Institutes
of Health). Band intensities were assessed and normalized to the
β-actin bands or Lamin B bands as indicated. The specific primary
antibodies used were as follows: Anti-NOX4 (1:400; cat. no.
HPA015475; Sigma-Aldrich), anti-NAD(P)H dehydrogenase quinone 1
(NQO1) (1:300; cat. no. N5288; Sigma-Aldrich), anti-Nrf2 (1:500;
cat. no. 12721; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-β-actin (1:1,000; cat. no. AV40173, Sigma-Aldrich) and
anti-Lamin B (1:400; cat. no. SAB1306342; Sigma-Aldrich)
antibodies.
Nrf2 interference
The ARPE-19 cells were cultured in 96-well plates at
a density of 2 × 104 cells/well. Non-targeting small
interfering (si)RNA (final concentration 50 nM; Ambion; Thermo
Fisher Scientific, Inc.) was used as a negative control. Human Nrf2
gene was silenced using Nrf2-specific siRNA (final concentra tion
30 nM) (Ambion; Thermo Fisher Scientific, Inc.). The cells were
transfected using siPORT Amine transfection reagent (Ambion; Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol
(19). Following transfection for
12 or 24 h, the expression levels of Nrf2 were determined by
western blotting.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Student's t-test was used to analyze differences between
two groups. The experimental results were analyzed using one-way
analysis of variance to compare the differences between three or
more groups. Statistical analyses were conducted using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). All the experiments were
performed at least in triplicate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Allicin protects RPEs from
H2O2-induced damage
In order to characterize the effects of allicin on
H2O2-induced damage in RPEs, the cells were
exposed to 250 or 500 µM H2O2 for 12
or 24 h, following preincubation with various doses of allicin (0,
5, 10, 20 or 40 µg/ml) for 4 h. Viability of the RPEs was
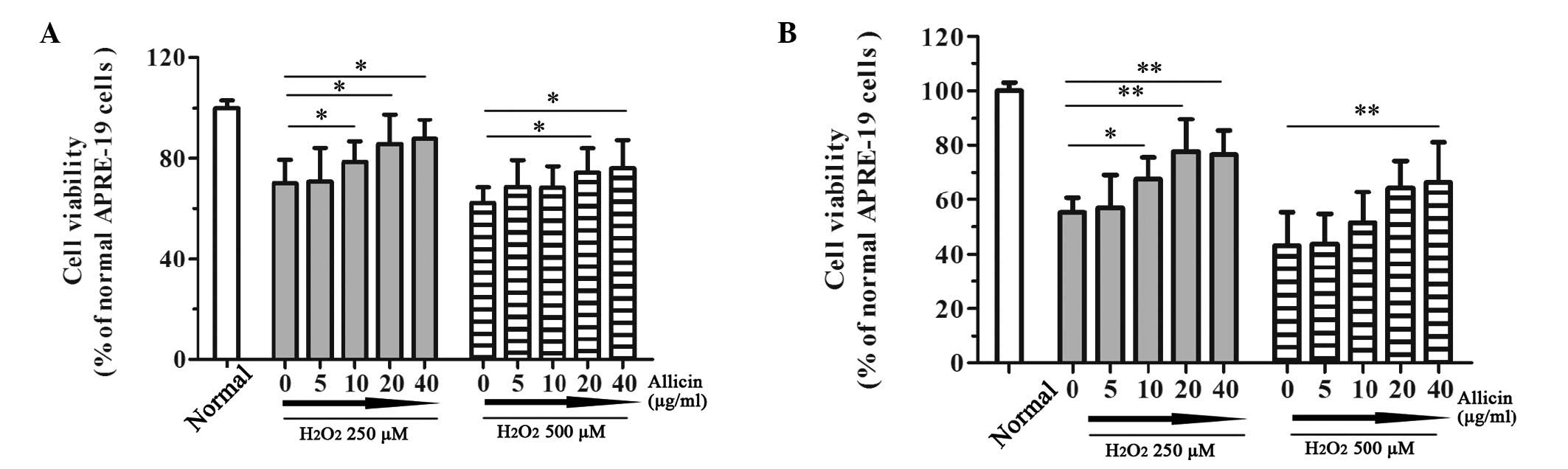
assessed using an MTT assay. Treatment with
H2O2 resulted in a significant loss of cell
viability after 12 or 24 h exposure (Fig. 1). Notably, treatment with allicin
reversed the effects of H2O2 on RPEs. As
shown in Fig. 1A, the effects of
allicin at various concentrations (5, 10, 20 or 40 µg/ml)
were determined following 12 h 250 µM
H2O2-induced loss of viability. The
percentage of viable cells was increased in response to 10–40
µg/ml allicin in a dose-dependent manner (P<0.05). These
results suggest that allicin may suppress the loss of cell
viability induced by 250 µM H2O2.
Furthermore, allicin exerted a significant protective effect
against 12 h 500 µM H2O2-induced
damage when administered at a concentration of 20 µg/ml
(P=0.039) or 40 µg/ml (P=0.032) (Fig. 1A). Following treatment with 250
µM H2O2 for 24 h, allicin
significantly attenuated the damaging effects on RPEs when
administered at 10 µg/ml (P=0.040), 20 µg/ml
(P=0.008) or 40 µg/ml (P=0.004). However, the protective
effect of allicin reached a plateau when the concentration of
allicin was increased to 20 µg/ml (Fig. 1B). In addition, allicin exerted
protective effects against 24 h 500 µM
H2O2-induced damage. These results suggest
that allicin may protect RPEs from
H2O2-induced damage.
Allicin reduces
H2O2-induced oxidative stress in RPEs
Oxidative stress is characterized by a pathological
state of excessive ROS production or abnormal ROS homeostasis
(6,20). The present study detected a
protective role for allicin with regards to combating
H2O2 stimulation in RPEs. Therefore, the
present study aimed to determine whether allicin suppressed the
H2O2-induced loss of cell viability by
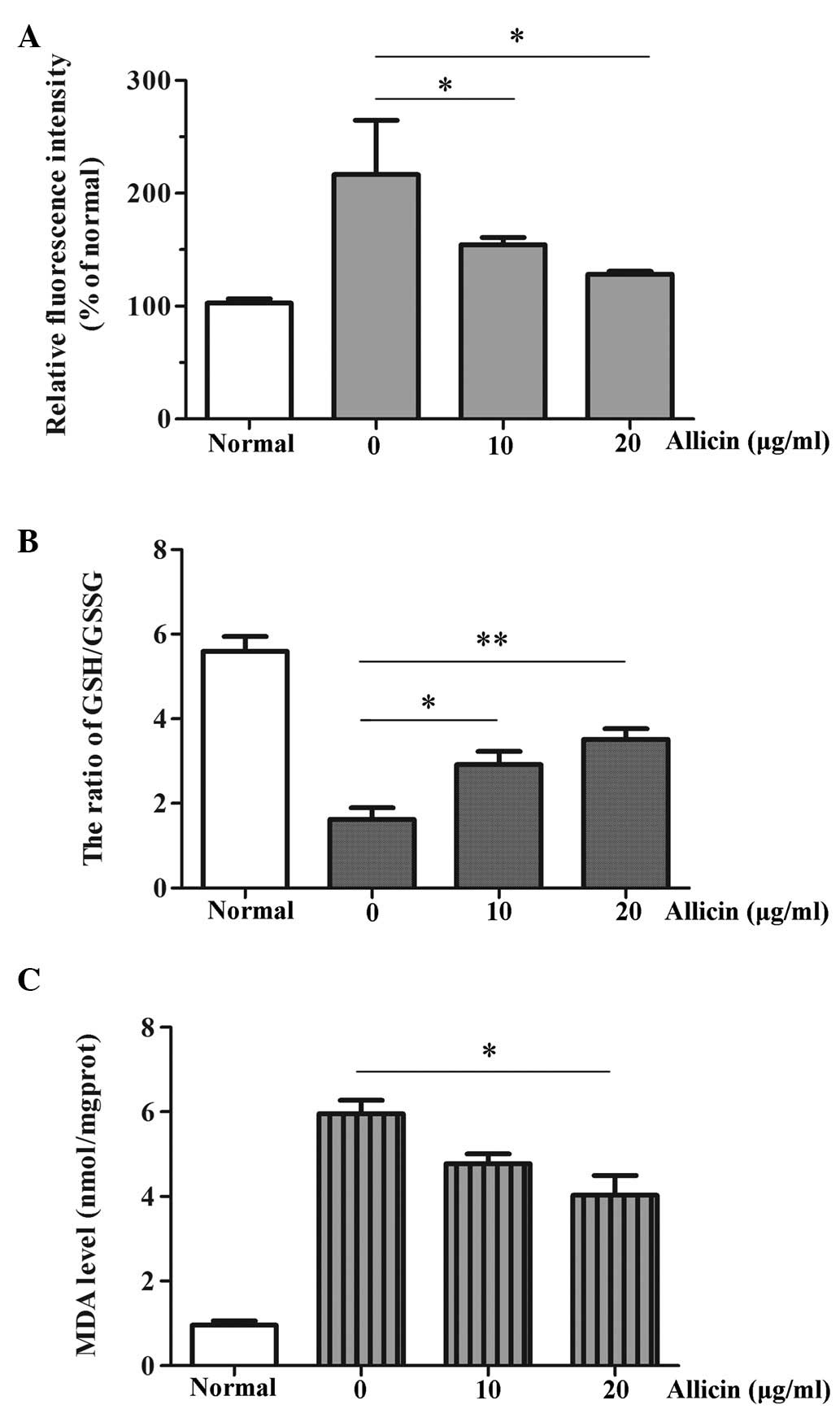
targeting ROS. The effects of allicin on intracellular ROS levels
in RPEs were determined using the DCFH-DA assay. Allicin (10 and 20
µg/ml) markedly reduced intracellular ROS levels (P=0.047
and P=0.033), as compared with in the cells not treated with
allicin (Fig. 2A). The GSH/GSSG
ratio reflects the extent of oxidative stress in cells (21). In the present study, RPEs
preincubated with allicin (10 µg/ml, P=0.037; 20
µg/ml, P=0.007) were better at combating
H2O2 injury, as compared with cells in the
H2O2 group (Fig.
2B). MDA levels are considered a biomarker of oxidative stress
(22). The present study measured
MDA levels in the ARPE-19 cells following
H2O2 stimulation and allicin preincubation
(0, 10 and 20 µg/ml). Treatment with
H2O2 resulted in a significant increase in
MDA levels, which was downregulated following allicin preincubation
in a dose dependent manner, especially when administered at 20
µg/ml (P=0.027) (Fig.
2C).
Allicin-mediated protection of RPEs is
associated with regulation of ROS-associated enzymes, SOD, NOX4 and
NQO1
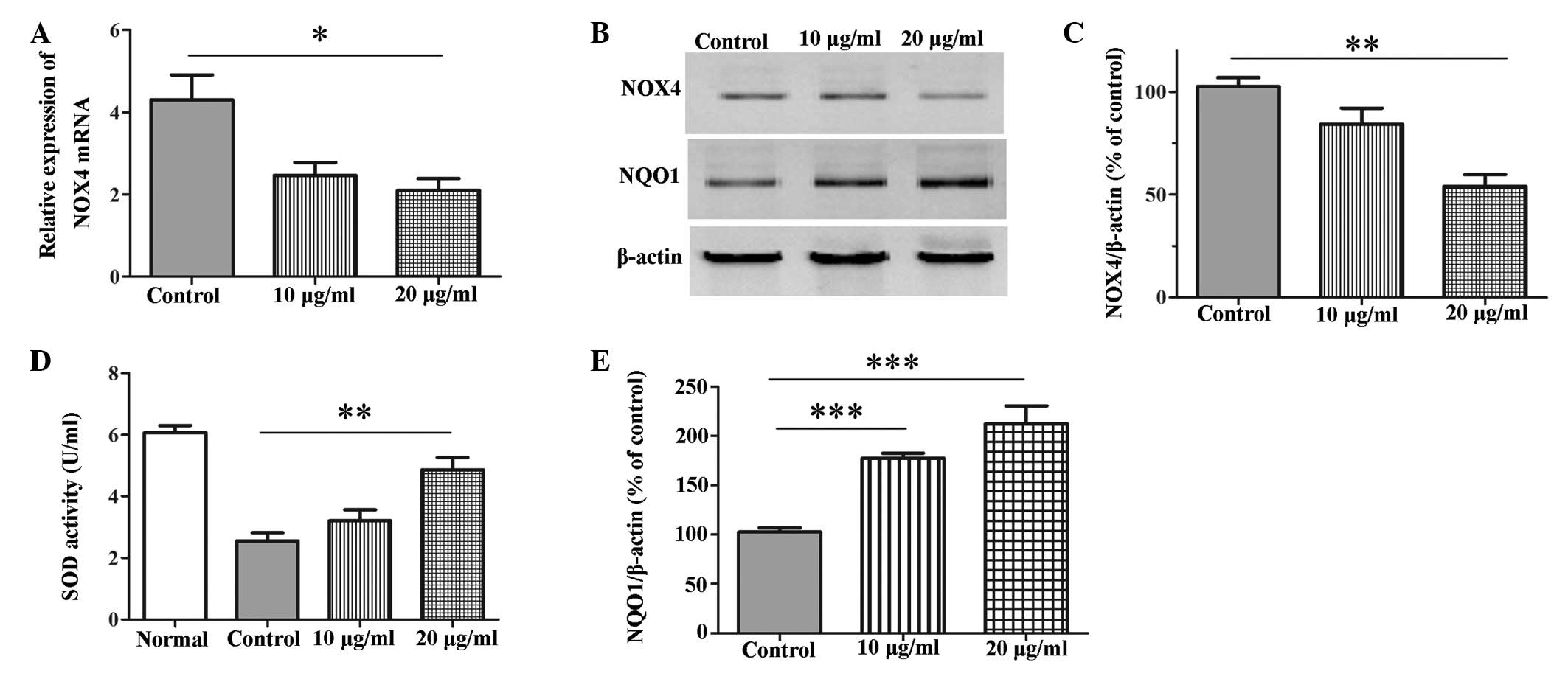
The present study detected the expression levels of
NOX4, which is the key enzyme of the ROS generation system
(23,24), SOD, which is an important biomarker
of oxidative stress (25), and
NQO1, which is an important antioxidant enzyme (26). Treatment with allicin (20
µg/ml) significantly downregulated the mRNA and protein
expression levels of NOX4 (mRNA, P=0.031; protein, P=0.003)
following exposure of the cells to 250 µM
H2O2 for 24 h (Fig. 3A–C). H2O2
significantly inhibited the levels of SOD, and pretreatment with
allicin significantly attenuated this inhibition when administered
at a concentration of 20 µg/ml (P=0.009; Fig. 3D). In addition, allicin was able to
markedly increase the protein expression levels of NQO1 when
administered at a concentration of 10 µg/ml or 20
µg/ml (P<0.001; Fig. 3B and
E).
Effects of Nrf2 regulation on
allicin-mediated protection against
H2O2-induced injury
To further understand the protective effects of
allicin against H2O2-induced damage, the
expression levels of Nrf2, which has an essential role eliminating
oxidants by reinforcing cellular antioxidant capacity, were
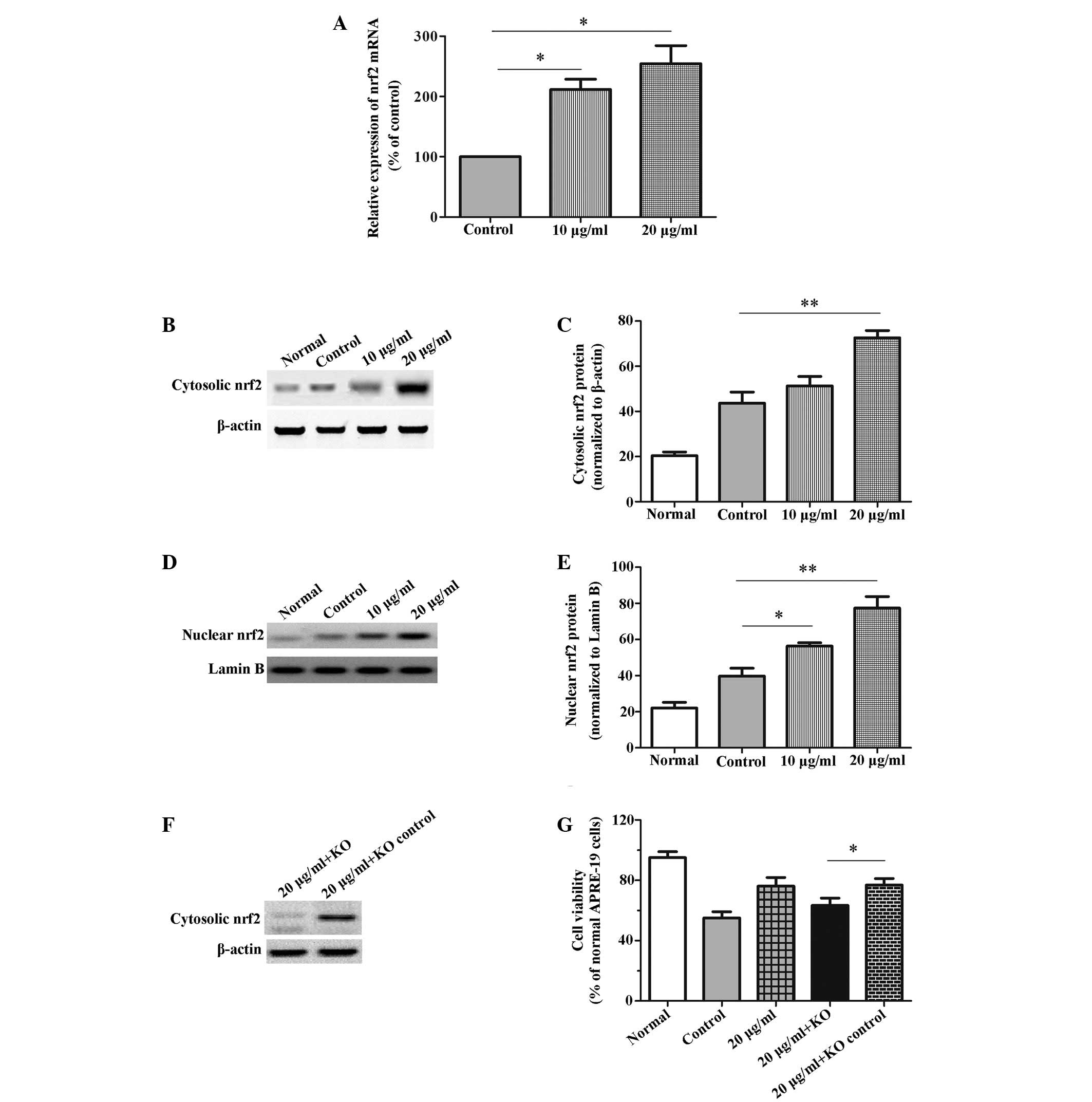
detected (27). As shown in
Fig. 4A, the relative mRNA
expression levels of Nrf2 were enhanced by allicin in a
dose-dependent manner (P<0.05), as compared with the
H2O2-treated control cells. Furthermore, the
cytosolic and nuclear protein expression levels of Nrf2 were
detected by western blotting. The cytosolic protein expression
levels of Nrf2 were significantly increased following treatment
with 20 µg/ml allicin (P=0.008; Fig. 4B and C). Furthermore, the nuclear
protein expression levels of Nrf2 were significantly elevated
following treatment with allicin at both 10 µg/ml (P=0.025)
and 20 µg/ml (P=0.008), as compared with the control cells
(Fig. 4D and E). Therefore it was
hypothesized that Nrf2 may be associated with the allicin-mediated
protection of cells against H2O2-induced
injury. To verify this hypothesis, differences in the viability of
RPEs with or without Nrf2 knockdown were detected in the presence
of H2O2 and allicin. As presented in Fig. 4F the Nrf2-siRNA effectively
silenced Nrf2 gene expression in the RPEs. Nrf2 knockdown
significantly attenuated the protection of allicin against
H2O2-mediated loss of viability (P=0.032).
However, Nrf2 knockdown did not completely suppress the protective
effects of allicin (Control group 53.34±5.01% vs. 20 µg/ml +
knockdown group 63.06±7.85%) (Fig.
4G). These data suggest that allicin may exert protective
effects against H2O2-induced damage in RPEs
not only via regulation of ROS-associated enzymes but also by
elevating the activity of the antioxidant Nrf2.
Discussion
Allicin (2-propene-1-sulfinothioic acid S-2-propenyl
ester) is a key component of garlic that is responsible for its
pungent smell (28). The roles of
allicin have been widely investigated with regards to
antimicrobial, immune-modulatory and anticancer activity (9). Previous studies have particularly
focused on the cardiovascular benefits of allicin, and its ability
to elevate antioxidant status by lowering oxidative stress
(29). Furthermore, allicin has
been suggested as a potential therapy for the treatment of
cognitive deficits in aging and Alzheimer's disease, due to its
antioxidative activity (15).
These previous findings have provided evidence suggesting that
supplementation of allicin may improve antioxidant status and slow
the progression of AMD; however, the effects of allicin on RPEs
against oxidative stress, and the underlying mechanisms, have yet
to be elucidated. Oxidative stress of RPEs has been considered a
crucial role with regards to the pathophysiology of AMD (4). Therefore, recent studies have focused
on strategies that protect RPEs from oxidative stress for the
treatment of AMD. Exposure to H2O2 is often
used as a classic model to convey the oxidative stress
susceptibility and antioxidant activity of RPEs (30,31).
The present study determined the protective effects of allicin on
RPEs exposed to H2O2. Notably, allicin was
able to attenuate the reduced viability induced by
H2O2. These results suggested that allicin
may exert protective effects against oxidative stress-induced
damage in RPEs. These data further our understanding of the
protective effects of allicin against oxidative stress not only
with regards to neuroprotection and cardioprotection (14,15,29,32),
but also vision protection.
Intracellular accumulation of ROS is associated with
oxidative stress and RPE dysfunction (33,34).
Reductions in the levels of intracellular ROS are able to protect
RPEs from oxidative stress (33,35).
The results of the present study demonstrated that allicin markedly
reduced H2O2-induced intracellular ROS levels
in RPEs. The ratio of GSH/GSSG is known to reflect the extent of
oxidative stress in cells (21).
The present study demonstrated that preincubation with allicin
increased the GSH/GSSG ratio, thus suggesting that allicin may
combat oxidative stress. In addition, the levels of MDA, a
biomarker of oxidative stress, were measured. MDA levels indirectly
reflect the severity of oxidative stress caused by free radicals
(13). In the present study,
allicin preincubation reduced the
H2O2-induced levels of MDA in ARPE-19 cells.
These data indicated that allicin may possess the ability to
indirectly scavenge oxygen free radicals. Therefore allicin may
decrease H2O2-induced oxidative stress in
ARPE-19 cells by decreasing intracellular ROS levels and scavenging
oxygen free radicals.
The present study also aimed to determine the
mechanisms underlying the protective effects of allicin against
oxidative stress in ARPE-19 cells. The present study demonstrated
that allicin may reduce the levels of intracellular ROS in ARPE-19
cells exposed to H2O2. Therefore, the
expression levels of NOX4, a key enzyme associated with ROS
generation, were measured (23,24).
Notably, following treatment with allicin, the NOX4 protein and
mRNA expression levels were attenuated. Furthermore, SOD, which is
an enzyme capable of indirectly scavenging oxygen free radicals
(16), was measured; allicin
significantly elevated the levels of SOD. These results further
supported the hypothesis that allicin may be able to indirectly
scavenge oxygen free radicals in ARPE-19 cells exposed to oxidative
stress. Chan et al (13)
reported that allicin inhibited intracellular ROS production,
rather than scavenging extracellular H2O2 or
free radicals in H9C2 cells. Conversely, allicin has been shown to
exhibit the ability to indirectly scavenge oxygen free radicals in
human umbilical vein endothelial cells (16), which is consistent with the
findings of the present study. Therefore, the present study
hypothesized that allicin may exert various biological abilities in
order to protect against oxidative stress in various cell lines and
tissues. Furthermore, NQO1 is an important enzyme, which has an
essential antioxidant role (25).
The protein expression levels of NQO1 were upregulated in
H2O2-stimulated ARPE-19 cells following
allicin pretreatment. These data indicated that protection of RPEs
is dependent on the regulation of enzymes that reduce intracellular
ROS production, scavenge free radicals and enhance antioxidant
ability.
Nrf2 is a ubiquitous transcription factor, which has
critical effects on maintenance of cellular homeostasis (36). It is well-known that Nrf2 mediates
an antioxidant response pathway that promotes the primary cellular
defense mechanism against oxidative stress induced by xeno biotic
exposure and other factors (36,37).
The antioxidative effects of allicin are associated with the Nrf2
antioxidant signaling pathway in cardiovascular diseases,
neurodegenerative diseases and cancer (15,38,39).
To further investigate the antioxidative effects of allicin on
ARPE-19 cells exposed to H2O2, the expression
levels of Nrf2 were detected. Nrf2 expression levels were
significantly elevated following treatment with allicin.
Furthermore, knockdown of Nrf2 significantly attenuated the
protective effects of allicin against
H2O2-mediated loss of viability. These
results indicated that the Nrf2-mediated antioxidant response
pathway is associated with the antioxidative effects of allicin on
RPEs exposed to oxidative stress.
In conclusion, allicin exerted protective effects
against oxidative stress-induced damage of RPEs via regulation of
the ROS system, including reducing intracellular ROS production,
scavenging free radicals, enhancing antioxidant ability, and
stimulating the Nrf2-mediated antioxidant response pathway. These
findings suggested that allicin may be considered a potential drug
for the prevention and treatment of AMD.
Acknowledgments
The present study was supported by the Renmin
Hospital of Wuhan University (grant no. RHWU-13-2012) and Inner
Mongolia People's Hospital (grant no. 2012-16-02).
References
|
1
|
Wang ZY, Shen LJ, Tu L, Hu DN, Liu GY,
Zhou ZL, Lin Y, Chen LH and Qu J: Erythropoietin protects retinal
pigment epithelial cells from oxidative damage. Free Radic Biol
Med. 46:1032–1041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Winkler BS, Boulton ME, Gottsch JD and
Sternberg P: Oxidative damage and age-related macular degeneration.
Mol Vis. 5:321999.PubMed/NCBI
|
|
3
|
Congdon NG, Friedman DS and Lietman T:
Important causes of visual impairment in the world today. JAMA.
290:2057–2060. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beatty S, Koh H, Phil M, Henson D and
Boulton M: The role of oxidative stress in the pathogenesis of
age-related macular degeneration. Surv Ophthalmol. 45:115–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dentchev T, Hahn P and Dunaief JL: Strong
labeling for iron and the iron-handling proteins ferritin and
ferroportin in the photoreceptor layer in age-related macular
degeneration. Arch Ophthalmol. 123:1745–1746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kietzmann T: Intracellular redox
compartments: Mechanisms and significances. Antioxid Redox Signal.
13:395–398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rishton GM: Natural products as a robust
source of new drugs and drug leads: Past successes and present day
issues. Am J Cardiol. 101:43D–49D. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chan SW: Panax ginseng, Rhodiola rosea and
Schisandra chinensis. Int J Food Sci Nutr. 63(Suppl 1): 75–81.
2012. View Article : Google Scholar
|
|
9
|
Borlinghaus J, Albrecht F, Gruhlke MC,
Nwachukwu ID and Slusarenko AJ: Allicin: Chemistry and biological
properties. Molecules. 19:12591–12618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kita T, Kume N, Minami M, Hayashida K,
Murayama T, Sano H, Moriwaki H, Kataoka H, Nishi E, Horiuchi H, et
al: Role of oxidized LDL in atherosclerosis. Ann NY Acad Sci.
947:199–205; discussion 205–206. 2001. View Article : Google Scholar
|
|
11
|
Arzanlou M, Bohlooli S, Jannati E and
Mirzanejad-Asl H: Allicin from garlic neutralizes the hemolytic
activity of intra- and extra-cellular pneumolysin O in vitro.
Toxicon. 57:540–545. 2011. View Article : Google Scholar
|
|
12
|
Izigov N, Farzam N and Savion N:
S-allylmercapto-N-acetylcysteine up-regulates cellular glutathione
and protects vascular endothelial cells from oxidative stress. Free
Radic Biol Med. 50:1131–1139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan JY, Tsui HT, Chung IY, Chan RY, Kwan
YW and Chan SW: Allicin protects rat cardiomyoblasts (H9c2 cells)
from hydrogen peroxide-induced oxidative injury through inhibiting
the generation of intracellular reactive oxygen species. Int J Food
Sci Nutr. 65:868–873. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abramovitz D, Gavri S, Harats D, Levkovitz
H, Mirelman D, Miron T, Eilat-Adar S, Rabinkov A, Wilchek M, Eldar
M and Vered Z: Allicin-induced decrease in formation of fatty
streaks (atherosclerosis) in mice fed a cholesterol-rich diet.
Coron Artery Dis. 10:515–519. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XH, Li CY, Lu JM, Tian RB and Wei J:
Allicin ameliorates cognitive deficits ageing-induced learning and
memory deficits through enhancing of Nrf2 antioxidant signaling
pathways. Neurosci Lett. 514:46–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Tang Y, Qian Y, Chen R, Zhang L,
Wo L and Chai H: Allicin prevents H2O2
-induced apoptosis of HUVECs by inhibiting an oxidative stress
pathway. BMC Complement Altern Med. 14:3212014. View Article : Google Scholar
|
|
17
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW 264.7 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koskela A, Reinisalo M, Hyttinen JM,
Kaarniranta K and Karjalainen RO: Pinosylvin-mediated protection
against oxidative stress in human retinal pigment epithelial cells.
Mol Vis. 20:760–769. 2014.PubMed/NCBI
|
|
20
|
Wasserman WW and Fahl WE: Functional
antioxidant responsive elements. Proc Natl Acad Sci USA.
94:5361–5366. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin M, Li L, Zhang Y, Zheng L, Xu M, Rong
R and Zhu T: Baicalin ameliorates H2O2
induced cytotoxicity in HK-2 cells through the inhibition of ER
stress and the activation of Nrf2 signaling. Int J Mol Sci.
15:12507–12522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ho E, Karimi Galougahi K, Liu CC, Bhindi R
and Figtree GA: Biological markers of oxidative stress:
Applications to cardiovascular research and practice. Redox Biol.
1:483–491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: Physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Block K, Gorin Y and Abboud HE:
Subcellular localization of Nox4 and regulation in diabetes. Proc
Natl Acad Sci USA. 106:14385–14390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu C, Pan F, Ge L, Zhou J, Chen L, Zhou
T, Zong R, Xiao X, Dong N, Yang M, et al: SERPINA3K plays
antioxidant roles in cultured pterygial epithelial cells through
regulating ROS system. PLoS One. 9:e1088592014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schelonka LP, Siegel D, Wilson MW,
Meininger A and Ross D: Immunohistochemical localization of NQO1 in
epithelial dysplasia and neoplasia and in donor eyes. Invest
Ophthalmol Vis Sci. 41:1617–1622. 2000.PubMed/NCBI
|
|
27
|
Nguyen T, Nioi P and Pickett CB: The
Nrf2-antioxidant response element signaling pathway and its
activation by oxidative stress. J Biol Chem. 284:13291–13295. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu YL, Ho CT, Chung JG, Rajasekaran R and
Sheen LY: Allicin induces p53-mediated autophagy in Hep G2 human
liver cancer cells. J Agric Food Chem. 60:8363–8371. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan JY, Yuen AC, Chan RY and Chan SW: A
review of the cardiovascular benefits and antioxidant properties of
allicin. Phytother Res. 27:637–646. 2013. View Article : Google Scholar
|
|
30
|
Geiger RC, Waters CM, Kamp DW and
Glucksberg MR: KGF prevents oxygen-mediated damage in ARPE-19
cells. Invest Ophthalmol Vis Sci. 46:3435–3442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zareba M, Raciti MW, Henry MM, Sarna T and
Burke JM: Oxidative stress in ARPE-19 cultures: Do melanosomes
confer cytoprotection? Free Radic Biol Med. 40:87–100. 2006.
View Article : Google Scholar
|
|
32
|
Zhu JW, Chen T, Guan J, Liu WB and Liu J:
Neuroprotective effects of allicin on spinal cord
ischemia-reperfusion injury via improvement of mitochondrial
function in rabbits. Neurochem Int. 61:640–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yamada Y, Tian J, Yang Y, Cutler RG, Wu T,
Telljohann RS, Mattson MP and Handa JT: Oxidized low density
lipoproteins induce a pathologic response by retinal pigmented
epithelial cells. J Neurochem. 105:1187–1197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Witmer AN, Vrensen GF, Van Noorden CJ and
Schlingemann RO: Vascular endothelial growth factors and
angiogenesis in eye disease. Prog Retin Eye Res. 22:1–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cakir Y and Ballinger SW: Reactive
species-mediated regulation of cell signaling and the cell cycle:
The role of MAPK. Antioxid Redox Signal. 7:726–740. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma Q and He X: Molecular basis of
electrophilic and oxidative defense: Promises and perils of Nrf2.
Pharmacol Rev. 64:1055–1081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cardozo LF, Pedruzzi LM, Stenvinkel P,
Stockler-Pinto MB, Daleprane JB, Leite M Jr and Mafra D:
Nutritional strategies to modulate inflammation and oxidative
stress pathways via activation of the master antioxidant switch
Nrf2. Biochimie. 95:1525–1533. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bat-Chen W, Golan T, Peri I, Ludmer Z and
Schwartz B: Allicin purified from fresh garlic cloves induces
apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 62:947–957.
2010. View Article : Google Scholar : PubMed/NCBI
|