Introduction
Kaempferol is a flavonoid compound that is found in
a variety of vegetables and fruits (1,2); its
chemical structure is presented in Fig. 1A. Kaempferol has been used in
traditional medicine and has attracted widespread attention due to
its various biological functions, including its role as an
antioxidant (3), anti-inflammatory
(4) and antitumor (5) compound. It has been demonstrated to
have number of antitumor effects, including preventing metastasis
in oral cancer (6) and inducing
apoptosis of colorectal (7),
breast (8,9) and prostate cancer (10,11)
and leukemia cells (12). A
previous study indicated that kaempferol may induce autophagic cell
death in SK-HEP-1 human hepatic cancer cells (13). However, to the best of our
knowledge, the molecular mechanisms behind the antitumor effects of
kaempferol on hepatocellular carcinoma (HCC) remain unknown.
The endoplasmic reticulum (ER) is involved in
numerous functions, including protein synthesis, folding and
secretion. Disturbances of ER function by stimuli, such as DNA
damage, hypoxia, nutritional deprivation and drug toxicity, lead to
the ER stress response, which subsequently triggers the unfolded
protein response (UPR). As a self-protection mechanism, the UPR
reduces protein synthesis and increases the expression of ER
molecular chaperones glucose-regulated protein 78 (GRP78) and GRP94
to facilitate the correct folding of proteins (14,15).
Protein kinase RNA-like ER kinase (PERK), inositol-requiring enzyme
1α (IRE1α) and activating transcription factor 6 (ATF-6) are the
three major transmembrane ER proteins. During ER stress, PERK and
IRE1α are activated and repress protein synthesis via the
phosphorylation of the translation initiation factor, eukaryotic
initiation factor 2α. Meanwhile, ATF-6 is transported to the Golgi
apparatus, where it is cleaved by Site-1 and Site-2 proteases
(14,15). Collectively, these factors activate
downstream signaling molecules that trigger a cascade of
reactions.
Excessive and prolonged ER stress leads to cellular
damage and eventually induces apoptosis. The C/EBP homologous
protein (CHOP) is the point of convergence for the three
aforementioned ER stress transducers (PERK, IRE1α and ATF-6) and is
also the most well-characterized factor in the transition of ER
stress to apoptosis (16,17). Proteins in the caspase family are
the primary drivers of apoptosis. Human caspase-4 is uniquely
located in the ER membrane, where it is specifically activated by
ER stress. Similar to caspase-12 in mice, caspase-4 activates
caspase-9, in addition to other molecules such as caspase-3,
eventually resulting in cell apoptosis (18,19).
A previous study indicated that kaempferol induces
apoptosis via the ER stress pathway in U2OS human osteosarcoma
cells (20). However, to the best
of our knowledge, the importance of ER stress in the antitumor
activity of kaempferol in HCC has not been previously elucidated.
The present study demonstrated that kaempferol triggers HepG2
apoptosis in a concentration- and time-dependent manner, and
indicated that the activation of the ER stress-CHOP pathway is
critical for kaempferol-induced apoptosis of HepG2 cells.
Materials and methods
Cells and cell culture
The HepG2 human hepatic cancer cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA). The cells were maintained in Dulbecco's modified Eagle's
medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) and antibiotics (100 U/ml penicillin and 100 mg/ml
streptomycin; Beyotime Institute of Biotechnology, Haimen, China)
and incubated at 37°C under a humidified atmosphere of 5%
CO2.
MTT assay for cell viability
HepG2 cells were seeded in 96-well culture plates at
a density of 1×104 cells/well. Subsequent to overnight
growth, the cells were incubated with 0, 5, 10, 25 50 and 100
µM kaempferol (Sigma-Aldrich, St. Louis, MO, USA) for 24 h.
A separate group of HepG2 cells was treated with 100 µM
kaempferol for time periods of 3, 6, 12 and 24 h. Kaempferol was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and the
final concentration of DMSO in the culture medium was maintained at
<0.1% (v/v). The vehicle (VE) and blank control (BC) groups were
established. For the viability assay, at the end of each treatment,
200 µl culture medium containing 0.5 mg/ml
3-(4,5-dimeth-ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Amresco LLC, Solon, OH, USA) was added to each well and the mixture
was incubated at 37°C for 4 h. The supernatant was removed,
formazan crystals were dissolved in 150 µl DMSO and the
absorbance was measured at 570 nm using a microplate reader
(Multiskan MK3; Bio-Rad Laboratories, Inc., Hercules, CA, USA). To
determine how kaempferol triggers apoptosis via molecular signaling
pathways, the following three additional treatment groups were
established: i) 4-Phenyl butyric acid (4-PBA; Sigma-Aldrich)
pretreatment group, where cells were pretreated with 4-PBA at 1 mM
for 30 min; ii) transfection group with CHOP small interfering (si)
RNA; and iii) plasmid group with a CHOP overexpressing plasmid
(Shanghai GenePharma Co., Ltd., Shanghai, China). HepG2 cells were
then exposed to 100 µM kaempferol for 24 h, and an MTT assay
was performed as abovementioned. Cell viability was calculated as
follows: [(Akaempferol treatment
group−ABC/AVE−ABC)] ×100,
where A represents absorbance.
LDH activity assay
A colorimetric lactate dehydrogenase (LDH) activity
assay kit (Applygen Technologies, Inc., Beijing, China) was used to
quantify the level of LDH released into the supernatant from the
damaged cells. This assay was performed subsequent to treatment
application. The supernatants from each treatment group were
collected and the assay was performed according to the
manufacturer's protocol.
Flow cytometric analysis
An Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double-staining assay (Nanjing KeyGen
Biotech, Co., Ltd., Nanjing, China) was used to quantify apoptosis.
HepG2 cells were cultivated in 60 mm culture plates for 24 h at
37°C. Following exposure to the indicated treatments, cells were
harvested by trypsinization (Sigma-Aldrich), washed with
phosphate-buffered saline (PBS), pelleted by centrifugation at 800
× g and 4°C for 5 min, and resuspended in 0.5 ml binding buffer
(Nanjing KeyGen Biotech, Co., Ltd.). The cells were incubated with
5 ml annexin V-FITC and 5 ml PI working solution for 15 min at room
temperature in the dark. The samples were analyzed on a FACScan
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using
FlowJo software (version 7.6; FlowJo LLC, Ashland, OR, USA). Double
staining of cells with annexin V-FITC and PI enabled the
identification of different cell populations based on their
staining patterns, as follows: Lower left quadrant, live cells
(FITC−PI−); lower right quadrant, early
apoptotic cells (FITC+PI−); upper right
quadrant, late apoptotic cells (FITC+PI+);
upper left quadrant, necrotic cells
(FITC−PI+).
CHOP siRNA treatment in vitro
At 24 h prior to transfection, HepG2 cells were
prepared in 12-well culture plates. Cells were transfected with 20
µM human CHOP siRNA and negative control siRNA using
Lipofectamine 2000 reagent for 6 h according to the manufacturer's
protocol (Shanghai GenePharma Co., Ltd.). CHOP siRNA sequences were
designed as follows: Forward (F) 5′-GAGCUCUGAUUGACCGAAUTT-3′ and
reverse (R) 5′-AUUCGGUCAAUCAGAGCUCTT-3′; control siRNA, F: 5′-UGA
GAG UGU CAG CAA UTT CCU-3′ and R: 5′-AUC GCU UCA AAG UUA GCG
CTT-3′. The transfected cells were then treated with 100 µM
kaempferol for 24 h.
CHOP overexpression plasmid treatment in
vitro
HepG2 cells were cultured in 12-well culture plates
of 1 ml volumes. CHOP overexpression plasmid (Shanghai GenePharma
Co., Ltd.) and the empty vector control plasmid were transfected
into HepG2 cells using Lipofectamine 2000 reagent, according to the
manufacturer's protocol (Shanghai GenePharma Co., Ltd.). Following
a 24 h transfection, HepG2 cells were treated with ER stress
inhibitor 4-PBA at 1 mM for 30 min at 37°C, and cultured with 100
µM kaempferol for an additional 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The mRNA levels of target genes were evaluated using
RT-qPCR. HepG2 cells were cultured at a concentration of
1×105 cells/well in 12-well culture plates. Cells were
cultured for 24 h prior to treatments. Total RNA was extracted from
HepG2 cells using TRIzol reagent and then reverse-transcribed into
cDNA by PrimeScript First Strand cDNA Synthesis kit (Takara Bio,
Inc., Otsu, Japan), following the manufacturer's protocol. The
hypoxanthine phosphoribosyl transferase (HPRT) gene was selected as
an endogenous control. PCR was performed in a reaction mixture (20
µl) containing 4 µl cDNA, 0.4 µl each primer
(10 µM), 5.2 µl diethylpyrocarbonate water and 10
µl SYBR Green (Takara Bio, Inc.) using a quantitative PCR
instrument (ABI Prism 7500; Applied Biosystems Inc., Waltham, MA,
USA). The reaction was processed with an initial 2 min denaturation
step at 50°C, followed by 95°C for 5 min, 95°C for 15 sec, and 60°C
for 30 sec, for 40 cycles, and then 55°C for 4 sec for 41 cycles.
The mRNA levels were calculated using the 2−ΔΔCq method
(21). The specific primer
sequences for these genes were as follows: HPRT, F
5′-TCAACGGGGGACATAAAAGT-3′ and R 5′-TGCATTGTTTTACCAGTGTCAA-3′;
CHOP, F 5′-CCTAGCTTGGCTGACAGAGG-3′ and R
5′-CTGCTCCTTCTCCTTCATGC-3′; GPR78, F 5′-AGTGGTGCCTACCAAGAAGTCTCA-3′
and R 5′-TGTCAGGGGTCTTTCACCTTCATA-3′; GPR94, F
5′-ACGTGGTCTGTTTGACGAATATGG-3′ and R
5′-TACACGGCGCACATAGAGCTTAAT-3′.
Western blot analysis
Subsequent to the indicated treatments, cells were
scraped off and washed with ice-cold PBS, and then lysed with
radioimmunoprecipitation assay buffer (Sigma-Aldrich) containing a
mixture of protease inhibitors. A total of 30 µg of protein
from each sample was separated by 12% sodium dodecyl
sulfate-polyacrylamide gel (Sigma-Aldrich) electrophoresis at 80 v
for 30 min and 120 v for 1 h, and then electrotransferred onto
nitrocellulose membranes (Bio-Rad Laboratories, Inc.) using the
Bio-Rad Laboratories, Inc. transfer blotting system. The membranes
were subsequently incubated with 5% skimmed milk in Tris-buffered
saline with Tween-20 (Beyotime Institute of Biotechnology) for 1 h
to block nonspecific binding and then overnight with the following
antibodies: Monoclonal rabbit anti-human GRP78 (1:1000; cat. no.
3177; Cell Signaling Technology, Inc., Danvers, MA, USA),
monoclonal rabbit anti-human GRP94 (1:1,000; cat. no. 20292; Cell
Signaling Technology, Inc.), monoclonal rabbit anti-human PERK
(1:1,000; cat. no. 5683; Cell Signaling Technology, Inc.),
monoclonal mouse anti-human partial ATF-6 (1:1,000; cat. no.
IMG-273; Imgenex Corporation, San Diego, CA, USA), monoclonal
rabbit anti-human IRE1α (1:1,000; cat. no. 3294; Cell Signaling
Technology, Inc.), monoclonal rabbit anti-human caspase-4 (1:1,000;
cat. no. 4450; Cell Signaling Technology, Inc.), monoclonal mouse
anti-human CHOP (1:1,000; cat. no. 2895; Cell Signaling Technology,
Inc.), monoclonal rabbit anti-human cleaved caspase-3 (1:1,000;
cat. no. 9664; Cell Signaling Technology, Inc.) and monoclonal
rabbit anti-human β-actin (1:1,000; cat. no. 4970; Cell Signaling
Technology, Inc.) at 4°C, followed by incubation with monoclonal
goat anti-rabbit IgG (1:2,000; cat. no. 14708; Cell Signaling
Technology, Inc.) secondary antibody for 1 h at room temperature.
Proteins were visualized using an enhanced chemiluminescence
commercial kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA).
The absorbance of each well containing the protein and reagent was
determined at a wavelength of 630 nm using a microplate reader
(Multiskan MK3; Thermo Fisher Scientific, Inc.) and a protein
concentration standard curve was established to calculate the
concentration of each protein sample.
Statistical analysis
Statistical analyses were performed using SPSS
statistical software (version 16.0: SPSS, Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation from a minimum
of three separate experiments. Statistical comparisons between
groups were performed using Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
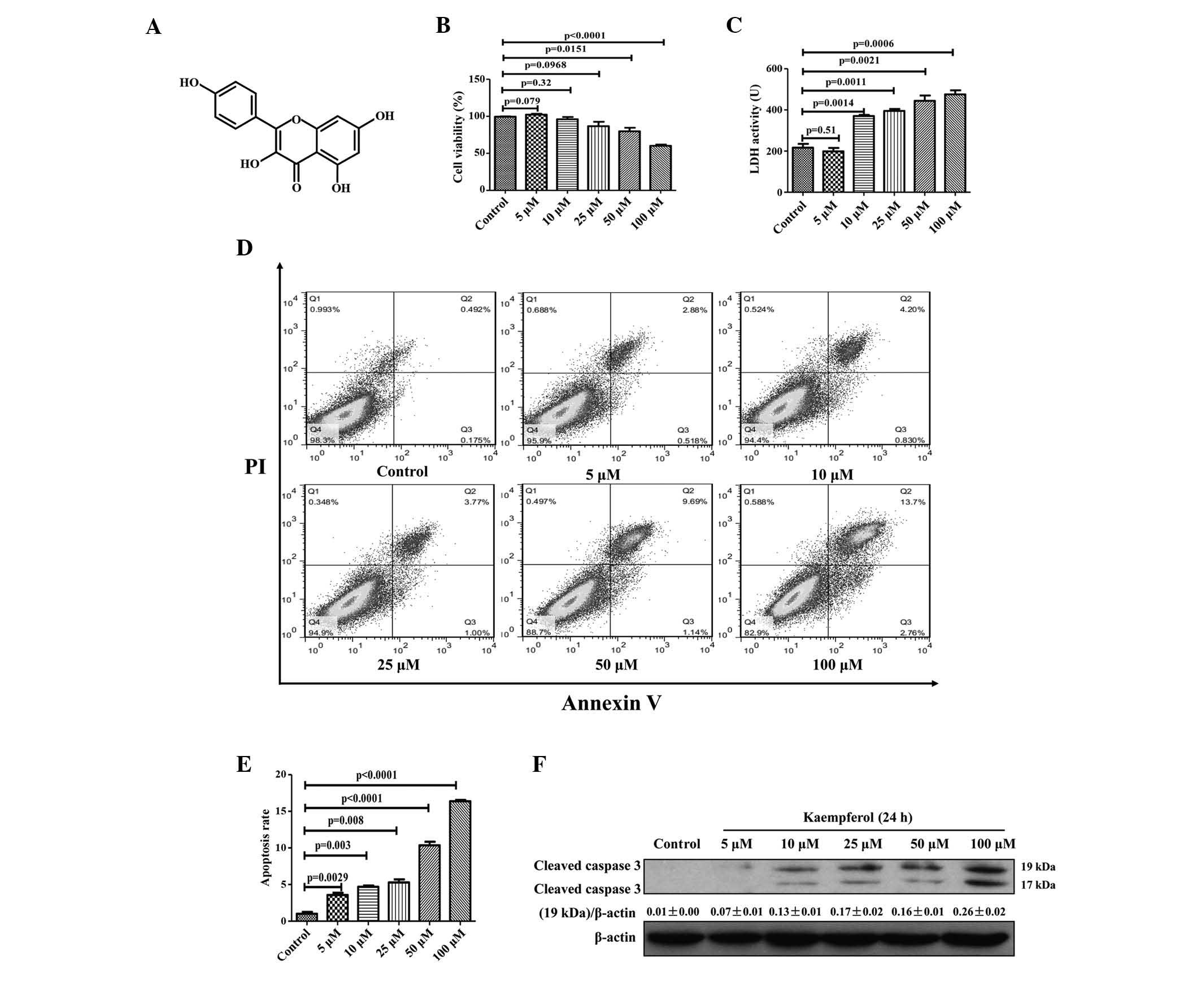
Kaempferol induces apoptosis of HepG2
cells in a dose-dependent manner
To investigate the effects of different
concentrations of kaempferol on apoptosis, HepG2 cells were treated
with 0–100 µM kaempferol for 24 h. Cell viability was
significantly reduced at 100 µM kaempferol concentration
compared with the control group (P<0.0001; Fig. 1B). Additionally, LDH activity
(P=0.0006) and the rate of apoptosis significantly increased at 100
µM kaempferol (P<0.0001; Fig. 1C–E). Western blotting also
indicated that kaempferol triggers expression of cleaved caspase-3
(Fig. 1F). The results suggest
that kaempferol induces apoptosis in HepG2 cells in a
dose-dependent manner.
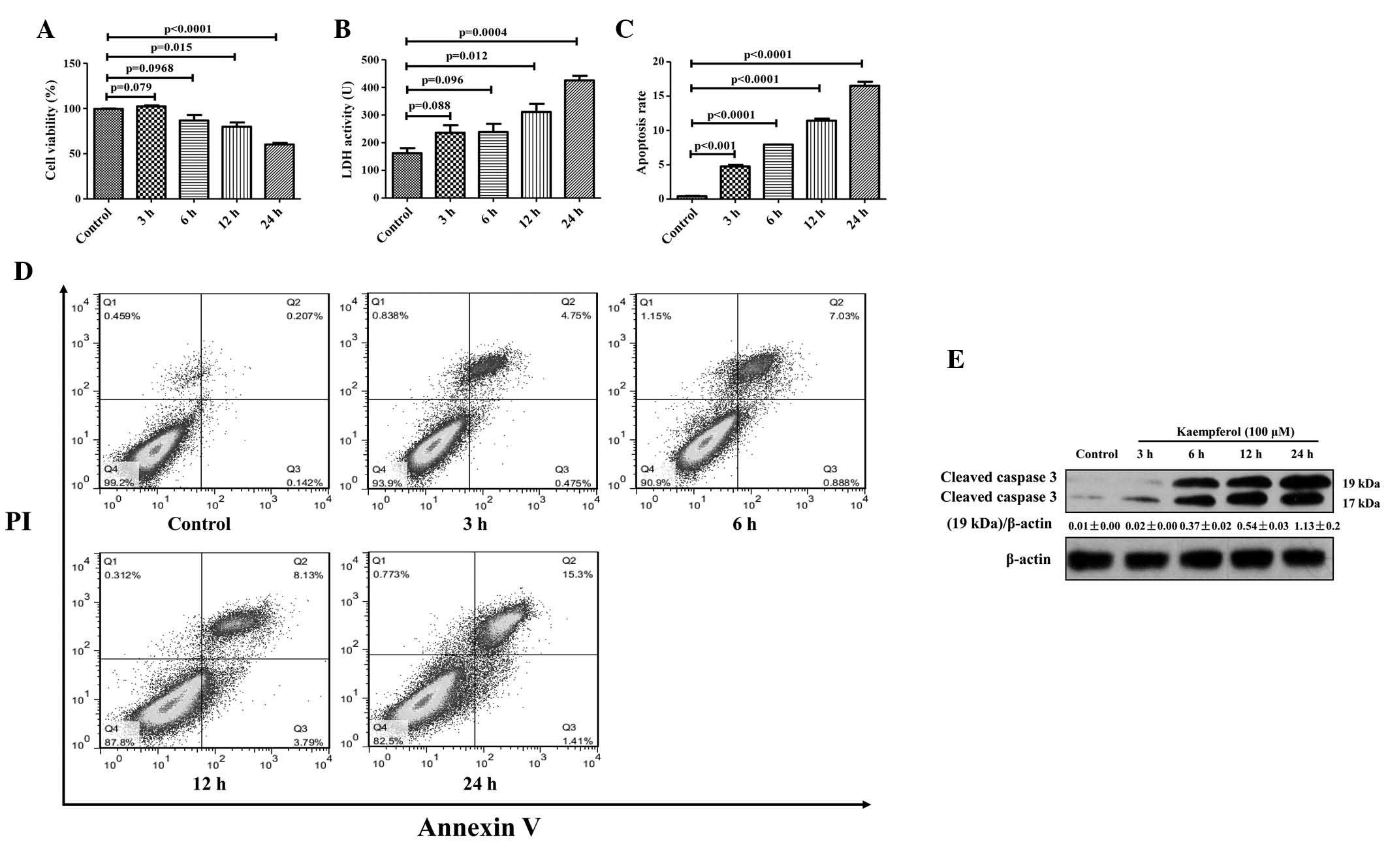
Kaempferol induces HepG2 apoptosis in a
time-dependent manner
To examine whether kaempferol triggers HepG2 cells
apoptosis in a time-dependent manner, HepG2 cells were treated with
100 µM kaempferol for 0, 3, 6, 12 and 24 h. Kaempferol
gradually inhibited the proliferation of HepG2 cells and promoted
cell death between 12 and 24 h. Following 24 h of 100 µM
kaempferol treatment, cell viability was significantly reduced
(P<0.0001; Fig. 2A). There was
greater LDH activity (P<0.05; Fig.
2B) and an increased rate of apoptosis (P<0.0001; Fig. 2C and D) in cells exposed to
kaempferol for ≥3 h compared with the control group. The expression
of cleaved caspase-3 was also elevated (Fig. 2E). These data indicate that
kaempferol induces apoptosis in HepG2 cells in a time-dependent
manner.
Kaempferol triggers ER stress response in
a dose- and time-dependent manner
In order to explore the molecular mechanisms of
kaempferol-induced apoptosis, the protein and mRNA expression
levels of ER stress markers, including GRP78, GRP94, PERK, partial
ATF-6, IRE1α, caspase-4 and CHOP were determined, using western
blotting and RT-qPCR, respectively. Kaempferol treatment led to an
increase of these protein and mRNA levels in a dose-and
time-dependent manner (Fig. 3A and
B). The protein and mRNA levels of GRP78, GRP94 and CHOP were
also significantly increased with in a dose-dependant manner
(Fig. 3C and D). Therefore, the
results indicate that kaempferol-induced apoptosis in HepG2 cells
is associated with the induction of the ER stress response.
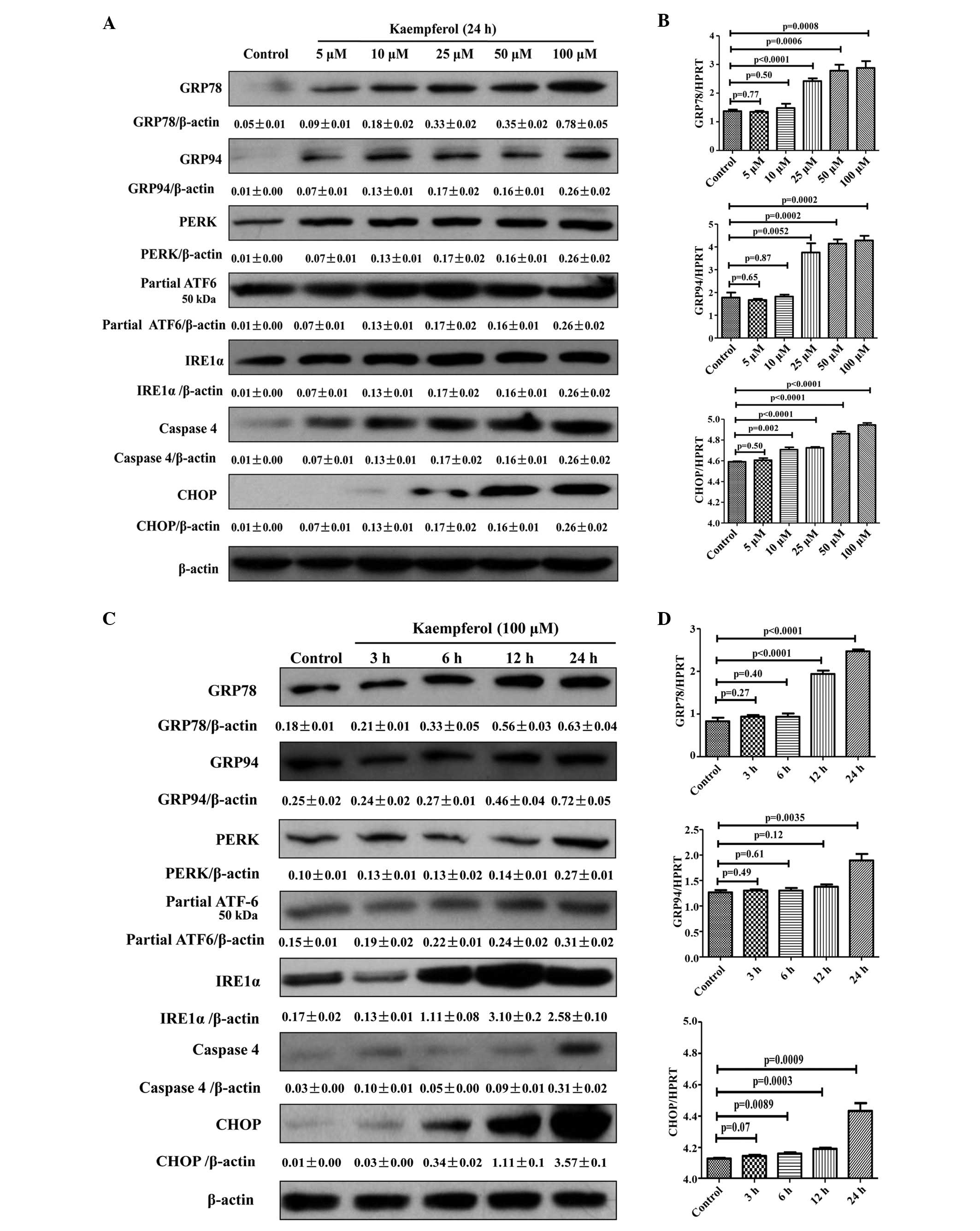 | Figure 3Dose- and time-dependent effects of
kaempferol on protein and mRNA levels of endoplasmic reticulum
stress markers. (A and B) For dose-dependent effects, HepG2 cells
were treated in the presence or absence of different concentrations
of kaempferol for 24 h. Western blotting and RT-qPCR were performed
with GRP78, GRP94, PERK, partial ATF-6, IRE1α, caspase-4 and CHOP
antibodies. (C and D) For time-dependent effects, HepG2 cells were
exposed to 100 µM kaempferol for different time periods.
Western blotting and RT-qPCR were performed with GRP78, GRP94,
PERK, partial ATF-6, IRE1α, caspase-4 and CHOP antibodies. Data are
expressed as the mean ± standard deviation of at least three
independent experiments. GRP78, glucose regulated protein 78;
GRP94, glucose regulated protein 94; PERK, protein kinase R-like
endoplasmic reticulum kinase; ATF-6, activation transcription
factor 6; IRE1α, inositol-requiring enzyme 1α; CHOP, C/EBP
homologous protein; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; HPRT, hypoxanthine phosphoribosyl
transferase. |
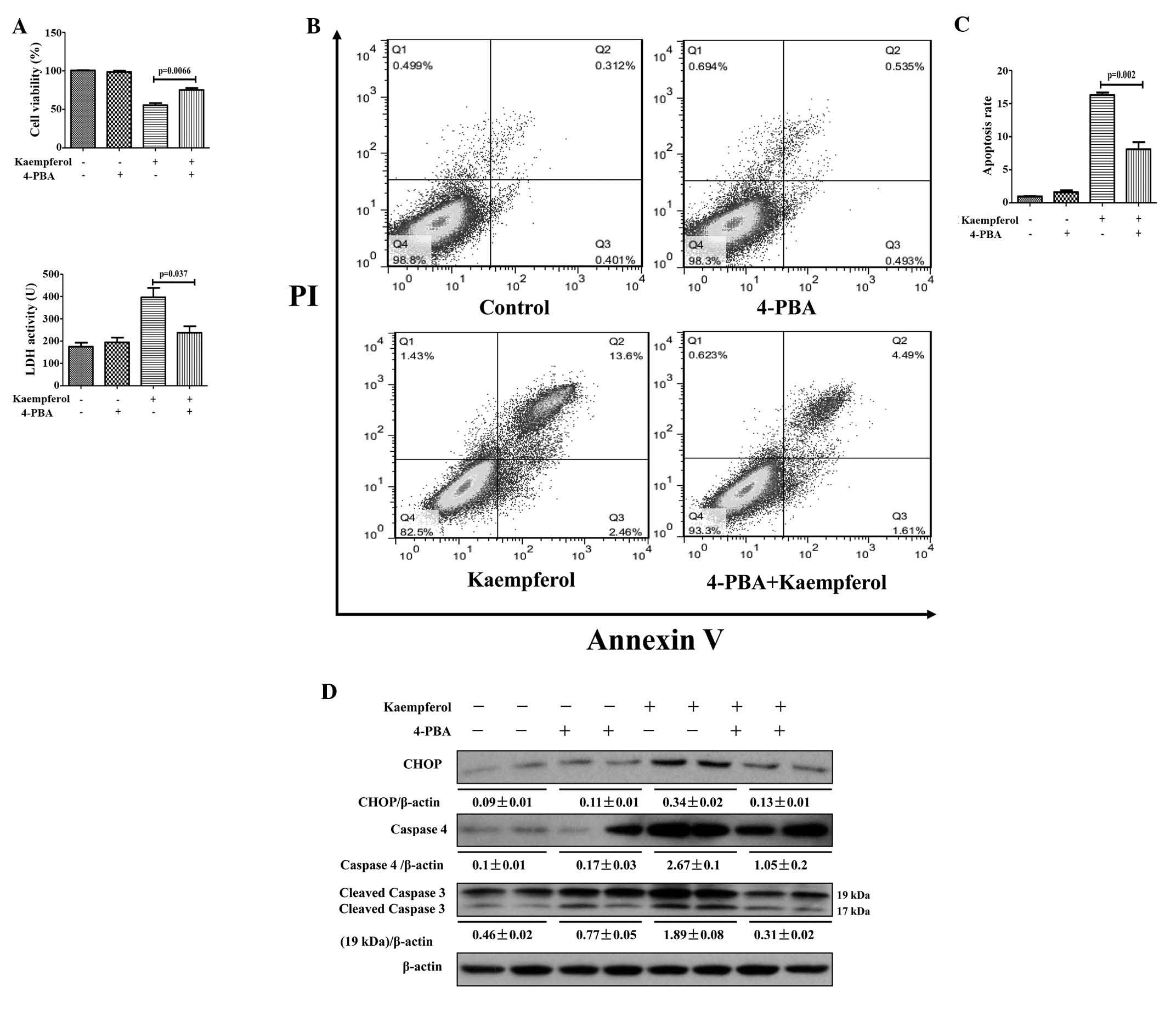
Inhibition of ER stress alleviates
kaempferol-induced HepG2 apoptosis
To confirm the role of the ER stress response as the
underlying molecular mechanism of kaempferol-induced apoptosis in
HepG2 cells, the cells were pretreated with the ER stress inhibitor
4-PBA to suppress ER stress. Compared with the kaempferol only
treatment group, cell viability was significantly higher in the
4-PBA pretreatment group (P=0.0066; Fig. 4A). Additionally, LDH activity and
apoptotic rates were lower in the 4-PBA pretreatment group compared
with the kaempferol group (P=0.037 and P=0.002; Fig. 4A–C). Furthermore, the protein
expression levels of ER stress markers were evaluated. The protein
expression levels of CHOP, caspase-4 and cleaved caspase-3 were
observed to be high in the kaempferol treatment group, whereas they
were reduced subsequent to the inhibition of ER stress by 4-PBA
(Fig. 4D). The results suggest
that kaempferol-induced apoptosis in HepG2 cells is mediated by the
ER stress response.
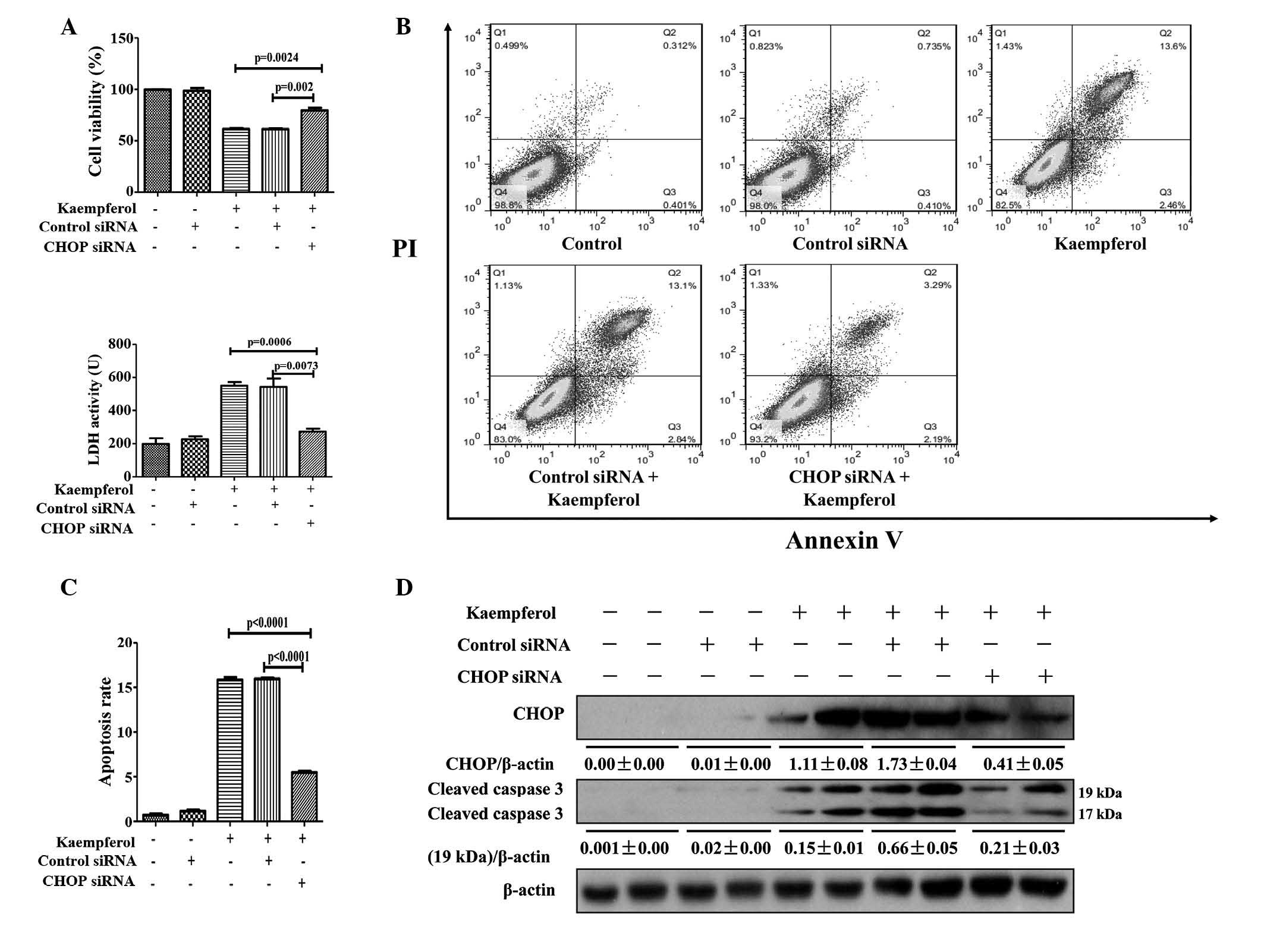
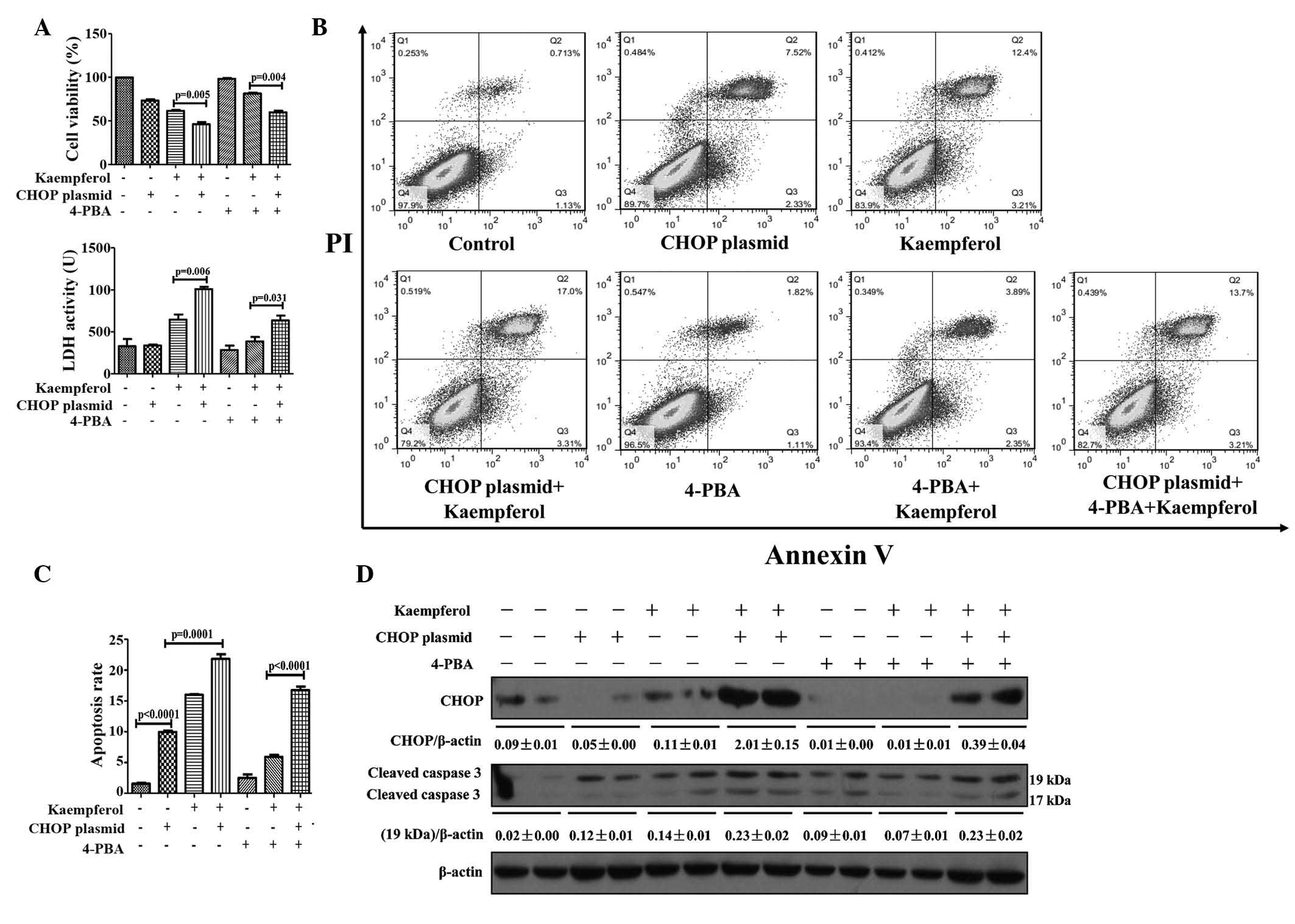
Kaempferol triggers ER stress to induce
HepG2 apoptosis via the CHOP pathway
CHOP is an extensively-characterized factor in the
transition of ER stress to apoptosis (16); therefore, its function in
kaempferol-induced apoptosis in HepG2 cells was investigated by
limiting its expression. Knockdown of CHOP with siRNA significantly
attenuated the reduction of cell viability compared with the
kaempferol only treatment group (P=0.0024; Fig. 5A). LDH activity (P=0.0006),
apoptotic rate (P<0.0001); and protein expression levels of CHOP
and cleaved caspase-3 were markedly reduced in the CHOP siRNA
treatment group compared with the kaempferol group (Fig. 5A–D). Following transfection with
the CHOP-overexpressing plasmid, the protein expression levels of
CHOP were markedly increased, resulting in a reversal of the
protective effect of 4-PBA on kaempferol-induced cell apoptosis
(Fig. 6A–D). These results
indicate that kaempferol promotes apoptosis of HepG2 cells via the
ER stress-CHOP pathway.
Discussion
Kaempferol belongs to the flavonoid family and is
found in various foods and traditional Chinese medicines (1,2). As
a result of its multiple uses, kaempferol has attracted widespread
interest, particularly due to its antitumor properties. The present
study indicates that kaempferol inhibits proliferation of HepG2
cells and promotes their apoptosis. The current study demonstrated,
for the first time to the best of our knowledge, that kaempferol
may induce apoptosis of hepatoma cells via the ER stress-CHOP
signaling pathway.
HCC is characterized by high mortality rates and
resistance to conventional treatments. Kaempferol has been proposed
as a potential agent for HCC treatment due to its antitumor
properties (13,22–24).
Previous studies have demonstrated that kaempferol may inhibit
hypoxia-inducible factor-1 activity and induce apoptosis of Huh7
and H4IIE hepatoma cells (22,23).
In addition, kaempferol induces autophagic cell death in SK-HEP-1
hepatoma cells through adenosine monophosphate-activated protein
kinase and protein kinase B signaling molecules (13). Kaempferol also mediated a reduction
in the proliferation rate of Hep3B hepatoma cells (24). HepG2 cells were selected for the
current study, as genes of interest in these cells may be easily
modified (silencing or overexpression), compared with other
hepatoma cells (25). In the
present study, the antitumor activity of kaempferol reduced the
viability of the tumor cells. Kaempferol damaged HepG2 cells,
resulting in intracellular LDH release into the supernatant, which
was detected as an increase in LDH activity. These results are in
agreement with previous studies (13,22–24),
which demonstrated that kaempferol had a distinct inhibitory effect
on hepatoma cellular growth. Although the suppressive effect of
kaempferol on the growth of hepatoma cells may be evident from
these studies, the molecular mechanisms involved remain to be fully
elucidated. In current study, the effect of inhibition of
proliferation of HepG2 cells was analyzed and ER stress was
identified as a novel mechanism in regulating kaempferol-induced
apoptosis of HCC.
The ER stress pathway is one of the three classical
apoptotic pathways. It is induced in response to numerous
conditions of stress (26,27). The inability to properly fold
proteins or remove misfolded proteins triggers the UPR to protect
cells against ER stress (28,29).
The UPR is considered to be a cell survival mechanism; however, if
ER stress cannot be alleviated, excessive and prolonged ER stress
may activate caspase-4, and then caspase-3, eventually resulting in
apoptosis (30). Previous studies
have indicated that kaempferol induces ER stress in different
cells. Kim et al (31)
demonstrated that kaempferol protects cells from
ischemia/reperfusion-induced cardiac damage through ER stress.
Huang et al (20)
demonstrated that kaempferol induces apoptosis via ER stress and a
mitochondrion-dependent pathway in U2OS human osteosarcoma cells.
In addition, Chandrika et al (32) also determined that kaempferol
induces colon cancer cell apoptosis via the ER stress pathway.
These results indicate that kaempferol-induced ER stress may be
triggered via different molecular pathways in different situations.
In the present study, the molecular mechanisms of
kaempferol-induced apoptosis via the ER stress pathway were
explored. Kaempferol induced apoptosis in HepG2 cells, as confirmed
by the apoptosis rate using flow cytometry and by protein
expression of cleaved caspase-3 using western blotting.
Furthermore, protein and mRNA levels of ER stress markers, GRP78,
GRP94, PERK, IRE1α, partial ATF-6, CHOP and caspase-4, suggested
that kaempferol promotes ER stress and induces ER stress-associated
apoptosis in HepG2 cells. The current results indicate that
promotion of excessive and prolonged ER stress is beneficial for
HCC therapy.
In the current study, ER stress inhibition by 4-PBA
protected HepG2 cells from kaempferol-induced apoptosis, and the
protein expressions of CHOP, caspase-4 and cleaved caspase-3 were
markedly reduced in the 4-PBA pretreatment group. The contribution
of CHOP to kaempferol-induced apoptosis was investigated by
transfecting HepG2 cells with CHOP siRNA. The results of the
current study indicated that CHOP siRNA; however not the negative
control siRNA, attenuated kaempferol-induced apoptosis. However,
apoptosis still occurred following treatment with 4-PBA or CHOP
siRNA, suggesting that kaempferol-induced apoptosis was not
completely ameliorated. This result suggests that an additional
mechanism may be involved in kaempferol-induced apoptosis in HepG2
cells. In addition, to confirm the role of CHOP in
kaempferol-induced ER stress, HepG2 cells were transfected with a
CHOP-overexpressiing plasmid. A marked increase in the apoptosis
rate and protein expression of cleaved caspase-3 in the transfected
cells subsequent to administration of kaempferol was observed. The
overexpression plasmid itself led to a lower apoptosis rate in
transfected HepG2 cells, but not the empty vector control plasmid,
strongly suggesting that high expression of CHOP promotes
kaempferol-induced apoptosis. These data indicate that activation
of the ER stress-CHOP pathway is one of the molecular mechanisms of
kaempferol-induced HepG2 apoptosis.
In conclusion, the present results have expanded on
those of previous studies and confirmed that kaempferol inhibits
hepatoma cell growth. Kaempferol-induced apoptosis in HepG2 cells
was at least partly mediated by ER stress; however, an additional
mechanism involved in kaempferol-induced apoptosis requires further
investigation. The current results also strongly suggest that the
ER stress-CHOP pathway is important in kaempferol-induced
apoptosis. Therefore, kaempferol is a potential therapeutic agent
for HCC treatment.
Acknowledgments
The current study was supported by China National
Key Project of the Twelfth Five-year Plan (grant no.
2012ZX10002005-003-003), the National Natural Science Foundation of
China (grant no. 81270532), the Beijing Excellent Talents Training
Fund (grant no. 2011D003034000022), the Technology Foundation for
Selected Overseas Chinese Scholar, Ministry of Personnel of Beijing
(2012) and Applied Research for the Clinical Characteristics of
Capital (grant no. Z1211070010112167), The Special Funded Projects
for 'Excellent Academic Staff' of Beijing Health Systems (grant
nos. 2013-3-175 and 2011-3-082) and the Cooperation Research
Project of CMU and Clinical (grant no. 13JL33).
References
|
1
|
Xiao J, Chen T and Cao H: Flavonoid
glycosylation and biological benefits. Biotechnol Adv.
S0734-9750(14)00092-5. 2014. View Article : Google Scholar
|
|
2
|
Georgiev V, Ananga A and Tsolova V: Recent
advances and uses of grape flavonoids as nutraceuticals. Nutrients.
6:391–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang YB, Lin MW, Chao Y, Huang CT, Tsai
YH and Wu PC: Anti-oxidant activity and attenuation of bladder
hyperactivity by the flavonoid compound kaempferol. Int J Urol.
21:94–98. 2014. View Article : Google Scholar
|
|
4
|
Gong JH, Shin D, Han SY, Kim JL and Kang
YH: Kaempferol suppresses eosionphil infiltration and airway
inflammation in airway epithelial cells and in mice with allergic
asthma. J Nutr. 142:47–56. 2012. View Article : Google Scholar
|
|
5
|
Singh M, Kaur M and Silakari O: Flavones:
An important scaffold for medicinal chemistry. Eur J Med Chem.
84:206–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CW, Chen PN, Chen MK, Yang WE, Tang
CH, Yang SF and Hsieh YS: Kaempferol reduces matrix
metalloproteinase-2 expression by down-regulating ERK1/2 and the
activator protein-1 signaling pathways in oral cancer cells. PLoS
One. 8:e808832013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li W, Du B, Wang T, Wang S and Zhang J:
Kaempferol induces apoptosis in human HCT116 colon cancer cells via
the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement
of p53 Upregulated Modulator of Apoptosis. Chem Biol Interact.
177:121–127. 2009. View Article : Google Scholar
|
|
8
|
Deepa M, Sureshkumar T, Satheeshkumar PK
and Priya S: Antioxidant rich Morus alba leaf extract induces
apoptosis in human colon and breast cancer cells by the
downregulation of nitric oxide produced by inducible nitric oxide
synthase. Nutr Cancer. 65:305–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radhika M, Ghoshal N and Chatterjee A:
Comparison of effectiveness in antitumor activity between
flavonoids and polyphenols of the methanolic extract of roots of
Potentilla fulgens in breast cancer cells. J Complement Integr Med.
9:242012.
|
|
10
|
Szliszka E, Zydowicz G, Janoszka B, Dobosz
C, Kowalczyk-Ziomek G and Krol W: Ethanolic extract of Brazilian
green propolis sensitizes prostate cancer cells to TRAIL-induced
apoptosis. Int J Oncol. 38:941–953. 2011.PubMed/NCBI
|
|
11
|
Gasmi J and Sanderson JT: Growth
inhibitory, antiandrogenic, and pro-apoptotic effects of punicic
acid in LNCaP human prostate cancer cells. J Agric Food Chem.
58:12149–12156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nandi D, Besra SE, Vedasiromoni JR, Giri
VS, Rana P and Jaisankar P: Anti-leukemic activity of Wattakaka
volubilis leaf extract against human myeloid leukemia cell lines. J
Ethnopharmacol. 144:466–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang WW, Tsai SC, Peng SF, Lin MW, Chiang
JH, Chiu YJ, Fushiya S, Tseng MT and Yang JS: Kaempferol induces
autophagy through AMPK and AKT signaling molecules and causes G2/M
arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human
hepatic cancer cells. Int J Oncol. 42:2069–2077. 2013.PubMed/NCBI
|
|
14
|
Chen S, Xuan J, Couch L, Iyer A, Wu Y, Li
QZ and Guo L: Sertraline induces endoplasmic reticulum stress in
hepatic cells. Toxicology. 322:78–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Xue R, Zhang Z, Yang X and Shi H:
Palmitic and linoleic acids induce ER stress and apoptosis in
hepatoma cells. Lipids Health Dis. 11:12012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marhfour I, Lopez XM, Lefkaditis D, Salmon
I, Allagnat F, Richardson SJ, Morgan NG and Eizirik DL: Expression
of endoplasmic reticulum stress markers in the islets of patients
with type 1 diabetes. Diabetologia. 55:2417–2420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McCloy RA, Shelley EJ, Roberts CG, Boslem
E, Biden TJ, Nicholson RI, Gee JM, Sutherland RL, Musgrove EA,
Burgess A and Butt AJ: Role of endoplasmic reticulum stress
induction by the plant toxin, persin, in overcoming resistance to
the apoptotic effects of tamoxifen in human breast cancer cells. Br
J Cancer. 109:3034–3041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xie Y, Tao X, Cheng Z, Guan Q, Yang W and
Zhu Y: Discrepancy of uterine leiomyoma and myometrium to
hypoxia-induced endoplasmic reticulum stress after uterine
occlusion therapy accounts for therapeutic effect. Arch Gynecol
Obstet. 289:1039–1045. 2014. View Article : Google Scholar
|
|
19
|
Gorman AM, Healy SJ, Jäger R and Samali A:
Stress management at the ER: Regulators of ER stress-induced
apoptosis. Pharmacol Ther. 134:306–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Mylonis I, Lakka A, Tsakalof A and Simos
G: The dietary flavonoid kaempferol effectively inhibits HIF-1
activity and hepatoma cancer cell viability under hypoxic
conditions. Biochem Biophys Res Commun. 398:74–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niering P, Michels G, Wätjen W, Ohler S,
Steffan B, Chovolou Y, Kampkötter A, Proksch P and Kahl R:
Protective and detrimental effects of kaempferol in rat H4IIE
cells: Implication of oxidative stress and apoptosis. Toxicol Appl
Pharmacol. 209:114–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berger A, Venturelli S, Kallnischkies M,
Böcker A, Busch C, Weiland T, Noor S, Leischner C, Weiss TS, Lauer
UM, et al: Kaempferol, a new nutrition-derived pan-inhibitor of
human histone deacetylases. J Nutr Biochem. 24:977–985. 2013.
View Article : Google Scholar
|
|
25
|
Guo L, Dial S, Shi L, Branham W, Liu J,
Fang JL, Green B, Deng H, Kaput J and Ning B: Similarities and
differences in the expression of drug-metabolizing enzymes between
human hepatic cell lines and primary human hepatocytes. Drug Metab
Dispos. 39:528–538. 2011. View Article : Google Scholar :
|
|
26
|
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M,
Vaillancourt E, Smith RO, Görgün CZ and Hotamisligil GS: Chemical
chaperones reduce ER stress and restore glucose homeostasis in a
mouse model of type 2 diabetes. Science. 313:1137–1140. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zode GS, Kuehn MH, Nishimura DY, Searby
CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM
and Sheffield VC: Reduction of ER stress via a chemical chaperone
prevents disease phenotypes in a mouse model of primary open angle
glaucoma. J Clin Invest. 121:3542–3553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim I, Xu W and Reed JC: Cell death and
endoplasmic reticulum stress: Disease relevance and therapeutic
opportunities. Nat Rev Drug Discov. 7:1013–1030. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim DS, Ha KC, Kwon DY, Kim MS, Kim HR,
Chae SW and Chae HJ: Kaempferol protects
ischemia/reperfusion-induced cardiac damage through the regulation
of endoplasmic reticulum stress. Immunopharmacol Immunotoxicol.
30:257–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandrika BB, Maney SK, Lekshmi SU, Joseph
J and Seervi M: Bax deficiency mediated drug resistance can be
reversed by endoplasmic reticulum stress induced death signaling.
Biochem Pharmacol. 79:1589–1599. 2010. View Article : Google Scholar : PubMed/NCBI
|